Abstract
On the basis mainly of pharmacological experiments, the p38α MAP kinase isoform has been established as an important regulator of immune and inflammatory responses. However, the role of the related p38γ and p38δ kinases has remained unclear. Here, we show that deletion of p38γ and p38δ impaired the innate immune response to lipopolysaccharide (LPS), a Toll-like receptor 4 (TLR4) ligand, by blocking the extracellular signal-regulated kinase 1/2 (ERK1/2) activation in macrophages and dendritic cells. p38γ and p38δ were necessary to maintain steady-state levels of tumor progression locus 2 (TPL2), the MKK kinase that mediates ERK1/2 activation after TLR4 stimulation. TNFα, IL-1β, and IL-10 production were reduced in LPS-stimulated macrophages from p38γ/δ-null mice, whereas IL-12 and IFNβ production increased, in accordance with the known effects of TPL2/ERK1/2 signaling on the induction of these cytokines. Furthermore, p38γ/δ-deficient mice were less sensitive than controls to LPS-induced septic shock, showing lower TNFα and IL-1β levels after challenge. Together, our results establish p38γ and p38δ as key components in innate immune responses.
The innate immune system is the front line of defense against invading pathogens and uses evolutionarily conserved systems of pathogen-associated pattern recognition (1, 2). For example, pathogen-specific molecules activate Toll-like receptors (TLRs) on innate immune cells, leading to secretion of proinflammatory cytokines and other mediators that promote elimination of infectious agents and the induction of tissue repair (1, 2). TLR stimulation by pathogen-associated molecules such as the bacterial lipopolysaccharide (LPS), a TLR4 ligand, activates various signaling pathways crucial for synthesis of proinflammatory molecules (2); one of these pathways is the p38 mitogen-activated protein kinase (MAPK) pathway (3, 4).
The four p38MAPK family members p38α, p38β, p38γ, and p38δ share highly similar protein sequences and are activated by dual phosphorylation mediated by the MAPK kinases MKK3 and MKK6 (3). On the basis of expression patterns, substrate specificities, and sensitivity to chemical inhibitors, p38MAPK can be further divided into two subsets, p38α/p38β and p38γ/p38δ (3). p38α and p38β are very similar proteins that appear to have overlapping functions. Whereas p38β is expressed at very low levels, p38α is abundant in most cell types and is the best-characterized isoform; most of the literature on p38MAPK refers to p38α. A better understanding of the essential role of p38α in inflammation and in numerous TLR4-triggered responses in macrophages and dendritic cells was provided by studies using a range of p38α inhibitors or the constitutive deletion of its physiological substrates or activators (4). Gene targeting to systemically knockout p38α is an embryonic lethal mutation (5–7). However, studies using conditional tissue-specific knockouts of p38α mice have revealed the importance of this isoform in the inflammatory response of cultured macrophages in vitro (8) and in the development of skin and gut inflammation (9, 10) in vivo. In contrast, p38β-null mice show no defects in lymphocyte development or cytokine production in response to LPS (11).
The less-studied p38γ and p38δ isoforms probably have specialized functions, given their restricted expression patterns; p38γ is abundant in skeletal muscle and p38δ in endocrine glands (3). Recent reports implicate p38γ and p38δ in metabolic diseases, cancer, and tissue regeneration, raising interest in this pathway as a therapeutic target for drugs. p38δ regulates insulin secretion and survival of pancreatic β-cells, implying a pivotal role for this kinase in diabetes (12). Moreover, studies in mice suggest that p38γ blocks premature differentiation of satellite cells, a skeletal muscle stem-cell population that participates in adult muscle regeneration (13). p38δ-null mice show reduced susceptibility to skin carcinogenesis (14). However, studies in immortalized mouse embryonic fibroblasts and in K-Ras–transformed cells lacking p38γ or p38δ have indicated that these kinases can also inhibit tumor development (15).
