Abstract
Liver fibrogenesis is associated with the transition of quiescent hepatocytes and hepatic stellate cells (HSC) into the cell cycle. Exit from quiescence is controlled by E-type cyclins (CcnE1, CcnE2). Thus, the aim of the current study was to investigate the contribution of E-type cyclins for liver fibrosis in man and mice.
Expression of CcnE1, but not of its homologue CcnE2 was induced in fibrotic and cirrhotic livers from human patients with different etiologies and in murine wildtype (WT) livers after periodical administration of the pro-fibrotic toxin carbon tetrachloride (CCl4). To further evaluate the potential function of E-type cyclins for liver fibrogenesis, we repetitively treated constitutive CcnE1−/− and CcnE2−/− knockout mice with CCl4 to induce liver fibrosis. Interestingly, CcnE1−/− mice were protected against CCl4–mediated liver fibrogenesis as evidenced by reduced collagen type I α1 expression and lack of septum formation. In contrast, CcnE2−/− mice showed accelerated fibrogenesis following CCl4 treatment. We isolated primary HSC from WT, CcnE1−/− and CcnE2−/− mice and analyzed their activation, proliferation and survival in vitro. CcnE1 expression in WT HSC was maximal when they started to proliferate, but decreased after the cells transdifferentiated into myofibroblasts. CcnE1−/− HSC showed dramatically impaired survival, cell cycle arrest and strongly reduced expression of alpha-smooth muscle actin, indicating deficient HSC activation. In contrast, CcnE2-deficient HSC expressed elevated level of CcnE1 and showed enhanced cell cycle activity and proliferation compared to WT cells.
Conclusions
CcnE1 and CcnE2 have antagonistic roles in liver fibrosis. CcnE1 is indispensable for activation, proliferation and survival of HSC and thus promotes synthesis of extracellular matrix and liver fibrogenesis.
Keywords: cell cycle, liver fibrosis, Carbon tetrachloride, Cyclin E2, cell cycle arrest, apoptosis
Liver fibrosis is a chronic wound healing process leading to liver scarring by excessive accumulation of extracellular matrix proteins including collagen. The main producers of liver collagen are myofibroblasts derived from activated hepatic stellate cells (HSC). Additionally, other cell types such as portal fibroblasts and bone marrow derived cells may contribute to extracellular matrix (ECM) production. Liver fibrosis develops on the basis of chronic liver injury induced e.g. by chronic viral hepatitis B or C infection, excessive alcohol abuse or fatty liver disease frequently associated with obesity (1). Although immune cells play an essential role in the modulation of liver fibrosis, its pathogenesis implicitly involves injury and proliferation of hepatic stellate cells, hepatocytes and potentially other cell species. Upon liver damage, dying hepatocytes stimulate remnant hepatocytes to re-enter the cell cycle in order to restore the original liver mass and function (2). Liver injury also stimulates HSC activation via complex mechanisms. This involves the conversion of a resting, vitamin A storing cell into a proliferating HSC without vitamin A droplets but capable of producing pro-inflammatory cytokines and ECM components such as collagen (3).
The transition from quiescent (G0) cells into the active phase of the cell cycle is predominantly controlled by E-type cyclins and their associated kinase, Cdk2 (4). In mammals, two E-cyclins are known termed Cyclin E1 (CcnE1) and Cyclin E2 (CcnE2), respectively (5, 6). Despite their anticipated essential function for developmental and regenerative processes, single genetic inactivation of CcnE1, CcnE2 or Cdk2 does not affect viability or development in mice (7–10). However, fibroblasts deficient for both E-cyclins are unable to re-enter the cell cycle from G0 (9). We recently demonstrated that CcnE1 and CcnE2 play antagonistic roles in the regenerating liver following partial hepatectomy (11). Accordingly, CcnE2−/− livers show increased and prolonged CcnE1/Cdk2 activity, resulting in earlier and sustained DNA synthesis, hepatomegaly and excessive endoreplication of hepatocytes, whereas ablation of CcnE1 provoked only a moderate delay of hepatocyte proliferation. Earlier work using rat HSC indicated that HSC activation is associated with increased gene expression of CcnE, Cyclin D and induction of polyploidy (12). However, the precise role of E-type cyclins for activation and proliferation of HSC and subsequent liver fibrogenesis has remained elusive.
In the present study, we aimed to investigate the contribution of E-type cyclins for liver fibrosis in vivo using constitutive CcnE1−/− and CcnE2−/− knockout mice and derived primary HSC. Our current work demonstrates that CcnE1, but not CcnE2 is essential for HSC survival, proliferation and liver fibrogenesis.
Materials and Methods
Human liver samples
Human liver samples were available from routine liver biopsies or from explanted cirrhotic livers due to transplantation as described recently (13). The study protocol was approved by the local ethics committee (ethics committee of University Hospital Aachen, RWTH Aachen), and the study was conducted according to the principles expressed in the Declaration of Helsinki.
Housing and breeding of mice
Animal husbandry and procedures were approved by the authority for environment conservation and consumer protection of the state North Rhine–Westfalia (LANUV, Germany) and the University Hospital Aachen Animal Care Facility’s guidelines. For our study we used mice of male gender with constitutive deletion of CcnE1 (CcnE1−/−) and CcnE2 (CcnE2−/−) and wildtype (WT) littermates from heterozygous breeding couples as recently reported (9, 11).
Isolation and Fluorescence Activated Cell Sorting (FACS) analysis of hepatic stellate cells (HSC)
HSC were prepared from adult male mice weighting about 25 g, according to the collagenase method (14) as described in the supplementary Methods section.
