Abstract
The ability to produce vigorous immune responses that spare self tissues and organs depends on elimination of autoreactive T and B cells. However, purging of immature and mature self-reactive T and B cells is incomplete and may require additional censorship by cells programmed to suppress immune responses 1. Regulatory T cells belonging to the CD4+ T cell subset may play a role in preventing untoward inflammatory responses, but T cell subsets programmed to inhibit the development of autoantibody formation and SLE-like disease have not been defined 2. Here we delineate a CD8+ regulatory T cell lineage that is essential for maintenance of self tolerance and prevention of autoimmune disease. Genetic disruption of the inhibitory interaction between these CD44+ ICOSL+ CD8+ T cells and their target Qa-1+ follicular T helper cells results in the development of a lethal SLE-like autoimmune disease. These findings define a sublineage of CD8 T cells programmed to suppress rather than activate immunity that represents an essential regulatory element of the immune response and a guarantor of self tolerance.
Interest in regulatory T cells has focused largely on FoxP3+ CD4+ cells 3. The possibility that development of CD8+ cells may give rise to a regulatory lineage has received less attention. Early observations detected a subpopulation of CD8 cells that suppressed T cell help to B cells 4 and recent studies have shown that Qa-1-restricted CD8 cells inhibit EAE by targeting autoreactive CD4 cells 5–7. Nonetheless, although Qa-1-deficient mice develop dysregulated immune responses to self and foreign antigens, they do not spontaneously develop autoimmune disease 8. However, deletion of the Qa-1 molecule disrupts interactions with two distinct receptors that have opposing effects on CD4-mediated immune responses. First, engagement of the TCR by Qa-1–peptide complexes leads to activation and expression of antigen-specific suppressor CD8 cells 9. Second, engagement of the CD94/NKG2A receptor expressed by NK cells by Qa-1/Qdm peptide complexes expressed by activated CD4 cells protects these CD4 cells from NK lysis and suppression by CD8+ Treg 7,10,11. We therefore generated Qa-1 knock-in mice, B6.Qa-1(D227K), containing a Qa-1 amino acid exchange mutation that disrupts Qa-1 binding to the TCR/CD8 co-receptor, but has no effect on engagement of the inhibitory NKG2A receptor on CD8 and NK cells (Supplementary Fig. 1).
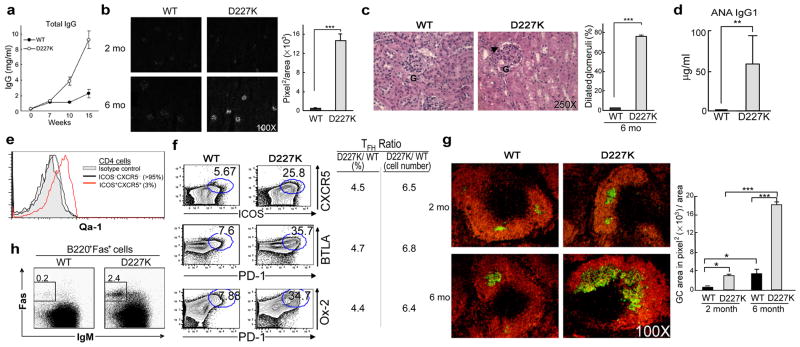
We first analyzed Qa-1 mutant mice for development of autoimmune disease. Analysis of sera from 4–6 mo old B6.Qa-1(D227K) mice and age-matched B6 controls revealed a 5-fold increase in total IgG (Fig. 1a) and a 20-fold increase in Ig deposition in renal glomeruli (Fig. 1b) associated with glomerulonephritis (Fig. 1c) and autoantibodies against nuclear antigens (Fig. 1d). To define potential target cells for Qa-1-dependent suppression 8, we analyzed Qa-1 expression by TH subsets. In the absence of activation by antigen, TFH cells (~5% of CD4 cells) expressed high levels of Qa-1, while non-TFH CD4 (Th0, Th17, Th1 and Th2) cells expressed barely detectable levels (Fig. 1e; Supplementary Fig. 2). These findings raised the possibility that TFH cells might be primary cellular targets of Qa-1 dependent regulation.
