Summary
The purpose of this study was to evaluate and report our anatomic results and complications associated with stent-assisted coil embolization of intracranial aneurysms using the Neuroform stent. From September 2003 to August 2007, 127 consecutive patients (ruptured 50, 39.4%; unruptured 77, 60.6%) underwent 129 stent-assisted coil embolization procedures to treat 136 aneurysms at our institution. Anatomic results at follow-up, procedure-related complications, and morbidity/mortality were retrospectively reviewed.
Stent deployment was successful in 128 out of 129 procedures (99.2%). Forty-seven patients presented with 53 procedure-related complications (37.0%, 47/127). Thromboembolic events (n=17, 13.4%) were the most common complications, followed by intraoperative rupture (n=8, 6.3%), coil herniation (n=5, 3.9%), and postoperative rupture (n=4, 3.1%). For thromboembolic events, acute intra-procedural in-stent thromboses were observed in two patients and subacute or delayed in-stent thromboses in three patients. Overall mortality rate was 16.5% (21/127) and procedure-related morbidity and mortality rates were 5.5% (7/127) and 8.7% (11/127) retrospectively. Patients with poor grade subarachnoid hemorrhage (Hunt and Hess grade IV or V; 25/127, 19.7%) exhibited 56% (14/25) overall mortality rate and 24% (6/25) procedure-related mortality rate. Immediate angiographic results showed complete occlusion in 31.7% of aneurysms, near-complete occlusion in 45.5%, and partial occlusion in 22.8%. Sixty nine patients in 70 procedures with 77 aneurysms underwent angiographic follow-up at six months or later. Mean follow-up period was 13.7 months (6 to 45 months). Complete occlusion was observed in 57 aneurysms (74.0%) and significant in-stent stenosis was not found.
Thromboembolism and intra/postoperative aneurysm ruptures were the most common complications and the main causes of procedure-related morbidity and mortality. Patients with poor grade subarachnoid hemorrhage showed poor clinical outcomes. Since most complications were induced by stent manipulation and deployment, it is mandatory to utilize these devices selectively and cautiously. While the follow-up angiographic results are promising, further studies are essential to evaluate safety, efficacy, and durability of the Neuroform stent.
Key words: stent, aneurysm, coil, Neuroform, complication
Introduction
Endovascular embolization of cerebral aneurysms using detachable coils, first introduced by Guglielmi1, has become a safe and effective alternative to direct surgical clipping2-9. Endovascular treatment of wide-necked and large/giant (>10 mm) aneurysms, however, remains challenging in spite of technical advancements10-13. Published complete occlusion rates of wide-necked and large/giant aneurysms ranges from 25 to 56.7%11,13-18. To improve treatment outcomes in these difficult cases, new devices and techniques such as three-dimensional coils, neck-bridging devices, multiple-microcatheter techniques, and balloon-assisted neck-remodeling techniques have been utilized19-24.
In the past, many clinicians utilized coronary stents for the endovascular treatment of wide-necked intracranial aneurysms 25-31. The device’s physical properties as well as the tortuous intracranial vasculature limited the use of coronary stents. The Neuroform stent (Boston Scientific/Target, Fremont, CA) answered the need for an aneurysmal neck-bridging device specifically designed for intracranial use. This self-expanding stent has a high degree of flexibility, trackability and conformability which facilitates movement through the intracranial vasculature. The purpose of this study is to evaluate and report our results and complications associated with stent-assisted coil embolization of intracranial aneurysms using the Neuroform stent.
Methods
From September 2003 to August 2007, a total of 129 stent-assisted coil embolization procedures were performed to treat 136 aneurysms in 127 consecutive patients at the University of Pittsburgh Medical Center’s Presbyterian Hospital. We retrospectively reviewed the medical records, radiographic studies and endovascular procedure reports for each case. Institutional Review Board (IRB) approval and informed consent were obtained from all the patients prior to aneurysm treatment.
Thirty one men (24.4%) and 97 women (75.6%) were included in this study. Mean age was 55.6 years with a range of 21 to 84. Nineteen patients (15.0%) were over 70 years old. Fifty patients (39.4%, 50/127) presented with subarachnoid hemorrhage (SAH) from a ruptured aneurysm, resulting in 52 procedures (40.3%, 52/129) for the treatment of 54 aneurysms (39.7%, 54/136). Forty-seven of these patients underwent one stent deployment procedure for a single aneurysm. In the setting of SAH with multiple aneurysms, identification of the ruptured aneurysm was based on clinical, CT and angiographic findings32-34. If multiple aneurysms were identified of similar size and shape at the same site in the face of SAH, all were regarded as ruptured and treated at the same time. Two patients, one with a middle cerebral artery (MCA) bifurcation aneurysm and a basilar apex (BA) aneurysm and the other with a posterior communicating (PCom) artery aneurysm and a BA aneurysm, underwent stent-assisted coil embolization for each aneurysm in a single session. Another patient with three anterior communicating (ACom) artery aneurysms underwent a single procedure for multiple aneurysms.
In 77 patients (60.6%, 77/127) in 77 procedures (59.7%, 77/129) with 82 unruptured aneurysms (60.3%, 82/136), 72 patients underwent one stent deployment procedure for a single aneurysm. Five patients (one patient with a BA aneurysm and a left superior cerebellar artery (SCA) aneurysm, three patients with two paraclinoid aneurysms, and one patient with a PCom artery aneurysm and a paraclinoid aneurysm) underwent one stent deployment across multiple aneurysms.
Aneurysm locations are reported in Table 1. Ninety-three aneurysms (68.4%, 93/136) were located in the anterior and 43 (31.2%, 43/136) in the posterior circulation. Fifty-four aneurysms (39.7%, 54/136) were ruptured and 82 (60.3%, 82/136) were unruptured. The most common location for ruptured aneurysms was the BA (n=12, 22.2%), followed by the PCom artery (n=9, 16.7%) and the MCA bifurcation (n=7, 13.0%). For unruptured aneurysms, the most common location was the paraclinoid internal carotid artery (n=32, 39.0%), followed by the PCom artery (n=12, 14.6%) and the BA (n=11, 13.4%). Overall, the most common location was the paraclinoid internal carotid artery (n=35, 25.7%), followed by the BA (n=23, 16.9%) and the PCom artery (n=21, 15.4%). One hundred and fourteen (83.8%, 114/136) of the aneurysms were treated de novo whereas 22 (16.2%, 22/136) represented previously-treated residual/recanalized lesions. Seventeen residual/recanalized aneurysms had been previously treated using coiling, four had undergone direct surgical clipping, and one had both. In the 22 residual/recanalized aneurysms, seven (31.8%) presented with SAH in this study (at the time of stent-assisted coil embolization) whereas 15 represented unruptured lesions. The most common location of the residual/recanalized aneurysm was the basilar apex (27.3%, 6/22), followed by the MCA bifurcation (18.2%, 4/22) and the paraclinoid internal carotid artery (ICA) (18.2%, 4/22). The location harboring the most residual/recanalization by volume was the MCA bifurcation (4/11, 36.4%), followed by the BA (6/23, 26.1%; Table 1).
Table 1.
Location of aneurysms.
| Location | Number | Number | Number |
|---|---|---|---|
| of total aneurysms | of ruptured aneurysms | of unruptured aneurysms | |
| (Number of previously | (Number of previously | (Number of previously | |
| treated aneurysms) | treated aneurysms) | treated aneurysms) | |
| n=136 (22) | n=54 (7) | n=82 (15) | |
|
| |||
| ACA/distal | 3 (1) | 2 (1) | 1 |
|
| |||
| ACA/ACom | 10 (1) | 6 | 4 (1) |
|
| |||
| ACA/A1 | 1 | 0 | 1 |
|
| |||
| MCA/bifurcation | 11 (4) | 7 (2) | 4 (2) |
|
| |||
| MCA/M1 | 1 | 0 | 1 |
|
| |||
| ICA/bifurcation | 7 (2) | 2 (1) | 5 (1) |
|
| |||
| ICA/anterior choroidal | 2 | 2 | 0 |
|
| |||
| ICA/PCom | 21 (3) | 9 | 12 (3) |
|
| |||
| ICA/paraclinoid | 35 (4) | 3 | 32 (4) |
|
| |||
| ICA/cavernous | 2 | 0 | 2 |
|
| |||
| BA/apex | 23 (6) | 12* (3) | 11 (3) |
|
| |||
| BA/SCA | 6 (1) | 1 | 5 (1) |
|
| |||
| BA/trunk | 3 | 1 | 2 |
|
| |||
| VB | 3 | 3 | 0 |
|
| |||
| V4 | 1 | 1 | 0 |
|
| |||
| V4/PICA | 7 | 5 | 2 |
|
| |||
| ACA, anterior cerebral artery; ACom, anterior communicating artery; a1, A1 segment of ACA; MCA, middle cerebral artery; | |||
| M1, M1 segment of MCA; ICA, internal carotid artery; PCom, posterior communicating artery; BA, basilar artery; | |||
| SCA, superior cerebellar artery; VBJ, vertebrobasilar junction; V4, intracranial vertebral artery; PICA, posterior cerebellar artery | |||
| *; two unruptured de novo aneurysms in the face of subarachnoid hemorrhage were included. | |||
Aneurysm geometry was evaluated by digital subtraction angiography and, if necessary, a three-dimensional reconstruction. All aneurysms were wide-necked, defined as a neck diameter >4 mm or aspect ratio <2. Aneurysms were measured according to their greatest diameter. Ninety-nine aneurysms (72.8%; ruptured 38, unruptured 61) were small (<10mm), 32 aneurysms (23.5%; ruptured 14, unruptured 18) were large (>10 mm, <25 mm), and five aneurysms (3.7%; ruptured 2, unruptured 3) were giant (>25 mm).