Statistical analysis
Data are expressed as mean ± standard deviation of the mean. Statistical significance was determined by two-way analysis of variance followed by a Student’s t test.
Results
Increased expression of the cell cycle mediator CcnE1 in human and murine liver fibrosis
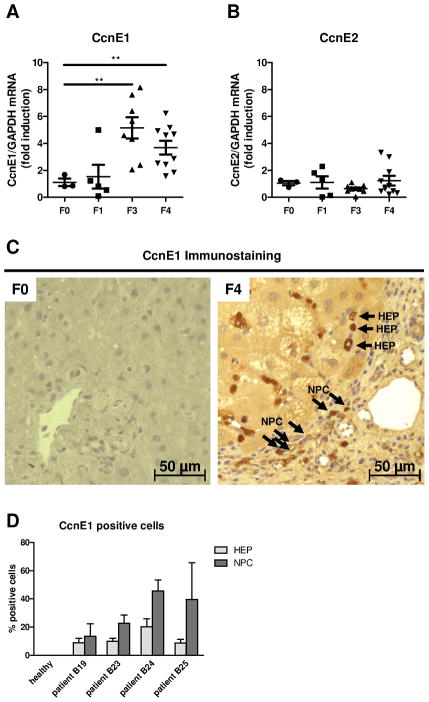
E-type cyclins CcnE1 and CcnE2 control the transition of quiescent cells into S-phase and subsequent cell proliferation (4). We hypothesized that liver fibrogenesis involves cell proliferation of hepatic cell populations and determined overall CcnE expression in liver biopsies from patients with liver fibrosis of different etiologies (see supplementary Table 1). CcnE1 mRNA expression was significantly up-regulated in patients with advanced hepatic fibrosis (F3) and liver cirrhosis (F4) compared to healthy control livers (F0) or patients with mild (F1) fibrosis (Figure 1A). In contrast, CcnE2 was not aberrantly expressed in liver fibrosis at any stage (Figure 1B). Immunostaining of liver biopsies confirmed over-expression of CcnE1 in liver cirrhosis (Figure 1C). Detailed analysis revealed substantial nuclear expression of CcnE1 in non-parenchymal cells but also in hepatocytes of cirrhotic livers, which was not observed in healthy liver samples (Figure 1D).
Figure 1. CcnE1, but not CcnE2 is induced in human liver fibrogenesis.
(A–B) Liver biopsies from healthy livers (F0) and patients with advanced liver fibrosis (F1, F3) or liver cirrhosis (F4) were analyzed for gene expression of (A) CcnE1 and (B) CcnE2 via quantitative real-time PCR. Target gene expression was normalized to GAPDH expression and calculated as fold induction compared to healthy controls. (C) CcnE1 protein expression was determined via immunohistochemistry on liver paraffin sections from healthy human livers (F0) and patients with liver cirrhosis (F4) showing sustained CcnE1 expression (signals in brown) in hepatocytes (HEP) and non-parenchymal cells (NPC). (D) Quantification of CcnE1 expressing cells in stained liver sections of 4 representative human patients with liver cirrhosis. A minimum of 10 randomly selected magnification fields per sample were analyzed for the percentage of CcnE1 positive hepatocytes (HEP) and non-parenchymal cells (NPC), respectively.
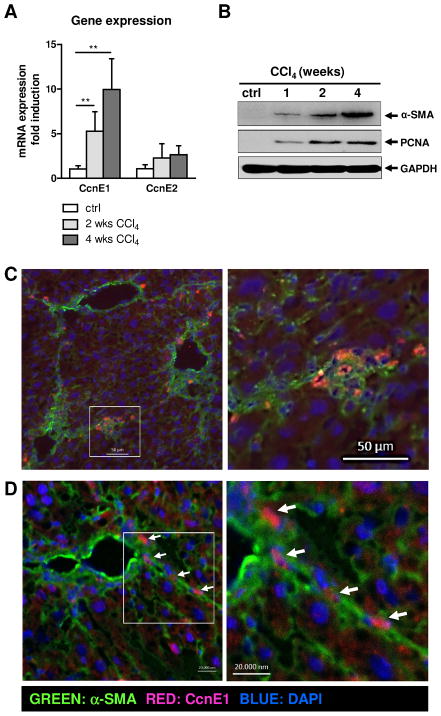
We next investigated the involvement of CcnE1 in experimental liver fibrosis in WT mice subjected to repetitive carbon tetrachloride (CCl4) injections. In agreement with the human samples, mRNA expression of CcnE1, but not of CcnE2 was induced in murine liver after CCl4 treatment and correlated with fibrosis progression (Figure 2A). This was associated with increased protein expression of the fibrosis marker α-SMA (expressed in activated HSC) along with the cell cycle mediator PCNA (Figure 2B). Co-staining of CcnE1 and α-SMA in fibrotic livers revealed an accumulation of CcnE1-expressing cells in areas of fibre formation (Figure 2C). Using confocal laser scanning microscopy we demonstrated nuclear CcnE1 localization predominantly in α-SMA-expressing cells (Figure 2D). These data demonstrated that liver fibrogenesis in man and mice involves increased cell cycle activity potentially driven by CcnE1 and suggested that CcnE1 is especially induced in HSC during this process.
Figure 2. CcnE1 is up-regulated in CCl4-induced murine liver fibrogenesis and co-localizes with α-SMA expressing cells.