Figure 1. B6.Qa-1(D227K) mice develop an autoimmune phenotype.
a) Serum IgG levels of B6.Qa-1(WT) and B6.Qa-1(D227K) mice (n=6), b) Kidney sections from 2 and 6 month old WT and D227K (n=4) mice stained with anti-mouse IgG antibody and quantified. c) Dilated capillary loops of glomeruli in kidney of 6 month old D227K mice and quantification are shown. d) ANA generation in WT and D227K (n=9) mice in 6–7 month old mice. e) Qa-1 expression on TFH cells (ICOS+CXCR5+) in steady state. f) Analysis of surface markers on TFH cells from 6 month old WT and D227K mice. g) Germinal centers in spleen and quantification of GC area (n=4/group). h) Isotype switched GC B cells (B220+Fas+IgM-) from 6 month old WT and D227K mice. Error bars represent mean ± SEM.
We asked whether TFH cell numbers increased after disruption of the inhibitory interaction between Qa-1-restricted CD8 cells and Qa-1+ TFH cells. B6.Qa-1(D227K) mice contained a 5–6 fold increase in TFH cells compared with age matched B6.Qa-1(WT) controls (Fig. 1f) and a 5-fold increase in germinal center (GC) area (Fig. 1g). Increased GC area was accompanied by a 15-fold increase in Fas+B220+ B cells (Fig. 1h), reminiscent of autoimmune-prone Sanroque and BXSB-Yaa mouse strains 10,11.
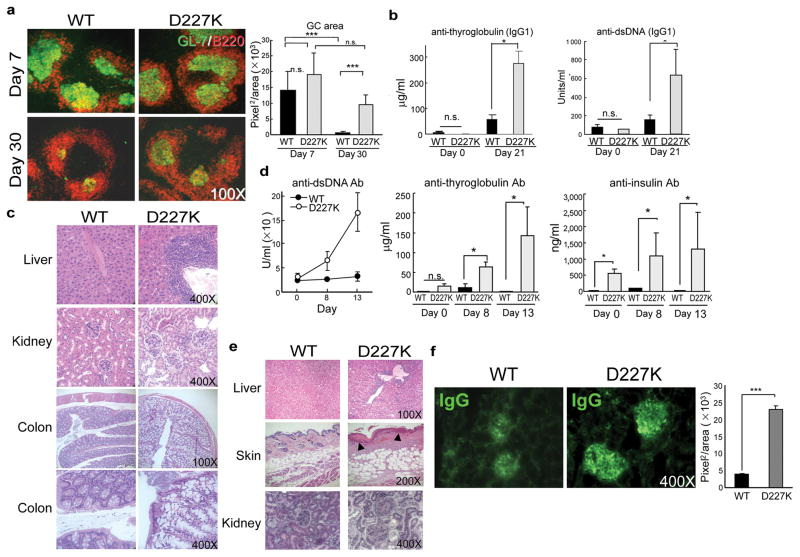
We then examined immune responses of Qa-1 mutant mice to foreign infectious and non-infectious antigens. T cell-dependent B cell immune responses in GC begin with cellular proliferation and end with selection of high affinity B cells that differentiate into memory and plasma cells. Although early primary responses of Qa-1 mutant mice (to KLH) were similar to Qa-1 WT mice (Fig. 2a), by day 30 the GC area of mutant mice increased ~10-fold over pre-immune GC, while the GC area of control Qa-1(WT) mice contracted to pre-immunization levels (Fig. 2a). Immunization of B6.Qa-1(D227K) mice with KLH also generated autoantibodies to thyroglobulin and dsDNA (Fig. 2b), accompanied by monocytic infiltration into liver, enlargement of kidney glomeruli and hyperplastic proximal colitis (Fig. 2c).
