Abstract
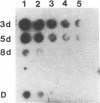
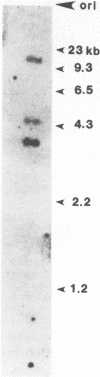
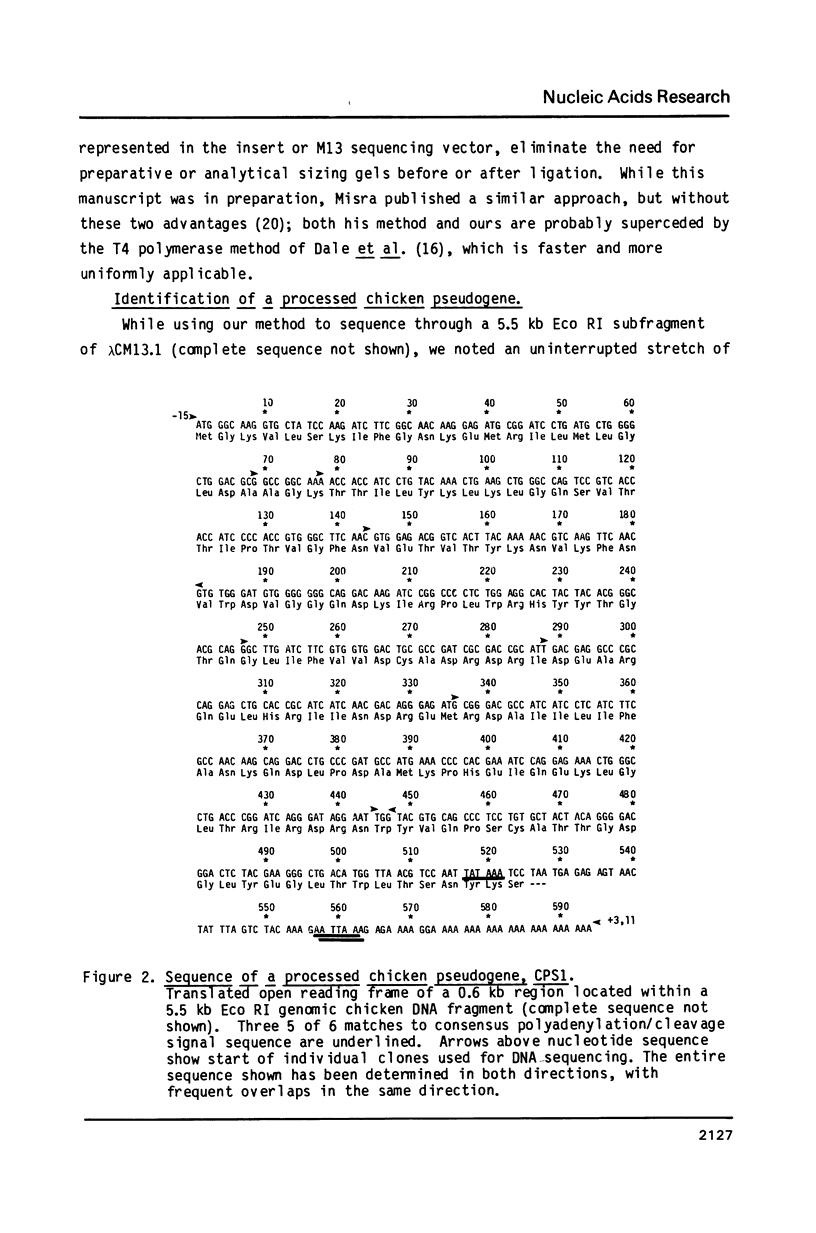
We describe the first polyA-containing processed pseudogene reported in the chicken. It includes a 0.52 kb open reading frame which could encode a 175 amino acid protein. The putative protein shows extensive homology to the ras oncogene superfamily, being most closely related to the yeast protein YP2. It is one of the two most divergent members of the ras superfamily yet described and is the most homologous of any ras-related protein to the G-protein alpha-transducin. The chicken genome contains at least one other gene highly homologous to CPS1; at least one member of the CPS1 family is active, but only early in chicken development. This pattern of expression, and the presence of mutations in regions known to activate human c-ras genes to oncogenicity, suggest that CPS1 may represent a new oncogene family.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Chang K. S., Schwartz R. J. Novel chicken actin gene: third cytoplasmic isoform. Mol Cell Biol. 1985 May;5(5):1151–1162. doi: 10.1128/mcb.5.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Gonda M. A., Ellis R. W., Scolnick E. M., Lowy D. R. Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4848–4852. doi: 10.1073/pnas.79.16.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield R. G., Jones S. S., Lo K. M., Weinberg R. A. Activation of Ha-ras p21 by substitution, deletion, and insertion mutations. Mol Cell Biol. 1985 Aug;5(8):1809–1813. doi: 10.1128/mcb.5.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Maizel J. V., Jr, Leder P. The evolution and sequence comparison of two recently diverged mouse chromosomal beta--globin genes. Cell. 1979 Nov;18(3):865–873. doi: 10.1016/0092-8674(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lochrie M. A., Hurley J. B., Simon M. I. Sequence of the alpha subunit of photoreceptor G protein: homologies between transducin, ras, and elongation factors. Science. 1985 Apr 5;228(4695):96–99. doi: 10.1126/science.3856323. [DOI] [PubMed] [Google Scholar]
- Madaule P., Axel R. A novel ras-related gene family. Cell. 1985 May;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Medynski D. C., Sullivan K., Smith D., Van Dop C., Chang F. H., Fung B. K., Seeburg P. H., Bourne H. R. Amino acid sequence of the alpha subunit of transducin deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K. A new strategy to create ordered deletions for rapid nucleotide sequencing. Gene. 1985;34(2-3):263–268. doi: 10.1016/0378-1119(85)90135-0. [DOI] [PubMed] [Google Scholar]
- Moos M., Gallwitz D. Structure of a human beta-actin-related pseudogene which lacks intervening sequences. Nucleic Acids Res. 1982 Dec 11;10(23):7843–7849. doi: 10.1093/nar/10.23.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder A., Leder P. Unusual alpha-globin-like gene that has cleanly lost both globin intervening sequences. Proc Natl Acad Sci U S A. 1980 May;77(5):2806–2809. doi: 10.1073/pnas.77.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Emigholz K., Monahan J. J. Increased amplification of pBR322 plasmid deoxyribonucleic acid in Escherichia coli K-12 strains RR1 and chi1776 grown in the presence of high concentrations of nucleoside. J Bacteriol. 