WT mice were treated with CCl4 twice per week. After 1, 2 and 4 weeks, respectively, animals were sacrificed and liver tissue was analyzed for cell cycle markers and the fibrosis mediator α-SMA. (A) Gene expression analysis for CcnE1 and CcnE2 via quantitative real-time PCR. **: p<0.01. (B) Immunoblot analysis of α-SMA and PCNA expression. (C) Co-immunofluorescence staining for CcnE1 and α-SMA on murine WT liver sections with CCl4-induced advanced liver fibrosis. Left: Overview showing excessive α-SMA expression (green) in fibrotic tissue; a region of interest is boxed. Right: Enlarged view. CcnE1 expressing cells are stained in red; co-expression of α-SMA and CcnE1 appears orange in merged images. (D) Co-localization of CcnE1 (red) in α-SMA expressing cells (green) was confirmed by confocal laser scanning microscopy. CcnE1 expression in fibres is highlighted by arrows.
Liver regeneration after CCl4 - mediated acute liver injury depends on CcnE1
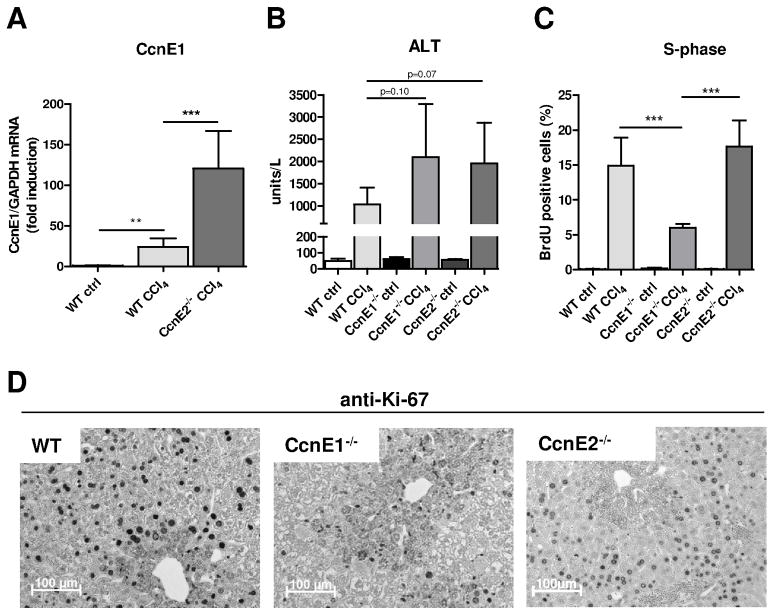
Single CCl4 administration triggers approximately 20-fold CcnE1 mRNA expression in WT mice within 48 h (Figure 2A). We recently reported that genetic ablation of CcnE2 results in over-expression of CcnE1 and excessive hepatocyte proliferation after partial hepatectomy (11). In agreement with our earlier findings, CcnE1 mRNA induction was approximately 5fold higher in CcnE2−/− mice compared to WT animals 48 h after single CCl4 administration (Figure 2A).
To evaluate the impact of CcnE1 for the onset of liver fibrosis, we compared the immediate proliferative response 48 h after single CCl4 treatment in WT, CcnE1−/− and CcnE2−/− mice by measuring BrdU incorporation (specific for DNA-synthesis) and the general proliferation marker Ki-67. Of note, CCl4 induced a similar level of necrotic liver injury in each group as evidenced by comparable induction of ALT activity (Figure 3B and supplementary Figure 1A). WT and CcnE2−/− livers revealed a similar proliferative response with substantial DNA synthesis of hepatic cells as evidenced by strong BrdU incorporation (Figure 3C and supplementary Figure 1B) and extensive Ki-67 expression. Under these conditions, CcnE1 was localized in hepatocytes of WT and CcnE2−/− mice (Supplementary Figure 1C). However, elevated CcnE1 levels as seen in CcnE2−/− liver did not result in enhanced hepatocyte proliferation after toxic injury. In contrast, CcnE1-deficiency resulted in a remarkable reduction of hepatocyte proliferation and DNA synthesis after acute CCl4 treatment (Figure 3C–D). Thus, CcnE1 plays an important role for liver regeneration after CCl4-mediated toxic liver injury.
Figure 3. Liver regeneration after CCl4 - mediated acute liver injury depends on CcnE1.
CcnE1−/−, CcnE2−/− and WT mice were treated with a single dose of CCl4 or corn oil (ctrl) and sacrificed 48 h after injection. (A) Quantification of hepatic CcnE1 mRNA expression in CCl4-treated WT- and CcnE2−/− mice. Expression was normalized to GAPDH and calculated as fold induction in comparison to oil-treated WT mice. (B) Determination of Alanine-Aminotransferase (ALT) activity in CCl4-treated mice. P-values are indicated and demonstrate equal induction of liver injury between the groups. (C–D) Determination of cell cycle activity 48 h after CCl4 treatment. (C) Quantification of BrdU incorporation. Values are calculated as percent BrdU-positive nuclei per high magnification field. (D) Ki-67 immunostaining of liver paraffin sections. Brown nuclei indicate proliferating cells.