In 50 patients with SAH, initial clinical status was assessed using the Hunt and Hess grade system.35 Six patients were grade I (12%); eleven grade II (22%); eight grade III (16%); sixteen grade IV (32%); and nine grade V (18%) (Table 2). In all patients, clinical outcomes were measured using the modified Rankin scale (mRS) which was obtained on admission day, three days post-procedure, and again at 90 days (Tables 2 and 3).
Table 2.
Clinical outcomes in patients with ruptured aneurysms (n=50).
| H-H | mRS 3 day | mRS 90 day | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| scale | n | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|
| |||||||||||||||
| I | 6 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | ||||||
|
| |||||||||||||||
| II | 11 | 7 | 1 | 1 | 1 | 1 | 8 | 1 | 1 | 1 | |||||
|
| |||||||||||||||
| III | 8 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | |||
|
| |||||||||||||||
| IV | 16 | 1 | 1 | 10 | 4 | 1 | 1 | 3 | 2 | 9 | |||||
|
| |||||||||||||||
| V | 9 | 6 | 3 | 1 | 2 | 1 | 5 | ||||||||
|
| |||||||||||||||
| H-H, Hunt and Hess grade; mRS, modified Rankin Scale | |||||||||||||||
Table 3.
Clinical outcomes in patients with unruptured aneurysms (n=77).
| mRS 0 day | mRS 3 day | mRS 90 day | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| score | n | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|
| |||||||||||||||
| 0 | 53 | 49 | 1 | 1 | 2 | 48 | 1 | 1 | 2 | 1 | |||||
|
| |||||||||||||||
| 1 | 8 | 7 | 1 | 7 | 1 | ||||||||||
|
| |||||||||||||||
| 2 | 11 | 8 | 2 | 1 | 1 | 9 | 1 | ||||||||
|
| |||||||||||||||
| 3 | 5 | 3 | 2 | 4 | 1 | ||||||||||
|
| |||||||||||||||
| 4 | |||||||||||||||
|
| |||||||||||||||
| 5 | |||||||||||||||
|
| |||||||||||||||
| 6 | |||||||||||||||
|
| |||||||||||||||
| mRS, modified Rankin Scale | |||||||||||||||
In patients with unruptured aneurysms, a dual antiplatelet regimen consisting of 325 mg of aspirin and 75 mg of clopidogrel (Plavix; Bristol-Meyers Squibb/Sanofi Pharmaceuticals, New York, NY) was administered orally for two days prior to the procedure. All procedures were performed under full heparinization with an activated clotting time of approximately 250 seconds. In patients with SAH, heparin was given after sheath placement to maintain an activated clotting time of 200-250 seconds. Fifteen milligrams (mg) of eptifibatide (Integrilin; Schering, Kenilworth, NJ) were administered intravenously once the stent was deployed and a loading dose of clopidogrel (600 mg) was given at the procedure’s conclusion via a naso- or orogastric tube. Heparin (500 units per hour IV) was continued for the next 12-15 hours in most cases. Patients continued taking 75 mg of clopidogrel daily for one month after the stenting procedure and 325 mg of aspirin for life.
All procedures were performed with patients under general anesthesia. Neurophysiologic monitoring was used for all cases, which included somatosensory evoked potentials, electroencephalography, and brain stem auditory evoked potentials. The right femoral approach was routinely used for placement of a 6F Envoy (Cordis, Miami, FL) or 7F Shuttle (Cook, Bloomington, IN) catheter. Microcatheters (Rapid Transit; Cordis, Miami, FL; Echelon; ev3, Irvine, CA) and Nexus/NXT (ev3, Irvine, CA) and/or Micrus (Micrus Co., Mountain View, CA) coils were primarily used. Cerebral angiography was performed to evaluate aneurysm morphology and to establish working projections. Typically, the undeployed stent was positioned across the aneurysms neck followed by placement of the microcatheter into the aneurysm fundus. The first coil was partially deployed into the aneurysm without detachment so as to anchor the microcatheter in place. Once the microcatheter was jailed by deploying the stent, the first coil was fully advanced and detached. The Neuroform stent was usually advanced over an exchange wire (Transcend 14/300 cm; Boston Scientific/Target, Fremont, CA; Accelerator 14/300cm, ev3, Irvine, CA) and deployed using either the provided pusher system or a coil pusher. All coils were introduced into the aneurysm by using simultaneous biplanar imaging. Coil placement proceeded until no additional coils could be placed, until angiographically complete occlusion, or until risks outweighed perceived benefits. Once the stent was deployed or shortly thereafter, 15 mg of eptifibatide was given intravenously unless contraindicated (i.e., intraoperative rupture).
In a total of 127 patients in 129 procedures with 136 aneurysms, 114 patients in 116 procedures with 123 aneurysms were treated with simultaneous stent deployment and aneurysmal coil embolization in the first session. One patient, complicated by uncontrolled intraoperative rupture, underwent Neuroform stent deployment and embolization with Onyx-18 (ev3, Irvine, CA). In one patient with complications related to the failure of Neuroform stent deployment, a Wingspan stent (Boston Scientific/Target, Fremont, CA) was deployed to pin herniated coil loops. In 11 patients in 11 procedures with 11 aneurysms, the stent was deployed in the first session and coil embolization was performed in the next session (Table 4). Seventeen patients were treated in a multi-staged fashion (Table 4). Fourteen patients underwent two sessions, two patients three sessions, and one patient four sessions.
Table 4.
Summary of cases treated in multiple stages.
| SAH | Size | Previous | Interval | Interval to | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/Sex | (H-H, | Location | Treatment/ | 1st | Immediate | to Next | Next | Immediate | Remark | Follow/up | Follow/up | |
| Fisher) | Interval | Session | Result | Sessions | Sessions | Result | (months) | Result | ||||
| (months) | (months) | |||||||||||
|
| ||||||||||||
| 49 F | 0 | L | BA | 0 | STENT | STENT | 1 | 2nd | PO | 6 | CO | |
| TRUNK | ||||||||||||
|
| ||||||||||||
| Fusiform, | ||||||||||||
| 2nd: STENT | 19mm | |||||||||||
| 40 M | 0 | L | PCOM | 0 | STENT | STENT | 1, 3 | 2nd, 3rd | AGAIN | posttraumatic, | 14 | No residual |
| 3rd: Clipping | BTO: not | |||||||||||
| tolerable | ||||||||||||
|
| ||||||||||||
| 25 M | 0 | L | paraclinoid | 0 | STENT | PO | 1 | 2nd | NC | 9, 23 | CO, CO | |
| + COIL | ||||||||||||
|
| ||||||||||||
| 38 F | 0 | S | paraclinoid | 0 | STENT | PO | 1 | 2nd | NC | 2, 6 | CO, CO | |
| + COIL | ||||||||||||
|
| ||||||||||||
| 57 F | 0 | S | paraclinoid | 0 | STENT | PO | 2 | 2nd | NC | 2, 18 | CO, CO | |
| + COIL | ||||||||||||
|
| ||||||||||||
| 43 F | 0 | S | ACOM | 0 | STENT | PO | 2 | 2nd | NC | |||
| + COIL | ||||||||||||
|
| ||||||||||||
| 48 F | 0 | S | paraclinoid | CLIPPING | STENT | PO | 3 | 2nd | PO | 5, 17 | CO, CO | |
| 149 | + COIL | |||||||||||
|
| ||||||||||||
| 30 M | 0 | S | MCAB | COILING | STENT | STENT | 1 | 2nd | NC | 6 | CO | |
| 14 | ||||||||||||
|
| ||||||||||||
| 53 F | 0 | L | paraclinoid | 0 | STENT | STENT | 1 | 2nd | NC | 7 | CO | |
|
| ||||||||||||
| 61 F | 0 | S | paraclinoid | COILING | STENT | STENT | 1 | 2nd | NC | 5, 11 | CO | |
| 6 | Recanalized | |||||||||||
|
| ||||||||||||
| 2nd: stent | ||||||||||||
| navigation | Deceased | |||||||||||
| 65 F | 0 | G | ICAB | 0 | STENT | STENT | 7 days, | 2nd, 3rd | failure | POD1 in 3rd | ||
| 13 days | 3rd: coiling, | session | ||||||||||
| intraoperative | ||||||||||||
| rupture | ||||||||||||
|
| ||||||||||||
| 62 F | 0 | S | paraclinoid | COILING | STENT | STENT | 2 | 2nd | NC | 3, 14, 26 | CO, CO, CO | |
| 11 | ||||||||||||
|
| ||||||||||||
| 56 F | 0 | L | paraclinoid | COILING | STENT | STENT | 2 | 2nd | NC | 6 | Recanalized | |
| 99, 92 | ||||||||||||
|
| ||||||||||||
| 55 M | Yes | G | ICAB | 0 | STENT | PO | 4 days, | 2nd, 3rd, | PO, PO, PO | Partially | 6 | Recanalized |
| (V, 4) | + COIL | 5, 8 | 4th | thrombosed | ||||||||
| giant aneurysm | ||||||||||||
|
| ||||||||||||
| CLIPPING | ||||||||||||
| 46 M | Yes | S | MCAB | 5, 1 | STENT | PO | 6 days | 2nd | STENT | small residual | 8 | NC |
| (I, 4) | COILING | AGAIN | sac: 2mm | |||||||||
| 4 days (PO) | ||||||||||||
|
| ||||||||||||
| 51 F | 0 | S | PCOM | COILING | STENT | STENT | 2 | 2nd | no treatment | decrease of | ||
| 14 | aneurysm size | |||||||||||
|
| ||||||||||||
| 44 F | 0 | S | BA APEX | COILING | STENT | STENT | 3 | 2nd | no treatment | decrease of | ||
| 25 | aneurysm size | |||||||||||
|
| ||||||||||||
| SAH, subarachnoid hemorrhage; H-H, Hunt and Hess grade; Fisher, Fisher grade; S, small; L, large; G, giant; | ||||||||||||
| ACOM, anterior communicating artery; BA APEX, basilar artery apex; BA TRUNK, basilar artery trunk; | ||||||||||||
| ICAB, internal carotid artery bifurcation; MCAB, middle cerebral artery bifurcation; PCOM, posterior communicating artery; | ||||||||||||
| CO, complete occlusion; NC, near-complete occlusion; PO, partial occlusion; BTO, balloon test occlusion; POD, postoperative day | ||||||||||||
The degree of aneurysm occlusion was classified into three subgroups: complete occlusion (CO) when the sac and the neck were densely packed, near-complete occlusion (NC) when the sac was occluded but a neck remnant was suspected, and partial occlusion (PO) when loose packing or opacification of the sac or the neck persisted (Figure 1). Angiographic follow-up at six months or later was obtained in 69 patients in 70 procedures with 77 aneurysms. Mean follow-up period was 13.7 months with a range from six to 45 months.
