Abstract
Autism is a neurodevelopmental disorder that is currently diagnosed by the presence of three behavioral criteria (1) qualitative impairments in reciprocal social interactions, (2) deficits in communication, including delayed language and noninteractive conversation, and (3) motor stereotypies, repetitive behaviors, insistence on sameness, and restricted interests. This chapter describes analogous behavioral assays that have been developed for mice, including tests for social approach, reciprocal social interactions, olfactory communication, ultrasonic vocalizations, repetitive and perseverative behaviors, and motor stereotypies. Examples of assay applications to genetic mouse models of autism are provided. Robust endophenotypes that are highly relevant to the core symptoms of autism are enabling the search for the genetic and environmental causes of autism, and the discovery of effective treatments.
Keywords: Autism, Behavior, Candidate genes, Communication, Genetics, Mice, Mouse models, Olfactory, Repetitive, Social, Vocalization
1 Introduction
Autism is a neurodevelopmental disorder that is defined by three behavioral criteria (1) qualitative impairments in social interactions, (2) deficits in communication, including delayed and noninteractive language, and (3) motor stereotypies, repetitive behaviors, insistence on sameness, and restricted interests (Kanner 1943; American Psychiatric Association 1994; Piven et al. 1997; Bodfish et al. 2000; Lord et al. 2000; Dawson et al. 2002; Cuccaro et al. 2003; Frith 2003; Volkmar and Pauls 2003; South et al. 2005; London 2007; Tager-Flusberg and Caronna 2007; Happe and Ronald 2008; Baron-Cohen 2009). Prevalence is currently estimated at 1:100 to 1:150 for autism spectrum disorders, a dramatic increase over the past decade that appears to be related primarily to improved detection (Fombonne 2009; Hertz-Picciotto and Delwiche 2009; King and Bearman 2009). The essential needs, for early behavioral intervention through special education programs for children and social services support for adults, place a high financial burden on society, as well as heavy personal demands on the families of autistic individuals.
No consistent biological markers for autism have been identified across individuals to date. Evidence from small numbers of autistic individuals suggests a variety of indicators, including cortical connectivity abnormalities (Williams and Minshew 2007), minimal activation of the fusiform gyrus and amygdala during social tasks (Pelphrey et al. 2002), insufficient GABAergic inhibitory neurotransmission (McDougle et al. 2005), mitochondrial dysfunctions (Zecavati and Spence 2009), high platelet serotonin (Anderson 2002), autoantibodies (Wills et al. 2009), and cognitive disabilities following prenatal exposure to valproate (Ornoy 2009) and environmental toxins (Halladay et al. 2009).
The causes of autism remain unknown; however, the strongest evidence is genetic. Concordance between monozygotic twins is as high as 90% for autism spectrum disorders, as compared to 5–10% concordance in dizygotic twins and siblings (Abrahams and Geschwind 2008; Lintas and Persico 2009). Male-to-female ratios are above 4:1 (Abrahams and Geschwind 2008). No single gene mutation has been implicated as uniformly causal. Rather, a variety of de novo and familial mutations have been documented, including a large number of genetic mutations and copy number variants, each in a small number of autistic individuals (Abrahams and Geschwind 2008; Cook and Scherer 2008; Levitt and Campbell 2009; Lintas and Persico 2009). Particularly interesting are clusters of candidate genes with similar functions, such as the synaptic cell adhesion protein families of neurexins, neuroligins, shanks, contactins, cadherins, and integrins (Jamain et al. 2003; Laumonnier et al. 2004; Jeffries et al. 2005; Lise and El-Husseini 2006; Autism Genome Project Consortium 2007; Durand et al. 2007; Garber 2007; Moessner et al. 2007; Alarcon et al. 2008; Arking et al. 2008; Jamain et al. 2008; Kim et al. 2008; Lawson-Yuen et al. 2008; Sudhof 2008). Several other neurodevelopmental disorders display comorbidity with autism. Syndromes caused by known single gene mutations, in which a significant proportion of individuals meet the diagnostic criteria for autism, include Fragile X (FMR1), Rett (MECP2), tuberous sclerosis (TSC), Angelman syndrome (UBE3A), and Phelan–McDermid syndrome (22q13.3) (Abrahams and Geschwind 2008). Hypotheses which focus on environmental causes, such as immune dysfunction and environmental toxins, are often conceptualized in terms of susceptibility genes and gene × environment interactions (Fombonne 2009; Halladay et al. 2009; Zecavati and Spence 2009).
Biomedical research has benefited from animal models of diseases, which provide translational systems to test hypotheses about causes and to develop treatments. The autism research field is at early stages in the development of appropriate assays with definitive relevance to the features of autism, and appropriate model systems for testing the many hypotheses about genetic and environmental causes of autism. As described in the sections below, several useful assays and model systems are now available. These preclinical research tools offer translational opportunities to test compounds for their ability to reverse autism-relevant behaviors in mouse models. Potential treatment targets include cell adhesion proteins that regulate the formation and development of synapses, intracellular signaling mechanisms mediating synaptic plasticity and pharmacological manipulation of neurotransmission through receptors for GABA, glutamate, serotonin, and oxytocin (Ehninger et al. 2008b).
As in other fields of biomedical research, mouse models for autism are being perfected across the required criteria for use in treatment development. Face validity refers to highly analogous endophenotypes in the human disease and the animal model. Construct validity refers to the induced cause being nearly identical in the animal models and the human disease. The first step is to introduce the hypothesized cause of the disease in the model organism. The consequences of the mutation, lesion, toxin, etc. are evaluated with assays that maximize similarities for the human disease and the model organism. Predictive validity refers to therapeutic efficacy. Treatments that reverse symptoms in the human disease should similarly reverse symptoms in the model organism.
To evaluate the roles of mutations in candidate genes for producing the symptoms of autism, the same gene that is mutated in an autistic individual is similarly mutated in the mouse genome (see Gondo et al. 2011 for further discussion). Molecular geneticists have generated thousands of lines of mice with targeted single gene mutations or humanized knock-in mutations relevant to human diseases, including autism spectrum disorders and comorbid neurodevelopmental disorders. Given the behavioral criteria for the diagnosis of autism, and the lack of consistent biomarkers, mouse behavioral assays with high face validity to the behavioral symptoms of autism provide the best tools to evaluate the functional outcomes of candidate gene mutations. The same assays can be used to evaluate environmental hypotheses. The fundamental challenge is to design mouse behavioral tasks with sufficient face validity to the core symptoms within each of the three diagnostic behavioral categories of autism (Crawley 2004, 2007a, b).
This chapter describes strategies from our laboratory and others to model the diagnostic and associated symptoms of autism in mice. Phenotypes obtained in mouse models with genetic and environmental manipulations are presented, relevant to several of the proposed molecular causes of autism spectrum disorders. Preliminary findings from mouse models of autism spectrum disorders that use hypothesis-driven treatments, which effectively reversed relevant phenotypes, are discussed.
2 Mouse Behavioral Tasks with Face Validity for the First Diagnostic Symptom of Autism, Qualitative Impairments in Reciprocal Social Interactions
Mice are a social species, which engage in easily scored social behaviors including approaching, following, sniffing, allogrooming, aggressive encounters, sexual interactions, parental behaviors, nesting and sleeping in a group huddle (Grant and MacIntosh 1963; Hofer et al. 2001; Miczek et al. 2001; Carter et al. 1992; Young et al. 2002; Winslow 2003; Terranova and Laviola 2005; Wersinger et al. 2007; Keller et al. 2006; Panksepp et al. 2007; Yang et al. 2007a, b, 2009; Chadman et al. 2008; McFarlane et al. 2008; Paylor et al. 2008; Scattoni et al. 2008a, b). Behavioral assays using dedicated equipment have been developed by our laboratory and others to quantify the types of social interactions that are unusual in autistic individuals. These include low spontaneous seeking of interactions with others, lack of social reciprocity, and failure to develop peer relationships appropriate to developmental ages.
