Abstract
Background
The proportion of patients with advanced chronic kidney disease (CKD) initiating dialysis at higher glomerular filtration rate (GFR) has increased over the past decade. Recent data suggest that higher GFR may be associated with increased mortality.
Study Design
A meta-analysis of cohort studies and trials.
Setting & Population
Patients with advanced CKD.
Selection Criteria for Studies
We performed a systematic literature search in MEDLINE, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, American Society of Nephrology abstracts, and bibliographies of retrieved articles to identify studies reporting on GFR at dialysis initiation and mortality.
Predictor
estimated or calculated GFR at dialysis initiation.
Outcome
Pooled adjusted hazard ratio (HR) of continuous GFR for all-cause mortality.
Results
Sixteen cohort studies and one randomized controlled trial were identified (n=1,081,116). By meta-analysis, restricted to the 15 cohorts (n=1,079,917), higher GFR at dialysis initiation was associated with a higher pooled adjusted HR for all-cause mortality (1.04; 95% CI, 1.03–1.05; P<0.001). However, there was significant heterogeneity (I2=97%; P<0.001). The association persisted among the 9 cohorts that adjusted analytically for nutritional covariates (HR 1.03; 95% CI 1.02, 1.04; P<0.001; residual I2=97%). The highest mortality risk was observed in hemodialysis cohorts (HR 1.05; 95% CI 1.02, 1.08; P<0.001) whereas there was no association between GFR and mortality in peritoneal dialysis cohorts (HR 1.04; 95% CI 0.99, 1.08, P=0.11; residual I2=98%). Finally, higher GFR was associated with a lower mortality risk in cohorts that calculated GFR (HR 0.80; 95% CI 0.71, 0.91; P=0.003), contrasting with a higher mortality risk in cohorts that estimated GFR (HR 1.04; 95% CI 1.03, 1.05; P<0.001; residual I2=97%).
Limitations
Paucity of randomized controlled trials; different methods for determining GFR; and substantial heterogeneity.
Conclusions
Higher estimated rather than calculated GFR at dialysis initiation is associated with a higher mortality risk among patients with advanced CKD, independent of nutritional status. Although there was substantial heterogeneity of effect size estimates across studies, this observation requires further study.
Keywords: ESRD, CKD, hemodialysis, peritoneal dialysis, early initiation, late initiation, GFR, mortality
The number of patients with stage-5 chronic kidney disease (CKD) is increasing worldwide1–4. In 2008, close to 110,000 patients initiated dialysis in the US4, and in 2020, 800,000 prevalent patients are projected to receive dialysis3. The care of patients with stage-5 CKD is associated with significant resource consumption and healthcare expenditure, close to 5.9% of the total Medicare budget in the US alone4. From 1996 to 2008, the proportion of patients initiating dialysis in the US at an estimated glomerular filtration rate (eGFR) of greater than 10 ml/min/1.73 m2 has more than doubled from 20% to 52%3. A similar trend has also been observed in Europe and Canada5,6. Likely reasons for this trend are the widespread adoption of clinical practice guidelines on management of advanced CKD, and the belief that malnutrition might develop in patients who start dialysis late7, thus affecting survival8.
Currently published clinical practice guidelines vary with respect to eGFR cutoffs below which dialysis therapy should be initiated but they unanimously recommend assessing for symptoms or signs of uremia8–13. However, typical uremic symptoms that constitute clear indications to initiate dialysis including, pericarditis, and encephalopathy, generally occur at very low of GFR14,15. In one study, symptoms such as nausea, vomiting, and progressive deterioration in nutritional status accounted for less than one-third of dialysis indications in the elderly16.
Patients with advanced CKD who transition to dialysis often experience significant physical and emotional stress. Health-related quality of life benefits observed following initiation of dialysis are debatable, and early initiation of dialysis is associated with higher costs17. Recent data also suggest that initiation of dialysis at higher GFR might be associated with worse clinical outcomes18–21. These controversial findings have prompted several commentaries22–27. Although a systematic review of 10 studies recently examined this question, the analysis was qualitative in nature and the results somewhat inconclusive28. To gain more information on this subject, we performed a quantitative meta-analysis of all available studies of patients with advanced CKD that examined the association of kidney function at the start of dialysis, as assessed by level of GFR, with all-cause mortality. We also explored potential sources of heterogeneity among studies.
METHODS
Data Sources and Searches
We searched MEDLINE (inception-March 2011), the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov to identify eligible studies using the medical subject headings (MeSH) search terms and keywords provided in Table S1 (available as online supplementary material). The search strategy was limited to human studies with no language restrictions. We also reviewed the American Society of Nephrology abstracts (2003–2010 meetings) and the bibliographies of retrieved articles.
Study Selection
In light of the paucity of randomized controlled trials, we focused primarily on cohort studies that examined the association of GFR at dialysis initiation with mortality. We included studies that compared early vs. late initiation of dialysis as defined in the individual reports. No restrictions were placed on sample size or study duration. Two authors (SA and MA) screened the electronic citations and then re-screened the full-text of potentially relevant articles.
Data Extraction and Quality Assessment
Data were extracted from full-text articles independently by 3 authors (SA, MA and PS). Disagreements were resolved through consensus and arbitration by a fourth author (BLJ). We extracted data on study characteristics including the country of origin, year of publication, study design, data source, accrual period, number of patients, initial dialysis modality (hemodialysis [HD], peritoneal dialysis [PD], or both), maximum duration of follow-up, and cohort characteristics including, mean age, percentage of men and diabetics, mean body mass index, and mean serum albumin. We also extracted data on the mean estimated GFR (eGFR) (estimated by the Modification of Diet in Renal Disease [MDRD] study equation), calculated GFR (cGFR) (defined as the average of creatinine and urea clearance from a 24 hour urine collection), or creatinine clearance (estimated by the Cockcroft-Gault equation). Outcomes of interest were the total number of deaths, all-cause mortality rates including those within GFR categories (if applicable), and the effect estimates of Cox-regression analyses examining the association of eGFR or cGFR (per 1-ml/min/1.73 m2 increment) with mortality, which consisted of adjusted odds ratios and hazard ratios (with 95% confidence intervals). We grouped the adjustment variables used in these regression analyses into demographic and socioeconomic factors, causes of kidney failure, co-morbid conditions, nutritional factors, anemia parameters, treatment variables, and miscellaneous. Corresponding authors of studies were contacted for data clarification and to provide additional analyses.