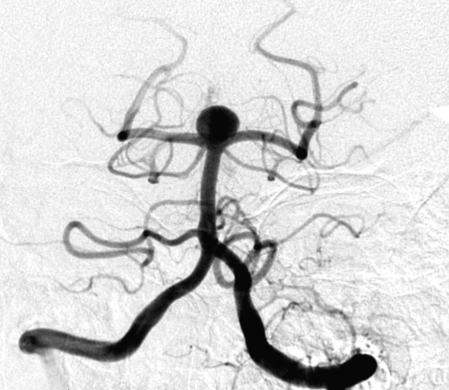
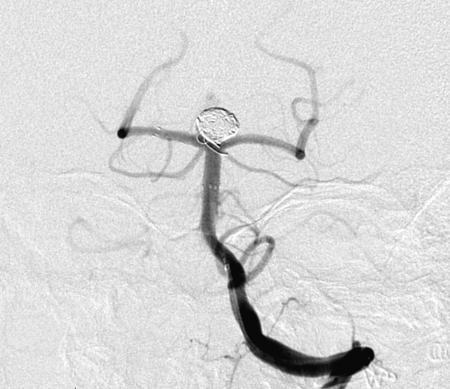
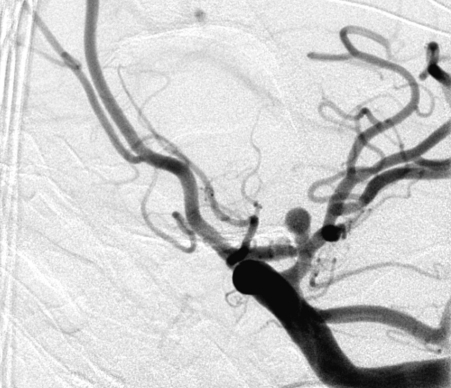
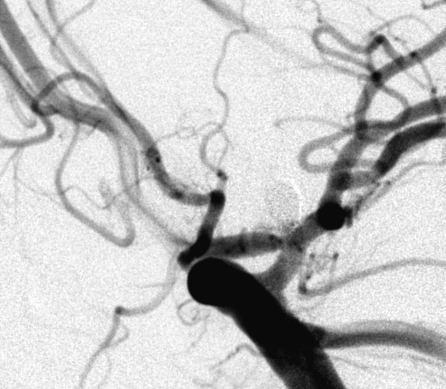
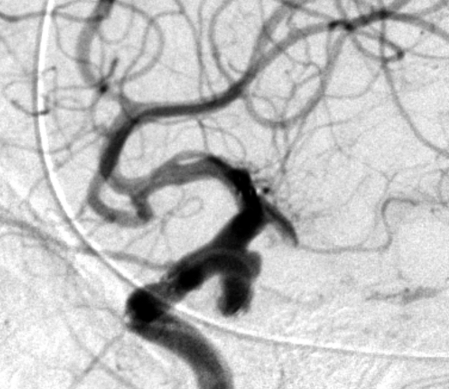
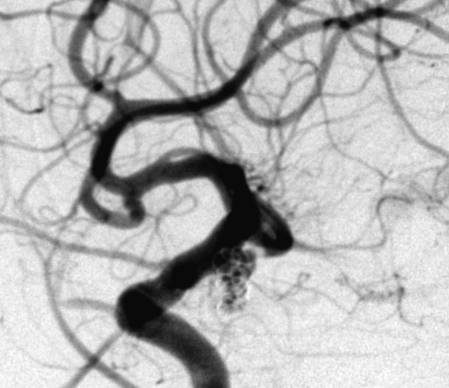
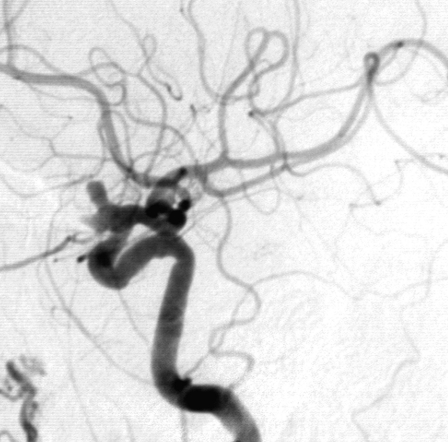
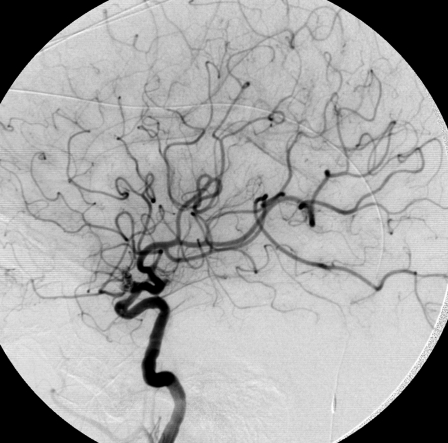
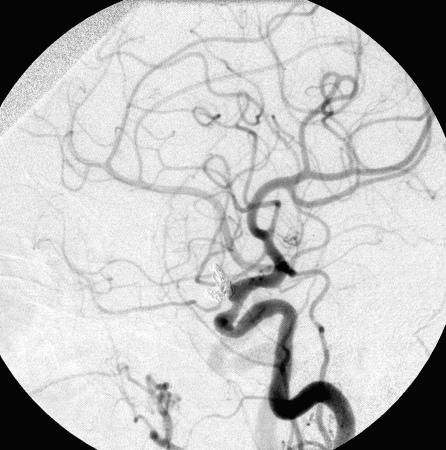
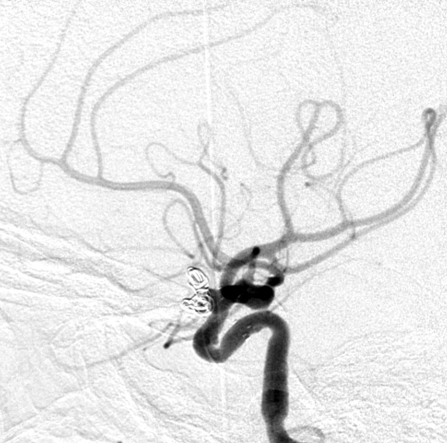
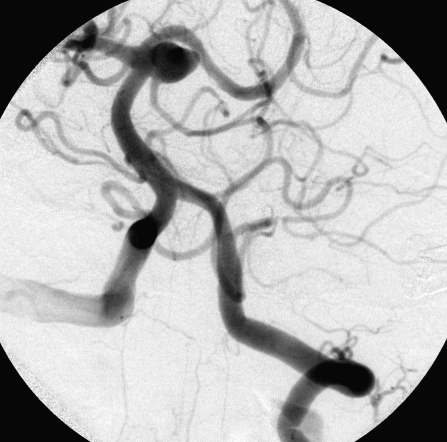
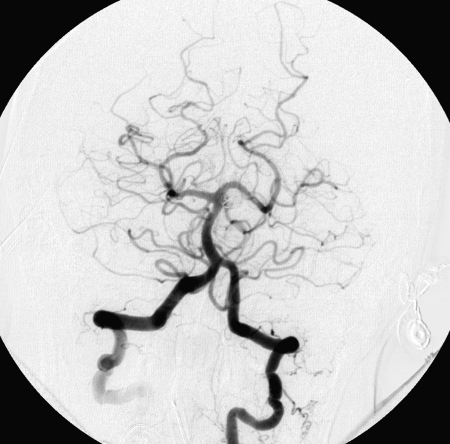
Figure 1.
Complete occlusion: Preoperative (A) and postoperative (B) angiogram. Near-complete occlusion: Preoperative (C) and postoperative (D) angiogram. Partial occlusion: Preoperative (E) and postoperative (F) angiogram.
A.
B.
C.
D.
E.
F.
All cases in which procedure-related complications occurred were noted. Procedure-related morbidity was defined as a complication leading to neurologic sequelae which persisted for at least 90 days after the procedure and/or a modified Rankin scale score that was higher at that time as compared to the initial score. Radiologic findings, clinical presentations, treatments, and clinical outcomes were analyzed.