We invented a simple social approach task in an automated three-chambered apparatus, illustrated in Fig. 1a, which compares the time that the subject mouse spends with a novel mouse versus the time that the subject mouse time spent with a nonsocial novel object (Moy et al. 2004, 2007, 2008b; Nadler et al. 2004; Kwon et al. 2006; Mineur et al. 2006; Crawley et al. 2007; Yang et al. 2007a, b, 2009; Chadman et al. 2008; McFarlane et al. 2008; Jamain et al. 2008; Ryan et al. 2008; Page et al. 2009; Radyushkin et al. 2009). If the group of mice spends more time in the side chamber with a novel mouse than time spent in the side chamber with the novel object, then sociability is demonstrated. If time spent in the two side chambers is not statistically different, or time with the novel object is greater than time with the novel mouse, then lack of sociability is demonstrated. A second parameter, time engaged in sniffing the novel object versus time sniffing the novel mouse, is scored by an observer to provide an independent corroborating measure of true social interactions. Most strains of mice spend more time with the novel mouse, representing normal sociability (Moy et al. 2007, 2008a, b; McFarlane et al. 2008; Yang et al. 2009). Equal time spent with the novel mouse and the novel object would represent impaired sociability in a mouse model of autism. Less time spent with the novel mouse than with the novel object may be analogous to the tendency of autistic children and adults to engage in nonsocial activities such as playing exclusively with a favorite toy train, rather than with the other children or adults in the room.
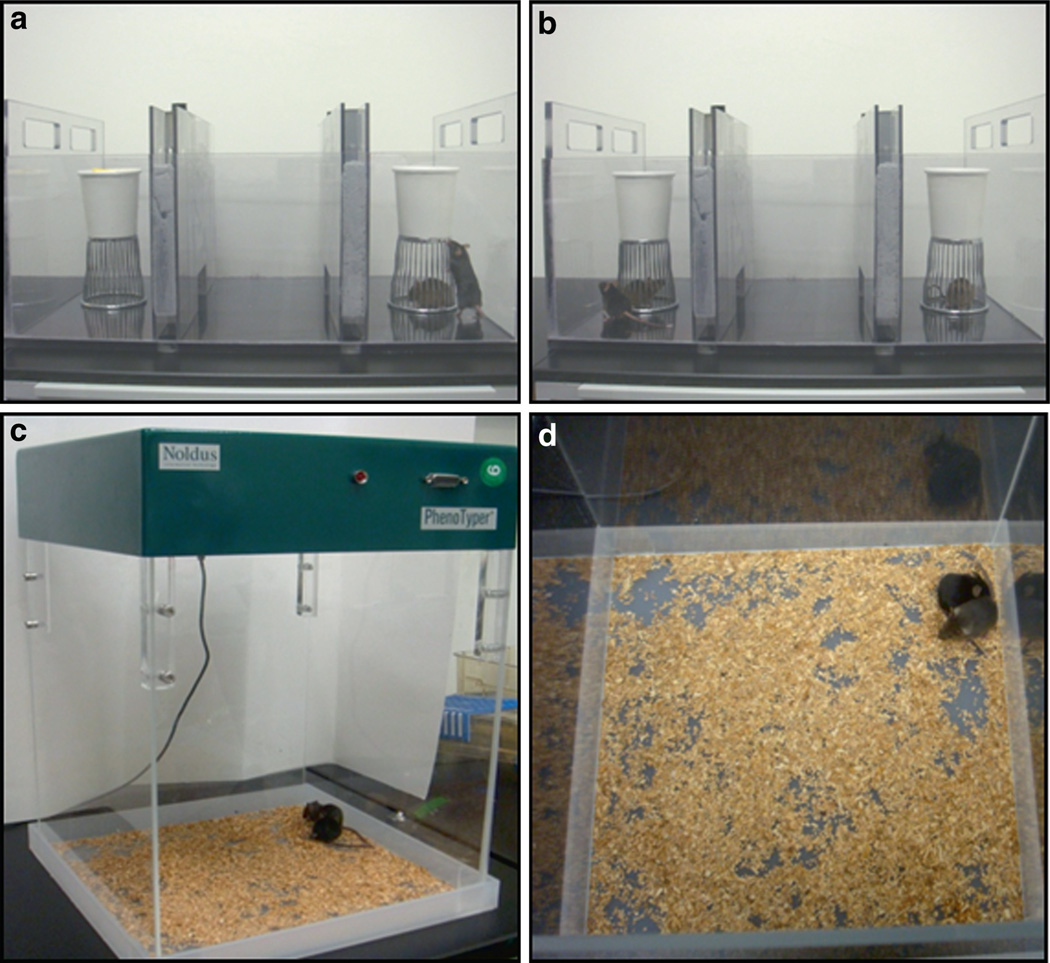
Fig. 1. Social approach and juvenile play.
Panel A illustrates the automated three-chambered apparatus, designed as an initial simple assay for sociability in mice (McFarlane et al. 2008; Moy et al. 2004, 2007; Nadler et al. 2004; Ryan et al. 2008; Yang et al. 2007a, b, 2009). Sets of photocell beams across the entrances between compartments are sequentially broken when the mouse moves between compartments. Software tallies the time spent in each chamber and number of entries into each chamber. The social approach test measures the sociability of the subject mouse as a simple yes-or-no parameter, defined as significantly more time spent in the side chamber containing a novel mouse than in the side chamber containing a novel object during a 10-min test session. The equipment can be used to assess preference for social novelty in a subsequent 10-min test session. Preference for social novelty is defined as significantly more time spent in the side chamber with a second novel mouse than in the side chamber with the first, now familiar, mouse (Panel B). Time spent sniffing the novel mouse versus the familiar mouse, or time spent sniffing the second novel mouse versus a familiar mouse, provides a corroborative measure of actual social interaction. Number of entries offers a control measure of general exploratory locomotion. Habituation to the empty test chamber takes place during a 10-min test session immediately before the sociability session. Innate side preference is evaluated during the habituation phase. If the group spends significantly more time on one side during habituation, the room configuration is changed to equalize time in each side chamber during general exploration. Panels C and D illustrate reciprocal social interactions between two freely moving mice. Reciprocal social interaction parameters provide more sensitive measures of relevant components of interactive sociability. Two juvenile mice, or two adult mice, usually of the same sex, are videotaped during a 10- to 30-min test session in the Noldus Phenotyper 3000 open field apparatus containing a thin layer of clean bedding. The invesigator scores the videotape using Noldus Observer event software for number of bouts and time spent in each behavioral category. Event categories include social behaviors such as sniffing, allogrooming, pushing past with physical contact, and crawling under and over. Control nonsocial behaviors are simultaneously scored, such as locomotion and self-grooming. Publications using this task are described in the text. Photographs contributed by the authors
The social approach task has been applied to investigate many lines of mice with mutations in candidate genes for autism, ranging from synaptic genes such as neuroligins to cancer genes such as Pten, and to genes associated with neurotransmission, including the serotonin transporter, GABA receptor subunits, vasopressin receptors, oxytocin, and vasoactive intestinal peptide, as well as to inbred strains of mice such as BTBR T+tf/J and BALB/c that display low social approach (Bolivar et al. 2007; Brodkin 2007; Crawley et al. 2007; Chadman et al. 2008; DeLorey et al. 2008; Jamain et al. 2008; Moy et al. 2008a, b, 2009; Stack et al. 2008; Page et al. 2009; Radyushkin et al. 2009; Zhou et al. 2009). Table 1 summarizes findings with this task from selected examples.
