Abstract
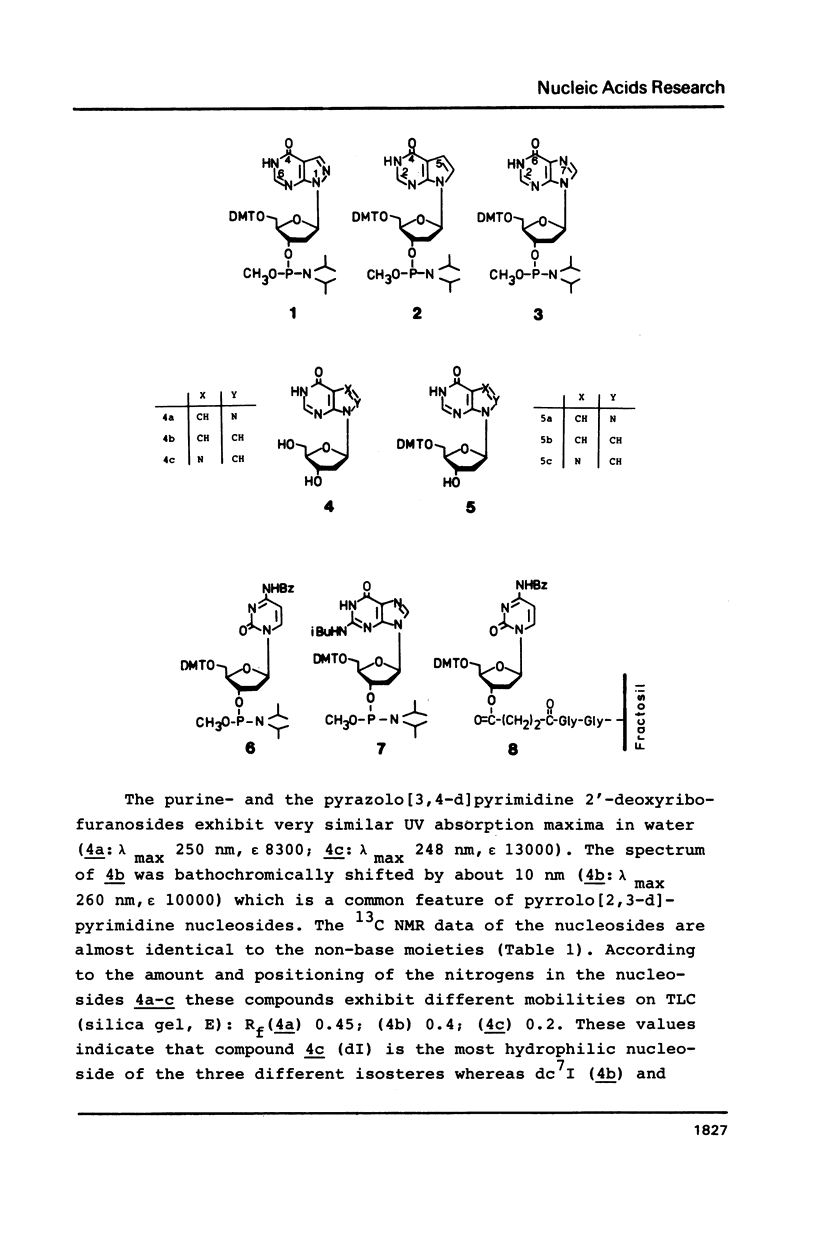
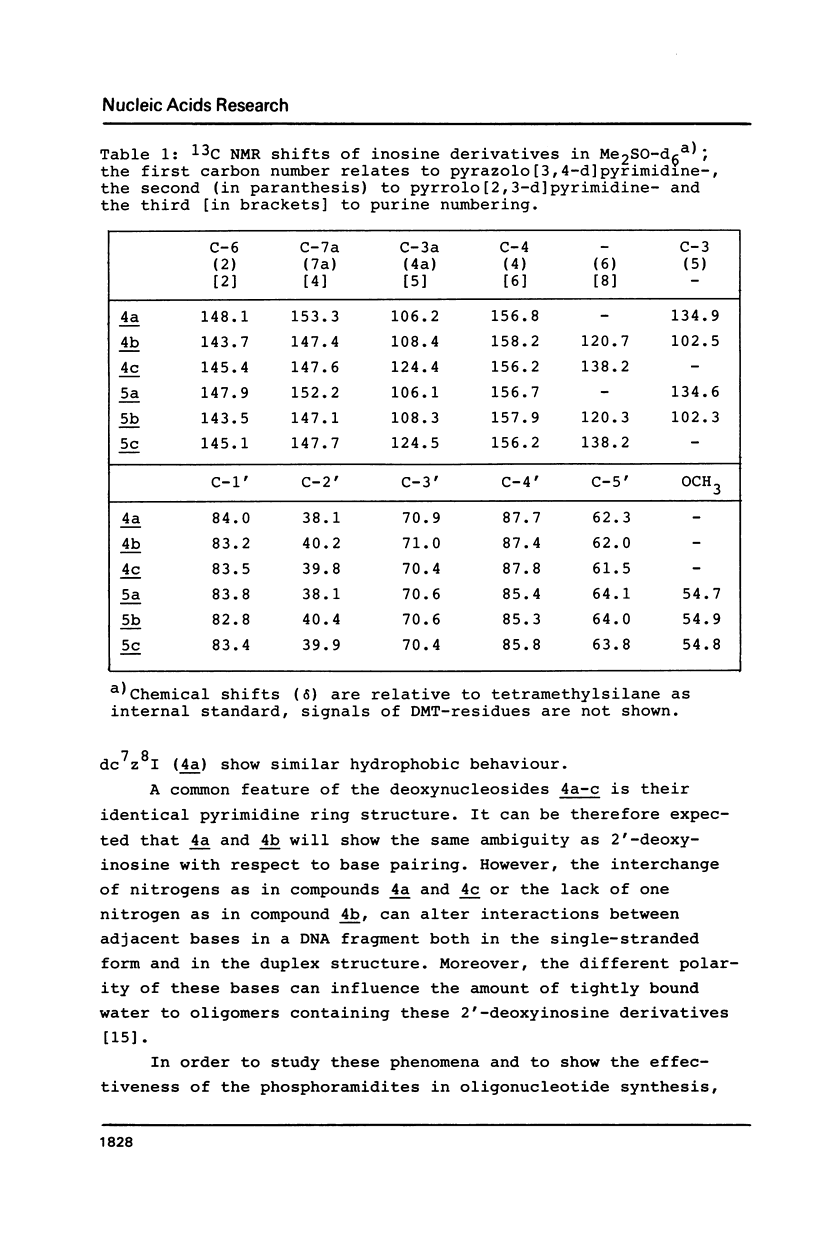
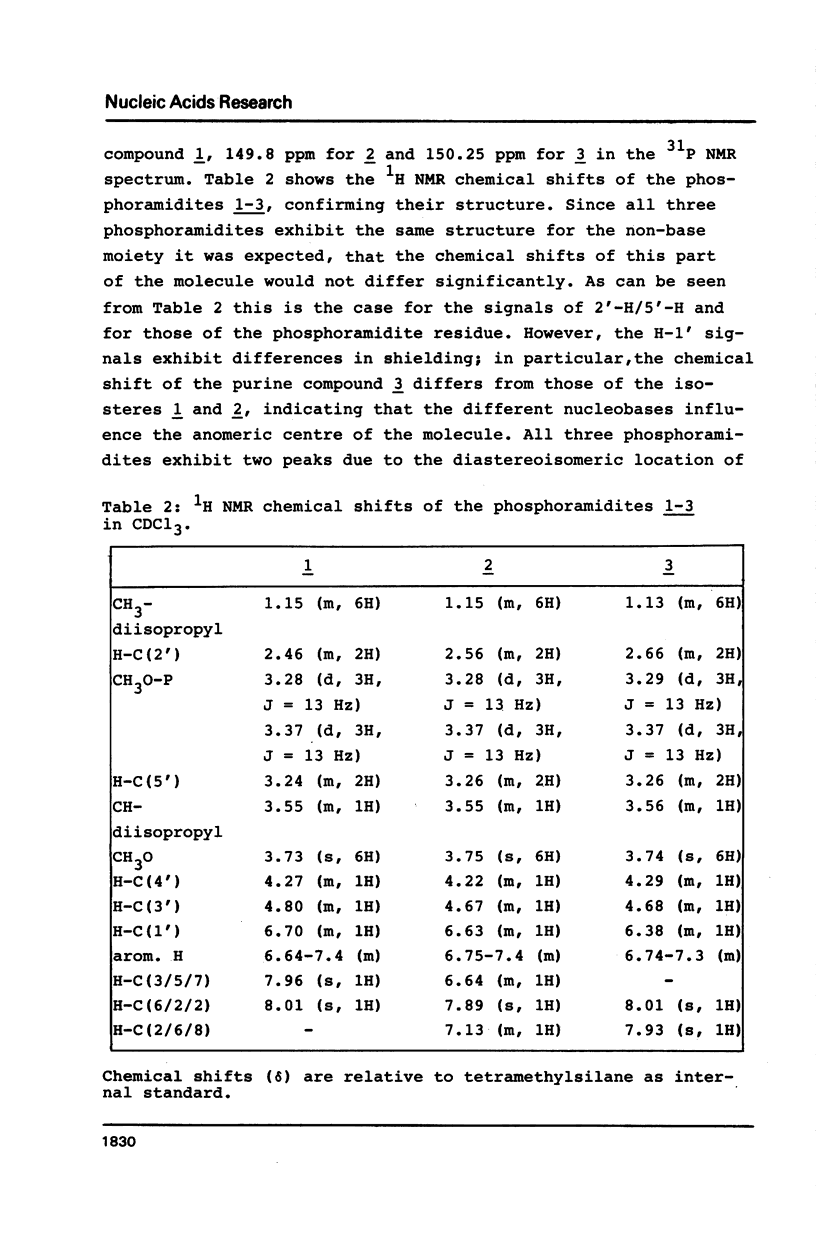
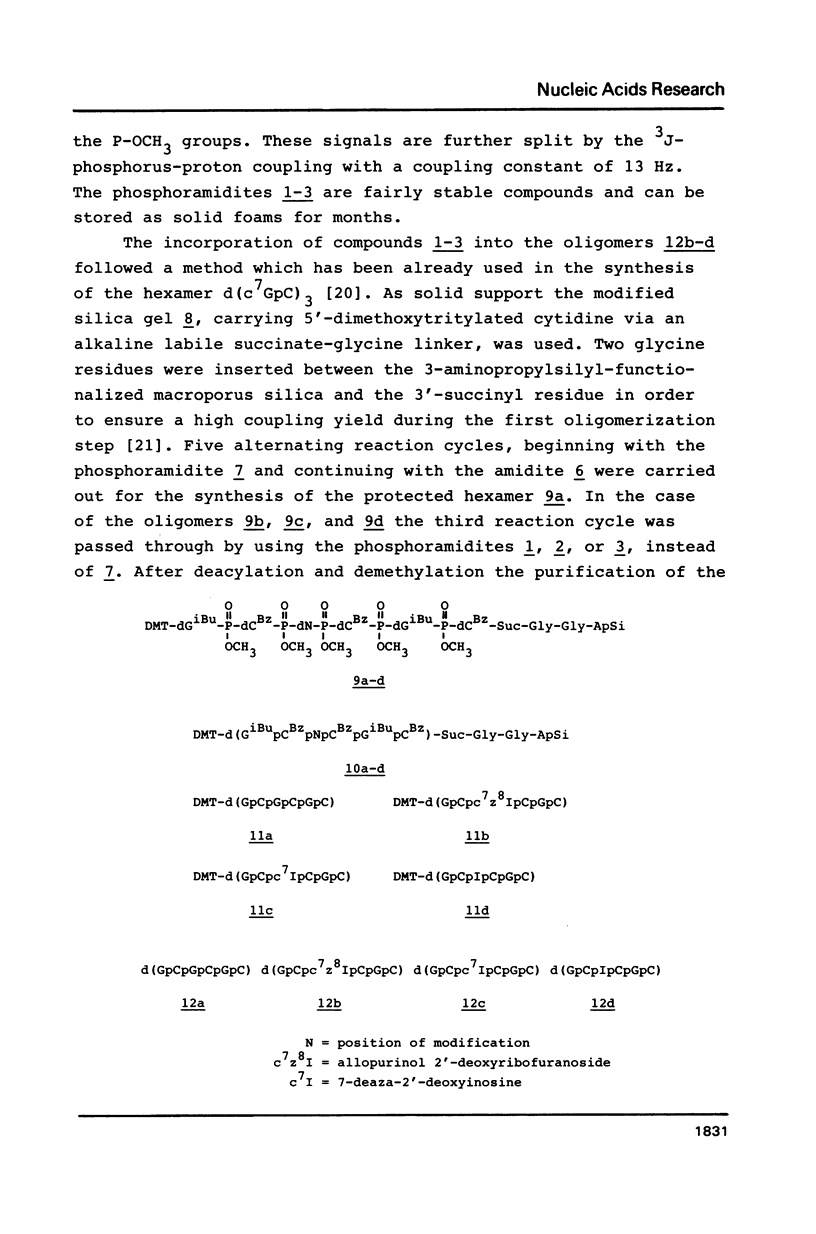
Novel phosphoramidites (1,2) of appropriately protected 2'-deoxyinosine isosteres (I*) such as allopurinol 2'-deoxyribofuranoside (4a) and 7-deaza-2'-deoxyinosine (4b) have been synthesized. They were employed together with the phosphoramidite of 2'-deoxyinosine in solid-phase synthesis of d(GCI*CGC) hexamers (12a-d). From thermodynamic data of these alternating hexamers it was shown that allopurinol 2'-deoxyribofuranoside destabilizes such duplexes less strongly than 2'-deoxyinosine. Additionally, the phosphoramidite of 7-deaza-2'-deoxyinosine (2) exhibits an extraordinary stability of the N-glycosylic bond. Since the new phosphoramidites are structurally related to 2'-deoxyinosine, they can be used in the construction of hybridization probes containing an ambiguous base.