Results
Immediate Angiographic Results
A Neuroform stent was successfully deployed in 126 patients in 128 procedures with 135 aneurysms out of 127 patients in 129 procedures with 136 aneurysms. In a total of 129 procedures, the technical success rate was 99.2% (128/129). Simultaneous stent deployment and aneurysmal coil embolization were conducted in 114 patients in 116 procedures on 123 aneurysms in the first session. In these patients, complete aneurysm occlusion was observed in 39 aneurysms (31.7%), near-complete occlusion in 56 (45.5%), and partial occlusion in 28 (22.8%).
Procedure-Related Complications
In a total of 127 patients, 47 patients exhibited 53 procedure-related complications for an overall complication rate of 37.0% (47/127). Among the 50 patients with ruptured aneurysms/SAH, 23 exhibited 26 complications for a complication rate of 46.0% (23/50). In the 77 patients with unruptured aneurysms, 24 exhibited 27 complications for a complication rate of 31.2% (24/77). Thromboembolism was the most common overall complication (13.4%, 17/127), followed by intraoperative rupture (6.3%, 8/127), coil herniation (3.9%, 5/127), postoperative rupture (3.1%, 4/127), catheter-induced vasospasm (3.1%), and stent migration (3.1%). In patients with ruptured aneurysms/SAH, thromboembolism was the most common complication (16.0%, 8/50), followed by intraoperative rupture (14.0%, 7/50), stent migration (6.0%, 3/50), and postoperative rupture (4.0%, 2/50). In patients with unruptured aneurysms, thromboembolism was also the most common (11.7%, 9/77), followed by coil herniation (5.2%, 4/77) and catheter-induced vasospasm (3.9%, 3/77) (Table 5).
Table 5.
Procedure-related complications.
| Complication | Number of cases | Incidence Rate | Morbidity (n=7) / Mortality (n=11) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rates from Complications | |||||||||
|
| |||||||||
| Total | Ruptured | Unruptured | Total | Ruptured | Unruptured | Total | Ruptured | Unruptured | |
| (n=53) | (n=26) | (n=27) | (n/127) | (n/50) | (n/77) | (n, n/127) | (n, n/50) | (n, n/77) | |
|
| |||||||||
| Thromboembolism | 17 | 8 | 9 | 13.4% | 16.0% | 11.7% | 5, 3.9% | 2, 4% | 3, 3.9% |
| 1, 0.8% | 1, 2% | ||||||||
|
| |||||||||
| Intraoperative | 8 | 7 | 1 | 6.3% | 14.0% | 1.3% | 7, 5.5% | 6, 12% | 1, 1.3% |
| rupture | |||||||||
|
| |||||||||
| Coil herniation | 5 | 1 | 4 | 3.9% | 2.0% | 5.2% | 1*, 0.8% | 1*, 2% | |
| through stent | |||||||||
|
| |||||||||
| Postoperative | 4 | 2 | 2 | 3.1% | 4.0% | 2.6% | 1, 0.8% | 2, 4% | 1, 1.3% |
| rupture | 3, 2.4% | 1, 1.3% | |||||||
|
| |||||||||
| Catheter-induced | 4 | 1 | 3 | 3.1% | 2.0% | 3.9% | |||
| vasospasm | |||||||||
|
| |||||||||
| Stent migration | 4 | 3 | 1 | 3.1% | 6.0% | 1.3% | |||
|
| |||||||||
| Iatrogenic dissection | 3 | 1 | 2 | 2.4% | 2.0% | 2.6% | |||
|
| |||||||||
| Coil fracture | 2 | 1 | 1 | 1.6% | 2.0% | 1.3% | |||
|
| |||||||||
| Inappropriate stent | 2 | 0 | 2 | 1.6% | 0 | 2.6% | |||
| deployment | |||||||||
|
| |||||||||
| Failure in | 1 | 0 | 1 | 0.8% | 0 | 1.3% | |||
| microcatheter | |||||||||
| placement after | |||||||||
| stenting | |||||||||
|
| |||||||||
| CCF | 1 | 1 | 0 | 0.8% | 2.0% | 0 | |||
|
| |||||||||
| ICH | 1 | 1 | 0 | 0.8% | 2.0% | 0 | 1, 0.8% | 1, 2% | |
|
| |||||||||
| Cranial nerve palsy | 1 | 0 | 1 | 0.8% | 0 | 1.3% | |||
|
| |||||||||
| CCF, carotid-cavernous fistula; ICH, intracerebral hemorrhage; *, one case of coil herniation led to thromboembolic event. | |||||||||
Clinical Outcome - Mortality
Clinical outcomes are summarized in Tables 2 and 3. In a total of 127 patients, overall mortality rate was 16.5% (21/127) and procedure-related mortality was 8.7% (11/127). In the 11 procedure-related mortalities, seven (63.6%) were due to intraoperative rupture, three (27.3%) were due to postoperative rupture, and one (9.1%) was due to thromboembolism. Table 6 summarizes the mortality cases.
Table 6.
Summary of mortality cases.
| Age/ | SAH | mRS | mRS | Systemic | Size | Location | Previous | Immediate | Major | Interval | Proce- |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | (H-H, | 0 | 3 | condition | Treatment/ | angiographic | Complications | to | dure | ||
| Fisher) | day | day | Interval | result | & Cause of Death | Death | -related | ||||
|
| |||||||||||
| 60/F | Yes | 0 | 6 | S | paraclinoid | NC | Postoperative | POD3 | Yes | ||
| (I, 2) | Rupture POD1 | ||||||||||
|
| |||||||||||
| 56/M | Yes | 1 | 6 | Hypertension | L | BA APEX | PO | Postoperative | POD1 | Yes | |
| (I, 2) | Coronary | Rupture POD0 | |||||||||
| Arterial Disease | |||||||||||
| Diabetes Mellitus | |||||||||||
|
| |||||||||||
| 80/F | Yes | 1 | 5 | Atrial Fibrillation | L | PCOM | Clipping | PO | ARDS | POD10 | |
| (III, 3) | Hypertension | 179 months | GI Bleeding | ||||||||
|
| |||||||||||
| 55/M | Yes | 2 | 1 | Hypertension | S | BA APEX | NC | Distal BA | POD16 | Yes | |
| (III, 4) | & bilateral PCA | ||||||||||
| Occlusion POD8 | |||||||||||
|
| |||||||||||
| 71/F | Yes | 4 | 5 | Hypertension | L | ACOM | CO | Metabolic | POD3 | ||
| (IV, 4) | Encephalopathy | ||||||||||
|
| |||||||||||
| 52/F | Yes | 5 | 5 | Rebled x 2 | G | paraclinoid | NC | Intraoperative | POD5 | Yes | |
| (IV, 3) | Rupture x 2 | ||||||||||
|
| |||||||||||
| 81/F | Yes | 5 | 5 | Hypertension | S | PCOM | Coiling, 65, | CO | IICP | POD9 | |
| (IV, 4) | 53 months | ARF | |||||||||
|
| |||||||||||
| 44/M | Yes | 5 | 5 | Hypertension | L | BA APEX | CO | IICP | POD6 | ||
| (IV, 4) | Morbid Obesity | ||||||||||
|
| |||||||||||
| 41/F | Yes | 5 | 5 | S | PCOM | NC | IICP | POD11 | |||
| (IV, 4) | Vasospasm | ||||||||||
|
| |||||||||||
| 55/F | Yes | 5 | 6 | Rebled | S | PCOM | PO | Intraoperative | POD1 | Yes | |
| (IV, 3) | Rupture | ||||||||||
|
| |||||||||||
| 75/M | Yes | 5 | 6 | Hypertension | S | PCOM | PO | Intraoperative | POD1 | Yes | |
| (IV, 3) | Rupture | ||||||||||
|
| |||||||||||
| 53/M | Yes | 5 | 6 | Rebled | S | PICA | CO | Intraoperative | POD2 | Yes | |
| (IV, 3) | Rupture | ||||||||||
|
| |||||||||||
| 37/M | Yes | 5 | 6 | Hypertension | S | V-B | CO | Intraoperative | POD1 | Yes | |
| (IV, 4) | Rupture | ||||||||||
|
| |||||||||||
| 71/F | Yes | 5 | 5 | Coronary | L | ACHOA | PO | IICP | POD10 | ||
| (V, 4) | Arterial Disease | Vasospasm | |||||||||
|
| |||||||||||
| 60/F | Yes | 5 | 5 | Hypertension | L | DACA | NC | IICP | POD7 | ||
| (V, 4) | Intracerebral | ||||||||||
| Hemorrhage | |||||||||||
|
| |||||||||||
| 65/M | Yes | 5 | 6 | Rebled | S | MCAB | NC | IICP | POD2 | ||
| (V, 4) | Intracerebral | ||||||||||
| Hemorrhage | |||||||||||
|
| |||||||||||
| 31/M | Yes | 5 | 6 | Rebled | S | PICA | CO | IICP | POD1 | ||
| (V, 4) | |||||||||||
|
| |||||||||||
| 68/F | Yes | 5 | 6 | S | ACOM | NC | Intraoperative | POD1 | Yes | ||
| (V, 4) | Rupture | ||||||||||
|
| |||||||||||
| 65/F | No | 0 | 6 | Hypertension | G | ICAB | NC | Intraoperative | POD1 | Yes | |
| Coronary | Rupture | ||||||||||
| Arterial Disease | (in the 3rd session) | ||||||||||
| Diabetes Mellitus | |||||||||||
|
| |||||||||||
| 54/F | No | 1 | 6 | S | BA-SCA | NC | Postoper. Rupture | POD1 | Yes | ||
| POD0 | |||||||||||
|
| |||||||||||
| 79/F | No | 3 | 4 | Atrial Fibrillation | L | BA APEX | NC | GI & Vaginal | 2 months | ||
| Hypertension | Bleeding | ||||||||||
| brain stem | |||||||||||
| compression | |||||||||||
|
| |||||||||||
| SAH, subarachnoid hemorrhage; H-H, Hunt and Hess grade; Fisher, Fisher grade; mRS, modified Rankin Scale; S, small; L, large; | |||||||||||
| G, giant; ACHOA, anterior choroidal artery; ACOM, anterior communicating artery; BA APEX, basilar artery apex; | |||||||||||
| BA-SCA, superior cerebellar artery; DACA, distal anterior cerebral artery; ICAB, internal carotid artery bifurcation; | |||||||||||
| MCAB, middle cerebral artery bifurcation; PCA, posterior cerebral artery; PCOM, posterior communicating artery; | |||||||||||
| PICA, posterior inferior cerebellar artery; V-B, vertebrobasilar junction; CO, complete occlusion; NC, near-complete occlusion; | |||||||||||
| PO, partial occlusion; POD, postoperative day; ARDS, adult respiratory distress syndrome; GI, gastrointestinal; | |||||||||||
| IICP, increased intracranial pressure; ARF, acute renal failure. | |||||||||||
In patients with ruptured aneurysms/SAH, overall mortality rate was 36.0% (18/50) and procedure-related mortality rate was 18.0% (9/50). Mortality rate was higher in patients whose initial clinical statuses were worse. In patients whose initial clinical statuses were poor (Hunt and Hess grade IV or V), overall mortality rate was 56.0% (14/25) and procedure-related mortality rate was 24.0% (6/25). In patients whose initial clinical statuses were good (Hunt and Hess grade I, II, or III), overall mortality rate was 16.0% (4/25) and procedure-related mortality rate was 12.0% (3/25). In patients with unruptured aneurysms, mortality rate was 3.9% (3/77) and procedure-related mortality was 2.6% (2/77).