1979 Apr;138(1):270–272. doi: 10.1128/jb.138.1.270-272.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of DNA sequence analysis programs for microcomputers. Nucleic Acids Res. 1982 Jan 11;10(1):51–59. doi: 10.1093/nar/10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson I. B., Ingram V. M. Gene evolution in the chicken beta-globin cluster. Cell. 1982 Mar;28(3):515–521. doi: 10.1016/0092-8674(82)90206-9. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Harris P. K., Woolford J. L., Jr, Teem J. L. The effect of temperature-sensitive RNA mutants on the transcription products from cloned ribosomal protein genes of yeast. Cell. 1981 Jun;24(3):679–686. doi: 10.1016/0092-8674(81)90094-5. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C., Wu R. Nonallelic members of the cytochrome c multigene family of the rat may arise through different messenger RNAs. Cell. 1983 Feb;32(2):473–482. doi: 10.1016/0092-8674(83)90467-1. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Bothwell A. L., Mueller-Hill B., Baltimore D. Multiple differences between the nucleic acid sequences of the IgG2aa and IgG2ab alleles of the mouse. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4495–4499. doi: 10.1073/pnas.78.7.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Shih T. Y., Stokes P. E., Smythers G. W., Dhar R., Oroszlan S. Characterization of the phosphorylation sites and the surrounding amino acid sequences of the p21 transforming proteins coded for by the Harvey and Kirsten strains of murine sarcoma viruses. J Biol Chem. 1982 Oct 10;257(19):11767–11773. [PubMed] [Google Scholar]
- Slamon D. J., Cline M. J. Expression of cellular oncogenes during embryonic and fetal development of the mouse. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7141–7145. doi: 10.1073/pnas.81.22.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Stein J. P., Munjaal R. P., Lagace L., Lai E. C., O'Malley B. W., Means A. R. Tissue-specific expression of a chicken calmodulin pseudogene lacking intervening sequences. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6485–6489. doi: 10.1073/pnas.80.21.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Nukada T., Nishikawa Y., Sugimoto K., Suzuki H., Takahashi H., Noda M., Haga T., Ichiyama A., Kangawa K. Primary structure of the alpha-subunit of transducin and its relationship to ras proteins. Nature. 1985 May 16;315(6016):242–245. doi: 10.1038/315242a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin E. F., Goldberg G. I., Tucker P. W., Smithies O. A mouse alpha-globin-related pseudogene lacking intervening sequences. Nature. 1980 Jul 17;286(5770):222–226. doi: 10.1038/286222a0. [DOI] [PubMed] [Google Scholar]
- Vanin E. F. Processed pseudogenes. Characteristics and evolution. Biochim Biophys Acta. 1984 Jul 18;782(3):231–241. doi: 10.1016/0167-4781(84)90057-5. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Yatsunami K., Khorana H. G. GTPase of bovine rod outer segments: the amino acid sequence of the alpha subunit as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4316–4320. doi: 10.1073/pnas.82.13.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]