The quality of the cohort studies was assessed using an adaptation of the Newcastle-Ottawa Scale29. This scale assesses the quality of observational studies, and allocates a maximum of 9 points for quality of selection (up to 4 points), comparability (up to 2 points), and outcome (up to 3 points) of study participants. Overall study quality was arbitrarily defined as poor (score 0–3), fair (score 4–6), or good (score 7–9). The Jadad score was used to assess the quality of randomized controlled trials.
Data Synthesis and Analysis
Most cohort studies performed multivariable Cox proportional-hazards regression analyses and reported the adjusted hazard ratio of eGFR or cGFR for all-cause mortality. Consequently, to minimize confounding, we performed a random-effects model meta-analysis of the pooled adjusted hazard ratio of estimated or calculated GFR (per 1-mL/min/1.73 m2 increment) reported in the cohort studies for all-cause mortality among patients initiating dialysis.
The heterogeneity of effect size estimates across studies was described with the I2 index and Q statistic P value. We investigated heterogeneity by performing univariate random-effects model meta-regressions of the adjusted hazard ratios against study characteristics including, methods of GFR assessment, initial dialysis modality, inclusion of nutritional covariates in the multivariable models, duration of follow-up (< vs. ≥ median), study sample size (< vs. ≥ 10,000), and quality scores. All the analyses were performed using Comprehensive Meta-Analysis version 2.0, and the metan and metareg commands of Stata 11 (College Station, TX). Publication bias was assessed using funnel plots and the Egger test30.
RESULTS
Study Characteristics and Quality
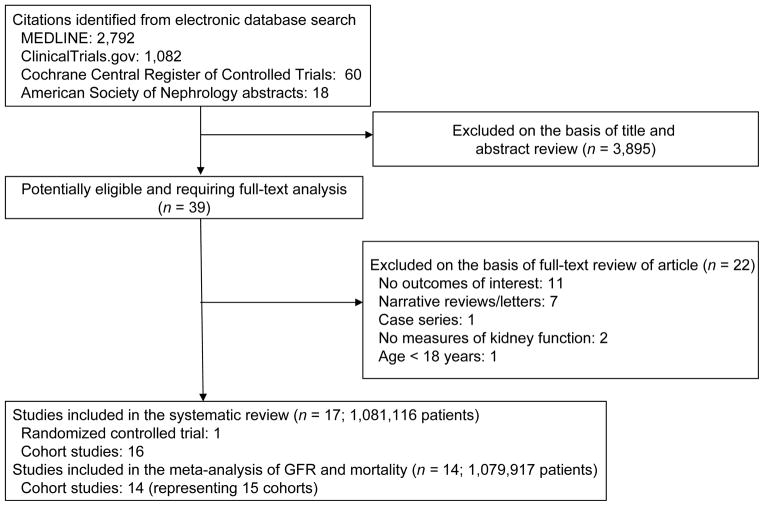
A total of 2,792 potentially relevant citations were identified and screened; 39 articles were retrieved for detailed evaluation, of which 17 fulfilled eligibility criteria (Figure 1)18–21,31–43. Characteristics of the studies are displayed in Table 1. There were 16 cohort studies and one randomized controlled trial. In the single randomized controlled trial, Initiating Dialysis Early and Late (IDEAL)19, 828 adults with progressive CKD were randomly assigned to initiate dialysis when the eGFR reached 10–14 mL/min/1.73 m2 or 5–7 mL/min/1.73 m2. 56% of study participants were initiated on HD and 44% on PD. The cohort studies spanned approximately 10 years, varied in sample size (100–896,546 patients) and maximum duration of follow up (1–11 years), and involved patients initiating HD, PD, or a mixture of the two modalities. Most studies had more men (range, 45–67%) with a mean age of 58 (range 46–67) years. The percentage of diabetics varied from 8.4–100%. Among studies that reported the baseline values, the meta-analyzed mean body mass index was 25.9 (95% CI, 25.6–26.2) kg/m2, and mean serum albumin 3.32 (95% CI, 3.30–3.35) gm/dL. Fourteen studies estimated the GFR using a variant (4-, 5- or 6-variable) of the MDRD Study equation18–21,34–43, and 1 study estimated GFR using the Cockcroft-Gault equation33. Two studies calculated GFR using the average of creatinine and urea clearance derived from a 24-hour urine collection (Table S2)31,32.
Figure 1.
Study selection flow diagram.
Table 1.
Characteristics of the studies included in the meta-analysis
| Author | Country | Year | Study Design |
Data Source | Accrual Period |
No. of Patients |
Initial Dialysis Modality |
Max F/U Length |
Mean Age (y) |
Mean eGFR |
Men (%) |
DM (%) |
Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Korevaar | NL | 2001 | PCS | NECOSAD | 1997–1999 | 253 | HD, PD | 3 y | 57 | 6.3 | 62 | NR | 6 |
| Traynor | GBa | 2002 | RCS | Glasgow Royal Infirmary’s Electronic Records | 1987–2000 | 235 | HD, PD | 10 y | 55* | 8.3 | 67 | 22 | 6 |
| Beddhu | US | 2003 | RCS | DMMS II | 1996–1997 | 2920 | HD, PD | 2 y | 59 | 8.2 | 53 | 42 | 5 |
| Kazmi | US | 2005 | RCS | CMS Program | 1996–1999 | 302,287 | HD, PD | 5 y | 62 | 8.4 | 53 | 48 | 5 |
| Wilson | CA | 2007 | RCS | Single Center | 2001–2005 | 271 | HD | 2 y | 66 | NR | 61 | 51 | 5 |
| Tang | HK | 2007 | PCS | Single Center | 2002–2004 | 233 | PD | 2 y | 58 | 9.1 | 51 | 42 | 6 |
| Shiao | TW | 2008 | RCS | Single Center | 1997–2005 | 275 | PD | 6 y | 51 | 4.8 | 45 | 19 | 3 |
| Sawhney | GBa + CA | 2009 | RCS | Scottish Renal Registry + BC Provincial Renal Agency | 2000–2005 | 7,299 | HD, PD | 5 y | 51 | 8.3 | 58 | NR | 5 |
| Coronel | ES | 2009 | RCS | Single Center | 1982–2004 | 100 | PD | 5 y | 53 | 8.3 | 65 | 100 | 3 |
| Stel | NL | 2009 | RCS | ERA-EDTA Registry | 1999, 2003 | 11,472 | HD, PD | 2 y | 64 | 7.9; 8.6 | 61 | 8.4† | 5 |
| Lassalle | FR | 2010 | RCS | French REIN Registry | 2002–2009 | 11,685 | HD, PD | 4 y | 67 | 8.8 | 62 | 36 | 5 |
| Hwang | TW | 2010 | RCS | The Bureau of National Health Insurance (TW) | 2001–2004 | 23,551 | HD | 1 y | 62 | 4.7 | 48 | 50 | 4 |
| Cooper | AU | 2010 | RCT | Multi-Center (AU & NZ) | 2000–2008 | 828 | HD, PD | 7 y | 60 | 8.1 | 66 | 43 | 3** |