Full text
PDF


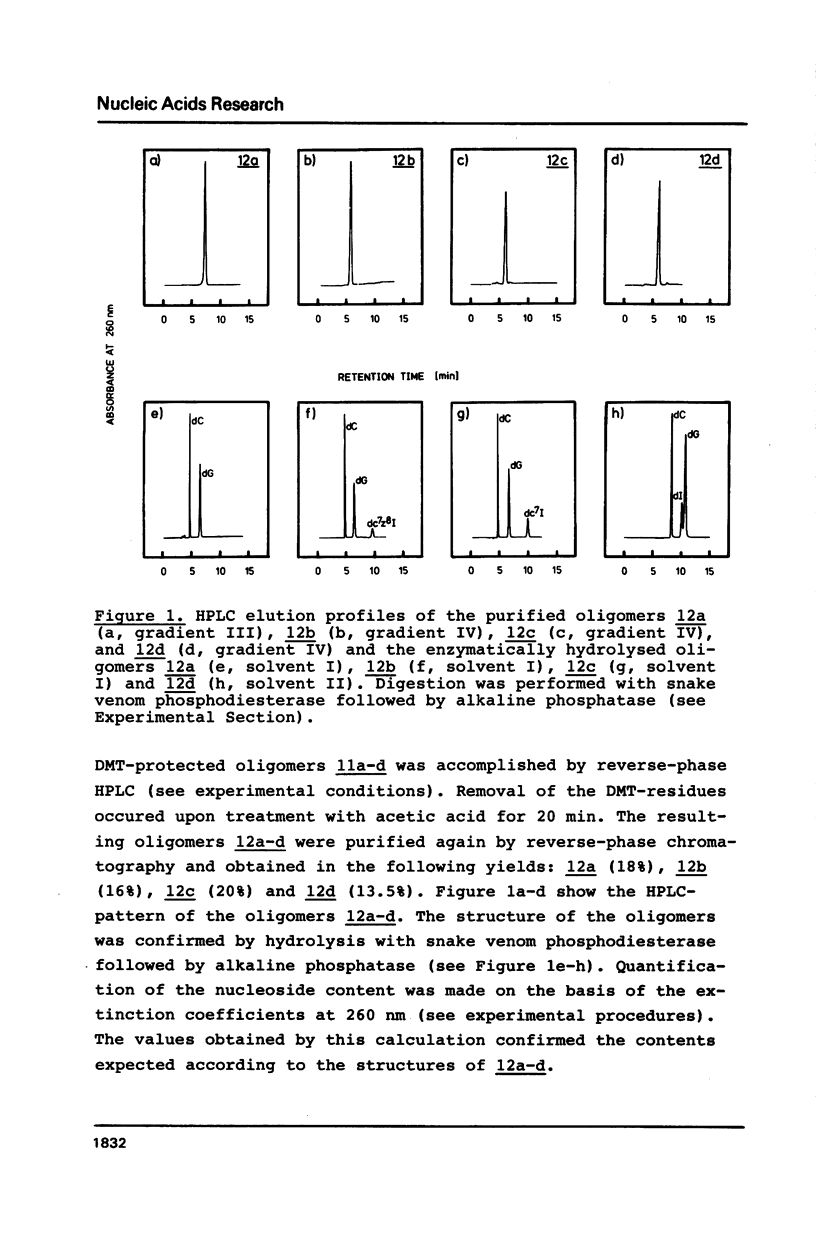

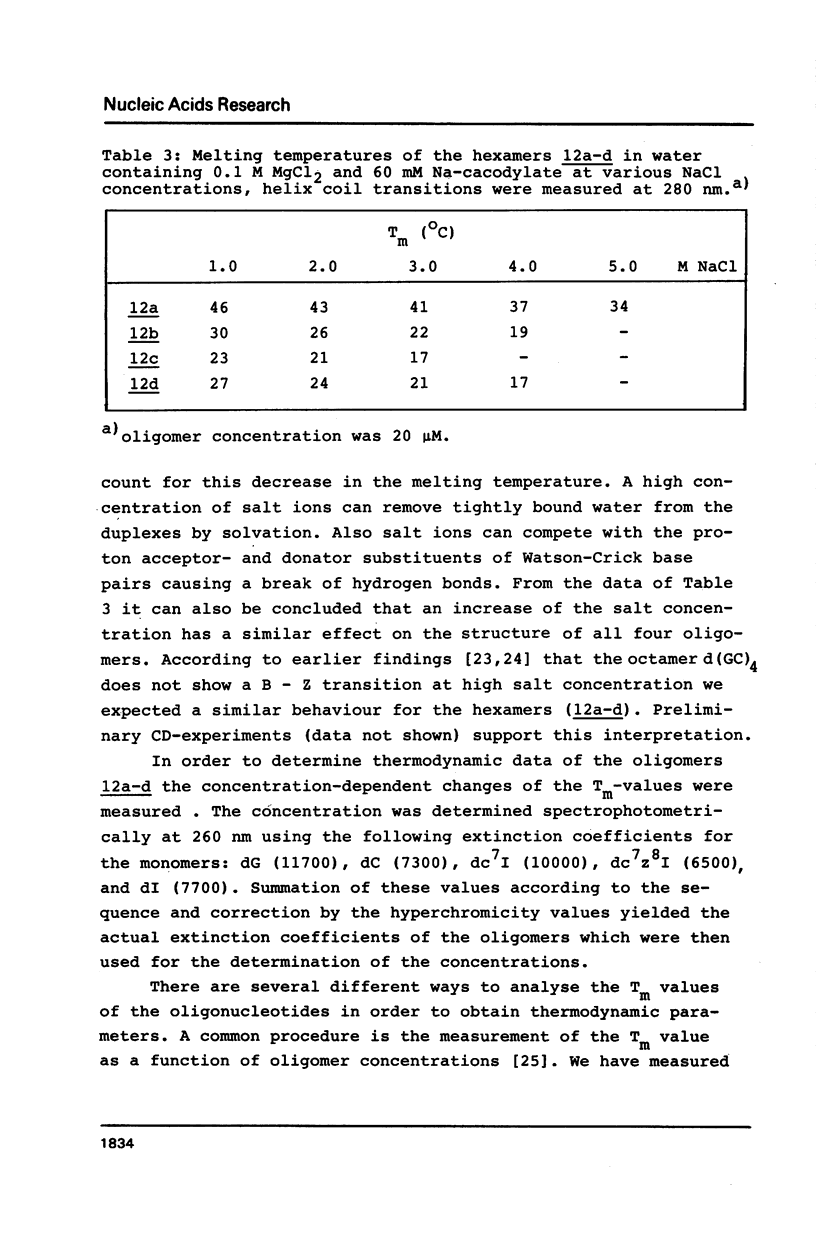

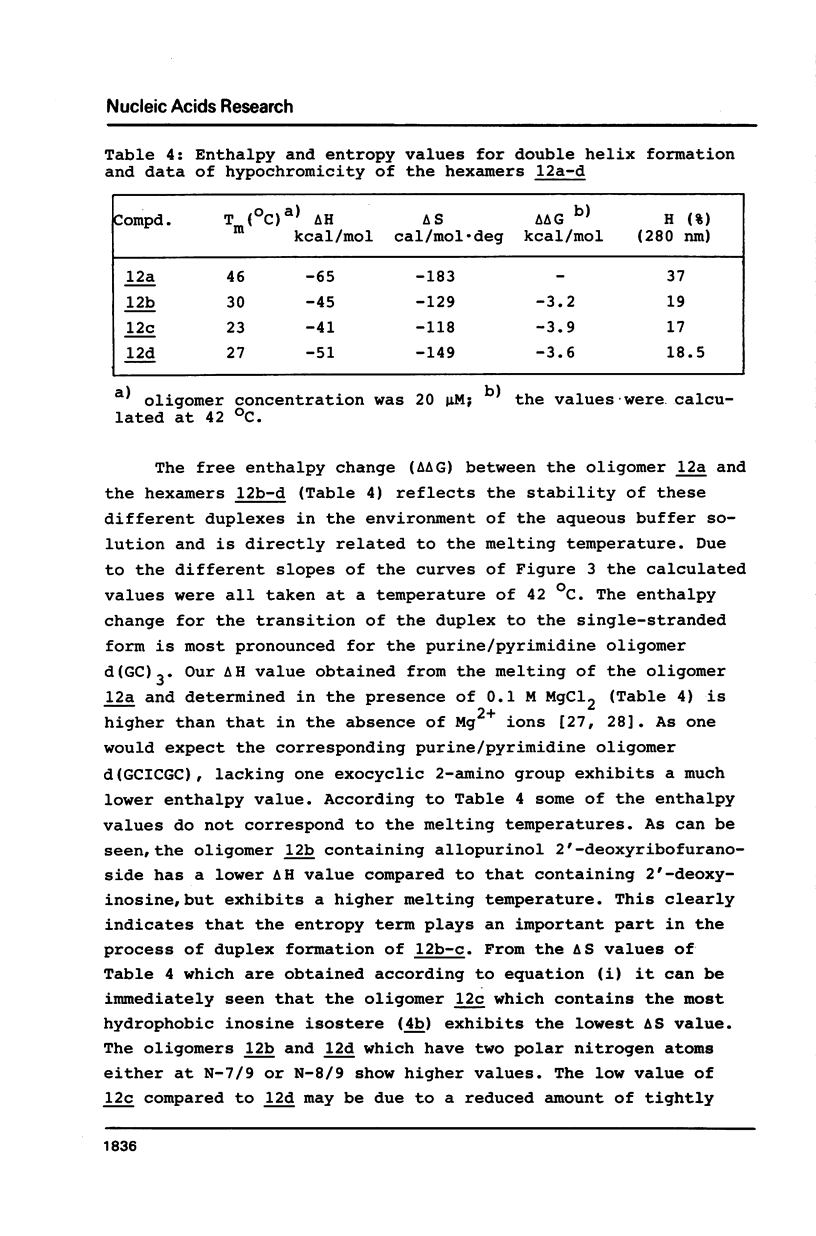








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albergo D. D., Marky L. A., Breslauer K. J., Turner D. H. Thermodynamics of (dG--dC)3 double-helix formation in water and deuterium oxide. Biochemistry. 1981 Mar 17;20(6):1409–1413. doi: 10.1021/bi00509a001. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Hubbard A. J., Jones A. S., Walker R. T. An investigation by 1H NMR spectroscopy into the factors determining the beta:alpha ratio of the product in 2'-deoxynucleoside synthesis. Nucleic Acids Res. 1984 Sep 11;12(17):6827–6837. doi: 10.1093/nar/12.17.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotick M. P., Szantay C., Bardos T. J. Synthesis of 5-S-substituted 2'-deoxyuridines. Study of the factors influencing stereoselectivity of the silyl modification of the Hilbert-Johnson reaction. J Org Chem. 1969 Dec;34(12):3806–3813. doi: 10.1021/jo01264a015. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Pohl F. M. Thermodynamics of the helix-coil transition of (dG-dC) oligomers. Eur J Biochem. 1974 Mar 1;42(2):495–504. doi: 10.1111/j.1432-1033.1974.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Seela F., Driller H. Solid-phase synthesis of the self-complementary hexamer d(c7GpCpc7GpCpc7GpC) via the O-3'-phosphoramidite of 7-deaza-2'-deoxyguanosine. Nucleic Acids Res. 1985 Feb 11;13(3):911–926. doi: 10.1093/nar/13.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. A., Peticolas W. L. Sequence dependence of conformations of self-complementary duplex tetradeoxynucleotides containing cytosine and guanine. Biochemistry. 1984 Jul 3;23(14):3202–3207. doi: 10.1021/bi00309a014. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Base pairing and fidelity in codon-anticodon interaction. Nature. 1976 Sep 23;263(5575):289–293. doi: 10.1038/263289a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]