Table 1.
Description of behavioral and biological phenotypes for five mouse models of autism
| Mouse model | Phenotypes | References |
|---|---|---|
| Fragile X | Fragile X syndrome is the most common genetic cause of mental retardation. Fmr1 mutant mice deficient in the FMRP protein displayed endophenotypes relevant to components of Fragile X. Phenotypes included elevated locomotor activity in an open field, anxiety-like behaviors in the mirrored chamber test, audiogenic seizures, macroochidism, and low prepulse inhibition of acoustic startle. Attentional deficits and impairment in inhibitory control (impulsivity) were detected in the five choice serial reaction time task. Fmr1 mutant mice engaged in more sniffing of a familiar partner in the partition test, and won fewer social dominance challenges. Impairments in spatial learning of the Morris water maze were accompanied by resistance to change during the reversal phase. Increased spine density and length were reported for the somatosensory cortex, while reduced dendritic spine density and length were reported for the hippocampus. Long-term potentiation was found to be impaired, and long-term depression enhanced, in Fmr1 mutant mice. | Bakker (1994), Beckel-Mitchener and Greenough (2004), Bilousova et al. (2009), D’Hooge et al. (1997), Dolen et al. (2007), Errijgers et al. (2008), Lauterborn et al. (2007), Moon et al. (2006), Paylor et al. (2008), Peier et al. (2000), Spencer et al. (2005) |
| Chr 15q11–13 | Deletions and duplications in the 15q11–13 chromosomal region are associated with Angelman’s syndrome, Prader–Willi syndrome, and autism. Mice with a paternally transmitted duplication on the homologous region of mouse chromosome 7 displayed impaired sociability in the three-chambered social approach task. Anxiety-like phenotypes were detected on the elevated plus-maze. More ultrasonic vocalizations were emitted by pups separated from their dams, while fewer vocalizations were emitted by adults in the resident-intruder test. PatDp/+ paternal duplication mice were normal on acquisition but failed on the reversal phase in both the Morris water and the Barnes maze, indicating an inability to reverse a spatial habit. Higher freezing in the altered context was reported for fear conditioning, indicating impaired learning and memory | Nakatani et al. (2009) |
| Pten | Mutations in phosphatase and tensin homolog deleted on chromosome ten (PTEN) are associated with cancers, seizures, macroencephaly, mental retardation, and autism. Mice with a conditional Pten mutation on a neuron-specific promoter exhibited seizures, macroencephaly, and dendritic hypertrophy. Increased acoustic startle and lower prepulse inhibition indicated hypersensitivity to sensory stimuli. Social interaction deficits were detected during juvenile reciprocal interactions, sociability in the three-chambered social approach task, preference for social novelty, and nest building. Impaired spatial learning with high thigmotaxis was found in Pten mutants in the Morris Water Maze test. Many of the neuroanatomical and behavioral abnormalities in Pten mutant mice were reversed by treatment with the mTOR inhibitor rapamycin. | Kwon et al. (2006), Zhou et al. (2009) |
| BTBR | BTBR T+ tfJ (BTBR) is a genetically homogenous, commercially available inbred strain of mice, included in the top tier of the Mouse Phenome Project (http://phenome.jax.org/). BTBR juveniles and adults display low levels of reciprocal social interactions, lack of sociability in the automated three-chambered social approach task, and reduced social transmission of food preference, as compared to social strains such as C57BL/6J and FVB/NJ. BTBR displays high levels of spontaneous self-grooming both as juvenile and as adults. More frequent and louder ultrasonic vocalizations were emitted by BTBR pups separated from their dams, while fewer vocalizations are emitted by adults in response to female urinary pheronome cues, as compared to C57BL/6J. Measures of general health, motor functions, and sensory abilities including olfaction are normal in BTBR, supporting an interpretation of highly selective abnormalities in traits relevant to the diagnostic symptoms of autism. | Bolivar et al. (2007), McFarlane et al. (2008), Moy et al. (2007), Scattoni et al. (2008a, b), Yang et al. (2007a, b, 2009), Wöhr et al. (2010) |
| Prenatal valproic acid | A small number of cases of autism were linked with valproic acid (VPA) medication taken by mothers during pregnancy. Rats from dams treated with VPA during pregnancy demonstrate reduced social interactions as juveniles, and low sociability as adults on the social approach task. VPA-treated rat pups display delays in olfactory discrimination during nest-seeking behavior. Repetitive/stereotyped patterns of behavior were observed in VPA rats placed in an open field. Lower sensitivity to painful stimuli as measured by tail flick and thermal paw withdrawal tests, but increased sensitivity to nonpainful stimuli as measured in tactile test (von Frey filaments) have been reported. VPA-treated rats display enhanced responses on eye blink conditioning, and impaired spatial learning in the Morris Water maze. Cerebellar pathology, increased complexity of apical dendritic arborization, and reduced excitatory synaptic responses have been observed in VPA-treated rats, as well as increased numbers of NMDA receptors and hyperconnectivity. Altered levels of monoamines, elevated brain serotonin, and disturbance of sleep patterns have been described. Mice treated prenatally with VPA have reduced expression of neuroligin-3, a cell adhesion protein involved in synapse formation, in hippocampus and somatosensory cortex. | Ingram et al. (2000), Kolozsi et al. (2009), Narita et al. (2002), Rinaldi et al. (2007), Rodier et al. (1996), Schneider and Przewlocki (2005), Snow et al. (2008), Stanton et al. (2007), Tsujino et al. (2007), Wagner et al. (2006) |
Reciprocal social interactions between two or more freely moving mice offer more sensitive assays for some of the specific types of social reciprocity deficits seen in autism spectrum disorders. Interactive sessions between two mice of the same sex and age are conducted in an arena, such as the Noldus Phenotyper 3000 illustrated in Fig. 1c, d. Sessions are videotaped and later scored by an observer, or by videotracking software (Cheh et al. 2006; Bolivar et al. 2007; Yang et al. 2007a, b, 2009; McFarlane et al. 2008). Measures that are reliably scored by human observers include following another mouse, sniffing each other, grooming each other, crawling over and under each other, sitting together in close physical contact, and sleeping together in a huddle. Investigators first define the behavioral parameters of interest, then quantify the number of bouts and/or cumulative time engaged in each behavior, across a test session as short as 10 min. Either reduced interactions or unusual interactions can thus be detected. Dyads of partners can be tested at any age postweaning, and pairs can be composed of any combinations of genotypes, strains, and genders. Representative examples are described in Table 1.
Social recognition and social memory in mice are evaluated by amount of time spent sniffing a novel mouse upon repeated exposures, to induce familiarity, and reinstatement of high levels of sniffing when a novel stimulus animal is introduced (Bielsky et al. 2004, 2005; Bielsky and Young 2004; Richter et al. 2005; Sanchez-Andrade et al. 2005; Wersinger et al. 2007; Wanisch et al. 2008). Social memory is assayed from videotapes in three-chambered environments, using a time delay of up to 30 min between presentations (Winslow and Insel 2002; Winslow 2003; Young et al. 2002). In the automated three-chambered apparatus illustrated in Fig. 1b, preference for social novelty is assayed by replacing the novel object with a second novel mouse for a 10 min social choice session following the 10 min sociability session (Moy et al. 2007, 2008a, b; Chadman et al. 2008; McFarlane et al. 2008). Representative examples are described in Table 1.