Figure 2. Germinal Center formation and antibody response in B6.Qa-1(WT) and B6.Qa-1(D227K) mice challenged with protein antigen or virus.
a) GC formation in spleen of WT or D227K mice (6––8 wk old) upon KLH/CFA immunization and quantification of GL-7+ area. b) Autoantibody generation and c) immunopathology in D227K mice upon multiple immunization (at day 0, 4 and 10) with antigen KLH/CFA. d) Autoantibody generation, e) histopathological analysis (day 28) in D227K mice after LCMV infection. f) IgG deposition in glomeruli from WT and D227K mice (day 28) after LCMV-Armstrong infection. 3–6 mice/group were used. Data shown represent mean ± SEM (20–30 kidney areas/group).
Since microbial infection can induce or exacerbate autoimmune disease 12–14, we tested the effects of LCMV-Armstrong viral infection. Although B6.Qa-1(WT) and B6.Qa-1(D227K) mice produced similar levels of anti-viral Ab (Supplementary Fig. 3a), the latter displayed an ~5-fold increase of GC+ B cells (Supplementary Fig 3b, left panel) and a >20-fold increase in splenic CD11b+ cells (Supplementary Fig. 3c) within two weeks of infection. Dysregulated cellular expression was accompanied by vigorous autoantibody responses to dsDNA, thyroglobulin and insulin by young (6 wk old) and older (6 mo old) mice (Fig. 2d and Supplementary Fig. 3d), indicating that autoantibody formation was not simply the residue of chronic autoimmunity. The response was marked by inflammatory cell infiltration into the liver, dermis and epidermis; ulceration of subdermal areas, hyperkeratosis (Fig 2e) and glomerulonephritis that resulted in death from renal dysfunction by 25–30 days post infection (Fig. 2f).
These findings indicate that a Qa-1 point mutation that impairs recognition of antigen by Qa-1-restricted CD8 Treg results in increased numbers of TFH and autoimmunity characterized by 1) tissue-specific autoantibodies, 2) lymphoid infiltration of peripheral organs, and 3) severe glomerulonephritis.
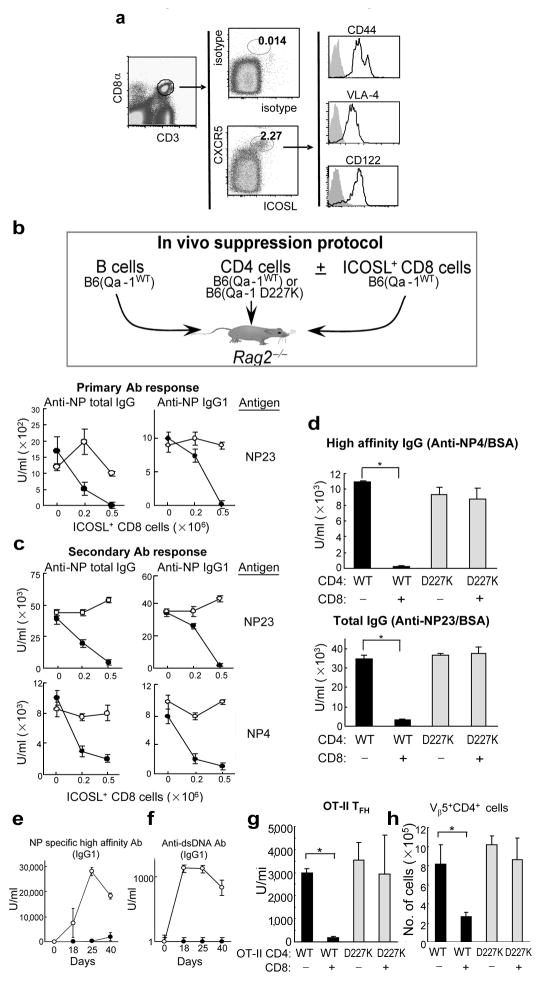
We then asked whether the abnormal phenotype of Qa-1 mutant mice reflected loss of a regulatory interaction between CD8+ Treg and CD4+ TFH. Although Qa-1-restricted CD8+ cells have been implicated in the regulation of diverse immune responses, insight into this process has been constrained by the lack of surface markers that distinguish them from conventional CD8 cells. Analysis of T cells one week after immunization with KLH/CFA revealed a subpopulation of activated CD8 cells that expressed ICOSL and CXCR5 on their surface according to FACS analysis along with CD44, VLA-4 and CD122 (Fig. 3a). Transfer of ICOSL+ CD8 cells but not ICOSL CD8 cells into Rag2−/− hosts resulted in dose-dependent inhibition of primary and secondary anti-NP Ab responses: anti-NP responses were inhibited >90% by as few