Abstract
T4 polynucleotide kinase rapidly loses activity during its reaction on duplex DNA termini. Addition of high concentrations of nonspecific polymers reverses or prevents this inactivation. In contrast, additions of related materials of lower molecular weight are relatively ineffective in stabilizing the kinase. Such a pattern suggests that the stabilizing effects of polymers on kinase activity are due to macromolecular crowding. An effect of crowding on the known tendency of the kinase to undergo oligomerization reactions is consistent with our observations.
Full text
PDF

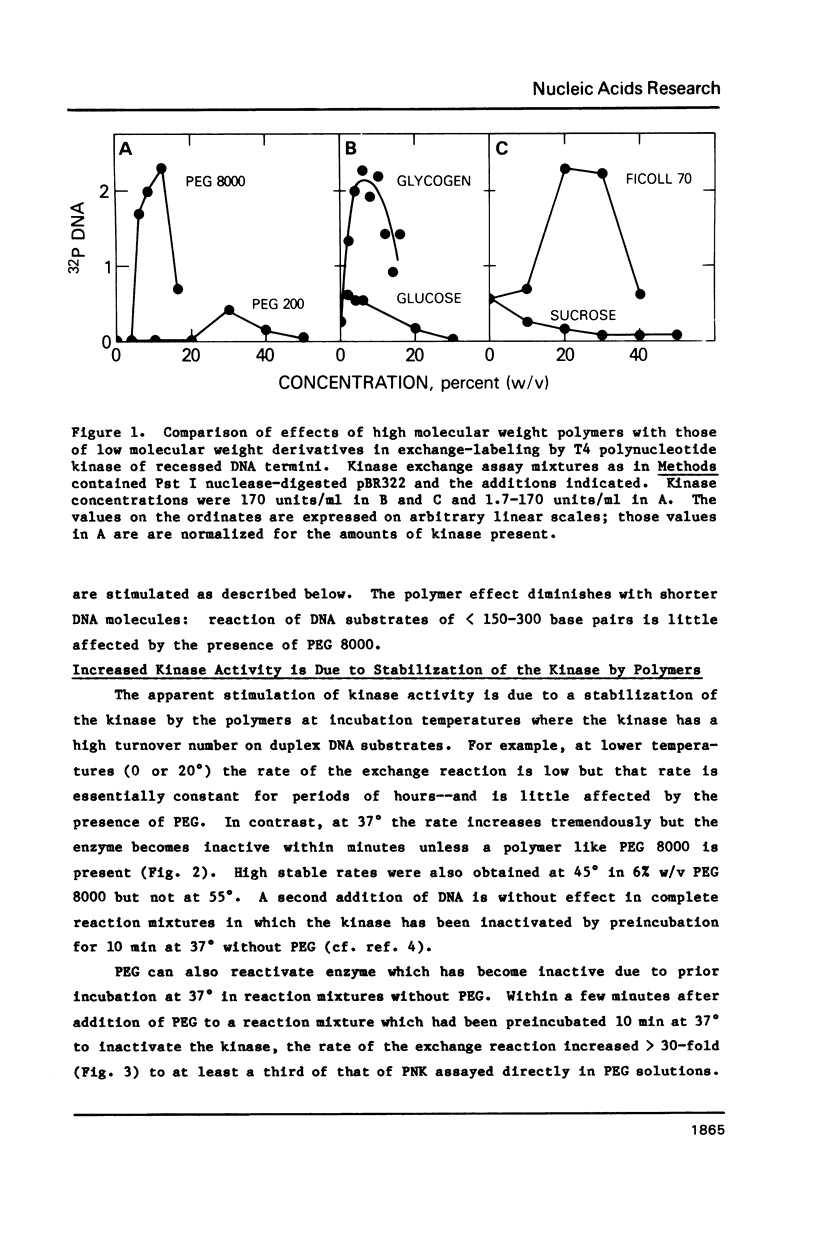
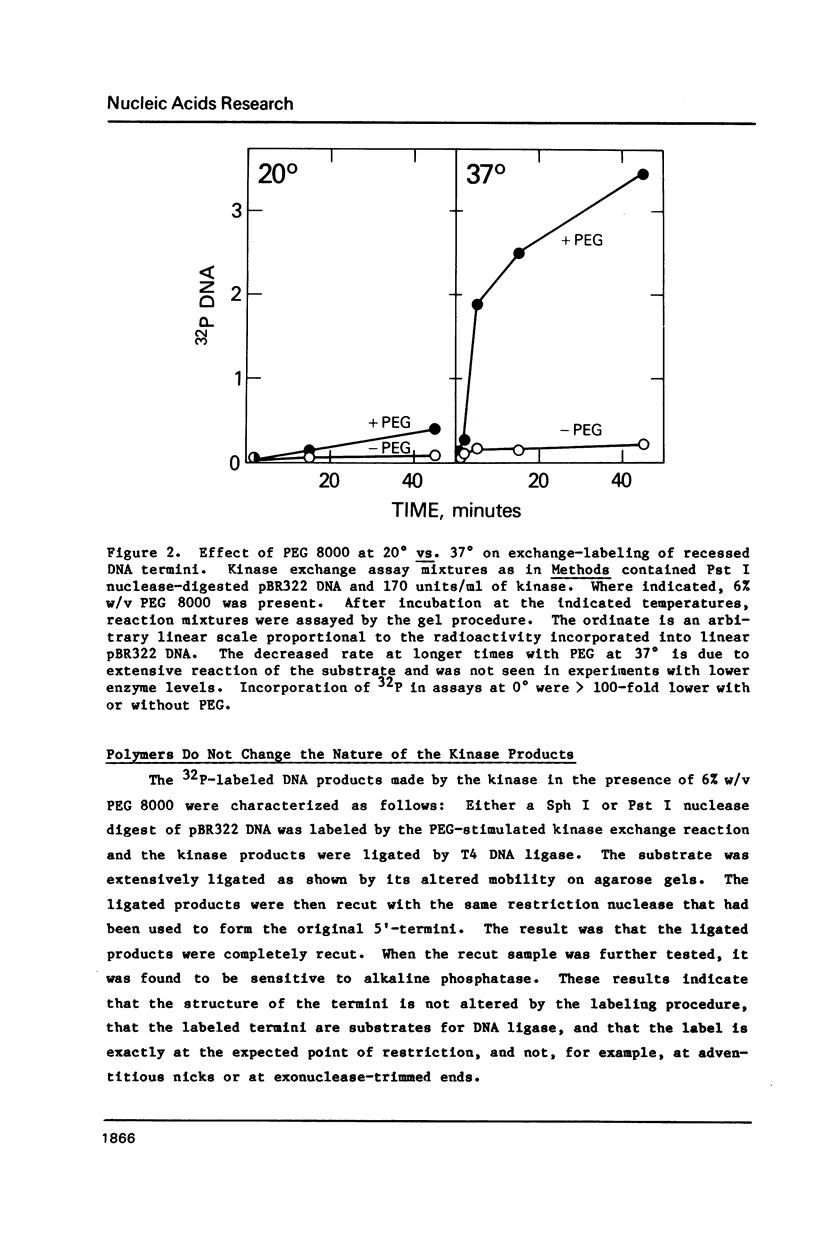
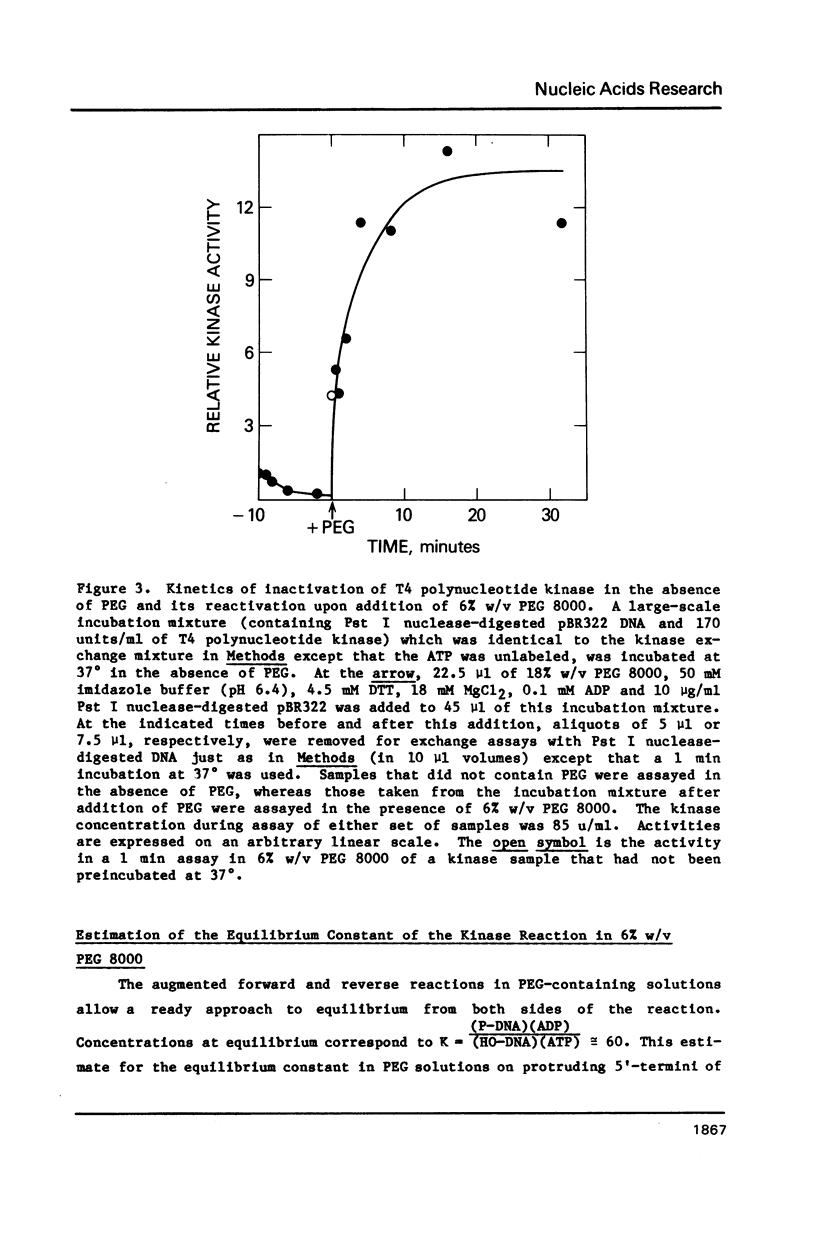



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Quantitation of the various termini generated by type II restriction endonucleases using the polynucleotide kinase exchange reaction. J Biol Chem. 