p38γ and p38δ are also expressed in immune cell lines and primary immune cells, and both are activated in myeloid cell lines in response to LPS (16, 17). However, the function of p38γ and p38δ in primary immune cell signaling and inflammatory responses has not been addressed. Here, we investigated this question using mice deficient in p38γ, p38δ, or both. We show that deletion of both p38γ and p38δ impaired the innate immune response to LPS by regulating the steady-state levels of ABIN-2 and its associated protein tumor progression locus 2 (TPL-2), the MKK kinase that mediates extracellular signal-regulated kinase 1/2 (ERK1/2) pathway activation. Consistently, p38γ/δ deficiency affected the production of cytokines by TLR4-stimulated macrophages and dendritic cells (DCs) and reduced LPS-induced septic shock similarly to the known effects of deleting TPL-2. Our results demonstrate that p38γ and p38δ are essential for innate immune responses and suggest that pharamacological modulation of their expression could be therapeutically beneficial in diseases involving chronic inflammation.
Results
p38γ and p38δ Regulate LPS-Induced Cytokine Production.
The p38 MAPK pathway is central to the regulation of cytokine production in macrophages, a primary outcome of TLR4 activation by LPS. To investigate the potential role of p38γ and p38δ in this process, we analyzed cytokine production by bone marrow-derived macrophages (BMDM) and bone marrow-derived dendritic cells (BMDC) generated from mice lacking both p38γ and p38δ. Real-time quantitative PCR (qPCR) and Western blot analyses demonstrated that p38γ and p38δ were expressed in wild-type (WT) BMDM and WT BMDC, although at a much lower level than p38α and p38β (SI Appendix, Table S1 and Fig. S1). Deficiency of p38γ and p38δ did not affect the in vitro differentiation of bone marrow progenitor cells to macrophages or dendritic cells because we obtained equivalent numbers of BMDM expressing the macrophage-specific marker F4/80, or of BMDC expressing the marker CD11c, from WT control and double-knockout mice (SI Appendix, Fig. S2).
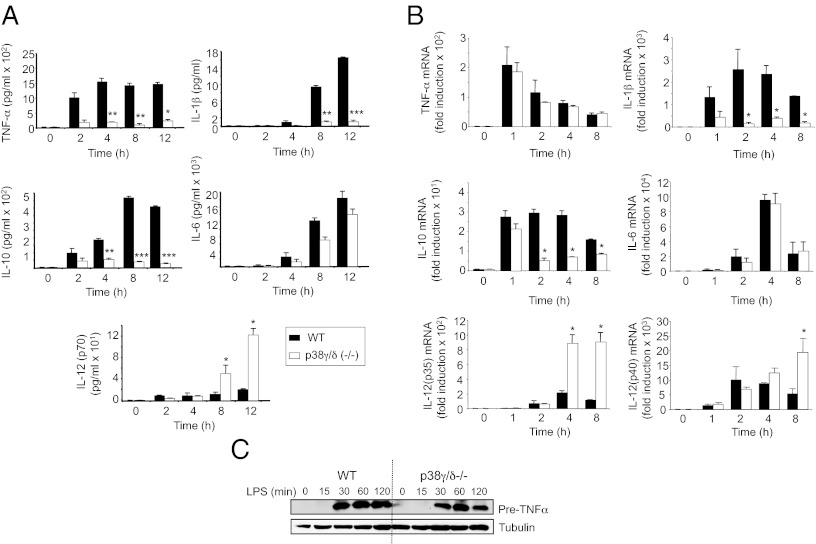
BMDM were stimulated with LPS; supernatants collected at the times indicated in Fig. 1; and TNFα, IL-1β, IL-6, IL-10, and IL-12 levels were measured. LPS induced the production of each these cytokines by WT macrophages (Fig. 1A). p38γ/p38δ deficiency impaired LPS induction of TNFα, IL-1β, or IL-10 compared with WT cells, whereas there was a significant increase in IL-12p70 production. p38γ/p38δ deficiency did not significantly alter IL-6 production (Fig. 1A).
Fig. 1.