Clinical Outcome - Morbidity
Procedure-related neurologic morbidity was observed in seven patients (5.5%, 7/127; Table 7). Three cases involved ruptured aneurysms and four involved unruptured aneurysms. In patients with ruptured aneurysms/SAH, procedure-related neurologic morbidity rate was 6.0% (3/50) and, in patients with unruptured aneurysms, 5.2% (4/77). Five patients suffered a thromboembolic event, one a postoperative rupture, and one an intracerebral hemorrhage.
Table 7.
Cases of procedure-related neurologic morbidity.
| Age/ | SAH | mRS | mRS | mRS | Systemic | Size | Location | Previous | Immediate | Compli- | Interval |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | (H-H, | 0 | 3 | 90 | condition | Treatment/ | angiographic | cations | to | ||
| Fisher) | day | day | day | Interval | result | complication | |||||
|
| |||||||||||
| 53/F | 0 | 0 | 3 | hypertension, | L | BA | NC | left PCA | 2 months | ||
| left PCA | SCA | occlusion | |||||||||
| stenosis, renal | |||||||||||
| artery stenosis | |||||||||||
|
| |||||||||||
| 63/F | 0 | 3 | 3 | Diabetes | L | paracli- | NC | Air | intra- | ||
| mellitus, | noid | embolism | operative | ||||||||
| hypertension | |||||||||||
|
| |||||||||||
| 47/F | Yes | 0 | 5 | 3 | S | BA | CO | left | |||
| (II, 2) | APEX | occipital | <24hr | ||||||||
| ICH | |||||||||||
|
| |||||||||||
| 62/F | 2 | 4 | 3 | dilated | G | MCAB | coiling | PO | postopera- | <24hr | |
| cardiomyopathy, | 3 months | tive | |||||||||
| coronary artery | rupture | ||||||||||
| disease, dyslipidemia | |||||||||||
|
| |||||||||||
| 66/F | Yes | 0 | 0 | hypertension, | S | BA- | NC | subacute | 1 month | ||
| (I, 2) | 2 | hypercholeste- | SCA | in-stent | |||||||
| rolemia | thrombosis | ||||||||||
|
| |||||||||||
| 65/F | Yes | 1 | 3 | 2 | hypertension | S | BA | NC | coil | intraope- | |
| (III, 4) | APEX | herniation | rative, | ||||||||
| left PCA | <24hr | ||||||||||
| infarction | |||||||||||
|
| |||||||||||
| 75/M | 0 | 3 | 2 | coronary artery | L | ACOM | NC | both ACA | 1 day | ||
| disease, diabetes | & left | ||||||||||
| mellitus, | parietal | ||||||||||
| hypertension | infarction | ||||||||||
|
| |||||||||||
| SAH, subarachnoid hemorrhage; H-H, Hunt and Hess grade; Fisher, Fisher grade; mRS, modified Rankin Scale; S, small; | |||||||||||
| L, large; ACOM, anterior communicating artery; BA APEX, basilar artery apex; BA-SCA, superior cerebellar artery; | |||||||||||
| MCAB, middle cerebral artery bifurcation; CO, complete occlusion; NC, near-complete occlusion; PO, partial occlusion; | |||||||||||
| PCA, posterior cerebral artery; ICH, intracerebral hemorrhage; ACA, anterior cerebral artery | |||||||||||
Follow-up Angiographic Results
In a total of 106 survivors in 108 procedures with 115 aneurysms, 69 patients (65.1%) in 70 procedures (64.8%) with 77 aneurysms (67.0%) underwent angiographic follow-up at six months or later. Mean follow-up period was 13.7 months with a range from six to 45 months. Thirty seven patients did not undergo follow-up angiography. Seventeen patients could not be located and 20 patients refused follow-up angiography for reason of old age, other medical conditions, and financial issues. On follow-up angiography, one patient exhibited 30% in-stent stenosis at six months with complete resolution on follow up at 24 months without intervention (Figure 2).
Figure 2.
A 61-year-old woman presented with an unruptured left ophthalmic artery aneurysm (A). She underwent stent-assisted coil embolization. The aneurysm was partially occluded and in-stent thrombosis was not identified on immediate postoperative angiogram (B). A follow-up angiogram at 6 months revealed insignificant (30%) in-stent stenosis (C). A follow-up angiogram at 24 months identified no in-stent stenosis and restoration of luminal integrity (D).
A.
B.
C.
D.
In a total of 77 aneurysms with angiographic follow-up, residual aneurysm was observed in 20 aneurysms (26.0%) while complete occlusion was found in 57 aneurysms (74.0%). In small aneurysms (<10 mm; n=67), 77.6% of aneurysms (n=52) showed complete occlusion at follow-up while in large aneurysms (>10 mm, <25 mm; n=9), 55.6% (n=5) showed complete occlusion (Table 8).
Table 8.
Follow-up angiographic results.
| Follow-up Results (n=77) | |||
|---|---|---|---|
|
| |||
| Remnant or Recanalized | Complete Occlusion | ||
| (n=20, 26.0%) | (n=57, 74.0%) | ||
|
| |||
| Aneurysm Size | Small (n=67) | 15 (22.4%) | 52 (77.6%) |
|
|
|||
| Large (n=9) | 4 (44.4%) | 5 (55.6%) | |
|
|
|||
| Giant (n=1) | 1 (100%) | 0 | |
|
| |||
| Initial Angiographic | PO (n=17) | 4 (23.5%) | 13 (76.5%) |
|
|
|||
| Results | NC (n=36) | 9 (25.0%) | 27 (75.0%) |
|
|
|||
| CO (n=24) | 7 (29.2%) | 17 (70.8%) | |
|
| |||
| Small, <10mm; Large, 10>mm, <25mm; Large, >25mm; PO, partial occlusion; NC, near-complete occlusion; CO, complete occlusion | |||
Thromboembolism
Thromboembolic events remained the most common complications, occurring in 17 patients (13.4%, 17/127). Ten cases occurred less than 24 hours after the procedure (58.8% of thromboembolic events, 10/17), two cases within five days (11.8%, 2/17), three cases within two weeks (17.6%, 3/17), one case at one month post procedure (5.9%, 1/17), and one case two months post procedure (5.9%).
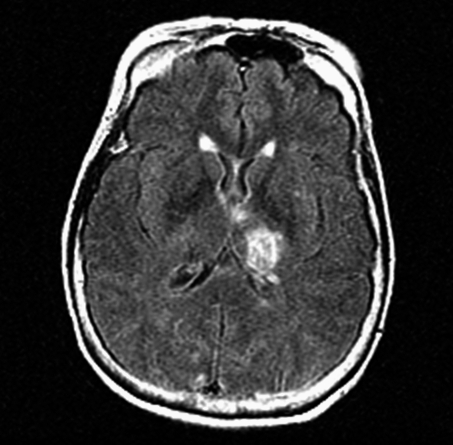
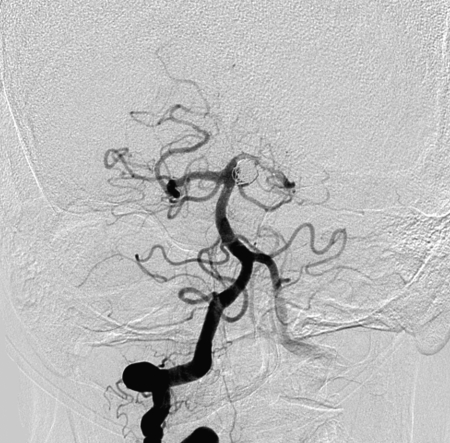
In-stent thromboses were observed in five patients (29.4% of thromboembolic events, 5/17). Two patient exhibited intra-procedural in-stent thrombosis. One autolyzed and the other was treated with intra-arterial (IA) tissue plasminogen activator (TPA). One patient with a ruptured BA aneurysm presented eight days post procedure with in-stent occlusion combined with distal basilar and bilateral posterior cerebral artery (PCA) occlusion. Despite IA TPA and mechanical manipulation, this patient died. One patient developed symptoms from in-stent stenosis one month after the procedure concomitant with cessation of antiplatelet agents due to gastrointestinal bleeding (Figure 3). Another patient exhibited symptomatic in-stent occlusion two months after stent-assisted coil embolization.