| Wright | US | 2010 | RCS | US Renal Data System | 1995, 2000–2006 | 611,913 | HD, PD | 5 y | 65 | 9.8 | 54 | 57 | 5 |
| Rosansky | US | 2011 | RCS | CMS Program | 1996–2006 | 81,176 | HD | 11 y | 46 | 7.1 | 58 | 0 | 7 |
| Clark | CA | 2011 | RCS | Canadian Organ Replacement Registry | 2001–2007 | 25,910 | HD | 7 y | 65 | 9.8 | 60 | 46 | 5 |
| Evans | SE | 2011 | PCS | Multi-Center | 1996–1998 | 708 | HD, PD | 7 y | 57 | 7.6 | 66 | NR | 7 |
Median age
Jadad score
reported in the 2003 cohort
PCS, prospective cohort study; RCS, retrospective cohort study; RCT, randomized controlled trial; DMMS II, Dialysis Morbidity Mortality Study Wave II; BC, British Columbia (Canada); HD, hemodialysis; PD, peritoneal dialysis; ERA-EDTA, European Renal Association–European Dialysis and Transplant Association; NL, The Netherlands; GB, Great Britain; US, United States; CA, Canada; HK, Hong Kong; TW, Taiwan; ES, Spain; FR, France; AU, Australia; SE, Sweden; NECOSAD, Netherland Cooperative Study on the Adequacy of Dialysis; NZ, New Zealand; CMS, Centers for Medicare and Medicaid Services; DM, diabetes mellitus; max, maximum; f/u, follow up; eGFR, estimated glomerular filtration rate
Scotland
Corresponding authors of 16 studies were contacted18–21,32–43, and 7 provided additional information18,20,21,33,35,40,41, including adjusted hazard ratios according to the initial dialysis modality40. One study included 2 cohorts with different accrual periods that were analyzed separately40. There was an overlap of patient populations between 2 cohort studies21,35; thus, in the larger and more recent report, those patients were excluded (overlap period of 1996–1999) to avoid duplication of the cohort21. Among cohort studies reporting adjusted hazard ratios for mortality, 15 modeled continuous GFR (per 1 mL/min/1.73 m2 increment)18,20,21,31–35,37,38,40–43, and 11 modeled pre-defined GFR categories (Table 2).
Table 2.
Adjusted hazard ratios of estimated GFR for all-cause mortality in the studies included in the meta-analysis.
| Author | eGFR* category | Mean eGFR | All-cause mortality** | Adjustment variables | ||||
|---|---|---|---|---|---|---|---|---|
| Demographic & socioeconomic factors | Cause of kidney failure | Comorbid conditions | Nutritional factors | Anemia parameters, treatment factors, & misc | ||||
| Korevaar | Timely starters a | 7.1 | 0.60 (0.35–1.05) | Age, sex | DM, GN, RVD, other | Wright/Khan co-morbidity index score | - | - |
| Late startersa | 4.9 | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 0.82 (0.72–0.94) | ||||||
| Traynor | ≥ 8.3 | 10.4b | 0.95c | Age, sex | - | Wright/Khan co-morbidity index score | Albumin, weight | Hb, WBC, vascular access, dialysis modality, MAP |
| < 8.3 | 6.7b | 1.00 (reference) | ||||||
| ≥ 8 (non-DM) | 9.8b | 0.62c | ||||||
| < 8 (non-DM) | 6.3b | 1.00 (reference) | ||||||
| Per 1-mL/min ↑ | - | 1.10 (1.00–1.20) | ||||||
| Beddhu | > 7.5 | 10.9 | NR | Age, sex, race, insurance status, smoking | - | CAD, CHF, LVH, CBVD, PVD, DM, malignancy, chronic lung disease, AIDS, functional impairment | Albumin, bicarbonate, BMI, clinical diagnosis of malnutrition | Hct, dialysis modality |
| ≤ 7.5 | 5.6 | NR | ||||||
| Per 1-unit ↑ | 1.03 (1.02–1.04) | |||||||
| Kazmi | > 10.0 | 13.8 | 1.42 (1.38–1.46) | Age, sex, race/ethnicity, employment, insurance status | DM, GN, HTN, other | CAD, CHF, HTN, CBVD, PVD, DM, COPD | Albumin, BMI | Hct, dialysis initiation y, network |
| 7.6–10.0 | 8.6 | 1.19 (1.15–1.21) | ||||||
| 5.0–7.5 | 6.3 | 1.09 (1.06–1.12) | ||||||
| < 5.0 | 3.9 | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.03 (1.03–1.04) | ||||||
| Wilson | > 10 | NR | 1.68 (0.65–4.32)c | Age, sex, race, employment status | - | CAD, PVD, antihypertensive use, DM | - | Predialysis nephrology care |
| 5–10 | NR | 1.58 (0.54–4.65)c | ||||||
| < 5 | NR | 1.00 (reference) | ||||||
| Tang | Elective starters (≤10) | 9.2 | 0.33 (0.11–0.76) | Age, sex | - | DM | - | First eGFR |
| Initial refusers | 8.9 | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 0.66 (0.45–0.97) | ||||||
| Shiao | ≥ 5 | 6.8 | NR | Age, sex | - | CAD, DM | Albumin, nPCR | Hb, WBC, total & renal CCr/wk, total & renal Kt/V, Catheter late implantation |
| < 5 | 3.5 | NR | ||||||
| Per 1-unit ↑ | - | 1.18 (1.02–1.37) | ||||||
| Sawhney | ≥ 15.0 | 18.6 | 1.65 (1.39–1.95) | Age, sex, standardized mortality ratio, registry | DM, GN, RVD, PKD, drug- induced, congenital, other, unknown | - | - | Hb, dialysis initiation y, dialysis modality |
| 10.0–14.9 | 11.6 | 1.37 (1.19–1.59) | ||||||
| 5.0–9.9 | 7.2 | 1.17 (1.02–1.34) | ||||||
| < 5.0 | 4.2 | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.02 (1.02–1.03) | ||||||
| Coronel | > 7.7 | 10.6 | 0.96d | Age | - | CAD, CHF, HTN, valvulopathy, CBVD, PVD | Albumin | - |
| ≤ 7.7 | 5.4 | 1.00 (reference) | ||||||
| Stel*** | ≥ 10.5 | 14.3e | 1.45 (1.32–1.62) | Age, sex | DM, GN, RVD, HTN, other | - | - | Dialysis modality, country |