3 Mouse Behavioral Tasks with Face Validity for the Second Diagnostic Symptom of Autism, Communication Deficits
The second category of core symptoms involves language delays, low ability to maintain interactive conversations, strictly literal use of words, and inability to understand nuances such as humor, sarcasm, facial expressions, and body language (Lord et al. 2000; Frith 2003; Tager-Flusberg and Caronna 2007). It will be difficult to design mouse tasks with high face validity to these uniquely human modes of communication. Communication in mice is primarily through olfactory cues (Bowers and Alexander 1967; Doty 1986; Schellinck et al. 1993; Isles et al. 2001; Brennan and Keverne 2004; Keverne 2004; Kavaliers et al. 2005; Wang et al. 2008; Arakawa et al. 2009; Restrepo et al. 2009; Roullet et al. 2010). Ultrasonic vocalizations emitted by mice in some social situations may represent another mode of communication (Maggio and Whitney 1985; White et al. 1998; Branchi et al. 2001; D’Amato and Moles 2001; Hofer et al. 2001; Gourbal et al. 2004; Panksepp et al. 2007; Scattoni et al. 2008a, b, 2009; Wöhr et al. 2010). Social olfactory tasks for mice generally measure sniffing of urinary pheronomones (Humphries et al. 1999; Hurst et al. 2001; Bakker 1994; Brennan and Keverne 2004; Hurst and Beynon 2004; Hurst et al. 2005; Arakawa et al. 2007; Yang and Crawley 2009) and behavioral responses toward scent marks (Cheetham et al. 2007; Arakawa et al. 2007, 2009; Roullet et al. 2010). Olfactory habituation/dishabituation involves presenting a sequence of nonsocial and social cues on cotton swabs and measuring time spent sniffing to same and to novel odors (Chadman et al. 2008; Stack et al. 2008; Yang et al. 2009; Yang and Crawley 2009). Scent marking involves measuring the number of urinary spots deposited by the subject mouse in proximity to urinary spots deposited by another mouse, or to a urine sample placed in an arena by the investigator (Hurst and Beynon 2004; Arakawa et al. 2007, 2008; Wang et al. 2008; Roullet et al. 2010). Open field arenas fitted with specialized urine-absorbing paper and ultrasonic microphones and representative scent markings by a male mouse in response to a spot of female urine in the center of the arena, are illustrated in Fig. 2.
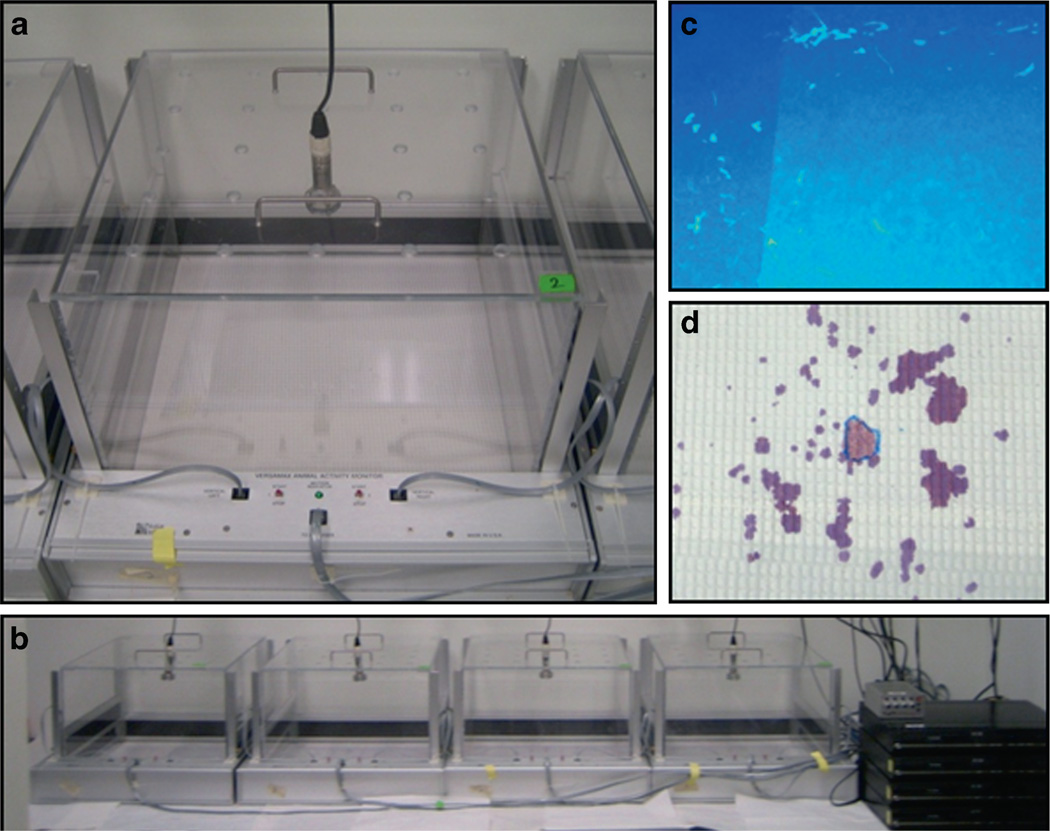
Fig. 2. Social olfactory scent marking and countermarking.
Figure 2 illustrates our apparatus for measuring urinary scent marking and countermarking behavior in an open field by adult mice. Scent marking in mice is often used as a mechanism for social dominance in marking territory and access to females, and may reflect the ability of mice to interpret and respond correctly to social cues. A sheet of paper with absorbent properties is placed in the bottom of the open field. The male mouse explores an open field in which a drop of urine from an estrus female has been placed in the center of the paper. The male will deposit his urine in spots located in proximity to the female urine sample and throughout the open field. Countermarking is another scent marking test that measures the scent marks deposited by an adult male in the presence of scent marks previously deposited by another male on the paper lining. As shown in Panels A and B, ultrasonic microphones positioned above each of four open fields simultaneously record ultrasonic vocalizations emitted by the subject mice during the session. At the end of the 5-min test session, the paper is placed under an ultraviolet lamp for visualization and quantitation of the deposited urinary traces (Panel C). Alternatively, the sheet of paper is treated with Ninhydrin spray. Scent marks appear as purple spots (Panel D). Number and location of scent marks are scored and analyzed for factors including proximity to a female urine spot (circled in blue) by the male subject mouse. See text for further descriptions. Photographs contributed by the authors
Ultrasonic vocalizations are emitted by mouse pups when separated from the nest, and detected by the parents to locate the straying pup and retrieve it to the nest (Zippelius and Schleidt 1956; Hofer et al. 2001; D’Amato and Moles 2001; Winslow and Insel 2002; Shu et al. 2005; Scattoni et al. 2008a, b). Pup calls in the range of 40–90 kHz are a robust, easily replicated phenomenon in mice, which represent communication in the sense that they elicit a response from the adult parents. However, intentionality on the part of the pup has not been demonstrated. Another issue is face validity. It is not obvious that low numbers of pup vocalizations represent the type of communication deficits seen in autism. Infants later diagnosed with autism display less crying in some cases, but louder and more frequent and inconsolable crying in some cases, and normal crying in other cases (Sheinkopf et al. 2000; Zwaigenbaum et al. 2005). Nevertheless, the number of ultrasonic vocalizations by separated pups has been widely used as an assay for communication in mouse models of autism, as described for some of the examples in Table 1.
Ultrasonic vocalizations in juvenile and adult mice may provide better models for intentional communication in mice. Vocalizations during social interactions by pairs of previously isolated juvenile mice, calls between pairs of adult females and vocalizations emitted by adult male mice sniffing female urine, are being analyzed for call numbers and call properties in mouse models of autism and other neuropsychiatric disorders (D’Amato and Moles 2001; Panksepp et al. 2007; Wang et al. 2008; Scattoni G2B paper if in press). In addition, vocalizations have proven to be a useful measure in mouse models of human speech disorders such as mutations in the FOXP2 gene (Shu et al. 2005; Fujita et al. 2008; Enard et al. 2009).