as 5×105 ICOSL+ CD8 cells (Fig. 3b). Infusion of the ICOSL− fraction of CD8 cells did not result in suppression of NP-specific IgG responses (Supplementary Figure 4). Analysis of the secondary response elicited after boosting reconstituted Rag2−/− hosts showed that as few as 2×105 ICOSL+ CD8+ T cells completely suppressed high affinity anti-NP Ab responses (Fig. 3c, lower panel) of Qa-1(WT) but not Qa-1 mutant CD4 cells. Qa-1+ CD4 cells were an essential cellular target of suppression, since CD4 cells expressing the B6.Qa-1(D227K) mutation allowed a full anti-NP response (Fig. 3c). Moreover, B cells were not targets of Qa-1-restricted suppression (Supplementary Figs. 5 and 6). ICOSL+ CD8 cells generated after in vitro stimulation with (Qa-1b+) Kb−/−Db−/− activated B and T cells suppressed ~99% of high affinity Ab and markedly reduced the numbers of TFH and GC B cells (Fig. 3d and Supplementary Fig. 7).
Figure 3. CD44+ ICOSL+ CD8+ cell population targets TFH to block generation of high affinity antibodies.
a) CD44+ICOSL+CXCR5+ CD8 cells 10 days after KLH/CFA immunization of WT mice (6–8 wk old). b, c) Dose dependent suppression of total and high affinity NP-specific antibodies. Rag2−/− mice were transferred with 2×106 naïve WT B cells along with 1×106 nasve WT (●) or D227K (○) CD4 cells with or without ICOSL+ enriched CD8 cells. Recipients were immunized i.p. with NP19-KLH/CFA and reimmunized i.p. with NP19-KLH/IFA at day 10. Serum was analyzed at day 7 for primary (b) and at day 18 for secondary (c) Ab responses. Data are representative of three independent experiments. d) Suppression of high affinity Ab responses by in vitro enriched ICOSL+ CD8+ T cells. Rag2−/− mice were transferred with B, CD4 and CD8 cells and immunized as described above. ICOSL+ CD8 cells were enriched in vitro. Secondary Ab response is shown at day 28 after transfer. Data represent one of two independent experiments. e, f) Ag-specific Ab and autoantibody generation in secondary hosts infused with WT (●) or D227K (○) CD4 cells. g) Qa-1-dependent suppression of monoclonal TFH cells by CD8 Treg (see legend to Supplementary Figure 10). (h) Number of recovered OT-II cells (Vβ5+CD4+) in spleen of Rag2−/− recipients. 3–6 mice/group were used. Error bars denote mean ± SEM.
If autoimmunity in Qa-1 mutant mice reflected a defective Qa-1-dependent regulatory interaction, Rag2−/− hosts reconstituted with Qa-1 mutant CD4 cells (and CD8+ Treg) might produce autoantibody upon challenge with antigen. Indeed, these hosts produced high titers of autoantibody to dsDNA, while recipients of Qa-1 WT CD4 cells did not (Figs. 3e,f and Supplementary Fig. 8). Taken together, these data demonstrate that an interaction between CD8+ Treg and Qa-1+ TFH cells inhibits production of both high affinity antibody and autoantibody.