Bone marrow-derived macrophages from p38γ/δ-deficient mice show altered cytokine production in response to LPS. (A) BMDM from WT (black bars) or p38γ/δ−/− (white) mice were exposed to LPS (100 ng/mL) for the indicated times, and culture supernatants harvested for luminex cytokine analysis of TNFα, IL-1β, IL-10, IL-6, and IL-12(p70). Values show mean ± SD for one representative experiment of three performed in duplicate. (B) qPCR of TNFα, IL-1β, IL-10, IL-6, IL-12(p35), and IL-12(p40) mRNA in total RNA from WT (black bars) or p38γ/δ−/− (white) BMDM stimulated with LPS (100 ng/mL). Results were normalized to 18S RNA expression and x-fold induction was calculated relative to WT expression at 0 h. Data show mean ± SD from one representative experiment of two in triplicate, with similar results. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 relative to WT BMDM exposed to LPS at each time. (C) WT and p38γ/δ−/− BMDM were exposed to LPS (100 ng/mL). Cell lysates (50 μg) were immunoblotted with a murine anti-TNFα antibody.
Quantitative PCR (qPCR) was used to determine whether the effects of p38γ/p38δ deficiency on induction of the analyzed cytokines were due to altered transcription. p38γ/p38δ deficiency reduced LPS induction of IL-1β and IL-10 mRNAs, whereas IL-12p35 and IL-12p40 mRNAs were increased (Fig. 1B). LPS induction of Il6 mRNAs was similar between WT and p38γ/δ−/− BMDM. The effects of p38γ/p38δ deficiency on the production of IL-1β, IL-10, and IL-12 therefore probably resulted from altered transcription.
p38γ/p38δ deficiency did not affect LPS induction of Tnfα mRNA (Fig. 1B). Although TNFα production was blocked in p38γ/δ−/− BMDM (Fig. 1A), immunoblotting of cell lysates demonstrated that production of pre-TNFα was similar to WT cells (Fig. 1C). Therefore, p38γ/δ did not control TNFα production at the transcriptional or translational level, but might regulate its maturation or secretion.
Neither p38γ nor p38δ deficiency alone affected LPS-induced TNFα, IL-10, IL-6, and IL-12p70 production, although IL-1β induction was partially impaired (SI Appendix, Fig. S3). This supports the idea that p38γ and p38δ functions are partially redundant.
To examine whether p38γ and p38δ also regulated LPS-induced cytokines in another immune cell type, we analyzed BMDC from WT or p38γ/δ−/− mice. Similarly to the effects detected in BMDM, p38γ/δ deficiency reduced LPS induction of TNFα, IL-1β, and IL-10 protein and mRNA production by BMDC compared with WT cells, whereas IL-6 was unaffected and IL-12p70 was increased (SI Appendix, Fig. S4).