Cyclin E1 is essential for CCl4-mediated liver fibrosis
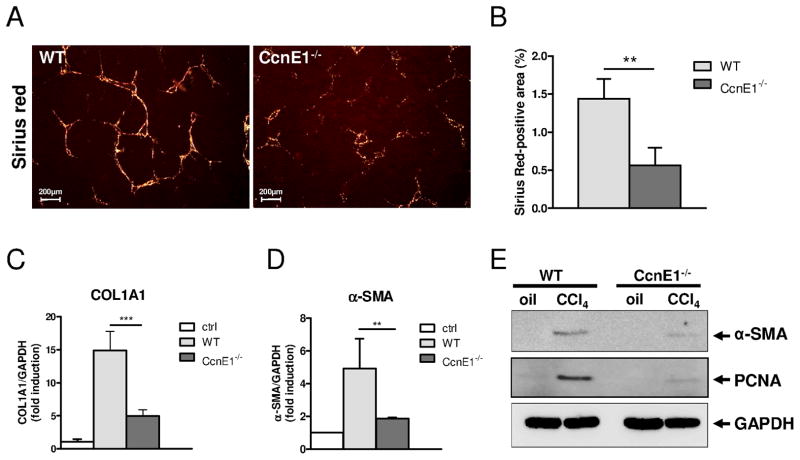
We next investigated the consequences of chronic CCl4-treatment in CcnE1−/− mice in comparison to WT controls. Repeated injections of CCl4 for 4 weeks induced prominent liver fibrosis with septum formation in WT animals as evidenced by Sirius red staining. In contrast, fiber formation was barely observed in CcnE1−/− mice (Figure 4A–B) suggesting a functional role of CcnE1. Additionally, WT mice revealed a substantial up-regulation of COL1A1 and α-SMA mRNA expression 4 weeks after CCl4 treatment, which were only moderately induced in CcnE1−/− livers (Figure 4C–D). The significant difference in α-SMA expression between WT and CcnE1−/− livers was further confirmed by Western Blot analysis suggesting that loss of CcnE1 protects against liver fibrosis in vivo (Figure 4E).
Figure 4. Cyclin E1 is essential for CCl4-mediated liver fibrosis.
CcnE1−/− knockout mice and WT controls were periodically (twice a week) treated with CCl4 for 4 weeks and sacrificed 48 h after the last injection. (A) Sirius red staining of liver paraffin sections after 4 weeks of CCl4-treatment. Collagen fibres are stained in red. (B) Quantification of Sirius red stained tissue area using image analysis software. (C) Quantification of COL1A1 mRNA expression in CCl4-treated liver samples by quantitative real-time PCR. (D) Quantification of α-SMA mRNA expression. (E) Protein expression of α-SMA and PCNA. Liver extracts from oil-treated WT- and CcnE1−/− mice were used as controls. **: p<0.01; ***: p< 0.001.
Furthermore, PCNA expression was significantly expressed in fibrotic WT livers, but faintly detectable in CcnE1−/− mice (Figure 4E). Detailed analysis of Ki-67 expression in liver sections revealed that ablation of CcnE1 did not significantly affect proliferation of hepatocytes, which was moderate but similar in WT- and CcnE1−/− liver (supplementary Figure 2A–B). However, proliferation of non-parenchymal cells (NPC) was significantly reduced in CcnE1−/− liver hinting at a cell type specific function of CcnE1 during liver fibrosis.
Loss of CcnE2 triggers CcnE1 over-expression and accelerated liver fibrogenesis during chronic CCl4 treatment
Several studies have suggested that CcnE1 and CcnE2 might have overlapping functions. In order to evaluate the role of CcnE2 for liver fibrosis we treated WT- and CcnE2−/− mice with CCl4 for 2 and 4 weeks, respectively. Surprisingly, after 4 weeks of treatment we detected comparable fiber formation and COL1A1 expression in CcnE2−/− mice and WT controls (supplementary Figure 3A–C) indicating that CcnE2 – in contrast to CcnE1 – is not essentially involved in fibrosis progression.
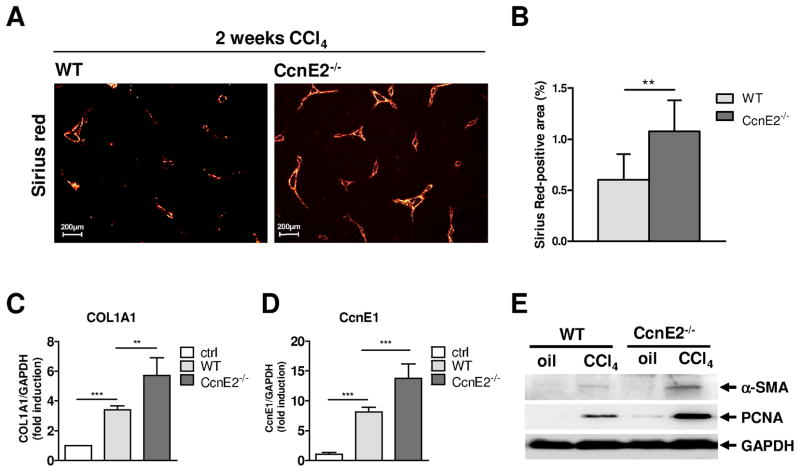
However, differences were found after 2 weeks of CCl4 treatment. WT livers showed minor collagen expression, whereas in CcnE2−/− livers first signs of bridging fibers were detectable (Figure 5A–B). Additionally, qRT-PCR showed significantly increased hepatic COL1A1 expression associated with significant CcnE1 mRNA up-regulation in CcnE2−/− livers compared to controls (Figure 5C–D). In line with these findings we detected increased proliferation of hepatocytes and non-parenchymal cells in CcnE2−/− liver as evidenced by Ki-67 and PCNA expression analysis (Figure 5E and supplementary Figure 3D–E). One sub-population of these highly proliferating CcnE2−/− cells were most likely activated HSCs since α-SMA mRNA and protein expression was also significantly increased in CcnE2−/− livers (Figure 5E and supplementary Figure 3F). Thus our findings implicate that accelerated fibrosis induction in CcnE2−/− mice might depend on enhanced proliferation and activation of HSC. However, despite accelerated fibrogenesis, liver injury in CcnE2−/− mice was not significantly increased compared to WT or CcnE1−/− mice (supplementary Figure 3G).