| 8.0–10.5 | 9.1e | 1.14 (1.04–1.25) | ||||||
| < 8.0 | 5.9e | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.04 (1.03–1.05) | ||||||
| ≥ 10.5 | 14.3e | 1.38 (1.19–1.61) | Age, sex | DM, GN, RVD, HTN, other | CAD, CBVD, PVD, DM, Malignancy | - | Dialysis modality, country | |
| 8–10.5 | 9.1e | 1.17 (1.01–1.36) | ||||||
| < 8.0 | 5.9e | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.02 (1.01–1.04) | ||||||
| Per 1-unit ↑ | - | 1.03 (1.03–1.04) | - | - | - | - | - | |
| Lassalle | > 20 | NR | NR | Age, sex | - | CAD, CHF, PVD, DM, dysrhythmia, malignancy, disability | Albumin, BMI | Hb, ESA use, planned/unplanned dialysis initiation, wait listing, transplant status |
| 16–20 | NR | NR | ||||||
| 11–15 | NR | NR | ||||||
| 6–10 | NR | NR | ||||||
| ≤ 5 | NR | NR | ||||||
| Per 1-unit ↑ | - | 1.02 (1.01–1.03) | ||||||
| Hwang | ≥ 6.52 | 7.7 | 2.44 (2.11–2.81) | Age, sex | DM, GN, HTN, chronic TIN, other, unknown | CAD, CHF, HTN, CVD, DM, malignancy, liver cirrhosis, TB, others | - | Hct, dialysis initiation year |
| 5.21–6.51 | 5.8 | 1.66 (1.43–1.93) | ||||||
| 4.28–5.20 | 4.7 | 1.21(1.04–1.41) | ||||||
| 3.29–4.27 | 3.8 | 1.18 (1.01–1.37) | ||||||
| < 3.29 | 2.6 | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.15 (1.14–1.17) | ||||||
| Cooper | 10–14 | 9 | 1.04 (0.83–1.30) | NA | ||||
| 5–7 | 7.2 | 1.00 (reference) | ||||||
| Wright | > 15.0 | 19.0f | 1.48 (1.47–1.50) | Age, sex, race, height | DM, GN, HTN, PKD, urological causes, other, unknown | CMI score, DM | Weight | Predialysis care duration, dialysis type, vascular access |
| 10.0–15.0 | 12.1 f | 1.16 (1.15–1.17) | ||||||
| 5.0–10 | 7.5 f | 1.00 (reference) | ||||||
| ≤ 5.0 | 3.9 f | 0.87 (0.86,0.88) | ||||||
| Per 1-unit ↑ | - | 1.04 (1.03–1.03) | ||||||
| Rosansky | ≥ 15.0 | 18.9 f | 1.74 (1.64–1.85) | Age, sex, race, ethnicity | GN, HTN, PKD, urological causes, other, unknown | - | Albumin, BMI | Hb, dialysis initiation year |
| 10–14.9 | 11.8 f | 1.47 (1.41–1.54) | ||||||
| 5–9.9 | 7.1 f | 1.23 (1.19–1.27) | ||||||
| < 5.0 | 3.7 f | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.04 (1.03–1.04) | ||||||
| Clark | >10.5 | 1.18 (1.13–1.23) | Age, sex, ethnicity | DM, GN, RVD, other, unknown | Modified CMI score, CAD, CHF, HTN, CVD, PVD, DM, lung disease, malignancy | Albumin | Vascular access, transplants status, late referral | |
| ≤ 10.5 | 7.1 | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.01 (1.01–1.02) | ||||||
| Evans | ≥ 7.5–20 | 10.8 | 1.19 (0.91–1.56) | Age, sex, smoking, alcohol use, education | DM, GN, HTN, other, unknown | CMI score | BMI | Clinical status, first eGFR, decline in eGFR, |
| < 7.5 | 5.5 | 1.00 (reference) | ||||||
| Per 1-unit ↑ | - | 1.03 (1.00–1.06) | ||||||
Timely starters defined as eGFR >10.5 mL/min/1.73 m2 (equivalent to standardized Kt/Vurea = 2.0) or eGFR ≤ 10.5 mL/min/1.73 m2 and in equivalent of total nitrogen appearance normalized to body weight (nPNA) > 0.8g/kg/d, and BMI >20 kg/m2;
Median eGFR at the start of dialysis;
Adjusted odds ratio;
Unadjusted odd ratio;
Mean eGFR of each category in the combined cohort;
Data received directly from corresponding author.
units are mL/min/1.73 m2 unless otherwise indicated
Adjusted hazard ratio (95% confidence interval)
analysis adjusted for general characteristics is based on 1999 data; the unadjusted analysis and the analysis adjusted for general characteristics and comorbidity are both based on 2003 data.
AIDS, acquired immune deficiency syndrome; eGFR, estimated glomerular filtration rate; ESA, erythropoietin-stimulating agent; BMI, body mass index; WBC, white blood cell; CCr/wk, weekly creatinine clearance; nPCR, normalized protein catabolic rate; CAD, coronary artery disease; CHF, congestive heart failure; LVH, left ventricular hypertrophy; HTN, hypertension; CBVD, cerebrovascular disease; PVD, peripheral vascular disease; DM, diabetes mellitus; GN, glomerulonephritis; RVD, renal vascular disease; PKD: polycystic kidney disease; TIN, tubulointerstitial nephritis; TB, tuberculosis; Hct, hematocrit; Hb, hemoglobin, MAP, mean arterial pressure; CMI, Charlson comorbidity index; misc, miscellaneous; NR, not reported; y, year.
We only meta-analyzed the Cox regression models that examined continuous GFR, and therefore excluded from the analysis 2 cohort studies36,39 and the IDEAL trial19. All 15 analyzable cohorts used multivariable adjustments, but only in 9 cohorts, analyses were adjusted for selected nutritional covariates including, weight, body mass index, serum albumin, and/or serum bicarbonate (Table 2). According to the Newcastle Ottawa Scale, most cohort studies were considered of fair (scale of 4–6) to good (scale of 7–9) quality (Table 1).
Association of GFR at Dialysis Initiation with All-Cause Mortality
The IDEAL trial found that over a median follow-up duration of 3.6 years, the early-start group had a hazard ratio of 1.04 (95% CI, 0.83–1.30) for all-cause mortality compared with the late-start group.