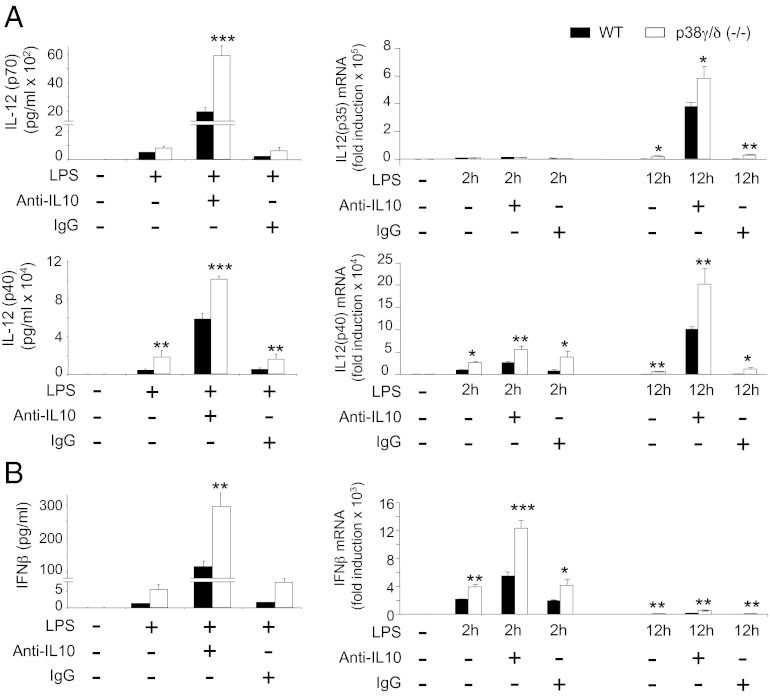
Because IL-10 has a profound inhibitory effect on cytokine production by TLR-stimulated macrophages (18), increased IL-12 levels in p38γ/δ−/− BMDM might have resulted from diminished IL-10 secretion. This was investigated by quantifying the synthesis of IL-12p70 and IL-12p40 proteins, as well as IL-12p35 and IL-12p40 mRNAs, in the presence of anti–IL-10 antibody (Fig. 2A). Because it has been suggested that IL-10 inhibits IFN-β production (18), IFN-β protein and IFN-β mRNA were also assayed under the same conditions (Fig. 2B). Incubation of cells with anti–IL-10 caused a marked increase in LPS-induced IL-12 and IFN-β production by both p38γ/δ−/− and WT BMDM compared with control cells incubated with IgG (Fig. 2). When incubated with either anti–IL-10 or control IgG, however, p38γ/δ−/− BMDM produced significantly higher IL-12 p40/p70 and IFN-β levels compared with WT BMDM (Fig. 2). These results show that p38γ and p38δ negatively regulated IL-12 and IFN-β production independently of IL-10, but that autocrine IL-10 limited TLR4-induced production of these cytokines. Secretion of IL-10 and TNFα, which was positively controlled by p38γ and p38δ (Fig. 1), was also reduced by autocrine IL-10 in both p38γ/δ−/− and WT BMDM (SI Appendix, Fig. S5).
Fig. 2.
Role of IL-10 in LPS-induced cytokine production. (A and B) BMDM from WT (black bars) or p38γ/δ−/− (white) mice were exposed to LPS (100 ng/mL) alone or in the presence of neutralizing antibody to IL-10 (anti-IL10) or an isotype control antibody (IgG) (both at 1 μg/mL, 12 h); culture supernatants were harvested for luminex cytokine analysis of (A) IL-12(p70) and IL-12(p40) and (B) IFNβ. Data are mean ± SD of one representative experiment of two in triplicate with similar results. Real-time qPCR analysis of (A) IL-12(p35), IL-12(p40), and (B) IFNβ in total RNA from WT (black bars) or p38γ/δ−/− (white) BMDM stimulated as before for the times indicated. Results show mean ± SD of triplicate wells normalized to GAPDH mRNA. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 relative to WT BMDM in each experimental condition.
Together these findings show an important function for p38γ and/or p38δ in the regulation of inflammatory cytokine production in both LPS-stimulated BMDM and BMDC.
p38γ and p38δ Are Required for ERK1/2 Pathway Activation in Response to LPS.