To further distinguish the TH lineage targeted by CD8+ Treg, we induced each of the major CD4+ TH subsets (TFH, Th1, Th2 and Th17) expressing either Qa-1(WT) or Qa-1(D227K) and sharing the OT-II TCR (Supplementary Fig. 9). Cells were transferred into Rag2−/− hosts along with B cells and sorted CD44+ CD8+ T cells before immunization with NP13-OVA in CFA. Two weeks later, the NP response induced by Qa-1(WT) TFH but not Qa-1(D227K) TFH was reduced by >90% (Fig 3g), and the numbers of Qa-1(WT) but not Qa-1(D227K) Vβ5+ CD4 cells were reduced by about 65–70% (Fig. 3h). The anti-NP response induced by Th1, Th2 and Th17 cells was not impaired (Supplementary Fig. 10).
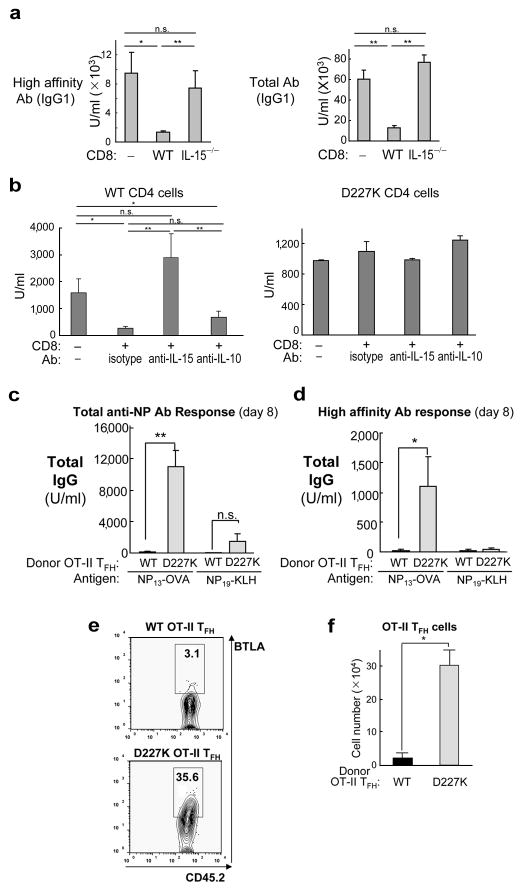
We then attempted to define the mechanism of CD8-dependent suppression. Perforin-deficient (Prf1−/−) CD8+ Treg fail to mediate significant Qa-1-restricted suppression of TFH targets in Rag2−/− hosts (Supplementary Fig. 11a), consistent with the observation that suppression is associated with reduced numbers of antigen-specific (OT-II) TFH in adoptive hosts (Figs. 3h and Supplementary Fig. 11b). Although dependent on Prf1, CD8+ Treg do not represent conventional CTL since, unlike the majority of CTL, they are fully dependent on IL-15 for activity 15 : CD8 T cells from IL-15−/− donors lack inhibitory activity (Fig. 4a). Analysis of IL-15−/− mice revealed a 15-fold reduction of central memory cells and a 2-fold reduction in effector memory cells, as well as a 5-fold reduction of CD44+ICOSL+CXCR5+ CD8 cells (Supplementary Fig. 12). Defective suppression by IL-15−/− CD8 cells may reflect more than a developmental block, since injection of anti-IL-15 Ab into adoptive Rag2−/− hosts also prevented suppression by CD8 Treg obtained from IL-15 WT B6 donors (Fig. 4b). These findings suggest that IL-15 may be required for CD8-dependent elimination of TFH, perhaps in concert with IL-21 expressed by activated TFH target cells 16. Although IL-10 has been implicated in suppression, this cytokine did not contribute to Qa-1-restricted suppression by CD8 Treg (Fig. 4b).