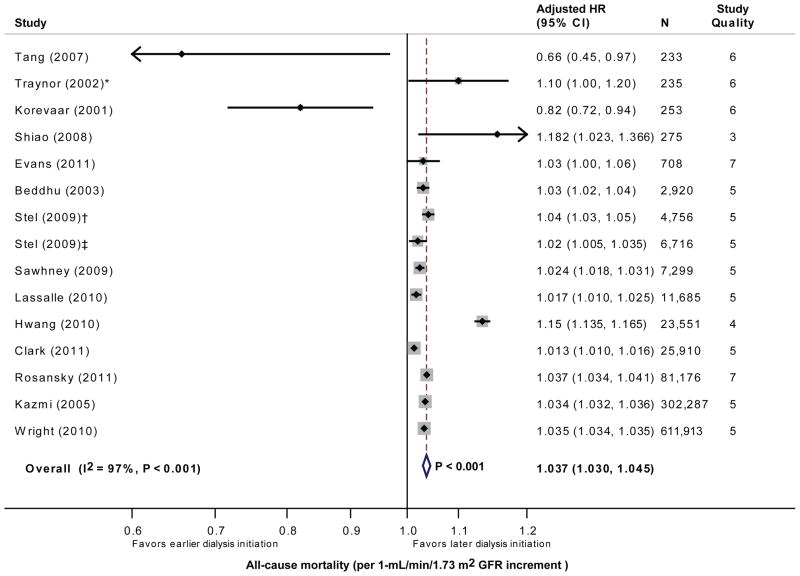
The 15 cohorts reporting the adjusted hazard ratio of continuous GFR included a total of 1,079,917 analyzable patients. By meta-analysis, higher GFR (per 1 mL/min/1.73 m2 increment) at dialysis initiation was associated with a significantly higher adjusted hazard ratio for all-cause mortality (HR, 1.04; 95% CI, 1.03–1.05; P<0.001; Figure 2). The test for heterogeneity was highly significant (I2=97%; P<0.001).
Figure 2.
Forest plot of the glomerular filtration rate (GFR) (per 1 mL/min/1.73 m2 increment) adjusted hazard ratio (with 95% confidence interval) for all-cause mortality. * The GFR is reported in mL/min and is not normalized to body surface area. The study by Stel et al included 2 cohorts with 2 accrual periods († cohort initiating dialysis in 1999; ‡ cohort initiating dialysis in 2003). The test for heterogeneity is significant (I2=97% and P<0.001 by Q test).
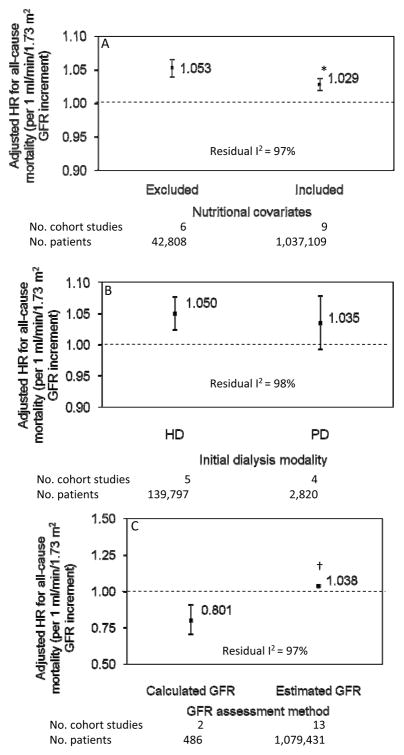
Subgroup analyses were explored. Among the 9 cohorts that included nutritional indicators in their multivariable models, GFR remained independently associated with a 3% risk increase in mortality (adjusted HR, 1.03; 95% CI, 1.02–1.04; P<0.001), but had a significantly smaller effect compared with the 6 cohorts that did not include nutritional covariates (P=0.008; residual I2=97%; Figure 3A). Among the 5 cohorts initiating HD (139,797 analyzable patients), the association between higher GFR and increased mortality was significant (adjusted HR, 1.05; 95% CI, 1.02–1.08; P<0.001), contrasting with the 4 cohorts restricted to PD as the initial dialysis modality (2,820 analyzable patients) where higher GFR was not associated with mortality (adjusted HR, 1.04; 95% CI, 0.99–1.08; P=0.1). However, there was no significant difference in the effect between these 2 subgroups (P=0.6; residual I2=98%; Figure 3B). Higher GFR was also associated with a lower mortality risk in the 2 cohorts (486 analyzable patients) that calculated GFR (adjusted HR=0.80; 95% CI 0.71, 0.91; P=0.003), contrasting with a higher mortality risk in the 13 cohorts (1,079,431 analyzable patients) that estimated GFR (adjusted HR=1.04; 95% CI 1.03, 1.05; P<0.001). The effect between these 2 subgroups was significant (P<0.001; residual I2=97%; Figure 3C). Cohorts that had a maximum follow-up period ≥5 years (adjusted HR=1.03; 95% CI 1.02, 1.04; P<0.001) had significantly smaller effect (P=0.04; residual I2=97%) compared to those with a follow-up period <5 years (adjusted HR=1.05; 95% CI 1.04, 1.06). Cohorts that included ≥10,000 patients (adjusted HR=1.04; 95% CI 1.03, 1.05; P<0.001) had near-significantly larger effect (P=0.07; residual I2=97%) compared to those with <10,000 patients (adjusted HR=1.03; 95% CI 1.02, 1.04).
Figure 3.
Subgroup analyses displaying the glomerular filtration rate (GFR) (per 1 mL/min/1.73 m2 increment) adjusted hazard ratio (with 95% confidence interval) for all-cause mortality stratified by (3A) use of nutritional covariates in the multivariable models; (3B) initial dialysis modality; and (3C) GFR assessment method. * P=0.008 vs. cohorts that excluded nutritional covariates; † P<0.001 vs. cohorts that calculated GFR. The test for heterogeneity is significant (residual I2=97%, 98% and 97%, respectively).
In a sensitivity analysis that excluded the 2 largest studies that contributed collectively 914,200 patients 21,35, higher GFR remained associated with mortality (adjusted HR=1.04; 95% CI 1.02, 1.05; P<0.001; residual I2=97%). In another sensitivity analysis where we excluded the report by Rosansky et al that might have had potential population overlap with the study by Wright et al, higher GFR remained associated with higher mortality (adjusted HR=1.04; 95% CI 1.03, 1.05; P<0.001; residual I2=97%).
Study quality did no significantly affect the pooled estimates (data not shown). Finally, funnel plots were symmetric suggesting less susceptibility to publication bias (Figure S1) and the Egger test was not significant (P=0.7).
