Abstract
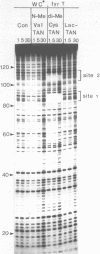
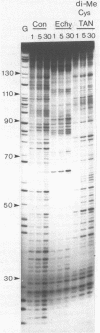
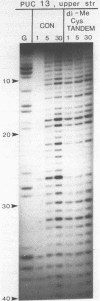
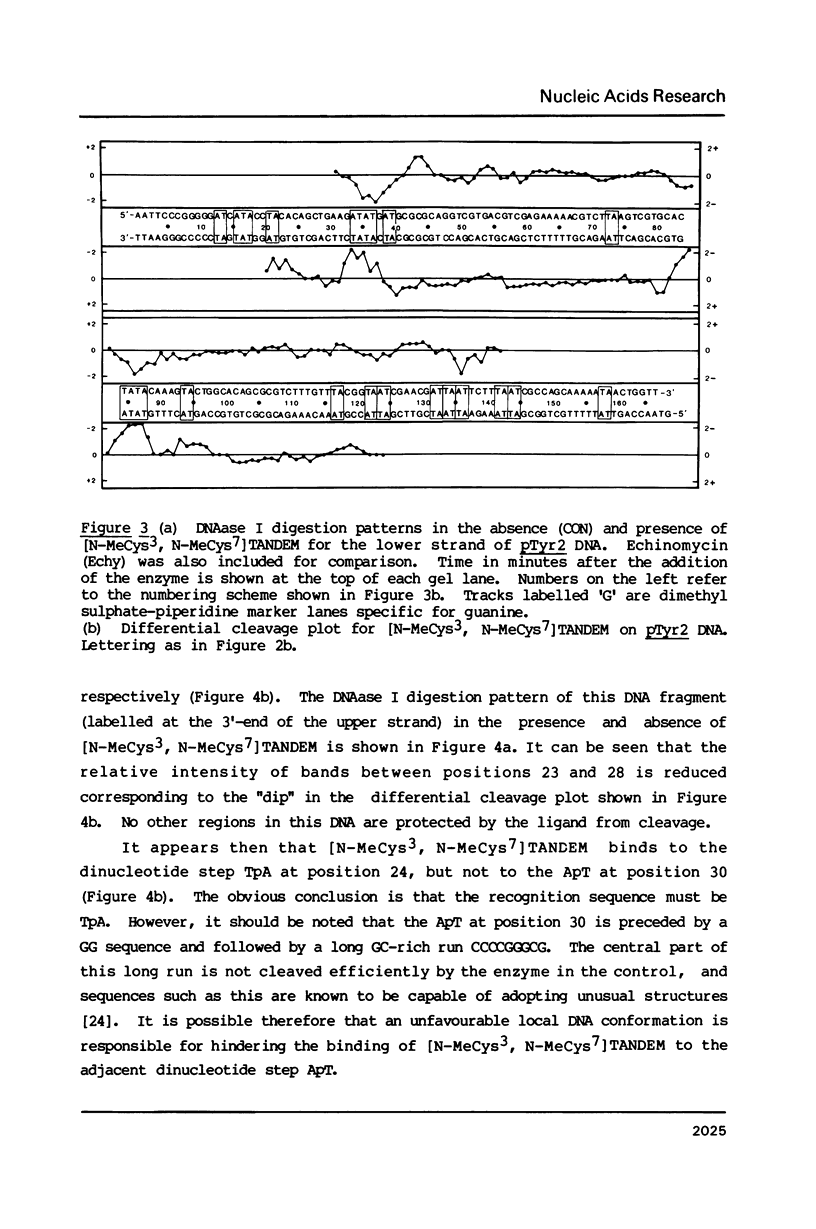
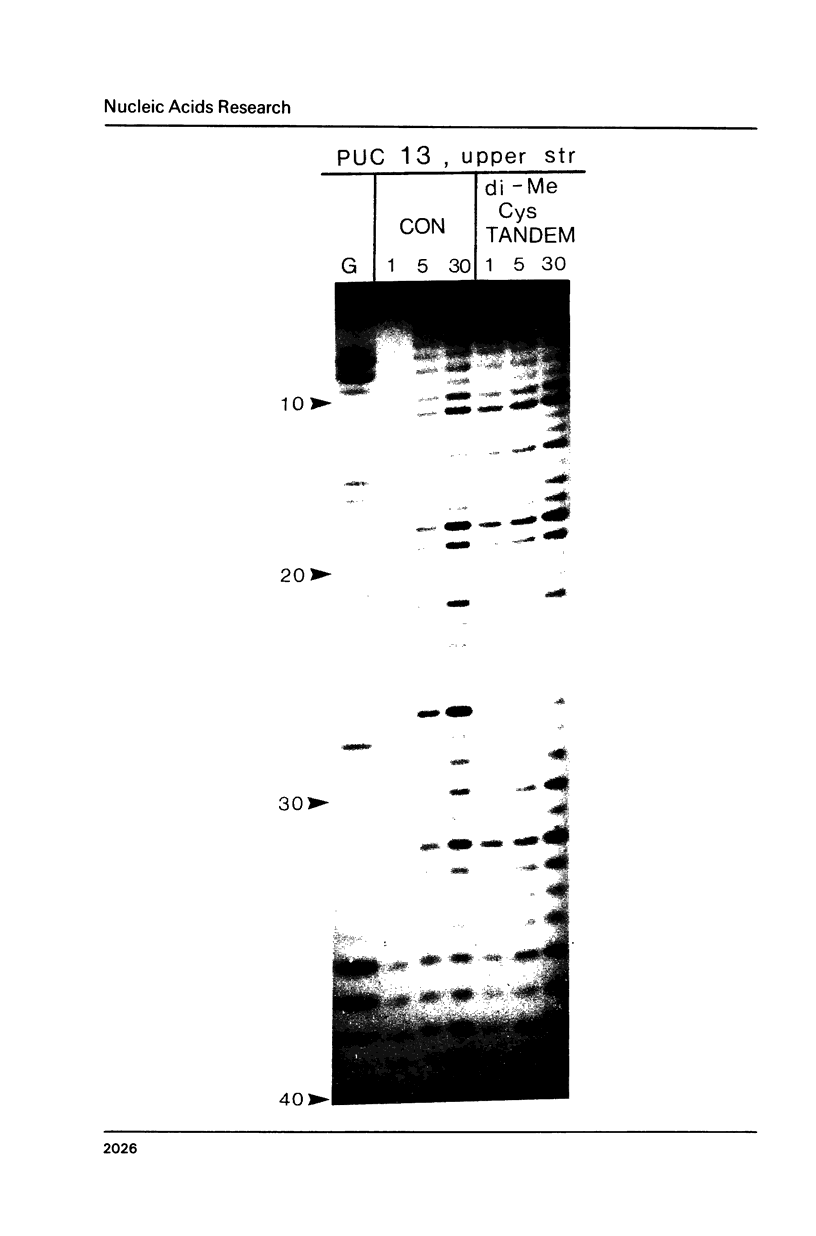
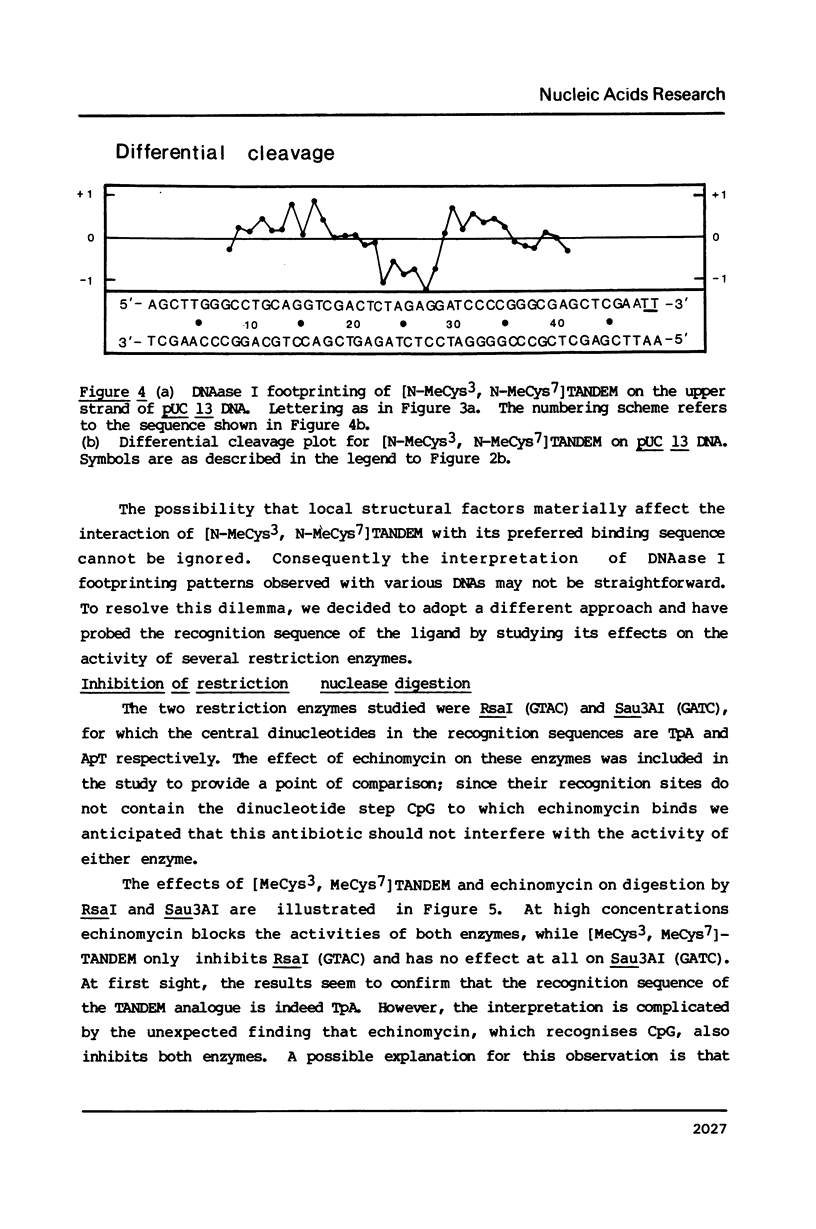
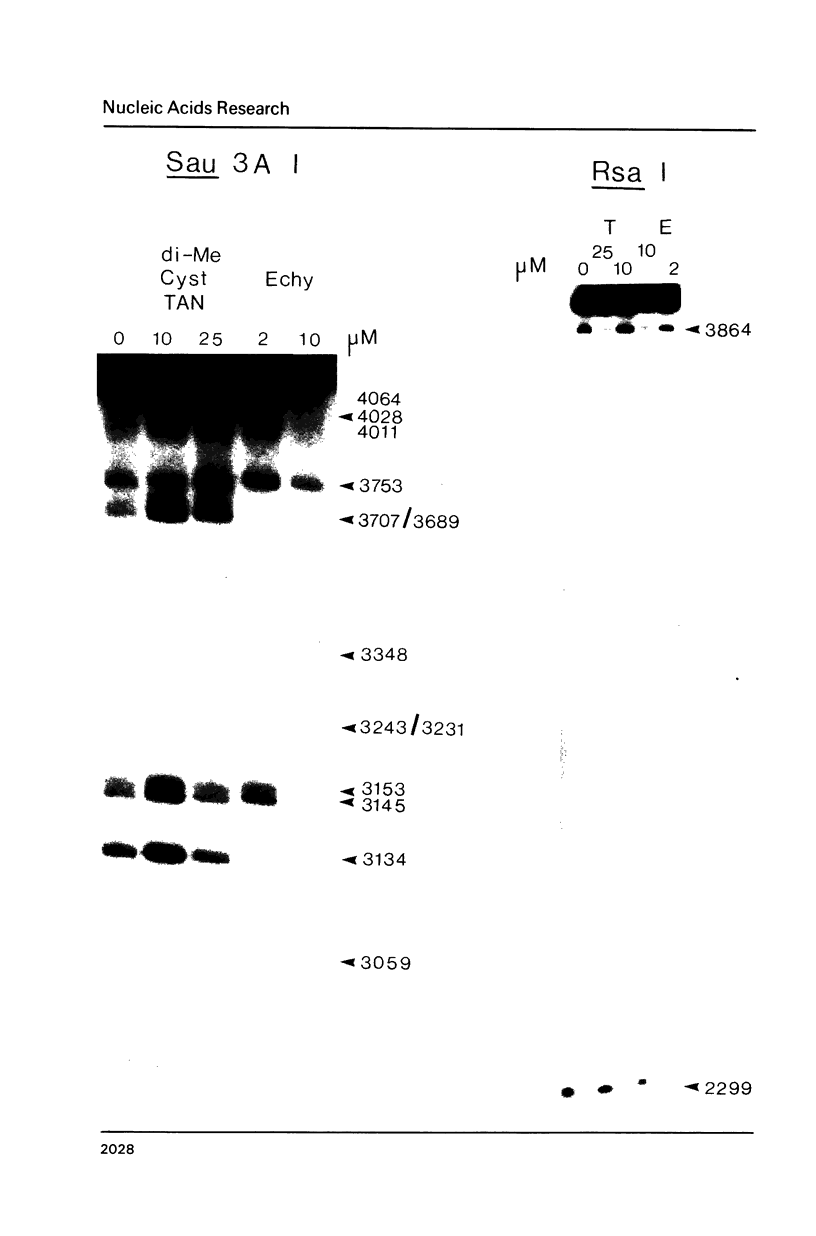
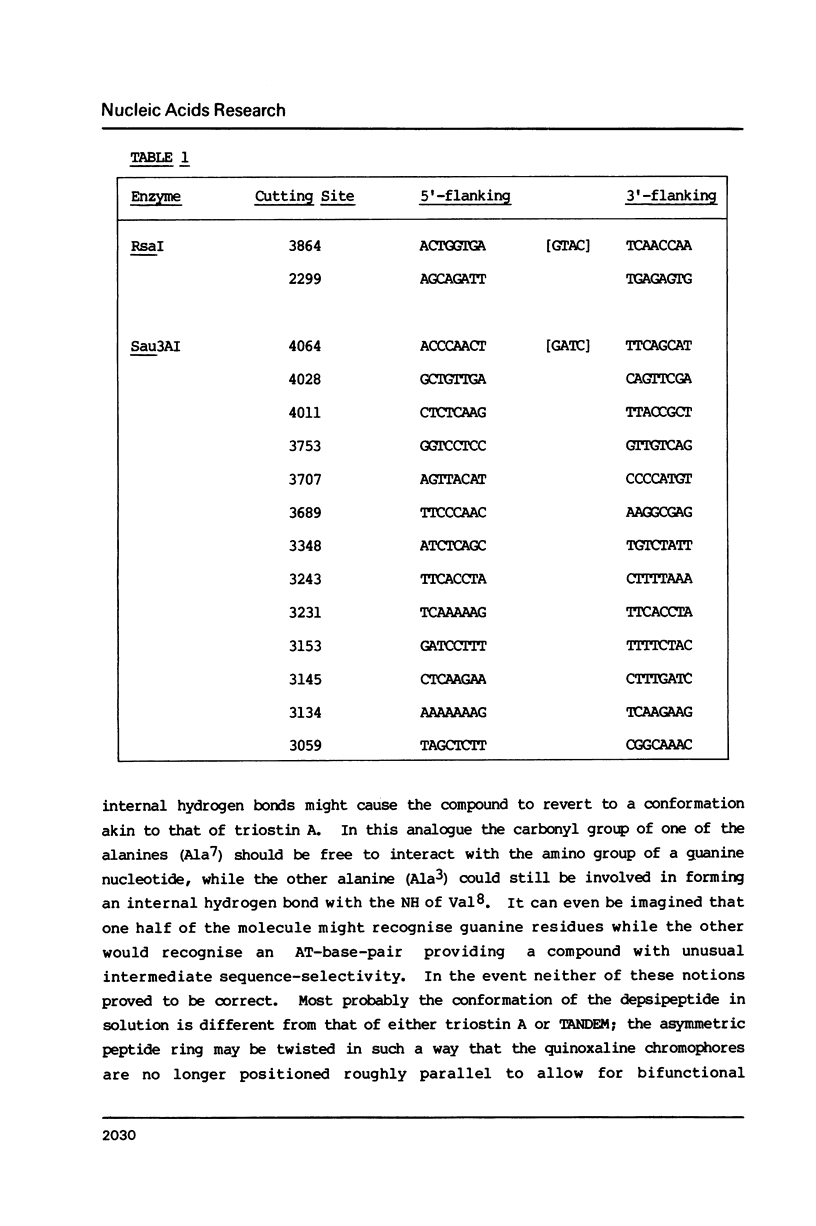
Two new analogues of TANDEM (des-N-tetramethyl triostin A) have been synthesised in an effort to elucidate the molecular basis of DNA nucleotide sequence recognition in this series of compounds. Their binding preferences have been investigated by DNAase I footprinting and differential inhibition of restriction nuclease attack. The presence of a single N-methyl group on only one valine residue (in [N-MeVal4] TANDEM) abolishes the ability to recognise DNA, presumably because this antibiotic analogue has suffered an unfavourable conformational change in the depsipeptide ring. A bis-methylated analogue, [N-MeCys3, N-MeCys7]TANDEM, was found to interact quite strongly with DNA and afforded binding sites, rich in AT residues, identical to those of TANDEM. Footprinting with various DNA fragments of known sequence showed that this analogue recognises sequences containing the dinucleotide TpA, although we cannot exclude the possibility that it binds to ApT as well. [N-MeCys3, N-MeCys7]TANDEM inhibits cutting by RsaI, a restriction enzyme that recognises GTAC but not by Sau3AI which recognises GATC. This provides further supportive evidence that the ligand (and, by extension, TANDEM itself) prefers binding to sequences containing the dinucleotide step TpA.