Figure 3.
A 66-year-old woman presented with subarachnoid hemorrhage. Her initial left vertebral artery angiogram on AP view revealed a wide-necked left superior cerebellar artery aneurysm (A). She underwent stent-assisted coil embolization (B). After discharge, she was prescribed 325 mg of aspirin and 75 mg of clopidogrel. However, she stopped medication due to gastrointestinal bleeding two weeks after the procedure. One month after the procedure, she suddenly exhibited right hemiparesis and hemihypesthesia. MRI revealed cerebral infarction (C) and left posterior cerebral artery in-stent thrombosis was identified on left vertebral angiogram (D).
A.
B.
C.
D.
Six thromboembolic cases resulted in persistent neurologic morbidity and mortality (35.3% of thromboembolic cases; 4.7% of all cases). One mortality and five morbidities were identified (Tables 6 and 7). The one mortality was due to in-stent occlusion on the eighth post-procedure day. Two morbidities were due to in-stent thromboses that presented one and two months after the procedure. Coil herniation and air embolism led to persistent neurologic morbidity in two cases. In one case with an unruptured ACom aneurysm, diffuse multi-focal infarctions were identified in both anterior cerebral artery (ACA) territories even though the procedure was uneventful. Overall, in 127 patients, the morbidity and mortality rates from thromboembolic events were 3.9% (5/127) and 0.8% (1/127), respectively.
Intraoperative Aneurysm Rupture
Intraoperative aneurysm ruptures occurred in eight (6.3%) of 127 patients. Two were spontaneous (without direct device manipulation in or into the aneurysm), two occurred during microwire/microcatheter manipulations within the aneurysm, and four occurred during coil insertion. Coil-induced ruptures were caused by the first coil in two cases, the fourth in one, and the sixth in one. Seven intraoperative ruptures occurred in patients with ruptured aneurysms (14.0%, 7/50) and one in a patient with an unruptured aneurysm (1.3%, 1/77). Preoperative clinical grades of the eight patients were Hunt and Hess grade 0 in one, III in one, IV in five, and V in one. All intraoperative ruptures were managed with intravenous protamine sulfate administration, external ventricular drain insertion if necessary, and further embolization. Seven patients died (Table 6) while one had no identifiable neurologic consequence. In 127 patients, the mortality rate from intraoperative aneurysm rupture was 5.5% (7/127).
Postoperative Aneurysm Rupture
Postoperative aneurysm rupture occurred in four (3.1%) of 127 patients. Two cases involved ruptured aneurysms (Hunt and Hess grade I) and two involved unruptured aneurysms (Table 5). Immediate angiographic results were near-complete occlusion in two cases and partial occlusion in two. All cases occurred within one day of the procedure. All postoperative ruptures led to morbidity and mortality. Three postoperative ruptures resulted in death and one in persistent neurologic morbidity (Table 6 and 7). The morbidity and mortality rates from postoperative aneurysm rupture were 0.8% (1/127) and 2.4% (3/127), respectively.
Coil Herniation
Coil herniation into the parent vessel despite stent placement was observed in five patients (3.9%, 5/127). One case (0.8%, 1/127) led to a thromboembolic event and neurologic morbidity (Table 7). In one case, coil herniation was caused by coil fracture. In three patients, an additional stent was deployed with stent-in-stent technique to pin protruded coil loops against the parent artery wall.
Stent Migration
Stent migration occurred in four (3.1%) of 127 patients. Stent migration was caused by microwire manipulation, microcatheter manipulation, coil hooking onto the stent during retrieval, and delivery device retrieval. In one case, because the aneurysm was still covered by the stent, no additional stent placement was required. The stent was retrieved in two cases and migrated into the parent vessel’s extracranial portion in one case. In three cases, an additional stent was deployed to cover the aneurysm.
Iatrogenic Dissection
Iatrogenic parent artery dissection occurred in three (2.4%) of 127 patients. These involved the distal cervical portion of the internal carotid artery in two cases and extracranial portion of the vertebral artery in one case. All dissections occurred during guide catheter manipulations. In the two cases of cervical internal carotid artery dissection, blood flow was compromised, requiring stent-assisted angioplasty to restore intraluminal integrity.
Discussion
Follow-up Angiographic Results
Angiographic follow-up in our study was achieved in 69 patients at a mean period of 13.7 months. In these 69 patients harboring 77 aneurysms, 57 complete aneurysm occlusions were documented (74.0%, 57/75; mean follow-up period, 13.7 months). In other series with stent-assisted coil embolization using the Neuroform stent, Biondi et Al.14 reported 17 out of 30 complete aneurysm occlusions (56.7%; follow-up period 9 months) and Fiorella et Al.36 reported progressive thrombosis in 25 out of 48 aneurysms (52.1%; follow-up period, 4.6 months). Weber et Al18 reported 15 out of 30 complete aneurysm occlusions (50%; follow-up period, six months) in their series using the Enterprise stent (Cordis, Miami, FL).
For several reasons, it is difficult to compare our angiographic results with those of other reported series. First, few papers documented delayed angiographic follow-up in stent-assisted coil embolization of intracranial aneurysms. Second, analysis of the radiographic and clinical data in our series was not performed by an independent investigator. Third, a standard definition of what constitutes complete occlusion is lacking and quantification of results is subjective37. Fourth, the mean follow-up period in this series was 13.7 months, which was longer than other series. The higher complete occlusion rate in this series may be attributable to the longer follow-up period. Fifth, we did not obtain follow-up in all eligible patients for several logistical reasons.
Morbidity and Mortality
In our series, procedure-related morbidity and mortality rates were 5.5% (7/127) and 8.7% (11/127). In other series with stent-assisted coil embolization, morbidity rates ranged from 4.8 to 20% and mortality rates ranged from 0 to 8.9%10,14,38-44. While in our series, the overall mortality and procedure-related mortality rates were relatively high (16.5%, 8.7%), this may be attributable to the greater number of SAH patients and poor grade SAH patients included in our study compared to others (patients with SAH, 39.4%, 50/127; Hunt and Hess grade IV or V, 19.7%, 25/127). The historical overall mortality rate for poor grade SAH patients undergoing any endovascular therapy is reported as ranging from 32.4 to 59%45-47. In our series, the overall mortality rate in poor grade SAH group was 56.0% (14/25) compared to 6.9% (7/102) in unruptured and good grade SAH patients.
Thromboembolism
The most frequent complication in this series was thromboembolism (13.4%, 17/127). Historical incidence rates of thromboembolism associated with coil embolization of cerebral aneurysms have ranged from 1.0% to 28%1,13,48-52. Other series looking at stent-assisted coil embolizations report incidence rates ranging from 4.8 to 21.1%38-40,44.
With regard to in-stent thrombosis, Lee et Al.42 reported an acute intraprocedural in-stent thrombosis rate of 4.5% (1/22). Historical incidence rates of subacute or delayed in-stent thrombosis range from 2.4 to 5.8%14,36,53,54. In our series, the rate of acute intra-procedural in-stent thrombosis was 1.6% (2/127) and the rate of subacute or delayed in-stent thrombosis was 2.4% (3/127).
According to Virchow’s triad, arterial thrombosis under pathologic or procedural circumstances is influenced by alterations in blood flow, endothelial thrombogenicity, and hematologic thrombogenicity (the thrombogenicity of circulating activated physiological and pathological hemostatic factors)55. Since platelets play a key role in arterial thrombosis, antiplatelet agents have an effect on hematologic and endothelial thrombogenicity. Because cerebral angiography itself carries a risk of thromboembolism, and most thromboembolic events associated with coil embolization are caused by device manipulation56,57, it is mandatory to modulate both hematologic and endothelial thrombogenicity before and during the procedure. Administration of antiplatelet agents, when possible, before the procedure may reduce the thromboembolic event rate. Generally, therapeutic platelet inhibition is achieved by using antiplatelet agents (aspirin, ticlopidine, and clopidogrel) for three to seven days unless bolus dosing is utilized, however longer periods may be needed to alter the endothelial surface. A combination of aspirin and clopidogrel for four weeks prior to a procedure or aspirin and ticlopidine for two weeks prior to a procedure may be more effective in preventing in-stent thrombosis58,59.
In patients with ruptured aneurysms, pre-procedural antiplatelet agent administration is a matter of debate, but in patients with unruptured aneurysms, adequate antiplatelet agent premedication is essential. It is recommended that adequate antiplatelet therapy should be sustained at least one month after the procedure60,61. In our series, one case of symptomatic in–stent thrombosis was associated with cessation of all antiplatelet agents due to gastrointestinal bleeding. A second case of symptomatic in-stent occlusion presented two months post procedure despite antiplatelet therapy.
While thromboembolic events associated with the Neuroform stent are problematic, they are not exclusive to this device. Balloon-assisted neck-remodeling technique can provide an alternative method for endovascular treatment of wide-necked aneurysms62-67. However, this technique has similar problems. Van Rooij et Al68 reported in their series of 681 patients with ruptured intracranial aneurysms that the combined morbidity and mortality rate from thromboembolic events with balloon-assisted neck-remodeling technique was 16.3% (8/49) and this technique was the only risk factor for intraoperative rupture and thromboembolic events. In our series, the combined morbidity and mortality rate for ruptured aneurysms secondary to thromboembolic events was 6.0% (3/50; two morbidities and one mortality). Unlike balloons, however, stents can permanently prevent coil protrusion into the parent artery, may redirect blood flow, may promote endothelialization at the aneurysm neck, and do not precipitate sudden intra-aneurysmal pressure changes14,40,44,62. Stents can also secure protruded coil loops against the parent artery wall in case of protrusion from the fundus after coiling is complete.
