Abstract
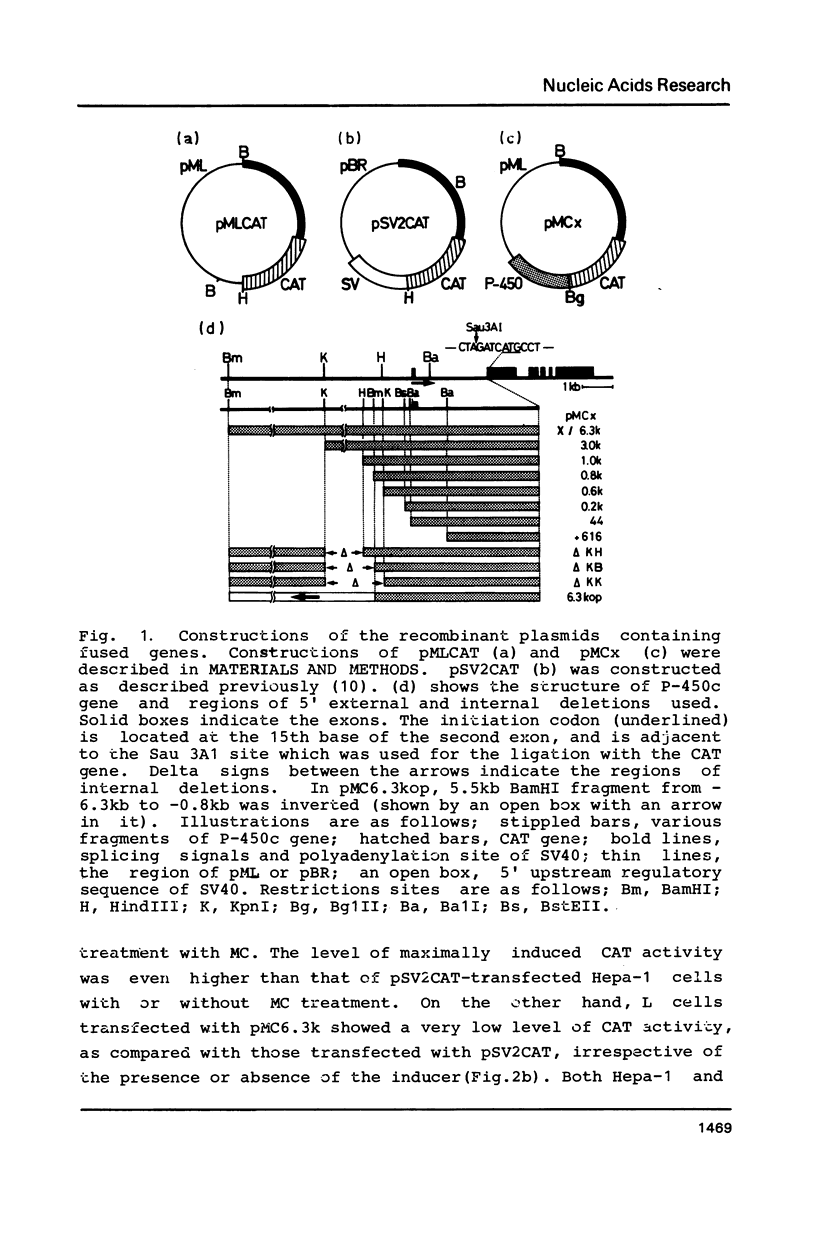
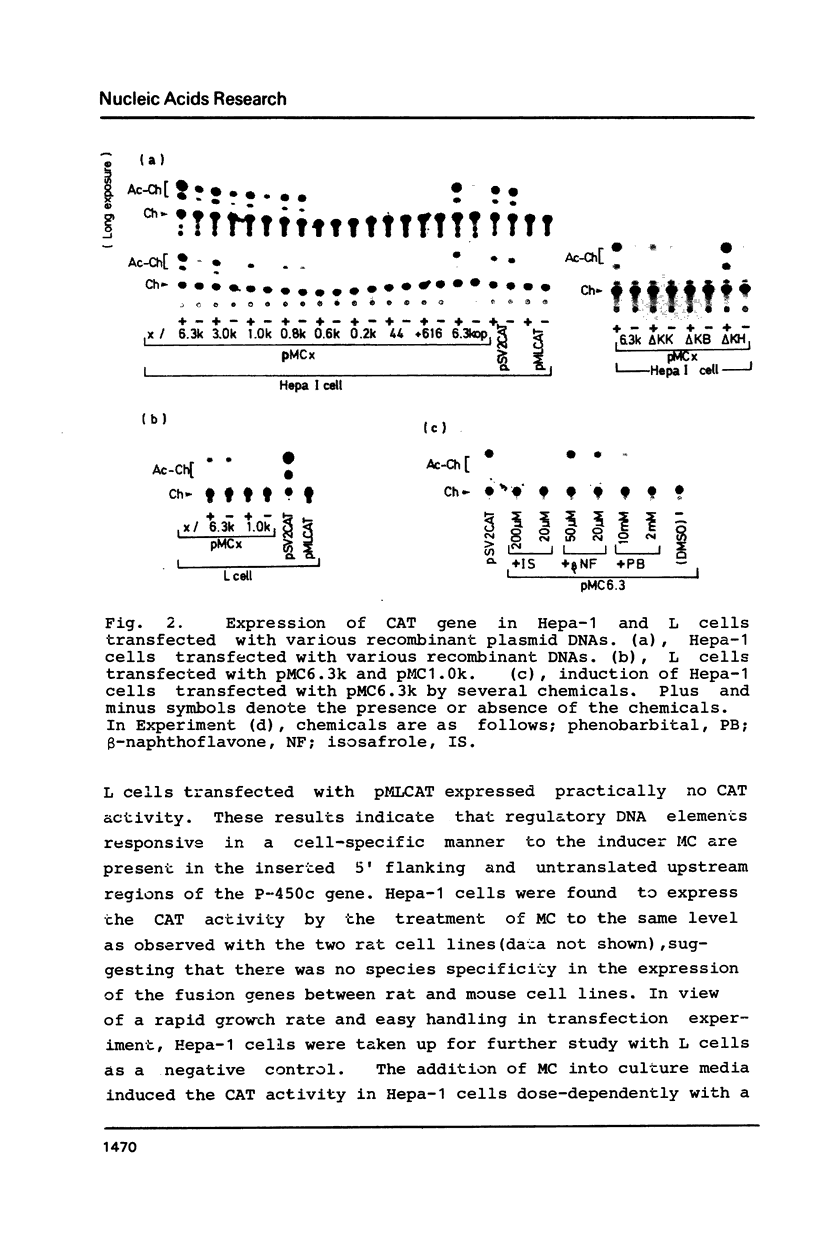
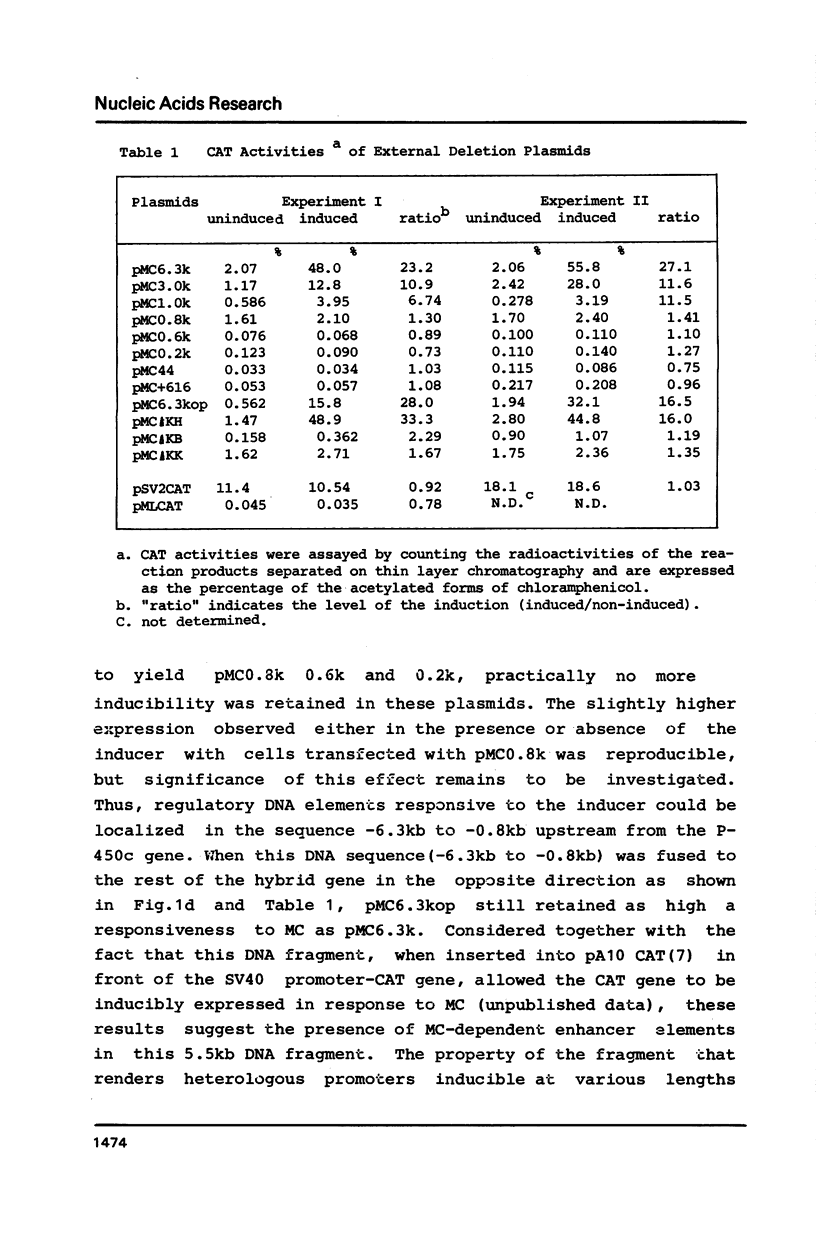
We have investigated regulatory DNA elements in the expression of the drug metabolizing P-450c gene of rats. After combining the 5' flanking and upstream untranslated regions of the isolated P-450c gene with structural gene for chloramphenicol acetyltransferase (CAT), the fusion genes were transfected into cultured cells (Hepa-1 and L cells) for the assay of transient expression of CAT activity. CAT activity was expressed inducibly in response to 3-methylcholanthrene only in Hepa-1 cells. At least three regions containing regulatory DNA elements were indentified; one, which is present in the sequence from -44b to -0.2kb immediately upstream of the TATA box, functions in the basal level of transcription, and the other two which were located in the sequence from -0.8kb to -1.0kb and from -1.0kb to -6.3kb, enhance in combination, transcription in response to inducers in a manner independent of their orientation.