Full text
PDF
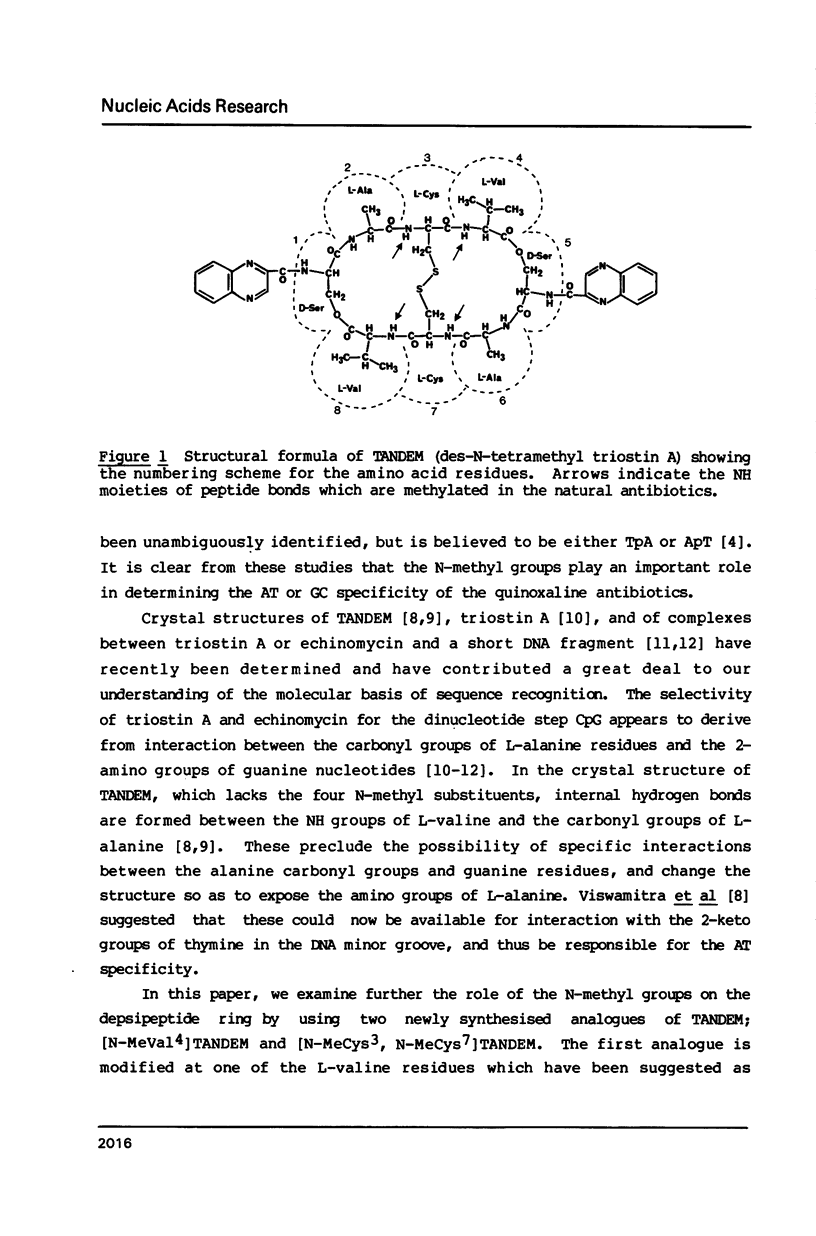



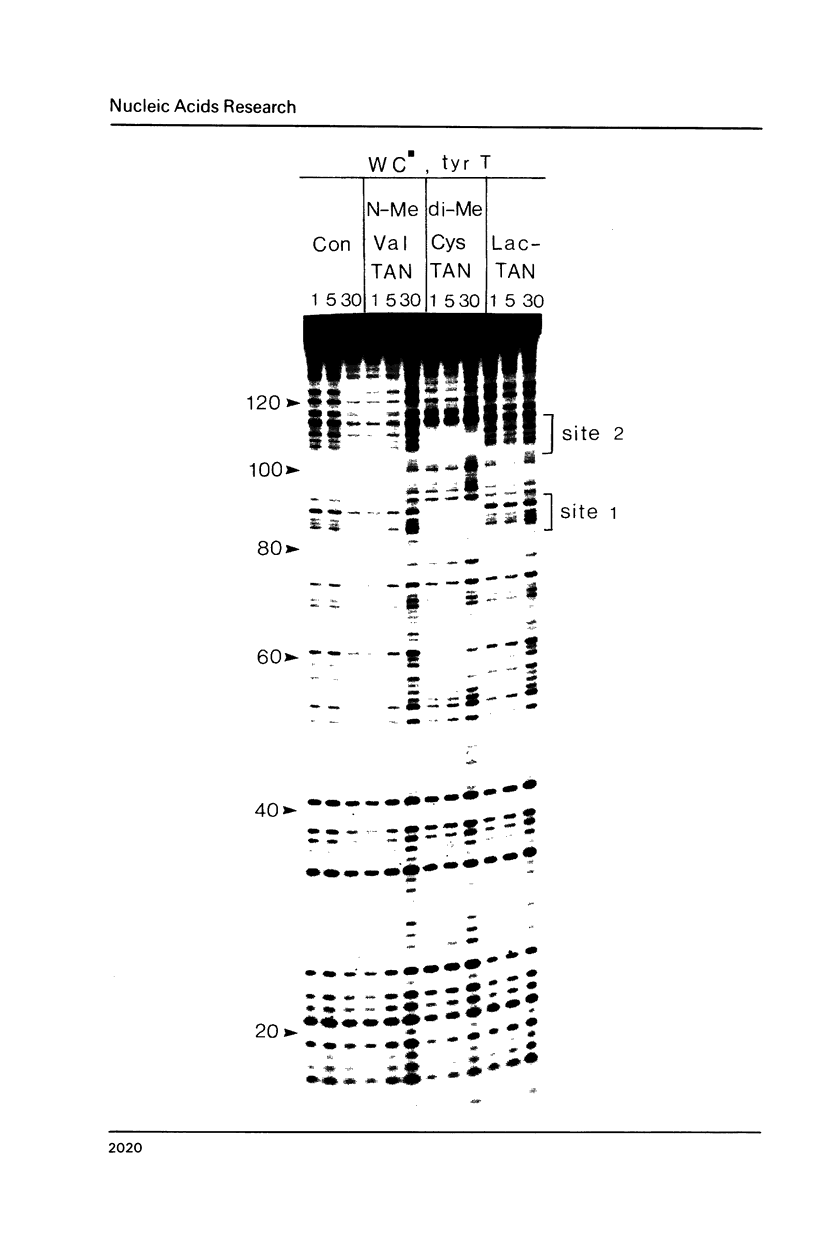
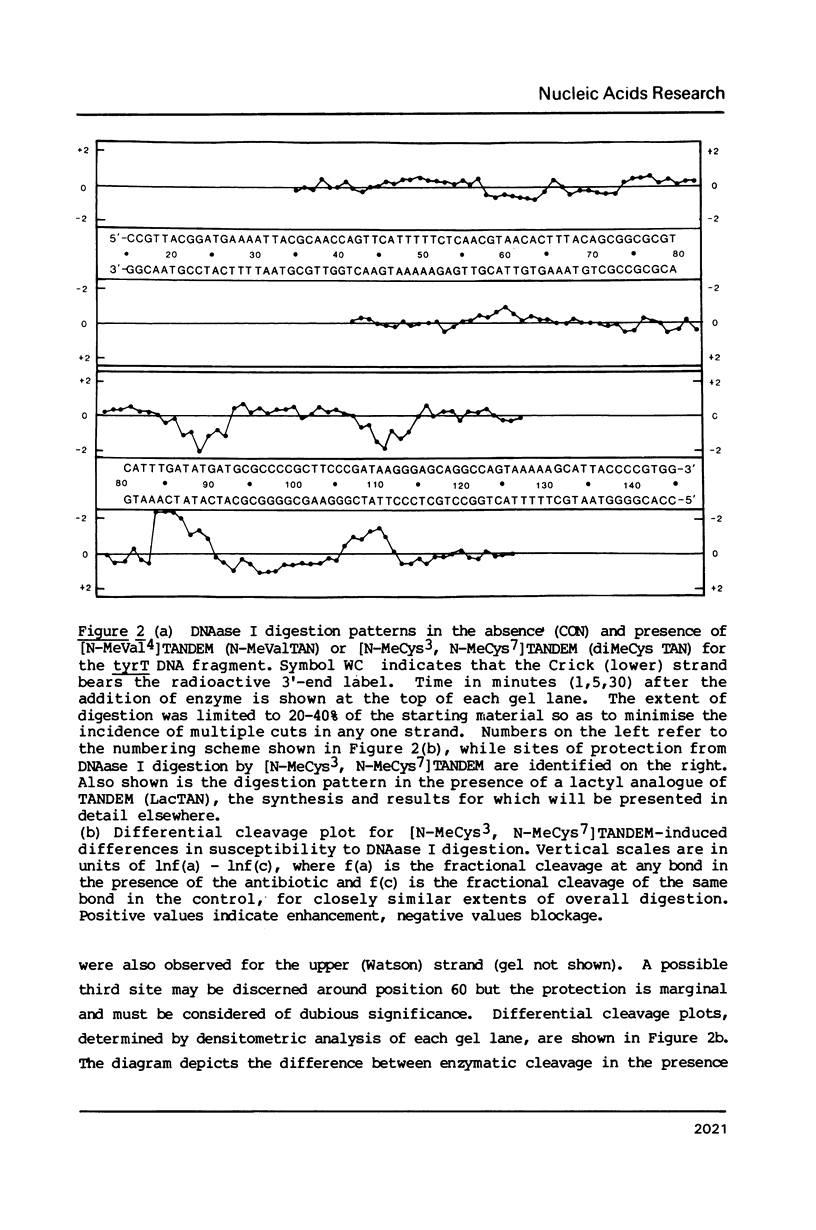


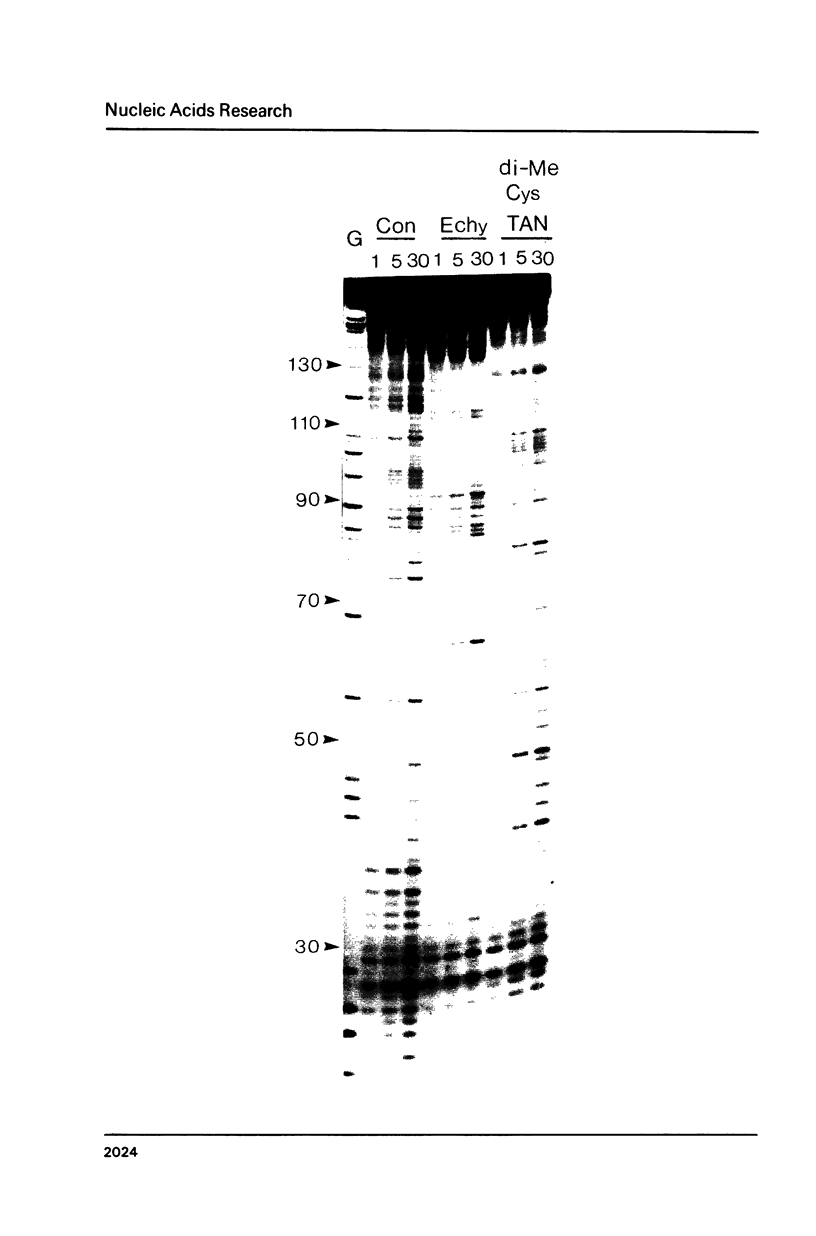




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardelli T. L., Olsen R. K. Synthesis of des-N-tetramethyltriostin A, a bicyclic octadepsipeptide related to the quinoxaline antibiotics. J Am Chem Soc. 1977 Apr 13;99(8):2806–2807. doi: 10.1021/ja00450a072. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. Structural junctions in DNA: the influence of flanking sequence on nuclease digestion specificities. Nucleic Acids Res. 1985 Jun 25;13(12):4445–4467. doi: 10.1093/nar/13.12.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Weeks J. R., Travers A. A. Negative supercoiling induces spontaneous unwinding of a bacterial promoter. EMBO J. 1985 Apr;4(4):1025–1032. doi: 10.1002/j.1460-2075.1985.tb03734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. H., Lee J. S., Morgan A. R., Olsen R. K. A method for the specific inhibition of poly[d(A-T)] synthesis using the A-T specific quinoxaline antibiotic TANDEM. Can J Biochem. 