Figure 4. Mechanism of Qa-1 restricted suppression by CD8 Treg.
a) Rag2−/− hosts were transferred with CD8 cells isolated from KLH/CFA immunized WT or IL-15−/− donor. Total and high affinity Ab was measured at day 18 (secondary response) after immunization. (b) IL-15-dependent and IL-10-independent suppression of Ab response by CD8 Treg: Rag2−/− recipients transferred with B, CD4 and CD8 Treg were immunized and reimmunized with NP-KLH/CFA and NP-KLH/IFA. Upon initial immunization and every 3 days thereafter, antibodies were injected i.v. (100 μg/mouse). (c) Transfer of high affinity Ab responses into normal mice: B6 mice were transferred with in vitro differentiated WT or D227K OT-II TFH cells. B6 recipients were immunized i.p. either with 100 μg NP13-OVA or NP19-KLH in CFA. NP specific total (c) and high affinity (d) Ab response was measured at day 7 after immunization. (e) Qa-1-dependent suppression of transferred TFH cells: In vitro differentiated WT or D227K OT-II TFH cells (CD45.2+; see skewing conditions in Additional Methods) were transferred into syngeneic CD45.1+ B6 recipients and immunized with 100 μg NP13-OVA/CFA. CD45.2+ OT-II TFH cells were analyzed 10 days later by FACS (e) and enumerated (f). 3–6 mice/group were used. Data shown represent mean ± SEM.
An inhibitory interaction between CD8 Treg and target TFH should require migration of CD8+ cells to lymphoid follicles. Rag2−/− hosts injected with CD4+ cells, B cells and protein/adjuvant (KLH/CFA) displayed organized lymphoid architecture containing secondary B cell follicles and T cell zones within 7–10 days. After injection of these mice with sorted CD44+CD8+ cells from B6.CD45.1 donors, histologic examination showed substantial homing to both the GL-7+ B cell areas and to adjacent CD4+ T cell zones: >80% of CD45.1+ CD8 cells located either within GC or the inner T cell zone (Supplementary Fig. 13). In addition, CXCR5+ CD8 cells purified by FACS sorting selectively migrated into lymphoid follicles (Supplementary Fig. 14), suggesting that homing of CXCR5+ CD44+ CD8+ cells to lymphoid follicles may allow inhibition of TFH activity by the migrant cells.
We then asked whether gene products associated with CD4+ regulatory T cell activity might be expressed by CD8+ (CXCR5+ ICOSL+) Treg. CD8+ Treg expressed neither CTLA-4 nor diminished levels of IL-7 receptor-α (CD127) (Supplementary Fig. 15), nor FoxP3 (Supplementary Figs. 15e, f). However, they expressed high levels of GITR and CD25 (Supplementary Fig. 15), possibly associated with cellular activation. In sum, although CD4+ and CD8+ Treg both inhibit immune responses, they belong to different T cell subsets, utilize dissimilar inhibitory mechanisms and probably are controlled by distinct genetic programs.
These findings suggested a new approach to a longstanding problem in basic and clinical immunology: the failure to transfer robust immune responses into syngeneic hosts. To test the hypothesis that this might reflect inhibition by Qa-1-restricted CD8 Treg in the host, we transferred OT-II TFH cells into naïve syngeneic B6 mice before NP13-OVA immunization, as described 17. TFH cells were >98% ICOS+/CD200+/BTLA+, and displayed enhanced levels of surface PD-1+ and Qa-1 for both Qa-1(WT) and Qa-1 mutant cells (Supplementary Figs. 16 and 17). Transfer of B6.Qa-1(D227K), but not B6.Qa-1(WT), OT-II TFH induced robust and specific high affinity anti-NP responses (Fig. 4c,d). Induction of high affinity anti-NP Ab responses by transferred B6.Qa-1(D227K) OT-II cells was specific, since challenge of these host mice with NP19-KLH rather than NP13-OVA stimulated little or no anti-NP Ab. This was associated with a pronounced (15-fold) reduction in the numbers of Qa-1 WT donor BTLA+ OT-II cells (30×104 vs. 2×104) (Figs. 4e, f), indicating that the adoptive response is tightly controlled by Qa-1-dependent suppression of donor TFH cells that is bypassed by CD4+ TFH cells expressing a mutant Qa-1.