DISCUSSION
In the present meta-analysis of cohort studies, we demonstrate that among patients with advanced CKD, higher GFR at the initiation of dialysis is associated with a higher mortality risk. Across studies, a 1-mL/min/1.73 m2 GFR increment was associated with a 4% higher adjusted hazard for all-cause mortality. This association persisted across a broad range of sensitivity and subgroup analyses. Indeed, restricting to studies that used nutritional covariates in their multivariable models demonstrated an attenuated but persistent association of GFR with a 3% risk increase in mortality. Furthermore, the mortality risk was the highest at 5% in studies restricted to patients initiating HD. By contrast, in studies restricted to patients initiating PD, higher GFR appeared to not be associated with mortality. However, the lack of significant difference in the effect between the 2 subgroups and the relatively small sample size of the pooled PD cohorts precludes definitive conclusions. Finally, the mortality risk was 20% lower in the few studies that derived the GFR from a 24-hour urine collection contrasting with a higher mortality risk among the many studies that estimated GFR.
The proportion of patients initiating dialysis at higher eGFR has been increasing over the past decade. This may be the result of a widespread adoption of clinical practice guidelines that provide eGFR cutoff values below which dialysis therapy should be considered8–12, coupled to the belief that early dialysis initiation might prevent the progressive decline in nutritional status7, and might possibly allow for better vascular access planning and avoidance of dialysis catheters44–46. Unfortunately, there is no definitive evidence to support this approach. In a post-hoc analysis of the IDEAL study, which is the only randomized controlled trial comparing early (eGFR of 10–14 mL/min/1.73 m2) with late (eGFR of 5–7 mL/min/1.73 m2) initiation of dialysis, the authors failed to demonstrate a survival benefit between the 2 groups19. However, 76% of the patients in the late-start group needed to initiate dialysis due to uremic symptoms when their eGFR was far above the 5 to 7 mL/min/1.73 m2. In fact, the mean eGFR at the start of dialysis in the early- and late-start group was 9.0 and 7.2 mL/min/1.73 m2, respectively. We can only speculate as to whether the absolute difference in eGFR between the two groups was too small to detect a survival difference. Furthermore, the wide 95% confidence interval of the HR with early dialysis initiation (1.04; 95% CI, 0.83, 1.30) observed in this trial is consistent with our observational data. Overall, the trial results suggest that the decision to initiate dialysis is not only determined by eGFR, but also by the clinical condition of the patient. In a subsequent pre-specified analysis of the IDEAL trial, early start was not cost-effective, with mean direct dialysis-related costs that were significantly greater in the early-start group by $10,77717. The present meta-analysis of cohort studies leaves us with the impression that higher eGFR at dialysis initiation, adjusted for confounders, might either be harmful or does not affect survival.
In the present report, we condensed the different “GFR” evaluation methods into a single rubric for the purpose of the analysis. The Cockcroft-Gault and MDRD Study equations, which incorporate the serum creatinine, tend estimate endogenous creatinine clearance not GFR, and are highly influenced by the muscle mass. Consequently, sarcopenia, which is the loss of muscle mass, especially in the elderly, is associated with lower serum creatinine, which would result in higher eGFR levels. Consequently, these equations are very poor estimates of GFR in advanced CKD. By contrast, 24-hour urine collections that measure endogenous creatinine or creatinine and urea clearance calculate GFR most accurately in advanced CKD, which was our population of interest. In one of our subgroup analyses, we demonstrated an association between higher calculated GFR (derived from a 24-hour urine collection), which is less likely to be influenced by the muscle mass, and lower mortality risk, contrasting with an association between higher estimated GFR (derived from the MDRD Study and Cockcroft-Gault equation) and higher mortality risk. Endogenous creatinine clearance (cGFR) seems to be the best markers, reflecting real kidney function. Therefore, the patients had the real worsening of kidney function; the initiation of dialysis did not result in high mortality. Although our results are consistent with a recent study that found an association between higher eGFR and increased mortality risk, but not cGFR 47, our analysis was restricted to a small sample of only 486 patients. In a large study of patients initiating dialysis, higher eGFR values were found to represent lower creatinine production rather than higher creatinine clearance, calling into question the reliability of estimating GFR with serum creatinine in advanced CKD 48. In that same study, the risk for mortality in early dialysis starters was greatly attenuated when endogenous creatinine clearance was used as the method to assess GFR48. As a consequence of these emerging data, a recently published updated guideline on when to start dialysis introduced cGFR derived from a 24-hour urine collection as the best GFR assessment in advanced kidney failure49.
A recently published qualitative systematic review that did not conduct a quantitative analysis was inconclusive regarding timing of dialysis initiation in patients with advanced CKD 28. However, the authors noted higher mortality rates in early-starters of HD but lower mortality in early-starters of PD28. Our subgroup analysis is supportive of this finding, whereby higher GFR in patients starting HD was associated with the highest adjusted mortality risk, whereas in the fewer studies restricted to PD populations, higher GFR appeared to not be associated with mortality. In a reanalysis of the CANUSA study, a 5 L/week/1.73 m2 (the equivalent of 0.5 mL/min/1.73 m2) increment in GFR (mean of urea and creatinine clearance) obtained 1 month after initiating PD, was associated with an adjusted 12% risk reduction in mortality, which disappeared once the 24-hour urine volume was forced into the model50. These authors suggested that the effect of GFR on mortality might be mediated in part by urine volume. This hypothesis needs to be tested. Although the IDEAL study failed to demonstrate a survival benefit of early vs. late start of dialysis19, the dialysis modality choice was made by the patient and treating physician. This might have confounded the results of the trial as the treatment modality might be associated with a different mortality risk.
Our data synthesis has several strengths. This is the first meta-analysis and largest systematic review of cohort studies of patients with advanced CKD (1,079,917 analyzable patients) that examines the association of GFR at dialysis initiation with mortality. In addition, the analyses used adjusted hazard ratios to minimize the confounding relation of GFR with mortality by including patient and treatment characteristics. Furthermore, particular emphasis was placed on studies that adjusted for nutritional indicators, as the main non-GFR determinant of serum creatinine is muscle mass, which is a reflection of nutritional status.