Full text
PDF




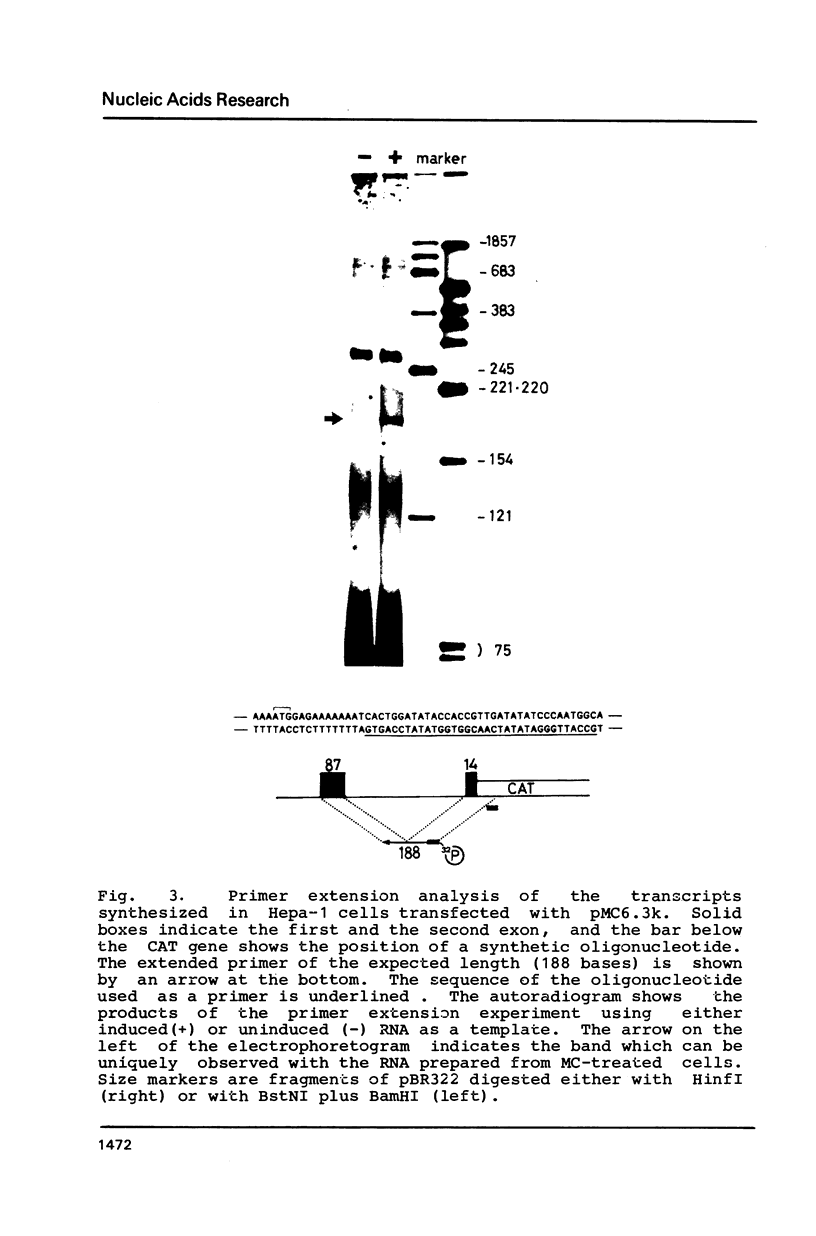




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Kimura S., Nebert D. W. Comparison of the flanking regions and introns of the mouse 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible cytochrome P1-450 and P3-450 genes. J Biol Chem. 1985 Apr 25;260(8):5040–5049. [PubMed] [Google Scholar]
- Gonzalez F. J., Mackenzie P. I., Kimura S., Nebert D. W. Isolation and characterization of full-length mouse cDNA and genomic clones of 3-methylcholanthrene-inducible cytochrome P1-450 and P3-450. Gene. 1984 Sep;29(3):281–292. doi: 10.1016/0378-1119(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P. Isolation and purification of cytochrome P-450, and the existence of multiple forms. Pharmacol Ther. 1979;6(1):99–121. doi: 10.1016/0163-7258(79)90057-3. [DOI] [PubMed] [Google Scholar]