Figure 5. Loss of CcnE2 triggers accelerated liver fibrogenesis and CcnE1 over-expression during chronic CCl4 treatment.
CcnE2−/− knockout mice and WT controls were periodically (twice a week) treated with CCl4 for 2 weeks and sacrificed 48 hours after the last injection. (A) Sirius red staining of liver paraffin sections after 2 weeks of CCl4-treatment. (B) Quantification of Sirius red stained tissue area using image analysis software. (C) Quantification of fibrosis by measurement of hepatic COL1A1 mRNA expression. Expression was normalized to GAPDH and calculated as fold induction compared to oil-treated WT-controls (ctrl). (D) Determination of hepatic CcnE1 mRNA expression showing over-expression of CcnE1 in CcnE2−/− liver after CCl4 treatment. (E)α-SMA and PCNA protein expression was determined by western blot analysis. **: p<0.01; ***: p< 0.001.
Cell cycle progression, myofibroblast-transdifferentiation and survival of hepatic stellate cells (HSC) depends on CcnE1 expression level
We next aimed to define the cellular mechanisms leading to accelerated fibrogenesis in CcnE2−/− mice and fibrosis protection in CcnE1−/− livers. Our first results indicated that HSCs might be the CcnE-dependent effector cell population. HSC are quiescent in healthy livers (G0, CcnE inactive), but start to proliferate (G0/G1-S-phase transition, CcnE dependent) upon pro-fibrotic stimulation.
To elucidate the function of E-type cyclins in activated HSCs, we isolated primary HSC from WT, CcnE1−/− and CcnE2−/− knock-out mice and investigated cell cycle progression, transdifferentiation and survival over a time period of ten days (d1–d10). Of notice, CcnE1−/− livers revealed a normal frequency of resident HSC (supplementary Figure 4A). Primary analysis of HSC was performed by FACS analysis of DNA content and immunofluorescence staining of Ki67 and α-SMA serving as markers for cell cycle activation and myofibroblast differentiation, respectively. As expected, the total number of living WT HSC increased continuously within the observation period of 10 days whereas the number of CcnE1−/− HSC remained constant at low levels (supplementary Figure 4B–C).
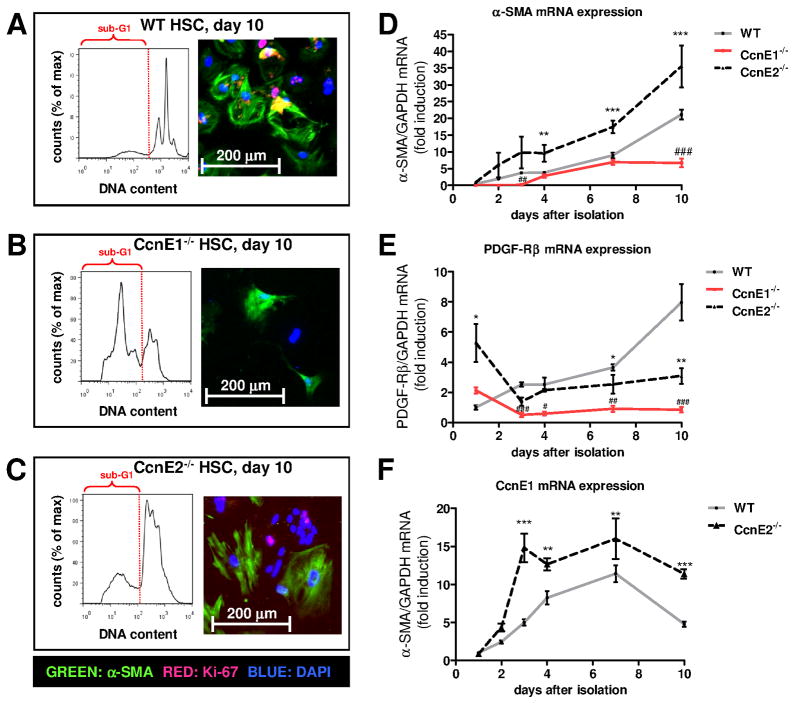
In agreement with these findings, WT HSC revealed marked occurrence of a 4n cell population after ten days indicating continuous cell cycle progression (G2/M phase, Figure 6A) with a tendency to form polyploid cells which is in agreement with earlier observations (12). These cells were characterized by expression of α-SMA and Ki-67 (Figure 6A and supplementary Figure 5A) indicating that they proliferate and transdifferentiate into myofibroblasts. In sharp contrast, CcnE1−/− HSC did not show 2n/4n conversion throughout the 10 days observation time demonstrating G1 cell cycle arrest of these cells. Instead, we observed a large sub-G1 population of apoptotic cells with reduced DNA content (<2n) due to DNA degradation (Figure 6B) and low total cell numbers throughout the observation period. Thus, quiescent ex vivo isolated CcnE1−/− HSC have a defect in entering the cell cycle and are prone to excessive cell death.
Figure 6. CcnE1 is essential for cell cycle progression and transdifferentiation of HSC.