Generation of high affinity Ab responses against pathogens that are not contaminated by anti-self Ab is a daunting biological task. The majority of B cells express self-reactive B cell receptors during the early phase of development 18. Receptor editing, deletion and/or induction of anergy does not prevent generation of autoreactive memory B cells 19,20, since somatic mutation of Ig V regions in B cells activated by cognate TFH cells generates autoreactive antibodies 21. Inhibition of the delivery of cognate help by disruption of the TFH/ B cell interaction is necessary to ensure self tolerance. Indeed, increased numbers of circulating TFH represent a cardinal feature of severe SLE 22.
Using mice that carry a single point mutation within a murine protein (Qa-1), we have uncovered a fundamental mechanism essential for maintenance of self tolerance and characterized the pathologic consequences that arise when this mechanism breaks down. B6.Qa-1(D227K) mice develop autoimmune disease at 2–3 months, which is characterized by tissue-specific autoantibodies and invasion of non-lymphoid tissues by monocytes and lymphocytes. Consistent with the finding that the genetic profile of CD4+ Treg is not expressed by CD8+ Treg (Supplementary Fig. 15), autoimmune disease in B6.Qa-1(D227K) mice occurs despite the presence of a full complement of CD4+ FoxP3+ Treg. A direct demonstration of this division of labor comes from the observation that TFH expressing the Qa-1 D227K mutation transfer high affinity Ab responses to non-lymphopenic mice, despite the fact that the host contains FoxP3+CD25+ Treg (Figs. 4c,d). These findings also suggest new approaches to adoptive transfer therapy based on diminished CD8+ Treg activity to allow robust antibody responses.
Additional Methods
Immunohistochemistry
7 μm acetone-fixed frozen sections from spleen or kidney were air-dried and stained with various antibodies for 30 min at room temperature in a moist chamber. For the identification of germinal centers, sections were labeled with PE conjugated anti-B220 antibodies (BD, RA3-6B2) and FITC conjugated anti-GL-7 (BD, clone GL7) antibodies. For assessment of IgG deposition in the kidney, >10 sections stained with FITC-conjugated anti-mouse IgG Ab (Sigma) were examined for each experimental condition, verified for reproducibility and quantified as positively-stained using ImageJ software.
Reagents and flow cytometry
Single-cell suspensions were prepared and maintained in the dark at 4°C for immunofluorescent analysis, washed in ice-cold FACS buffer (2% fatal calf serum, 0.1% NaN3 in PBS) and incubated with each antibody for 30 min and washed with FACS buffer before analyzing. Anti-CD4, anti-CD8, anti-B220, anti-CD44, anti-CD62L, anti-Fas, anti-Qa-1b, anti-CD45.2, anti-PD-1, anti-CD11b, anti-CXCR5, anti-IFNγ, anti-IL10, anti-IL4, anti-ICOS, anti-Vb5.1/5.2, anti-NKG2A, anti-IgM (BD Bioscience) or anti-CD8β, anti-CTLA4, anti-CCR7, anti-GITR, anti-ICOSL, anti-CD127, anti-CD25, anti-KLRG1 and anti-VLA4 (eBioscience) were used followed by analysis of cells using a FACSCalibur (BD Biosciences) and FlowJo software (TriStar).
ELISA for antibodies
For detection of NP specific antibodies, ELISA plates were coated with 0.5 μg/ml NP4-BSA or 1 μg/ml NP23-BSA (Biosearch Technologies) and serum harvested 14 days after immunization with NP19-KLH in CFA and reimmunization with NP19-KLH in IFA was used as a standard. 1:4000 dilution of this immune serum was defined as 100 units/ml. Total IgG, IgG1 and IgG2a were detected by incubating plates with biotinylated anti-mouse IgG, IgG1 or IgG2a followed by streptavidin-peroxidase. Anti-dsDNA and anti-nuclear antibodies in mouse sera were determined by ELISA (Alpha Diagnostic International) and porcine thyroglobulin (Sigma) and porcine insulin (Sigma) were used to detect relevant Ab. IFNγ and IL-4 were measured using OptEIA ELISA kit (BD Biosciences); IL-21 and IL-17 were measured using ELISA Duo set (R&D Systems).