1979 Apr 10;254(7):2561–2564. [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe K. Effect of salts and polyamines on T4 polynucleotide kinase. Biochemistry. 1975 Mar 25;14(6):1225–1229. doi: 10.1021/bi00677a021. [DOI] [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe R. K., Kleppe K. Phosphorylation of double-stranded DNAs by T4 polynucleotide kinase. Biochemistry. 1976 May 4;15(9):1858–1865. doi: 10.1021/bi00654a011. [DOI] [PubMed] [Google Scholar]
- Lillehaug J. R. Physicochemical properties of T4 polynucleotide kinase. Eur J Biochem. 1977 Mar 1;73(2):499–506. doi: 10.1111/j.1432-1033.1977.tb11343.x. [DOI] [PubMed] [Google Scholar]
- Minton A. P. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55(2):119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Minton K. W., Karmin P., Hahn G. M., Minton A. P. Nonspecific stabilization of stress-susceptible proteins by stress-resistant proteins: a model for the biological role of heat shock proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7107–7111. doi: 10.1073/pnas.79.23.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Tal M., Traub A., Hurwitz J. The enzymatic phosphorylation of ribonucleic acid and deoxyribonucleic acid. II. Further properties of the 5'-hydroxyl polynucleotide kinase. J Biol Chem. 1966 Jun 25;241(12):2933–2943. [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H. Kinetic studies on the reaction catalyzed by polynucleotide kinase from phage T4-infected Escherichia coli. Biochim Biophys Acta. 1976 Jan 23;422(1):109–119. doi: 10.1016/0005-2744(76)90012-7. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Kleppe K., Khorana H. G. Reversal of bacteriophage T4 induced polynucleotide kinase action. Biochemistry. 1973 Dec 4;12(25):5050–5055. doi: 10.1021/bi00749a004. [DOI] [PubMed] [Google Scholar]