Primary HSC were isolated from WT, CcnE1−/− and CcnE2−/− mice. A total number of 150.000 cells were seeded per 6-well plate and cultivated for up to 10 days. (A–C) Ten days after cultivation, attached cells were harvested, stained with propidium iodide (PI) and subjected to FACS analysis or analyzed by immunofluorescence staining for Ki-67 (red) and α-SMA (green). Representative FACS histograms demonstrate the DNA content of HSC subpopulations. The sub-G1 population is highlighted and indicates apoptotic cells with DNA content <2n. (A) WT HSC. (B) CcnE1−/− HSC. (C) CcnE2−/− HSC. (D–F) Time-dependent mRNA expression analysis from primary HSC derived from WT, CcnE1−/− and CcnE2−/− mice. (D)α-SMA mRNA expression. **: p<0.01; ***: p<0.001 (WT versus CcnE2−/−); #: p<0.05; ###: p<0.001 (WT versus CcnE1−/−). (E) PDGF receptor beta (PDGF-Rβ) mRNA expression. *: p<0.05; **: p<0.01; (WT versus CcnE2−/−); ##: p<0.01; ###: p<0.001 (WT versus CcnE1−/−). (F) CcnE1 mRNA expression in WT and CcnE2−/− cells; **: p<0.01; ***: p<0.001.
Using CcnE2−/− HSC completely opposite effects were observed showing already highly polyploid cells after isolation undergoing a further, time-dependent increase in DNA synthesis and polyploidization (Figure 6C). The complete data including all investigated time points is shown in supplementary Figure 6 and demonstrates that in WT cells, Ki-67 expression started at day 4 after seeding, whereas transdifferentiated (α-SMA-positive) myofibroblasts were first detected after 7 days. After 10 days, the majority of HSC were activated and reached confluence (supplementary Figure 5A). CcnE2−/− HSC showed accelerated transactivation starting day 3 after seeding with overall stronger Ki-67 expression pointing at an enhanced cell cycle activity of these cells (supplementary Figure 6C, 6F).
mRNA quantification revealed substantial α-SMA induction - and thus transactivation - after 7 days in WT HSC, but already after 3 days in CcnE2−/− cells (Figure 6D). Importantly, overall α-SMA expression in CcnE2−/− HSC significantly exceeded WT levels at all time-points investigated. In contrast, overall α-SMA levels in CcnE1−/− HSC were lower compared to WT cells and especially lacked induction after 7 days. These findings suggested that CcnE1 is essential for HSC transactivation. To further test our hypothesis we measured expression of PDGF receptor beta (PDGF-Rβ), which is usually induced when HSC are activated and start to transdifferentiate into myofibroblasts (15). While PDGF-Rβ was up-regulated in WT HSC, no increase was evident in CcnE1−/− cells (Figure 6E). Interestingly, loss of CcnE2 resulted in an approximately fivefold up-regulation of basal PDGF-Rβ expression suggesting that quiescent CcnE2−/− HSC are already primed for accelerated activation.
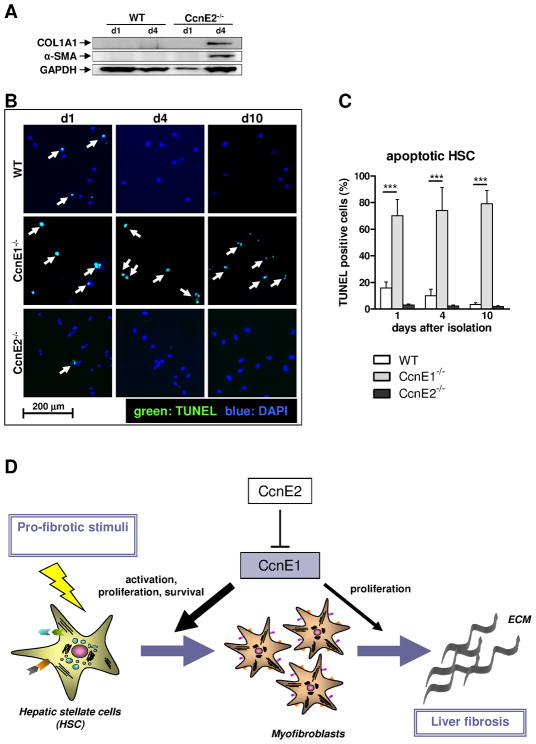
We next compared CcnE1 mRNA expression levels in WT and CcnE2−/− HSC throughout the transdifferentiation process. Interestingly, CcnE1 expression was significantly elevated in CcnE2−/− HSC at all time-points investigated (Figure 6F). CcnE1 peak expression in WT cells was found at day 7 after seeding, whereas comparable expression levels were detected in CcnE2−/− HSC between days 3–10. Interestingly, in both groups maximal CcnE1 expression was detected before the first appearance of transdifferentiated, α-SMA positive myofibroblasts, suggesting that CcnE1 might be involved in HSC transactivation. We therefore performed expression analysis of HSC-derived pro-fibrotic proteins which confirmed accelerated onset of α-SMA and collagen I expression in CcnE2−/− HSC compared to WT controls (Figure 7A). Of notice, protein data could not be obtained from CcnE1−/− HSCs due to poor survival and thus low protein yields.
Figure 7. CcnE1 expression is crucial for HSC survival and its transdifferentiation into myofibroblasts.
(A) Protein expression analysis of collagen 1 and α-SMA was determined in WT- and CcnE2−/− HSC at day 1 (d1) and day 4 (d4) after isolation. Expression of GAPDH was used as loading control. (B) TUNEL analysis of ex vivo isolated primary HSC. TUNEL-positive, apoptotic cells are stained in green and highlighted by arrows. Nuclei of total cells were stained with DAPI and are shown in blue. (C) Quantification of apoptotic cells shown in (D). ***: p<0.001. (D) Proposed model to explain the pro-fibrogenic function of CcnE1. CcnE1 is negatively regulated by CcnE2. After pro-fibrotic stimulation e.g. toxic liver injury, CcnE1 is up-regulated at an early stage and drives the initial cell cycle activation of hitherto quiescent HSC. HSC activation results in transdifferentiation into myofibroblast, which produce extracellular matrix (ECM) and are essential for fibrogenesis. Maximal CcnE1 expression in HSC occurs prior to transdifferentiation. Thus CcnE1 is essential for early HSC activation and survival, but presumably less important for proliferation of differentiated myofibroblasts.