There are several limitations that should be noted. Multiple definitions were used to quantify severity of kidney impairment including the MDRD Study equation (and its variants), the Cockroft-Gault equation, and 24-hour urine collection. The studies included varied greatly in duration of follow-up. Our meta-analysis was subject to several potential biases including lead-time bias, survival bias, and publication bias, as well as confounding and variable methodological quality inherent to the use of observational studies. There was also a potential overlap in the populations of two US-based cohort studies that utilized the same dialysis registry18,20,21,33,35,40,41. However, a sensitivity analysis excluding the smaller-size study18,20,21,33,35,40,41, yielded similar results. The symmetric funnel plots coupled to a non-significant Egger test are suggestive of less susceptibility to publication bias. Confounding by indication is frequently encountered in observational studies, and is a more concerning bias and an important limitation of the analysis51. Indeed, the profile of early dialysis starters, as defined by a higher GFR level, might be related to the risk of adverse outcomes rather than the treatment variable of interest (i.e., higher GFR). Consequently, it remains unclear if persons with higher comorbidity profiles were more likely to initiate dialysis earlier. Since poor nutritional status at dialysis initiation is a known predictor of mortality in patients with CKD52–54, we attempted to minimize this potential bias by using hazard ratios that were adjusted for several patient characteristics including nutritional indicators. However, the observational design of our analysis limits causal inference, and full adjustment for known and unknown confounders. This is an important limitation as some of the comorbidity assessment tools used in national data registries that collect, analyze, and distribute information on dialysis patients might under-report certain diagnoses55,56.
Finally, the absence of differences in the pooled effect estimates according to study quality argues against biases resulting from flaws in the design of the individual studies included in our meta-analysis. Overall, these well-founded concerns call into question the design of clinical trials that rely on creatinine-based eGFR in patients with advanced CKD for treatment allocation.
In conclusion, the present meta-analysis of 15 cohort studies with 1,079,917 analyzable patients with advanced CKD found that higher GFR level at initiation of dialysis is associated with a higher adjusted mortality risk in observational studies, independent of nutritional status. This association might be strongest among patients initiating HD. Although there was substantial heterogeneity of effect size estimates across studies, The association between higher GFR and mortality in patients with advanced CKD requires further study, and calls for the design of a large trial to formally test the appropriate timing of dialysis initiation preferably using better kidney function assessment tools such as measured or calculated GFR or non-creatinine-based GFR markers to minimize misclassification bias and nutritional confounding. Such trial should ideally be restricted to a particular dialysis modality due to the potential confounding effect of the modality on outcomes. In the meantime, the timing of dialysis initiation in individuals with advanced CKD will continue to focus on the burden of uremic symptoms, treatment availability, physician preferences, and patient choices25.
Supplementary Material
Table S1: MeSH and keywords used for the systematic literature search.
Table S2: Assessment of GFR in the studies included in the meta-analysis.
Figure S1: Funnel graph for the assessment of potential publication bias.
Acknowledgments
Support: This work was made possible in part through Dr. Susantitaphong’s International Society of Nephrology funded Fellowship. This work was supported in part by Grant number UL1 RR025752 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Financial Disclosure: Dr. Jaber serves as scientific advisor for NxStage Medical, Inc. The remaining authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Couchoud C, Stengel B, Landais P, et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant. 2006 Feb;21(2):411–418. doi: 10.1093/ndt/gfi198. [DOI] [PubMed] [Google Scholar]
- 2.Klebe B, Irving J, Stevens PE, et al. The cost of implementing UK guidelines for the management of chronic kidney disease. Nephrol Dial Transplant. 2007 Sep;22(9):2504–2512. doi: 10.1093/ndt/gfm248. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley R, Herzog C, et al. Excerpts from the United States Renal Data System 2007 annual data report. Am J Kidney Dis. 2008 Jan;51(1 Suppl 1):S1–320. doi: 10.1053/j.ajkd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011 Jan;57(1 Suppl 1):A8, e1–526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Stel VS, Tomson C, Ansell D, et al. Level of renal function in patients starting dialysis: an ERA-EDTA Registry study. Nephrol Dial Transplant. 2010 Oct;25(10):3315–3325. doi: 10.1093/ndt/gfq209. [DOI] [PubMed] [Google Scholar]
- 6.Couchoud C, Guihenneuc C, Bayer F, Stengel B. The timing of dialysis initiation affects the incidence of renal replacement therapy. Nephrol Dial Transplant. 2010 May;25(5):1576–1578. doi: 10.1093/ndt/gfp675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper BA, Aslani A, Ryan M, Ibels LS, Pollock CA. Nutritional state correlates with renal function at the start of dialysis. Perit Dial Int. 2003 May-Jun;23(3):291–295. [PubMed] [Google Scholar]
- 8.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006 Jul;48(Suppl 1):S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 9.United Kingdom Renal Association. The Fifth Edition of the Clinical Practice Guidelines: Planning, Initiating and Withdrawal of Renal Replacement Therapy. http://www.renal.org/clinical/GuidelinesSection/RenalReplacementTherapy.aspx%23Intro.
- 10.Section I. Measurement of renal function, when to refer and when to start dialysis. Nephrol Dial Transplant. 2002;17(Suppl 7):7–15. doi: 10.1093/ndt/17.suppl_7.7. [DOI] [PubMed] [Google Scholar]
- 11.Kelly J, Stanley M, Harris D. The CARI guidelines. Acceptance into dialysis guidelines. Nephrology (Carlton) 2005 Oct;10(Suppl 4):S46–60. doi: 10.1111/j.1440-1797.2005.00486_1.x. [DOI] [PubMed] [Google Scholar]
- 12.Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008 Nov 18;179(11):1154–1162. doi: 10.1503/cmaj.080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evidence-based practice guideline for the treatment of CKD. Clin Exp Nephrol. 2009 Dec;13(6):537–566. doi: 10.1007/s10157-009-0237-8. [DOI] [PubMed] [Google Scholar]
- 14.Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci. 2003 Apr;325(4):228–236. doi: 10.1097/00000441-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004 Dec;107(1):1–16. doi: 10.1016/j.clineuro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Kurella Tamura M, O’Hare AM, McCulloch CE, Johansen KL. Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis. 2010 Dec;56(6):1117–1126. doi: 10.1053/j.ajkd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris A, Cooper BA, Li JJ, et al. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis. 2011 May;57(5):707–715. doi: 10.1053/j.ajkd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010 Aug;25(8):2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 19.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010 Aug 12;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 20.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011 Mar 14;171(5):396–403. doi: 10.1001/archinternmed.2010.415. [DOI] [PubMed] [Google Scholar]