4 Mouse Behavioral Tasks with Face Validity for the Third Diagnostic Symptom of Autism, Repetitive Behaviors with Restricted Interests
Stereotyped, repetitive behaviors, and the restricted range of interests and activities that characterize autism (Bodfish et al. 2000; Lord et al. 2000; Cuccaro et al. 2003; Frith 2003; South et al. 2005) are amenable to modeling with available rodent tasks that incorporate reasonable face validity. Mice engage in motor stereotypies including vertical jumping, backflipping, circling, digging, marble burying rearing, repeated sniffing of one location or object, barbering, excessive self-grooming, and excessive running (Creese and Iversen 1975; Turner et al. 2001; Lee et al. 2002; Pogorelov et al. 2005; Korff and Harvey 2006; Lewis et al. 2007; Crawley 2007b; Welch et al. 2007; Yang et al. 2007a, b; McFarlane et al. 2008, Moy et al. 2008a). Excessive stereotyped grooming was reported for several mouse lines with mutations in developmental genes. Sapap3 (Welch et al. 2007) and Hoxb8 knockout mice (Greer and Capecchi 2002) exhibit extreme self-grooming that leads to hair loss and skin injuries. The inbred strain BTBR T+tf/J exhibits the normal pattern of grooming but at very high levels, representing a prolonged repetitive behavior (Yang et al. 2007a, b, 2009; McFarlane et al. 2008).
Assays for repetitive behaviors include perseverations, such as the inability to change to a spatial habit (Ralph et al. 2001; Brigman et al. 2006; Chen et al. 2007; Moy et al. 2008a). Spatial habits in mice can be induced by first locating the reinforcer consistently in one place, e.g., the food pellet is always in the left arm of a T-maze, or the hidden platform is always in the northwest quadrant of a water maze, during the initial training sessions. If the food pellet is later moved to the right arm of the T-maze, or the hidden platform is later moved to the southeast quadrant of the water maze, most mice can learn to change their spatial habit during the reversal training sessions. A mouse model of autism is predicted to acquire the initial habit but not the reversal. Examples are described in Table 1. An interesting report with a Fragile X model engaged in an operant task indicates a resistance to change, in that general performance was disrupted after an error was made (Moon et al. 2006).
Restricted interest assays for mice are under development. One is a holeboard array, in which mice usually nosepoke into all of the holes in the floor of an open field, including holes baited with various objects and odors (Moy et al. 2008a). A mouse model of autism would be predicted to show nosepoke activity into only one or a small subset of holes, representing restricted interest in one location or type of bait. Spontaneous alternation in a Y-maze, which represents a common exploratory strategy in mice, could be used to measure restricted exploration to only one arm of the Y-maze. Another approach is an attentional task, in which a mouse model of autism would be predicted to excel at maintaining focused attention, and ignoring distractors.
5 Mouse Behavioral Tasks with Face Validity to Associated Symptoms of Autism
Associated symptoms of autism that occur in varying percentages of cases include mental retardation, seizures, anxiety, hyperreactivity to sensory stimuli, and sleep disturbances. Endophenotypes with face validity to these associated symptoms may be easier to model in mice. However, analogs of associated symptoms raise questions about the extent to which they are essential to include in a mouse model of autism. For example, standard anxiety-related tasks for mice include the elevated plus-maze, elevated zero maze, light/dark exploration, emergence test, and Vogel thirsty lick conflict test (Cryan and Holmes 2005; Crawley et al. 2007). Anxiety-related phenotypes have been reported for the Nlgn2, 5Htt, Fmr1, Avpr1b, and other lines of mice with mutations that may be relevant to autism (Wersinger et al. 2002; Holmes et al. 2003; Spencer et al. 2005; Blundell et al. 2009). Seizures in mice are scored with tonic–clonic rating scales and electroencephalogram recordings. High levels of seizures and seizure susceptibility have been reported for some lines of mice with mutations relevant to neurodevelopmental disorders, e.g., GABA receptor Gabrb3 subunit and Pten knockouts (DeLorey et al. 1998; Zhou et al. 2009).
Compelling findings relevant to associated symptoms offer important leads to pursue, in defining genes underlying a broad set of neurodevelopmental abnormalities that may converge in the etiology of autism. However, at a practical level, traits such as seizures, high anxiety-like behaviors, or circadian disruptions produce confounds in the interpretations of other behavioral findings such as social deficits. For example, mice with high anxiety-like tendencies will remain in the center chamber of the three-chambered automated social approach apparatus and will not explore either the side chamber with the novel mouse or the side chamber with the novel object. Mice experiencing frequent seizures or lack of sleep may similarly be generally inactive in all tasks requiring exploratory locomotion. The role of learning deficits on social behaviors remains to be determined in mice, as well as in humans.
6 Biological Assays
As described above, clinical studies have reported unusual neuroanatomical features in some autistic individuals as compared to typically developing children and adults. Reported differences include larger head circumference at young ages, larger or smaller volumes of cortical gray matter and white matter, in thickness of long cortical connectivity pathways and intrahemispherical connections including the corpus callosum, loss of cerebellar Purkinje cells, reduced amygdala size, and less activation of brain regions during social tasks in fMRI studies (Herbert et al. 2003; Minshew and Williams 2007: Pelphrey et al. 2002; Spezio et al. 2007; Amaral et al. 2008; McAlonan et al. 2008). Analogous morphometric analyses are beginning to be applied to mouse models (Radyushkin et al. 2009). Hypotheses about impairments in synaptic connections, dendritic spines, and electrophysiological measures of synaptic plasticity are being tested in mouse models of neurodevelopmental disorders (Beckel-Mitchener and Greenough 2004; Dolen et al. 2007; Lauterborn et al. 2007; Ehninger et al. 2008a; Sudhof 2008; Zhou et al. 2009). Table 1 and the section below include descriptions of biological and behavioral phenotypes that have been reported in mutant mouse models of autism spectrum disorders.
7 Comprehensive Phenotypes of Selected Mouse Models of Autism Spectrum Disorders
7.1 Fragile X
Fragile X syndrome, the major form of mental retardation with a known genetic basis, is caused by highly expanded CGG trinucleotide repeats within the X-linked FMR1 gene, an RNA binding protein (Bassell and Warren 2008). Approximately 25% of individuals with Fragile X syndrome also meet the diagnostic criteria for autism (Abrahams and Geschwind 2008). Fmr1 knockout mice display several behavioral phenotypes relevant to autism spectrum disorders, depending on the genetic background into which the mutation is bred (Errijgers et al. 2008; Moy et al. 2009). As shown in Table 1, well-replicated phenotypes include hyperactivity, high anxiety-like behaviors, low prepulse inhibition of acoustic startle and mild impairments on water maze learning (Bakker 1994; D’Hooge et al. 1997; Peier et al. 2000; Spencer et al. 2005; Errijgers et al. 2008). Fmr1 knockout mice display abnormally high densities of long, thin, immature dendritic spines and impaired long-term potentiation (Beckel-Mitchener and Greenough 2004; Lauterborn et al. 2007). Gene therapy with normal human FRM1 rescued the hyperactivity, prepulse inhibition deficit, anxiety-like behaviors, and the social anxiety-like behaviors in Fmr1 knockout mice (Peier et al. 2000; Paylor et al. 2008; Spencer et al. 2008). Treatments with minocycline, brain-derived neurotrophic factor, and mGluR5 receptor antagonists reduced the dendritic spine abnormalities and long-term potentiation deficits in Fmr1 knockout mice (Lauterborn et al. 2007; Dolen and Bear 2008; Bilousova et al. 2009).