Intraoperative and Postoperative Rupture
Intraoperative aneurysm rupture was the second most common complication in our series (6.3%, 8/127). Historical rates of intraoperative aneurysm rupture associated with coil embolization range from 1.9 to 16% for ruptured aneurysms and from 0 to 1.3% for unruptured aneurysms12,13,15,69-72. In our series, rupture rates were 14.0% (7/50) and 1.3% (1/77) for ruptured and unruptured aneurysms, respectively. Aneurysm size is thought to be inversely associated with an increased incidence of intraoperative rupture. Vin˜uela et Al13 reported that nine of eleven intra-operatively ruptured aneurysms measured 4-10 mm in diameter and Sluzewski et Al71 reported that six of seven measured 2-10 mm.
In our series, six of eight ruptures involved small lesions (<10 mm). Six cases of intraoperative aneurysm rupture in this series were induced by device (wire/catheter/coil) manipulations and two occurred spontaneously without direct device manipulation. All spontaneous intraoperative ruptures occurred in patients with acute subarachnoid hemorrhage. Possible causes of rupture include stretching of the aneurysm wall after stent deployment, anticoagulation effect and fundal perforation.
Naidech et Al73 reported that a poor Hunt and Hess grade was a strong predictor of rebleeding. In our cases of spontaneous intraoperative aneurysm rupture, all patients were Hunt and Hess grade IV. Once an aneurysm ruptured, the outcome was dismal. Seven of eight (87.5%) patients died. In poor grade SAH patients (Hunt and Hess grades IV or V), all six patients died.
Postoperative aneurysm rupture occurred in four patients (3.1%, 4/127), resulting in universally poor clinical outcomes (three died; one disabled). In our series, rupture rates were 4.0% (2/50) and 2.6% (2/77) for ruptured and unruptured aneurysms. All postoperative ruptures occurred within one day after the procedure. Two of four postoperative ruptures involved small lesions (<10 mm), one involved a large (>10 mm, <25 mm), and one involved a giant aneurysm (>25 mm). In two postoperative ruptures with ruptured aneurysms/SAH, initial clinical status was good (Hunt and Hess grade I). One lesion was small (3 mm) and relatively spherical. The other was large (12.6 mm) and irregular in shape. In two postoperative ruptures with unruptured aneurysms, one patient presented with progressively worsening severe headache and angiography revealed a small (8.6 mm), but irregular shaped aneurysm. The other patient exhibited a partially thrombosed giant aneurysm. Incomplete occlusion is thought to be associated with postoperative rupture. In our four postoperative ruptures, immediate post-coiling angiographic results showed partial occlusion in two patients and near-complete occlusion in two patients.
Conclusions
Key findings in this review of 129 stent-assisted coil embolization procedures in 127 patients with 136 aneurysms were as follows:
1) Forty seven patients presented with 53 procedure-related complications (37.0%, 47/127).
2) Overall mortality rate was 16.5% (21/127) and procedure-related morbidity and mortality rates were 5.5% (7/127) and 8.7% (11/127), retrospectively.
3) Thromboembolism (n=17, 13.4%) and intra/postoperative aneurysm ruptures (n=8/4, 6.3%/3.1%) were the most common complications and the main causes of procedure-related morbidity and mortality.
4) Patients presenting with a clinically poor grade SAH had dismal clinical outcomes (overall mortality rate, 56%; procedure-related mortality rate, 24%).
5) Since most complications were induced by device manipulation, it is mandatory to handle devices cautiously.
6) Follow-up angiographic results in 65.1% of eligible patients showed complete occlusion in 74.0%.
7) Further studies are essential to evaluate the safety, efficacy, and durability of the Neuroform stent.
References
- 1.Guglielmi G, Viñuela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach, II: preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 2.Brilstra EH, Rinkel GJ, et al. Treatment of intracranial aneurysms by embolization with coils: a systemic review. Stroke. 1999;30:470–476. doi: 10.1161/01.str.30.2.470. [DOI] [PubMed] [Google Scholar]
- 3.Johnston HC, Higashida RT, et al. Committee on cerebrovascular imaging of the American heart association council on cardiovascular radiology: recommendation for the endovascular treatment of the intracranial aneurysms: a statement for healthcare professionals from the committee on cerebrovascular imaging of the America heart association council on cardiovascular radiology. Stroke. 2002;33:2536–2544. doi: 10.1161/01.str.0000034708.66191.7d. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SC, Wilson CB, et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol. 2000;48:11–19. doi: 10.1002/1531-8249(200007)48:1<11::aid-ana4>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Johnston SC, Zhao S, et al. Treatment of unruptured cerebral aneurysms in California. Stroke. 2001;32:597–605. doi: 10.1161/01.str.32.3.597. [DOI] [PubMed] [Google Scholar]
- 6.Koivisto T, Vanninen R, et al. Outcome of early endovascular versus surgical treatment of ruptured cerebral aneurysms: a prospective randomized study. Stroke. 2000;31:2369–2377. doi: 10.1161/01.str.31.10.2369. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux A, Kerr R, et al. International Subarachnoid Aneurysms Trial (ISAT) Collaborative Group: International Subarachnoid Aneurysms Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 8.Raftopoulos C, Mathurin P, et al. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurg. 2000;93:175–182. doi: 10.3171/jns.2000.93.2.0175. [DOI] [PubMed] [Google Scholar]
- 9.Vannien R, Koivisto T, et al. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils. a prospectively randomized study. Radiology. 1999;211:325–336. doi: 10.1148/radiology.211.2.r99ap06325. [DOI] [PubMed] [Google Scholar]
- 10.Akpek S, Arat A, et al. Self-expandable stent-assisted coiling of wide-necked intracranial aneurysms: A single-center experience. Am J Neuroradiol. 2005;26:1223–1231. [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa M, Murayama Y, et al. Natural history of the neck remnant of a cerebral aneurysms treated with the Gugliemi detachable coil system. J Neurosurg. 2000;93:561–568. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 12.Ng P, Khangure MS, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: Analysis of midterm angiographic and clinical outcomes. Stroke. 2002;33:210–217. doi: 10.1161/hs0102.100486. [DOI] [PubMed] [Google Scholar]
- 13.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 14.Biondi A, Janardhan V, et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. 2007;61:460–468. doi: 10.1227/01.NEU.0000290890.62201.A9. [DOI] [PubMed] [Google Scholar]
- 15.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery. 1998;43:1016–1025. doi: 10.1097/00006123-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Murayama Y, Suzuki Y, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part I: in vitro study. Am J Neuroradiol. 1999;20:1986–1999. [PMC free article] [PubMed] [Google Scholar]
- 17.Richling B, Gruber A, et al. GDC-system embolization for brain aneurysms-location and follow-up. Acta Neurochir. 1995;134:177–183. doi: 10.1007/BF01417686. [DOI] [PubMed] [Google Scholar]
- 18.Weber W, Bendszus M, et al. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: initial clinical and angiographic results in 31 aneurysms. Neuroradiology. 2007;49:555–561. doi: 10.1007/s00234-007-0232-2. [DOI] [PubMed] [Google Scholar]
- 19.Kwon OK, Kim SH, et al. Embolization of wide-necked aneurysms with using three or more microcatheters. Acta Neurochir. 2006;148:1139–1145. doi: 10.1007/s00701-006-0876-4. [DOI] [PubMed] [Google Scholar]
- 20.Levy DI, Ku A, et al. Balloon-assisted coil placement in wide-necked aneurysms: technical note. J Neurosurg. 1997;86:724–727. doi: 10.3171/jns.1997.86.4.0724. [DOI] [PubMed] [Google Scholar]
- 21.Malek AM, Higashida RT, et al. Treatment of an intracranial aneurysm using a new three-dimensional shape Guglielmi of detachable coil: technical case report. Neurosurgery. 1999;44:1142–1245. doi: 10.1097/00006123-199905000-00125. [DOI] [PubMed] [Google Scholar]
- 22.Mericle RA, Wakhloo AK, et al. Temporary balloon protection as an adjunct to endovascular soiling of wide-necked cerebral aneurysms: technical note. Neurosurgery. 1997;41:975–978. doi: 10.1097/00006123-199710000-00045. [DOI] [PubMed] [Google Scholar]
- 23.Moret J, Cognard C, et al. Reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results-report of 56 cases. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- 24.Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology. 2001;221:318–326. doi: 10.1148/radiol.2212010474. [DOI] [PubMed] [Google Scholar]
- 25.Higashida RT, Smith W, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of he basilar artery: case report and review of the literature. J Neurosurg. 1997;87:944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 26.Horowitz MB, Levy EI, et al. Transluminal stent-assisted coil embolization of a vertebral confluence aneurysm: technique report. Surg Neurol. 2001;55:291–296. doi: 10.1016/s0090-3019(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 27.Lanzino G, Wakhloo AK, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91:538–546. doi: 10.3171/jns.1999.91.4.0538. [DOI] [PubMed] [Google Scholar]