1982 Feb;60(2):131–136. doi: 10.1139/o82-018. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Olsen R. K., Waring M. J. Equilibrium and kinetic studies on the binding of des-N-tetramethyltriostin A to DNA. Biochim Biophys Acta. 1982 Mar 29;696(3):315–322. doi: 10.1016/0167-4781(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Olsen R. K., Waring M. J. Interaction between synthetic analogues of quinoxaline antibiotics and nucleic acids: role of the disulphide cross-bridge and D-amino acid centres in des-N-tetramethyl-triostin A. Br J Pharmacol. 1980 Sep;70(1):25–40. doi: 10.1111/j.1476-5381.1980.tb10900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. DNA structural variations produced by actinomycin and distamycin as revealed by DNAase I footprinting. Nucleic Acids Res. 1984 Dec 21;12(24):9271–9285. doi: 10.1093/nar/12.24.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratini A. V., Kopka M. L., Drew H. R., Dickerson R. E. Reversible bending and helix geometry in a B-DNA dodecamer: CGCGAATTBrCGCG. J Biol Chem. 1982 Dec 25;257(24):14686–14707. [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Requirement for an upstream element for optimal transcription of a bacterial tRNA gene. Nature. 1983 Sep 15;305(5931):248–250. doi: 10.1038/305248a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Waring M. J. Interaction between synthetic analogues of quinoxaline antibiotics and nucleic acids. Changes in mechanism and specificity related to structural alterations. Biochem J. 1978 Jul 1;173(1):129–144. doi: 10.1042/bj1730129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. M., Drew H. R., Waring M. J. Sequence-specific binding of echinomycin to DNA: evidence for conformational changes affecting flanking sequences. Nucleic Acids Res. 1984 Jun 25;12(12):4865–4879. doi: 10.1093/nar/12.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. M., Olsen R. K., Waring M. J. Sequence preferences in the binding to DNA of triostin A and TANDEM as reported by DNase I footprinting. FEBS Lett. 1984 Oct 29;176(2):414–420. doi: 10.1016/0014-5793(84)81209-0. [DOI] [PubMed] [Google Scholar]