7.2 Chromosome 15q11–13 Duplication
Chromosomal duplications and deletions, termed copy number variants, appear with higher frequencies in autism than in the general population (Sebat et al. 2007). The most frequent duplication appears to be at the 15q11–13 locus, and is usually maternally transmitted (Cook et al. 1997; Kwasnicka-Crawford et al. 2007). Mice with 15q11–13 duplications displayed lack of sociability in the three-chambered social approach task, and normal acquisition but failure to reverse a spatial habit in the Morris water maze (Nakatani et al. 2009), as shown in Table 1. Comprehensive analyses of general health, sensory abilities, and motor functions confirmed normal physical abilities, including olfactory (Nakatani et al. 2009). It is interesting to note that paternal transmission of the 15q11–13 duplication in mice produced these autism-relevant phenotypes to a great extent than the maternal transmission of the duplication, in contrast to the maternal duplication of 15q11–13 more frequently causing autism in humans.
7.3 Pten
Phosphatase and tensin homolog on chromosome ten (PTEN) is a tumor suppressor gene implicated in cancers (Diaz-Meco and Abu-Baker 2009). PTEN mutations additionally result in macrocephaly, and some individuals meet the diagnostic criteria for autism (Varga et al. 2009). Mice with Pten null mutations generated with a conditional neuronal promoter displayed macroencephaly, neuronal hypertrophy, poor spatial learning, higher anxiety-like behaviors, higher open field activity, elevated acoustic startle, and lack of preference for social novelty (Kwon et al. 2006). Signaling proteins downstream from Pten include PI3K, AKT, TSC, and mTOR (Zhou et al. 2009). Remarkably, long-term treatment with the mTOR inhibitor rapamycin reversed the neuronal soma hypertrophy, dentate gyrus enlargement, and social interaction deficits in 2-month-old Pten knockout mice (Zhou et al. 2009).
7.4 BTBR
A broad survey of inbred strains of mice from the top tier of The Mouse Phenome Project (http://phenome.jax.org/) revealed an obscure strain, BTBR T+tf/J (BTBR), which displayed specific deficits on social approach and high levels of repetitive self-grooming, described in Table 1, along with normal scores on measures of general health, sensory abilities, and motor functions (Moy et al. 2007). Failure to display sociability in the automated three-chambered social approach task has been replicated in multiple cohorts of BTBR, across three laboratories, and in both the light and the dark phases of the circadian cycle (Moy et al. 2007; Bolivar et al. 2007; McFarlane et al. 2008; Yang et al. 2007a, b, 2009). Low reciprocal social interactions have been found in pairs of juvenile BTBR as compared to pairs of juvenile C57BL/6J, a commonly used inbred strain with high social approach (McFarlane et al. 2008; Yang et al. 2007a, b, 2009). Relevant to the second diagnostic symptom of autism, unusual patterns of ultrasonic vocalizations have been reported for BTBR (Scattoni et al. 2008a, b). Relevant to the third diagnostic symptom of autism, normal patterns but very long bouts of repetitive self-grooming in BTBR have been detected in multiple cohorts in various environments (McFarlane et al. 2008; Yang et al. 2007a, b, 2009). Similar social approach deficits and unusual vocalizations have been detected in another inbred strain, BALB/cJ (Brodkin 2007; Panksepp et al. 2007). While inbred strains do not test specific hypotheses about autism candidate gene mutations, they provide opportunities to discover background genes mediating social, communication, and repetitive behaviors. Robust phenotypes in BTBR offer translational tools to evaluate treatments for low sociability and high repetitive behaviors. For example, repetitive self-grooming in BTBR was reduced by acute treatment with an mGluR5 antagonist, MPEP (Silverman et al. 2010).
7.5 Prenatal Valproic Acid
Valproic acid (VPA) is a drug used in the treatment of epilepsy and mood disorder (Ornoy 2009). Administered during pregnancy, VPA can induce fetal valproate syndrome in the offspring, characterized by neural tube defects such as spina bifida, craniofacial abnormalities (Ardinger et al. 1988; Arpino et al. 2000; DiLiberti et al. 1984; Wide et al. 2004), and behavioral and cognitive dysfunctions associated with autism (Christianson et al. 1994; Moore et al. 2000; Rasalam et al. 2005; Williams et al. 2001; Williams and Hersh 1997). In rodents, prenatal exposure to VPA results in deficit in social interaction, repetitive/stereotyped patterns of behavior, a lower sensitivity to pain but increased sensitivity to nonpainful stimuli, (Schneider and Przewlocki 2005), disturbed sleep pattern (Tsujino et al. 2007), and alterations in eye blink conditioning (Stanton et al. 2007). In addition, rats treated in utero with VPA show cerebellar pathology (Ingram et al. 2000; Rodier et al. 1996), increased complexity of apical dendritic arborization (Snow et al. 2008), elevated levels of brain serotonin (Tsujino et al. 2007), and enhanced hyperconnectivity (Rinaldi et al. 2008). Adult mice previously treated in utero with VPA have reduced neuroligin 3 mRNA expression in some brain areas (Kolozsi et al. 2009). Interestingly, prenatal exposure to VPA produced greater behavioral and physiological abnormalities in male rats than in female rats (Schneider et al. 2008). Behavioral deficits relevant to autism (abnormalities in social behavior, stereotypy) were reversed when VPA-treated rats were exposed to an enriched environment (Schneider et al. 2006).
8 Conclusions
Considerable progress has been made in the translational use of mouse models to investigate molecular hypotheses about the causes of the behavioral symptoms of autism spectrum disorders. The nascent field of mouse models of autism will mature in concert with the clinical field in diagnosing specific symptoms and subcategories of autism. Evaluation instruments for both the mouse models and the human syndrome are in place, but considerably more development and refinement is needed. Interactive conversations between clinical investigators and behavioral neuroscientists will aid the process of generating mouse assays most relevant to the core endophenotypes of autism. Synergistic discoveries of genetic mutations in autistic individuals, genes underlying social, communicative, and repetitive behaviors in mice, and the functional consequences of autism candidate gene mutations in mice, are likely to move the field forward significantly in the next few years.
Abbreviations
- 5Htt
Serotonin transporter mutant line of mice
- Avpr1b
Arginine vasopressin receptor 1b null mutant line of mice
- B6
C57BL/6J inbred strain of mice
- BTBR
BTBR T+tf/J inbred strain of mice
- Fmr1
Fragile X Fmr1 null mutant line of mice
- Nlgn2
Neuroligin 2 mutant line of mice
- VPA
Valproic acid [Di-n-dipropylacetic acid]
Contributor Information
Florence I. Roullet, Email: firoullet@gmail.com.