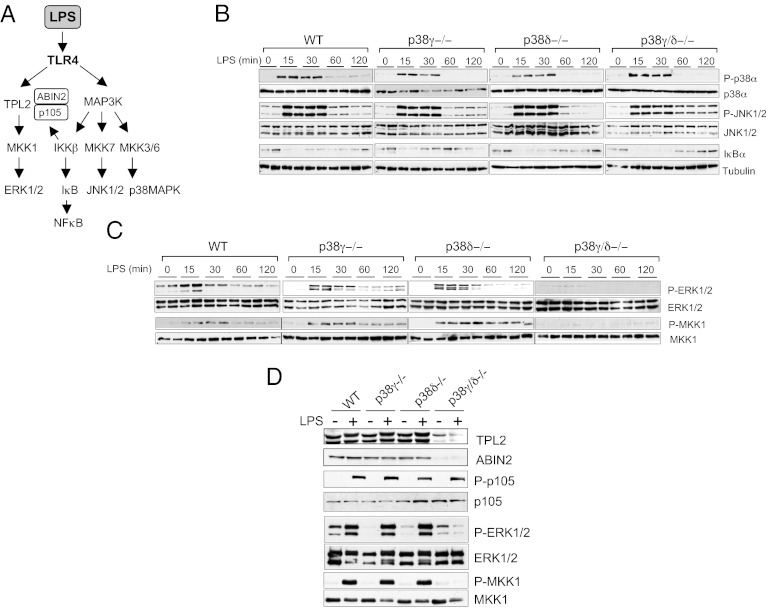
TLR4 stimulation of myeloid cells by LPS activates all three major MAPK pathways, c-Jun N-terminal kinase (JNK), p38α, and ERK1/2, as well as the canonical NF-κB–signaling pathway (Fig. 3A), which together regulate cytokine production (1, 19). p38γ and/or p38δ deficiency did not affect the LPS-induced transient activation of p38α and JNK1/2, as determined by immunoblotting with phospho-specific antibodies (Fig. 3B). TLR4-induced proteolysis of the NF-κB inhibitor IκBα was also unaffected by the lack of p38γ, p38δ, or p38γ/δ (Fig. 3B). In contrast, phosphorylation of both ERK1/2 kinases and their activator MKK1 was substantially reduced in p38γ/δ-null BMDM compared with WT or single p38 knockouts (Fig. 3C). This effect in ERK1/2 pathway activation in p38γ/δ−/− cells compared with single knockout cells indicates that p38γ and p38δ appeared to be redundant with respect to their requirement for LPS activation of ERK1/2. ERK1/2 activation was also impaired in LPS-stimulated p38γ/δ−/− BMDC, whereas JNK phosphorylation, p38α phosphorylation, and IκBα degradation were similar to that in WT cells (SI Appendix, Fig. S6).
Fig. 3.
p38γ and p38δ are necessary for LPS activation of ERK1/2 but not of other signaling pathways. (A) Scheme of various signaling pathways activated in macrophages after LPS stimulation. (B–D) BMDM from WT, p38γ−/−, p38δ−/−, or p38γ/δ−/− mice were stimulated with 100 ng/mL LPS for the times indicated (B and C) or for 15 min (D). Cell lysates (50 μg) were immunoblotted with antibodies to (B) active phosphorylated p38α (P-p38α) and active phosphorylated JNK1/2 (P-JNK1/2) or IκBα and (C) active phosphorylated ERK1/2 (P-ERK1/2) and MKK1 (P-MKK1). (B and C) Total protein levels of tubulin, p38MAPK, JNK1/2, ERK1/2, and MKK1 were also measured in the same lysates as loading controls. Duplicate lanes are shown; similar results were obtained in at least three independent experiments. (D) Cell lysates immunoblotted with anti–TPL-2, anti–ABIN-2, anti-phospho p105 (P-p105), anti-p105, anti–P-ERK1/2, anti–ERK1/2, anti–P-MKK1, or anti–MKK1.