- McCall M., Brown T., Kennard O. The crystal structure of d(G-G-G-G-C-C-C-C). A model for poly(dG).poly(dC). J Mol Biol. 1985 Jun 5;183(3):385–396. doi: 10.1016/0022-2836(85)90009-9. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Mace H. A., Berman M. L. RNA polymerase interactions with the upstream region of the E. coli tyrT promoter. Cell. 1983 Nov;35(1):265–273. doi: 10.1016/0092-8674(83)90229-5. [DOI] [PubMed] [Google Scholar]
- Ughetto G., Wang A. H., Quigley G. J., van der Marel G. A., van Boom J. H., Rich A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985 Apr 11;13(7):2305–2323. doi: 10.1093/nar/13.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. M., Dervan P. B. Echinomycin binding sites on DNA. Science. 1984 Sep 14;225(4667):1122–1127. doi: 10.1126/science.6089341. [DOI] [PubMed] [Google Scholar]
- Viswamitra M. A., Kennard O., Cruse W. B., Egert E., Sheldrick G. M., Jones P. G., Waring M. J., Wakelin L. P., Olsen R. K. Structure of TANDEM and its implication for bifunctional intercalation into DNA. Nature. 1981 Feb 26;289(5800):817–819. doi: 10.1038/289817a0. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]