- 28.Mericle RA, Lanzino G, et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery. 1998;43:1229–1234. doi: 10.1097/00006123-199811000-00130. [DOI] [PubMed] [Google Scholar]
- 29.Phatoursos CC, Sasaki TY, et al. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery. 2000;47:107–115. doi: 10.1097/00006123-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Sekhon LH, Morgan MK, et al. Combined endovascular stent implantation and endovascular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery. 1998;43:380–384. doi: 10.1097/00006123-199808000-00127. [DOI] [PubMed] [Google Scholar]
- 31.Wakhloo AK, Lanzino G, et al. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery. 1998;43:377–379. doi: 10.1097/00006123-199808000-00126. [DOI] [PubMed] [Google Scholar]
- 32.Almaani WS, Richardson AE. Multiple intracranial aneurysms: Identifying the ruptured lesion. Surg Neurol. 1978;9:303–305. [PubMed] [Google Scholar]
- 33.Nehls DG, Flom RA, et al. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg. 1985;63:342–348. doi: 10.3171/jns.1985.63.3.0342. [DOI] [PubMed] [Google Scholar]
- 34.Sengupta RP, Lassman LP, et al. Identification of the source of bleeding in multiple intracranial aneurysms. Vasc Surg. 1974;8:177–183. [PubMed] [Google Scholar]
- 35.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–19. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 36.Fiorella D, Albuquerque FC, et al. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: Results at initial (3-6-mo) follow-up. Neurosurgery. 2005;56:1191–1202. doi: 10.1227/01.neu.0000159645.86823.af. [DOI] [PubMed] [Google Scholar]
- 37.Katz JM, Tsiouris AJ, et al. Advances in endovascular aneurysm treatment: Are we making a difference? Neuroradiology. 2005;47:695–701. doi: 10.1007/s00234-005-1413-5. [DOI] [PubMed] [Google Scholar]
- 38.Benitez RP, Silva MT, et al. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery . 2004;54:1359–1368. doi: 10.1227/01.neu.0000124484.87635.cd. [DOI] [PubMed] [Google Scholar]
- 39.dos Santos Souza MP, Agid R, et al. Microstent-assisted coiling for wide-necked intracranial aneurysms. Can J Neurol Sci. 2005;32:71–81. doi: 10.1017/s0317167100016917. [DOI] [PubMed] [Google Scholar]
- 40.Fiorella D, Albuquerque FC, et al. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6–17. doi: 10.1227/01.neu.0000097194.35781.ea. [DOI] [PubMed] [Google Scholar]
- 41.Han PP, Albuquerque FC, et al. Percutaneous intracranial stent placement for aneurysms. J Neurosurg. 2003;99:23–30. doi: 10.3171/jns.2003.99.1.0023. [DOI] [PubMed] [Google Scholar]
- 42.Lee YJ, Kim DJ, et al. Stent-assisted coil embolization of intracranial wide-necked aneurysms. Neuroradiology. 2005;47:680–689. doi: 10.1007/s00234-005-1402-8. [DOI] [PubMed] [Google Scholar]
- 43.Lylyk P, Ferrario A, et al. Buenos Aires experience with the Neuroform self-expanding stent for the treatment of intracranial aneurysms. J Neurosurg. 2005;102:235–241. doi: 10.3171/jns.2005.102.2.0235. [DOI] [PubMed] [Google Scholar]
- 44.Yahia AM, Gordon V, et al. Complications of Neuroform Stent in Endovascular Treatment of Intracranial Aneurysms. Neurocrit Care. 2008;8:19–30. doi: 10.1007/s12028-007-9001-7. [DOI] [PubMed] [Google Scholar]
- 45.Jain R, Deveikis J, Thompson BG. Endovascular management of poor-grade aneurysmal subarachnoid hemorrhage in the geriatric population. Am J Neuroradiol. 2004;25:596–600. [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki S, Jahan R, et al. Contribution of endovascular therapy to the management of poor-grade aneurysmal subarachnoid hemorrhage: Clinical and angiographic outcomes. J Neurosurg. 2006;105:664–670. doi: 10.3171/jns.2006.105.5.664. [DOI] [PubMed] [Google Scholar]
- 47.Weir RU, Marcellus ML, et al. Aneurysmal subarachnoid hemorrhage in patients with Hunt and Hess grade 4 or 5: treatment using the Guglielmi datachable coil system. Am J Neuroradiol. 2003;24:585–590. [PMC free article] [PubMed] [Google Scholar]
- 48.Moret J, Congrad C, et al. The “remodeling technique” in the treatment of wide neck intracranial aneurysms: Angiographic results and clinical follow-up in 56 cases. Interventional Neuroradiology. 1997;3:21–35. doi: 10.1177/159101999700300103. [DOI] [PubMed] [Google Scholar]
- 49.Murayama Y, Viñuela F, et al. Embolization of incidental cerebral aneurysm by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207–214. doi: 10.3171/jns.1999.90.2.0207. [DOI] [PubMed] [Google Scholar]
- 50.Nelson PK, Levy DI. Balloon assisted coil embolization of wide-neck aneurysms of the internal carotid artery: medium-term angiographic and clinical follow-up in 22 patients. Am J Neuroradiol. 2001;22:19–26. [PMC free article] [PubMed] [Google Scholar]
- 51.Park HK, Horowitz M, et al. Periprocedural morbidity and mortality associated with endovasculat treatment of intracranial aneurysms. Am J Neuroradiol. 2005;26:506–514. [PMC free article] [PubMed] [Google Scholar]
- 52.Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. Am J Neuroradiol. 1998;19:1541–1547. [PMC free article] [PubMed] [Google Scholar]
- 53.Fiorella D, Albuquerque FC, et al. In-stent stenosis as a delayed complication of Neuroform stent-supported coil embolization of an incidental carotid terminus aneurysm. Am J Neuroradiol. 2004;25:1764–1767. [PMC free article] [PubMed] [Google Scholar]
- 54.Fiorella D, Albuquerque FC, et al. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34–42. doi: 10.1227/01.NEU.0000219853.56553.71. [DOI] [PubMed] [Google Scholar]
- 55.Morris P. Interventional and endovascular therapy of the nervous system. New York: Springer; 2002. [Google Scholar]
- 56.Bendszus M, Koltzenburg M, et al. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;353:1594–1597. doi: 10.1016/S0140-6736(99)07083-X. [DOI] [PubMed] [Google Scholar]
- 57.Soeda A, Sakai N, et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. Am J Neuroradiol. 2003;24:127–132. [PMC free article] [PubMed] [Google Scholar]
- 58.Moussa I, Oetgen M, et al. Effectiveness of clopidogrel and aspirin versus ticlopidine and aspirin in preventing stent thrombosis after coronary artery stent implantation. Circulation. 1999;99:2364–2366. doi: 10.1161/01.cir.99.18.2364. [DOI] [PubMed] [Google Scholar]
- 59.Quinn MJ, Fitzgerald DJ. Ticlopidine and Clopidogrel. . Circulation. 1999;100:1667–1673. doi: 10.1161/01.cir.100.15.1667. [DOI] [PubMed] [Google Scholar]
- 60.Moshfegh K, Redondo M, et al. Antiplatelet effect of clopidogrel compared with aspirin after myocardial infarction: enhanced inhibitory effect of combination therapy. J Am Coll Cardiol. 2000;36:699–705. doi: 10.1016/s0735-1097(00)00817-2. [DOI] [PubMed] [Google Scholar]
- 61.Savcic M, Hauert J, et al. Clopidogrel loading dose regimens: kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost. 1999;25(2):15–19. [PubMed] [Google Scholar]
- 62.Akiba Y, Murayama Y, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysm: part I-experimental evaluation. Neurosurgery. 1999;45:519–527. doi: 10.1097/00006123-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 63.Cottier JP, Pasco A, et al. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. . Am J Neuroradiol. 2001;22:345–351. [PMC free article] [PubMed] [Google Scholar]
- 64.Lefkowitz MA, Gobin YP, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysm: part II-clinical results. Neurosurgery. 1999;45:531–537. doi: 10.1097/00006123-199909000-00024. [DOI] [PubMed] [Google Scholar]
- 65.Lubicz B, Leclerc X, et al. HyperForm remodeling-balloon for endovascular treatment of wide-neck intracranial aneurysms. Am J Neuroradiol. 2004;25:1381–1383. [PMC free article] [PubMed] [Google Scholar]
- 66.Malek AM, Halbach VV, et al. Balloon-assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery. 2000;46:1397–1406. doi: 10.1097/00006123-200006000-00022. [DOI] [PubMed] [Google Scholar]
- 67.Ross IB, Dhillon GS. Complications of endovascular treatment of cerebral aneurysms. Surg Neurol. 2005;64:12–18. doi: 10.1016/j.surneu.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 68.van Rooij WJ, Sluzewski M, et al. Procedure complications of coiling of ruptured intracranial aneurysms: incidence and risk factors in consecutive series of 681 patients. Am J Neuroradial. 2006;27:1498–1501. [PMC free article] [PubMed] [Google Scholar]
- 69.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41:1235–1246. doi: 10.1097/00006123-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Ricolfi F, Le Guerinel C, et al. Rupture during treatment of recently ruptured aneurysms with Guglielmi electrodetachable coils. Am J Neuroradiol. 1998;19:1653–1658. [PMC free article] [PubMed] [Google Scholar]
- 71.Sluzewski M, Bosch JA, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg . 2001;94:238–240. doi: 10.3171/jns.2001.94.2.0238. [DOI] [PubMed] [Google Scholar]
- 72.Tummala RP, Chu RM, et al. Outcomes after aneurysm rupture during endovascular coil embolization. Neurosurgery. 2001;49:1059–1067. doi: 10.1097/00006123-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Naidech AM, Janjua N, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. . Arch Neurol. 2005;62:410–416. doi: 10.1001/archneur.62.3.410. [DOI] [PubMed] [Google Scholar]