To better characterize the findings in CcnE1−/− HSC, we performed TUNEL-analysis of seeded HSC from all groups and controls up to 10 days after isolation. These experiments revealed that CcnE1−/− HSC were more prone to undergo apoptosis which was not evident in CcnE2−/− cells or controls (Figure 7B–C). Accordingly, CcnE1 is essential for triggering proliferation, transdifferentiation and survival of HSC.
Discussion
Liver fibrosis is a chronic wound healing process leading to liver scarring directing progressively deteriorating organ function. In this context chronic liver injury triggers a proliferative response of hepatocytes, but also of non-parenchymal liver cells including matrix producing cells such as activated hepatic stellate cells and myofibroblasts. Therefore, liver fibrogenesis involves cell cycle re-entry of quiescent cells such as hepatocytes and HSC. Surprisingly, little information exists how cell cycle mediators such as cell cycle dependent kinases and cyclins contribute to the progression of liver fibrosis (16). Genetic inactivation of single D-type (CcnD1–3) and E-type (CcnE1, CcnE2) cyclins or their associated kinases (e.g. Cdk2, 4, 6) did not affect general cellular processes such as embryonic development presumably due to overlapping or even redundant functions (17). However, it has been postulated that these cyclins and Cdks may also perform cell type specific functions (18) and in line with this hypothesis we recently described non-redundant functions for CcnE1 and CcnE2 in hepatocytes during liver regeneration following partial hepatectomy (PH) (11).
In the present study, we found that CcnE1, but not CcnE2 was up-regulated in human patients with advanced liver fibrosis suggestive of a specific function of CcnE1 during liver fibrogenesis. To address this hypothesis we treated CcnE1−/− and CcnE2−/− mice acute or chronically with CCl4 and analyzed the impact on the proliferative response of hepatocytes and non-parenchymal liver cells.
Ubiquitous ablation of CcnE1 in this fibrosis model revealed several unexpected findings defining CcnE1 as an essential pro-fibrotic mediator. After acute toxic liver injury, the overall proliferative response of CcnE1-deficient hepatic cells was dramatically impaired. Several studies including our own work demonstrated that CcnE1 is dispensable for proliferation of continuously cycling cells and regenerating hepatocytes after surgical partial liver resection (9, 11, 19). From our present data it is now evident that the requirement of proliferating hepatocytes for CcnE1 depends on the proliferation stimulus. While CcnE1 is dispensable for hepatocyte proliferation in a pro-inflammatory environment (hepatectomy), it is apparently essential after toxic liver injury (CCl4) in vivo. In agreement with this hypothesis we recently observed a prolonged cell cycle arrest of CcnE1−/− hepatocytes in vivo after treatment with the hepatotoxic agent diethylnitrosamine (data not shown).
Intriguingly, constitutive ablation of CcnE1 – but not inhibition of CcnE2 - protected from CCl4-mediated liver fibrosis, which was related to impaired cell cycle activity of non-parenchymal liver cells. Hence, we focussed on hepatic stellate cells, as this cell population is central for the process of liver fibrosis as the major source of extracellular matrix proteins (20). A recent study suggested that down-regulation of CcnE1 is related to delayed cell cycle progression of the human HSC line LX-2 (21). In line with these findings, we demonstrated that complete ablation of CcnE1 induces a dramatic cell cycle arrest of HSC and hyper-sensitivity to apoptosis and overall poor survival. Although the mechanism triggering apoptosis in CcnE1-deficient cells remains elusive, HSC apoptosis clearly acts anti-fibrotic (22). Thus, reduced liver fibrosis in CCl4-treated CcnE1−/− mice is most likely explained by impaired viability and cell cycle arrest of HSCs after pro-fibrogenic stimulation.
Our study also revealed an unexpected role of CcnE2 for liver fibrogenesis. In line with our earlier studies (11) loss of CcnE2 resulted in accelerated gene activation of CcnE1 in hepatocytes and HSC suggesting that CcnE2 is an inhibitor of CcnE1 expression. At present, the underlying mechanism is unclear; however, CcnE2 was shown to be 1.5–10 fold higher expressed compared to CcnE1 in at least 3 independent studies (23). We speculate that CcnE2 might sequester and thus inactivate transcriptional activators of CcnE1. Besides up-regulating CcnE1, loss of CcnE2 resulted in early liver fibrogenesis and more importantly in accelerated HSC activation and proliferation. Although it is tempting to speculate that this might be a direct consequence of elevated CcnE1 expression, we cannot exclude alternative mechanisms. Indeed, we also observed fivefold basal up-regulation of PDGF-Rβ in CcnE2−/− HSC which indicates, that these cells are already primed to activation and proliferation. We thus conclude that CcnE2 does not share overlapping functions with CcnE1 in HSC but acts anti-fibrotic.
Based on our experiments we suggest an essential role of CcnE1 for HSC activation and fibrosis induction as illustrated in Figure 7D: In WT cells the peak of CcnE1 expression occurs before maximum expression of α-SMA and PDGF-Rβ. We conclude that CcnE1 drives pro-fibrogenic mechanisms predominantly via targeting and activation of hitherto quiescent hepatic stellate cells. Previous work demonstrated that E-type cyclins are dispensable for continuous proliferation of embryonic fibroblasts - sharing some similarities with hepatic myofibroblasts -, whereas they are essential for the exit from quiescence (9). In our present study the same phenomenon seems to operate in HSC, except that they rely only on CcnE1 for cell cycle re-entry as CcnE2 is not induced during liver fibrogenesis. Accordingly, CcnE1 deficient HSC are unable to normally re-enter the cell cycle from G0.