- 21.Wright S, Klausner D, Baird B, et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010 Oct;5(10):1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosansky S, Glassock RJ. ‘Early’ dialysis start based on eGFR is no longer appropriate. Nat Rev Nephrol. 2010 Dec;6(12):693–694. doi: 10.1038/nrneph.2010.131. [DOI] [PubMed] [Google Scholar]
- 23.Van Biesen W, Vanholder R. When to start chronic dialysis: tunnel vision induced by numbers? Nephrol Dial Transplant. 2010 Aug;25(8):2405–2407. doi: 10.1093/ndt/gfq391. [DOI] [PubMed] [Google Scholar]
- 24.Manns BJ, Quinn RR. Early dialysis of no benefit to the patient or the health care system. Am J Kidney Dis. 2011 May;57(5):649–650. doi: 10.1053/j.ajkd.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Weiner DE, Stevens LA. Timing hemodialysis initiation: a call for clinical judgment. Am J Kidney Dis. 2011 Apr;57(4):562–565. doi: 10.1053/j.ajkd.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Tattersall J. Is it really better to start dialysis as late as possible? Nephrol Dial Transplant. 2009 Oct;24(10):2972–2974. doi: 10.1093/ndt/gfp410. [DOI] [PubMed] [Google Scholar]
- 27.Stel VS, Jager KJ. Glomerular filtration rate and initiation of dialysis. CMAJ. 2011 Jan 11;183(1):24–25. doi: 10.1503/cmaj.101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantero-Munoz P, Ruano-Ravina A, Otero-Gonzalez A, Sanchez-Guisande D, Gonzalez Rodriguez L. Influence of early dialysis among patients with advanced chronic renal disease: results of a systematic review. Nephrol Dial Transplant. 2010 Aug;25(8):2414–2421. doi: 10.1093/ndt/gfq227. [DOI] [PubMed] [Google Scholar]
- 29.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang SC, Ho YW, Tang AW, et al. Delaying initiation of dialysis till symptomatic uraemia--is it too late? Nephrol Dial Transplant. 2007 Jul;22(7):1926–1932. doi: 10.1093/ndt/gfm109. [DOI] [PubMed] [Google Scholar]
- 32.Korevaar JC, Jansen MA, Dekker FW, et al. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet. 2001 Sep 29;358(9287):1046–1050. doi: 10.1016/S0140-6736(01)06180-3. [DOI] [PubMed] [Google Scholar]
- 33.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002 Aug;13(8):2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 34.Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003 Sep;14(9):2305–2312. doi: 10.1097/01.asn.0000080184.67406.11. [DOI] [PubMed] [Google Scholar]
- 35.Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005 Nov;46(5):887–896. doi: 10.1053/j.ajkd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Wilson B, Harwood L, Locking-Cusolito H, et al. Optimal timing of initiation of chronic hemodialysis? Hemodial Int. 2007 Apr;11(2):263–269. doi: 10.1111/j.1542-4758.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- 37.Shiao CC, Huang JW, Chien KL, Chuang HF, Chen YM, Wu KD. Early initiation of dialysis and late implantation of catheters adversely affect outcomes of patients on chronic peritoneal dialysis. Perit Dial Int. 2008 Jan-Feb;28(1):73–81. [PubMed] [Google Scholar]
- 38.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrol Dial Transplant. 2009 Oct;24(10):3186–3192. doi: 10.1093/ndt/gfp189. [DOI] [PubMed] [Google Scholar]
- 39.Coronel F, Cigarran S, Herrero JA. Early initiation of peritoneal dialysis in diabetic patients. Scand J Urol Nephrol. 2009;43(2):148–153. doi: 10.1080/00365590802602903. [DOI] [PubMed] [Google Scholar]
- 40.Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009 Oct;24(10):3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 41.Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011 Jan 11;183(1):47–53. doi: 10.1503/cmaj.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010 Apr;77(8):700–707. doi: 10.1038/ki.2010.14. [DOI] [PubMed] [Google Scholar]
- 43.Evans M, Tettamanti G, Nyren O, Bellocco R, Fored CM, Elinder CG. No survival benefit from early-start dialysis in a population-based, inception cohort study of Swedish patients with chronic kidney disease. J Intern Med. 2011 Mar;269(3):289–298. doi: 10.1111/j.1365-2796.2010.02280.x. [DOI] [PubMed] [Google Scholar]
- 44.Raithatha A, McKane W, Kendray D, Evans C. Catheter access for hemodialysis defines higher mortality in late-presenting dialysis patients. Ren Fail. 2010;32(10):1183–1188. doi: 10.3109/0886022X.2010.517347. [DOI] [PubMed] [Google Scholar]
- 45.Herget-Rosenthal S, Quellmann T, Linden C, Hollenbeck M, Jankowski V, Kribben A. How does late nephrological co-management impact chronic kidney disease? - an observational study. Int J Clin Pract. 2010 Dec;64(13):1784–1792. doi: 10.1111/j.1742-1241.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 46.de Jager DJ, Voormolen N, Krediet RT, Dekker FW, Boeschoten EW, Grootendorst DC. Association between time of referral and survival in the first year of dialysis in diabetics and the elderly. Nephrol Dial Transplant. 2011 Feb;26(2):652–658. doi: 10.1093/ndt/gfq438. [DOI] [PubMed] [Google Scholar]
- 47.Grootendorst DC, Michels WM, Richardson JD, et al. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant. 2011 Jun;26(6):1932–1937. doi: 10.1093/ndt/gfq667. [DOI] [PubMed] [Google Scholar]
- 48.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Pappas LM, Cheung AK. Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol. 2003 Apr;14(4):1000–1005. doi: 10.1097/01.asn.0000057856.88335.dd. [DOI] [PubMed] [Google Scholar]
- 49.Tattersall J, Dekker F, Heimburger O, et al. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol Dial Transplant. 2011 Jul;26(7):2082–2086. doi: 10.1093/ndt/gfr168. [DOI] [PubMed] [Google Scholar]
- 50.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001 Oct;12(10):2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 51.Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sorensen HT, Blot WJ. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther. 2002 May-Jun;9(3):199–205. doi: 10.1097/00045391-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Leinig CE, Moraes T, Ribeiro S, et al. Predictive value of malnutrition markers for mortality in peritoneal dialysis patients. J Ren Nutr. 2011 Mar;21(2):176–183. doi: 10.1053/j.jrn.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 53.Anees M, Ibrahim M. Anemia and hypoalbuminemia at initiation of hemodialysis as risk factor for survival of dialysis patients. J Coll Physicians Surg Pak. 2009 Dec;19(12):776–780. [PubMed] [Google Scholar]
- 54.Cooper BA, Penne EL, Bartlett LH, Pollock CA. Protein malnutrition and hypoalbuminemia as predictors of vascular events and mortality in ESRD. Am J Kidney Dis. 2004 Jan;43(1):61–66. doi: 10.1053/j.ajkd.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 55.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000 Mar;11(3):520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010 Jan;77(2):141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: MeSH and keywords used for the systematic literature search.
Table S2: Assessment of GFR in the studies included in the meta-analysis.
Figure S1: Funnel graph for the assessment of potential publication bias.