Jacqueline N. Crawley, Email: crawleyj@mail.nih.gov.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association WDC. Diagnostic and Statistical Manual of Mentral Disorders (DSM-IV) Washington DC: APA; 1994. [Google Scholar]
- Anderson GM. Genetics of childhood disorders: XLV. Autism, part 4: serotonin in autism. J Am Acad Child Adolesc Psychiatry. 2002;41:1513–1516. doi: 10.1097/00004583-200212000-00025. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Social features of scent-donor mice modulate scent marking of C57BL/6J recipient males. Behav Brain Res. 2009;205:138–145. doi: 10.1016/j.bbr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardinger HH, Atkin JF, Blackston RD, Elsas LJ, Clarren SK, Livingstone S, Flannery DB, Pellock JM, Harrod MJ, Lammer EJ, et al. Verification of the fetal valproate syndrome phenotype. Am J Med Genet. 1988;29:171–185. doi: 10.1002/ajmg.1320290123. [DOI] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino C, Brescianini S, Robert E, Castilla EE, Cocchi G, Cornel MC, de Vigan C, Lancaster PA, Merlob P, Sumiyoshi Y, Zampino G, Renzi C, Rosano A, Mastroiacovo P. Teratogenic effects of antiepileptic drugs: use of an international database on malformations and drug exposure (MADRE) Epilepsia. 2000;41:1436–1443. doi: 10.1111/j.1528-1157.2000.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Autism Genome Project Consortium. Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, Lajonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F , et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker CE. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Baron-Cohen S. Autism: the empathizing-systemizing (E-S) theory. Ann NY Acad Sci. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckel-Mitchener A, Greenough WT. Correlates across the structural, functional, and molecular phenotypes of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10:53–59. doi: 10.1002/mrdd.20009. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. TheV1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JM, Alexander BK. Mice: individual recognition by olfactory cues. Science. 1967;158:1208–1210. doi: 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14:R81–R89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006;120:984–988. doi: 10.1037/0735-7044.120.4.984. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, Williams JR, Witt DM, Insel TR. Oxytocin and social bonding. Ann N Y Acad Sci. 1992 Jun 12;652:204–211. doi: 10.1111/j.1749-6632.1992.tb34356.x. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni MLS, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham SA, Thom MD, Jury F, Ollier WE, Beynon RJ, Hurst JL. The genetic basis of individual-recognition signals in the mouse. Curr Biol. 2007;17:1771–1777. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116:166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen KS, Kobayashi D, Barbour R, Motter R, Games D, Martin SJ, Morris RG. Active beta-amyloid immunization restores spatial learning in PDAPP mice displaying very low levels of beta-amyloid. J Neurosci. 2007;27:2654–2662. doi: 10.1523/JNEUROSCI.3710-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson AL, Chesler N, Kromberg JG. Fetal valproate syndrome: clinical and neuro-developmental features in two sibling pairs. Dev Med Child Neurol. 1994;36:361–369. doi: 10.1111/j.1469-8749.1994.tb11858.x. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455:919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Lindgren V, Leventhal BL, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am J Hum Genet. 1997;60:928–934. [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Medicine. Testing hypotheses about autism. Science. 2007a;318:56–57. doi: 10.1126/science.1149801. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007b;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL, Abramson RK, Ravan SA, Wright HH, DeLong GR, Pericak-Vance MA. Factor analysis of restricted and repetitive behaviors in autism using the autism diagnostic interview-R. Child Psychiatry Hum Dev. 2003;34:3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Moles A. Ultrasonic vocalizations as an index of social memory in female mice. Behav Neurosci. 2001;115:834–840. doi: 10.1037//0735-7044.115.4.834. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, Kooy RF, Oostra BA, Willems PJ, De Deyn PP. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997;76:367–376. doi: 10.1016/s0306-4522(96)00224-2. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco MT, Abu-Baker S. The Par-4/PTEN connection in tumor suppression. Cell Cycle. 2009;8:2518–2522. doi: 10.4161/cc.8.16.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLiberti JH, Farndon PA, Dennis NR, Curry CJ. The fetal valproate syndrome. Am J Med Genet. 1984;19:473–481. doi: 10.1002/ajmg.1320190308. [DOI] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Odor-guided behavior in mammals. Experientia. 1986;42:257–271. doi: 10.1007/BF01942506. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008a;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008b;60(6):950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard W, Gehre S, Hammerschmidt K, Holter SM, Blass T, Somel M, Bruckner MK, Schreiweis C, Winter C, Sohr R, Becker L, Wiebe V, Nickel B, Giger T, Muller U, Groszer M, Adler T, Aguilar A, Bolle I, Calzada-Wack J, Dalke C, Ehrhardt N, Favor J, Fuchs H, Gailus-Durner V, Hans W, Holzlwimmer G, Javaheri A, Kalaydjiev S, Kallnik M, Kling E, Kunder S, Mossbrugger I, Naton B, Racz I, Rathkolb B, Rozman J, Schrewe A, Busch DH, Graw J, Ivandic B, Klingenspor M, Klopstock T, Ollert M, Quintanilla-Martinez L, Schulz H, Wolf E, Wurst W, Zimmer A, Fisher SE, Morgenstern R, Arendt T, de Angelis MH, Fischer J, Schwarz J, Paabo S. Ahumanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Errijgers V, Fransen E, D’Hooge R, De Deyn PP, Kooy RF. Effect of genetic background on acoustic startle response in fragile X knockout mice. Genet Res. 2008;90:341–345. doi: 10.1017/S0016672308009415. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: explaining the Enigma. Oxford, UK: Wiley-Blackwell; 2003. [Google Scholar]
- Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, Momoi T. Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc Natl Acad Sci USA. 2008;105:3117–3122. doi: 10.1073/pnas.0712298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. Neuroscience. Autism’s cause may reside in abnormalities at the synapse. Science. 2007;317:190–191. doi: 10.1126/science.317.5835.190. [DOI] [PubMed] [Google Scholar]
- Gondo Y, Murata T, Makino S, Fukumura R, Ishitsuka Y. Mouse mutagenesis and disease models for neuropsychiatric disorders. Curr Topics Behav Neurosci. 2011 doi: 10.1007/7854_2010_106. [DOI] [PubMed] [Google Scholar]
- Gourbal BE, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91:381–385. doi: 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- Grant EC, MacIntosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–259. [Google Scholar]
- Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Amaral D, Aschner M, Bolivar VJ, Bowman A, DiCicco-Bloom E, Hyman SL, Keller F, Lein P, Pessah I, Restifo L, Threadgill DW. Animal models of autism spectrum disorders: information for neurotoxicologists. Neurotoxicology. 2009;30:811–821. doi: 10.1016/j.neuro.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, Ronald A. The ‘fractionable autism triad’: a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18:287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS., Jr Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20:84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Shair HN, Masmela JR, Brunelli SA. Developmental effects of selective breeding for an infantile trait: the rat pup ultrasonic isolation call. Dev Psychobiol. 2001;39:231–246. doi: 10.1002/dev.1000. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Humphries RE, Robertson DH, Beynon RJ, Hurst JL. Unravelling the chemical basis of competitive scent marking in house mice. Anim Behav. 1999;58:1177–1190. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Thom MD, Nevison CM, Humphries RE, Beynon RJ. MHC odours are not required or sufficient for recognition of individual scent owners. Proc Biol Sci. 2005;272:715–724. doi: 10.1098/rspb.2004.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22:319–324. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- Isles AR, Baum MJ, Ma D, Keverne EB, Allen ND. Urinary odour preferences in mice. Nature. 2001;409:783–784. doi: 10.1038/35057323. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries AR, Curran S, Elmslie F, Sharma A, Wenger S, Hummel M, Powell J. Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A. 2005;137:139–147. doi: 10.1002/ajmg.a.30780. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Pfaff DW. Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci Biobehav Rev. 2005;29:1347–1359. doi: 10.1016/j.neubiorev.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Sexual experience does not compensate for the disruptive effects of zinc sulfate–lesioning of the main olfactory epithelium on sexual behavior in male mice. Chem Senses. 2006;31:753–762. doi: 10.1093/chemse/bjl018. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, Pauls DL, Daly MJ, MacDonald ME, Morton CC, Quade BJ, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Bearman P. Diagnostic change and the increased prevalence of autism. Int J Epidemiol. 2009;38:1224–1234. doi: 10.1093/ije/dyp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolozsi E, Mackenzie RN, Roullet FI, deCatanzaro D, Foster JA. Prenatal exposure to valproic acid leads to reduced expression of synaptic adhesion molecule neuroligin 3 in mice. Neuroscience. 2009;163:1201–1210. doi: 10.1016/j.neuroscience.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology. Psychiatr Clin North Am. 2006;29:371–390. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Kwasnicka-Crawford DA, Roberts W, Scherer SW. Characterization of an autism-associated segmental maternal heterodisomy of the chromosome 15q11-13 region. J Autism Dev Disord. 2007;37:694–702. doi: 10.1007/s10803-006-0225-8. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008;16:614–618. doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- Lee JW, Ryoo ZY, Lee EJ, Hong SH, Chung WH, Lee HT, Chung KS, Kim TY, Oh YS, Suh JG. Circling mouse, a spontaneous mutant in the inner ear. Exp Anim. 2002;51:167–171. doi: 10.1538/expanim.51.167. [DOI] [PubMed] [Google Scholar]
- Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119:747–754. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas C, Persico AM. Autistic phenotypes and genetic testing: state-of-the-art for the clinical geneticist. J Med Genet. 2009;46:1–8. doi: 10.1136/jmg.2008.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lise MF, El-Husseini A. The neuroligin and neurexin families: from structure to function at the synapse. Cell Mol Life Sci. 2006;63:1833–1849. doi: 10.1007/s00018-006-6061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. The role of the neurobiologist in redefining the diagnosis of autism. Brain Pathol. 2007;17:408–411. doi: 10.1111/j.1750-3639.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- McAlonan GM, Suckling J, Wong N, Cheung V, Lienenkaemper N, Cheung C, Chua SE. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger’s syndrome. J Child Psychol Psychiatry. 2008;49:1287–1295. doi: 10.1111/j.1469-7610.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Erickson CA, Stigler KA, Posey DJ. Neurochemistry in the pathophysiology of autism. J Clin Psychiatry. 2005;66(Suppl 10):9–18. [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Beaudin AE, Verosky S, Driscoll LL, Weiskopf M, Levitsky DA, Crnic LS, Strupp BJ. Attentional dysfunction, impulsivity, and resistance to change in a mouse model of fragile X syndrome. Behav Neurosci. 2006;120:1367–1379. doi: 10.1037/0735-7044.120.6.1367. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, Dean JC. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008a;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008b;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N. Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res. 2002;52(4):576–579. doi: 10.1203/00006450-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol. 2009;28:1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci USA. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Yuva-Paylor LA, Nelson DL, Spencer CM. Reversal of sensorimotor gating abnormalities in Fmr1 knockout mice carrying a human Fmr1 transgene. Behav Neurosci. 2008;122:1371–1377. doi: 10.1037/a0013047. [DOI] [PubMed] [Google Scholar]
- Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet. 2000;9:1145–1159. doi: 10.1093/hmg/9.8.1145. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Pogorelov VM, Rodriguiz RM, Insco ML, Caron MG, Wetsel WC. Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology. 2005;30:1818–1831. doi: 10.1038/sj.npp.1300724. [DOI] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Doucette W, Whitesell JD, McTavish TS, Salcedo E. From the top down: flexible reading of a fragmented odor map. Trends Neurosci. 2009;32:525–531. doi: 10.1016/j.tins.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Wolf G, Engelmann M. Social recognition memory requires two stages of protein synthesis in mice. Learn Mem. 2005;12(4):407–413. doi: 10.1101/lm.97505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci USA. 2007;104:13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex. 2008;18:763–770. doi: 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wöhr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res. 2011;216(1):19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory. J Reprod Dev. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008a;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008b;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellinck HM, Smyth C, Brown R, Wilkinson M. Odor-induced sexual maturation and expression of c-fos in the olfactory system of juvenile female mice. Brain Res Dev Brain Res. 1993;74:138–141. doi: 10.1016/0165-3806(93)90094-q. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Schneider T, Turczak J, Przewlocki R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism. Neuropsychopharmacology. 2006;31:36–46. doi: 10.1038/sj.npp.1300767. [DOI] [PubMed] [Google Scholar]
- Schneider T, Roman A, Basta-Kaim A, Kubera M, Budziszewska B, Schneider K, Przewlocki R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology. 2008;33:728–740. doi: 10.1016/j.psyneuen.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Mundy P, Oller DK, Steffens M. Vocal atypicalities of preverbal autistic children. J Autism Dev Disord. 2000;30:345–354. doi: 10.1023/a:1005531501155. [DOI] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35(4):976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow WM, Hartle K, Ivanco TL. Altered morphology of motor cortex neurons in the VPA rat model of autism. Dev Psychobiol. 2008;50:633–639. doi: 10.1002/dev.20337. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. J Autism Dev Disord. 2005;35:145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Graham DF, Yuva-Paylor LA, Nelson DL, Paylor R. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122:710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 2007;37:929–939. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- Stack CM, Lim MA, Cuasay K, Stone MM, Seibert KM, Spivak-Pohis I, Crawley JN, Waschek JA, Hill JM. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol. 2008;211:67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Peloso E, Brown KL, Rodier P. Discrimination learning and reversal of the conditioned eyeblink reflex in a rodent model of autism. Behav Brain Res. 2007;176:133–140. doi: 10.1016/j.bbr.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Caronna E. Language disorders: autism and other pervasive developmental disorders. Pediatr Clin North Am. 2007;54:469–481. vi. doi: 10.1016/j.pcl.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Curr Protocols Toxicol. 2005;13:10.1–10.10. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Nakatani Y, Seki Y, Nakasato A, Nakamura M, Sugawara M, Arita H. Abnormality of circadian rhythm accompanied by an increase in frontal cortex serotonin in animal model of autism. Neurosci Res. 2007;57:289–295. doi: 10.1016/j.neures.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Turner CA, Presti MF, Newman HA, Bugenhagen P, Crnic L, Lewis MH. Spontaneous stereotypy in an animal model of Down syndrome: Ts65Dn mice. Behav Genet. 2001;31:393–400. doi: 10.1023/a:1012226603255. [DOI] [PubMed] [Google Scholar]
- Varga EA, Pastore M, Prior T, Herman GE, McBride KL. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009;11:111–117. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Pauls D. Autism. Lancet. 2003;362:1133–1141. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK. A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord. 2006;36(6):779–793. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]
- Wanisch K, Wotjak CT, Engelmann M. Long-lasting second stage of recognition memory consolidation in mice. Behav Brain Res. 2008;186(2):191–196. doi: 10.1016/j.bbr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol Behav. 1998;63:467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Wide K, Winbladh B, Kallen B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr. 2004;93:174–176. doi: 10.1080/08035250310021118. [DOI] [PubMed] [Google Scholar]
- Williams PG, Hersh JH. A male with fetal valproate syndrome and autism. Dev Med Child Neurol. 1997;39:632–634. doi: 10.1111/j.1469-8749.1997.tb07500.x. [DOI] [PubMed] [Google Scholar]
- Williams DL, Minshew NJ. Understanding autism and related disorders: what has imaging taught us? Neuroimaging Clin N Am. 2007;17:495–509. ix. doi: 10.1016/j.nic.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol. 2001;43:202–206. [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT. Mouse social recognition and preference. Chapter 8, Unit 8.16. Curr Protoc Neurosci. 2003 doi: 10.1002/0471142301.ns0816s22. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2010 doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Chapter 8, Unit 8.24. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell HK, Young WS, McFarlane HG, Crawley JN. Similar social approach behaviors in BTBR T+tf/J, C57BL/6J, and vasopressine receptor 1B knockout mice tested on conventional versus reverse light cycles, and in replications across cohorts. Front Behav Neurosci. 2007a;1:9. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Pitkow LJ, Ferguson JN. Neuropeptides and social behavior: animal models relevant to autism. Mol Psychiatry. 2002;7(suppl 2):S38–S39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]
- Zecavati N, Spence SJ. Neurometabolic disorders and dysfunction in autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:129–136. doi: 10.1007/s11910-009-0021-x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippelius HM, Schleidt WM. Ultraschall-aute bei jungen Mausen. Naturwissenschaften. 1956;43:502–503. [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]