LCMV infection and determination of Ab titer
6–8 week old or 6 month old B6.Qa-1(WT) or B6.Qa-1(D227K) mice were infected i.p. with 1×106 PFU LCMV-Armstrong. At day 8, mice were reinfected i.p. with 1×105 PFU LCMV-Armstrong. Serum samples were prepared at day 8 (prior to reinfection) and day 13 and subjected to the ELISA test (Biotech Trading Partners).
Generation of TFH in vitro and adoptive transfer
Cells from spleen and lymph nodes were collected from WT OT-II or B6.Qa-1(D227K) OT-II mice. 2×105 cells/ml OT-II CD4 cells were stimulated with 1 μg/ml OT-II peptides (ISQAVHAAHAEINEAGR) in the presence of 2×106 irradiated total splenocytes. For the differentiation of OT-II CD4 cells to follicular helper T cell phenotype, 50 ng/ml IL-21, 10 μg/ml anti-IFNγ Ab, 10 μg/ml anti-IL-4 Ab, 20 μg/ml rIL-6, and 20 μg/ml anti-TGFβ (1D11) Abs were added into the culture. At day 5, live CD4+ cells were harvested from the culture by percoll gradient centrifugation. OT-II cells were transferred i.v. into naïve WT B6 mice or Rag2−/− mice. Recipient mice were immunized i.p. with either 100 μg NP13-OVA in CFA or 100 μg NP19-KLH in CFA.
Statistical analyses
Statistical analyses were performed using Wilcoxon-Mann-Whitney rank sum test for comparison of two conditions and Kruskal-Wallis test for comparison of more than two conditions. P value < 0.05 was considered statistically significant (*= <0.05, **= <0.01, ***= <0.001).
Supplementary Material
Acknowledgments
We thank R. Bronson (DF/HCC Rodent Histopathology Core) for histology analysis, R. Gelman and J. Yang for help with statistical analyses, C. Daniel and T. Kreslavsky for cytometry support and critical discussions, M. Iannacone and U. von Andrian for LCMV, X. Wang for technical assistance, M. Call, D.A. Alvarez Arias, T. Kadakia and A. Angel for manuscript and figure preparation. This work was supported in part by NIH Research Grant AI 037562, the Lupus Research Institute and a gift from the Schecter Research Foundation to HC; NRSA Fellowships (DFCI/NCI T32 CA070083) to H-JK and (HSPH/NCI T32 CA009382) to XT; and a Belgian American Educational Foundation Fellowship to BV.
Footnotes
AUTHOR CONTRIBUTIONS: H-JK and HC conceived and planned experiments; H-JK, BV, XT and LL performed experiments; H-JK and HC analyzed data and wrote the paper.
References
- 1.Cantor H. T-cell receptor crossreactivity and autoimmune disease. Advan Immunol. 2000;75:209–233. doi: 10.1016/s0065-2776(00)75005-x. [DOI] [PubMed] [Google Scholar]
- 2.Cantor H. Reviving suppression? Nat Immunol. 2004;5:347–349. doi: 10.1038/ni0404-347. [DOI] [PubMed] [Google Scholar]
- 3.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble A, Zhao ZS, Cantor H. Suppression of immune responses by CD8 cells: II. Qa-1 on activated B-cells stimulates CD8 cell suppression of Th2-dependent antibody responses. J Immunol. 1998;160:566–571. [PubMed] [Google Scholar]
- 5.Jiang H, Zhang SL, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, et al. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci U S A. 2007;104:20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu D, et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci U S A. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubier JA, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangster MY, et al. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol. 2000;164:1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 13.Panoutsakopoulou V, et al. Analysis of the Relationship between Viral Infection and Autoimmune Disease. Immunity. 2001;15:137–147. doi: 10.1016/s1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 14.Hunziker L, et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 19.Yurasov S, Nussenzweig MC. Regulation of autoreactive antibodies. Curr Opin Rheumatol. 2007;19:421–426. doi: 10.1097/BOR.0b013e328277ef3b. [DOI] [PubMed] [Google Scholar]
- 20.Hao Z, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci U S A. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson N, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.