Our results raises the question if our findings are model specific and may only apply for the CCl4 -fibrosis model. Although we cannot exclude that some of our results (e.g. cell cycle arrest of CcnE1−/− hepatocytes) are CCl4-specific, our data from ex vivo analyzed primary HSC describe a general, model-independent biological function showing that CcnE1 is an essential cell cycle and survival factor for HSC.
In summary we demonstrate that CcnE1 is a novel key mediator of hepatic fibrosis in mice as it provides essential functions for the proliferation and survival of HSCs. Future work will evaluate if targeted inhibition of CcnE1 might be a therapeutic option to prevent liver fibrosis.
Supplementary Material
Acknowledgments
Financial support:
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), SFB TRR57, project P04 (to C.L. and C.T) and grant R01 CA108950 (to P.S.).
The excellent assistance of Sibille Sauer-Lehnen, Carmen C. Tag and the Core Unit “Q3 - Cell Isolation” of the SFB/TRR57 with the isolation of primary hepatic stellate cells is gratefully acknowledged. We are also grateful to Kanishka Hiththetiya, and Christiane Esch for the technical support with the histological analysis of liver samples. Confocal microscopy was performed in the Interdisciplinary Center for Clinical Research (IZKF) Aachen within the Faculty of Medicine at RWTH Aachen University with kind support of Gerhard Müller-Newen.
List of Abbreviations
- α-SMA
alpha smooth muscle actin
- CCl4
Carbon tetrachloride
- CcnE1
Cyclin E1
- CcnE2
Cyclin E2
- COL1A1
Collagen type I α1
- ECM
extracellular matrix
- HSC
hepatic stellate cells
- Ki-67
cell cycle specific protein encoded by the MKI67 gene
- NPC
non-parenchymal cells
- PCNA
Proliferating cell nuclear antigen
- WT
wildtype
Contributor Information
Yulia A. Nevzorova, Email: nevzorovaj@googlemail.com.
Jörg-Martin Bangen, Email: jbangen@ukaachen.de.
Wei Hu, Email: whu@ukaachen.de.
Ute Haas, Email: uhaas@ukaachen.de.
Ralf Weiskirchen, Email: rweiskirchen@ukaachen.de.
Nikolaus Gassler, Email: ngassler@ukaachen.de.
Sebastian Huss, Email: sebastian.huss@uk-koeln.de.
Frank Tacke, Email: ftacke@ukaachen.de.
Piotr Sicinski, Email: Peter_Sicinski@dfci.harvard.edu.
Christian Trautwein, Email: ctrautwein@ukaachen.de.
Christian Liedtke, Email: cliedtke@ukaachen.de.
References
- 1.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 2.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng Y, Yu Q, Whoriskey W, Dick F, Tsai KY, Ford HL, Biswas DK, et al. Expression of cyclins E1 and E2 during mouse development and in neoplasia. Proc Natl Acad Sci U S A. 2001;98:13138–13143. doi: 10.1073/pnas.231487798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauper N, Beck AR, Cariou S, Richman L, Hofmann K, Reith W, Slingerland JM, et al. Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene. 1998;17:2637–2643. doi: 10.1038/sj.onc.1202477. [DOI] [PubMed] [Google Scholar]
- 7.Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, Amati B. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003;22:4794–4803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 9.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 10.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Nevzorova YA, Tschaharganeh D, Gassler N, Geng Y, Weiskirchen R, Sicinski P, Trautwein C, et al. Aberrant cell cycle progression and endoreplication in regenerating livers of mice that lack a single E-type cyclin. Gastroenterology. 2009;137:691–703. 703 e691–696. doi: 10.1053/j.gastro.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudas J, Saile B, El-Armouche H, Aprigliano I, Ramadori G. Endoreplication and polyploidy in primary culture of rat hepatic stellate cells. Cell Tissue Res. 2003;313:301–311. doi: 10.1007/s00441-003-0768-3. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 15.Borkham-Kamphorst E, Herrmann J, Stoll D, Treptau J, Gressner AM, Weiskirchen R. Dominant-negative soluble PDGF-beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Invest. 2004;84:766–777. doi: 10.1038/labinvest.3700094. [DOI] [PubMed] [Google Scholar]
- 16.Kim MR, Kim HS, Lee MS, Lee MJ, Jang JJ. Cell cycle protein profile of the hepatic stellate cells(HSCs)in dimethylnitrosamine-induced rat hepatic fibrosis. Exp Mol Med. 2005;37:335–342. doi: 10.1038/emm.2005.43. [DOI] [PubMed] [Google Scholar]
- 17.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 18.Berthet C, Kaldis P. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene. 2007;26:4469–4477. doi: 10.1038/sj.onc.1210243. [DOI] [PubMed] [Google Scholar]
- 19.Geng Y, Lee YM, Welcker M, Swanger J, Zagozdzon A, Winer JD, Roberts JM, et al. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–139. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 21.Sekiya Y, Ogawa T, Iizuka M, Yoshizato K, Ikeda K, Kawada N. Down-regulation of cyclin E1 expression by microRNA-195 accounts for interferon-beta-induced inhibition of hepatic stellate cell proliferation. J Cell Physiol. 2011 doi: 10.1002/jcp.22598. [DOI] [PubMed] [Google Scholar]
- 22.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldon CE, Musgrove EA. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010;5:2. doi: 10.1186/1747-1028-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.