- Hankinson O., Andersen R. D., Birren B. W., Sander F., Negishi M., Nebert D. W. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 1985 Feb 10;260(3):1790–1795. [PubMed] [Google Scholar]
- Hannah R. R., Nebert D. W., Eisen H. J. Regulatory gene product of the Ah complex. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3-methylcholanthrene binding to several moieties in mouse liver cytosol. J Biol Chem. 1981 May 10;256(9):4584–4590. [PubMed] [Google Scholar]
- Hynes N., van Ooyen A. J., Kennedy N., Herrlich P., Ponta H., Groner B. Subfragments of the large terminal repeat cause glucocorticoid-responsive expression of mouse mammary tumor virus and of an adjacent gene. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3637–3641. doi: 10.1073/pnas.80.12.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Cathala G., Slater E., Baxter J. D. Activation of a heterologous promoter in response to dexamethasone and cadmium by metallothionein gene 5'-flanking DNA. Cell. 1984 Feb;36(2):371–379. doi: 10.1016/0092-8674(84)90230-7. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Gotoh O., Tagashira Y., Sogawa K., Fujii-Kuriyama Y. Titration of mRNAs for cytochrome P-450c and P-450d under drug-inductive conditions in rat livers by their specific probes of cloned DNAs. J Biol Chem. 1984 Aug 25;259(16):10145–10149. [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Fujii-Kuriyama Y. Distinct organization of methylcholanthrene- and phenobarbital-inducible cytochrome P-450 genes in the rat. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5066–5070. doi: 10.1073/pnas.81.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Harada T., Fujii-Kuriyama Y. Complete nucleotide sequence of a methylcholanthrene-inducible cytochrome P-450 (P-450d) gene in the rat. J Biol Chem. 1985 Apr 25;260(8):5026–5032. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]