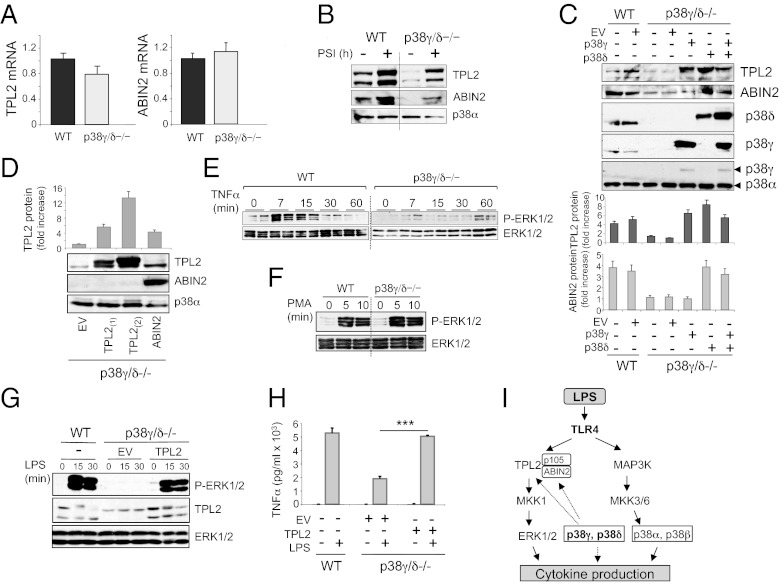
These results suggested that lack of p38γ/δ affected a kinase upstream of MKK1. In response to LPS, MKK1/ERK1/2 activation in macrophages is regulated by the MKK kinase TPL-2 (also known as cancer Osaka thyroid) (20–22). In unstimulated cells, TPL-2 is stoichiometrically complexed with the NF-κB inhibitory protein NF-κB1 p105 and the ubiquitin-binding protein ABIN-2 (A20-binding inhibitor of NF-κB2), both of which are needed to maintain TPL-2 protein stability (20, 23–25) (Fig. 3A). In unstimulated and LPS-stimulated p38γ/δ−/− BMDM, steady-state levels of TPL-2 and ABIN-2 proteins were substantially reduced compared with WT cells or single p38 knockouts (Fig. 3D; SI Appendix, Fig. S7), whereas NF-κB1 p105 protein levels were normal (Fig. 3D). NF-κB1 p105 phosphorylation in LPS-stimulated p38γ/δ−/− BMDM was also similar to WT cells (Fig. 3D). The reduction in TPL-2 and ABIN-2 proteins resulted from posttranscriptional effects because Tpl2 and Abin2 mRNA levels were unaffected by p38γ/δ deficiency (Fig. 4A). TPL-2 and ABIN-2 protein expression was also substantially reduced in BMDC (SI Appendix, Fig. S6).
Fig. 4.
Deletion of p38γ and p38δ decrease TPL-2 and ABIN-2 protein levels in cells. (A) Quantitative PCR of Tpl-2 mRNA or Abin-2 mRNA in total RNA from WT or p38γ/δ−/− BMDM. Results were normalized to 18S RNA expression. Data show mean ± SD from one representative experiment of two with similar results. (B) BMDM from WT or p38γ/δ−/− mice were incubated with the proteasome inhibitor PSI (60 μM) for 0 or 8 h before lysis with SDS-containing buffer A. Immunoblotting was carried with anti-total TPL-2, anti–ABIN-2, or anti-total p38α antibody as protein loading control. (C) WT and p38γ/δ−/− MEF were transiently transfected with plasmids encoding p38γ, p38δ, or with no insert (EV). (D) p38γ/δ−/− MEF were transiently transfected with plasmids encoding ABIN-2, TPL2 (as positive control), or with no insert (EV). (C and D) Lysates were immunoblotted with the antibodies indicated, and the band intensity of blots in D and E were quantified and the relative amount of TPL-2 or ABIN-2 in the lysates was calculated. Histogram show mean ± SD of two to three independent experiments. BMDM from WT or p38γ/δ−/− mice were stimulated with (E) 100 ng/mL TNFα or (F) 100 ng/mL PMA. Immunoblotting was carried out with antibodies to active phosphorylated-ERK1/2 (P-ERK1/2) or total protein (ERK1/2) as loading control. E shows duplicate lanes. Results were similar in two independent experiments. (G and H) Uninfected WT BMDM or p38γ/δ−/− BMDM infected with recombinant retroviruses encoding TPL-2 or with no insert (EV). Cells were stimulated with LPS at various times (G), and immunoblots were carried out as in E and F. (H) BMDM were stimulated with LPS for 6 h, and qPCR of TNFα was performed as in Fig. 1. Data show mean ± SD from one representative experiment of two in triplicate, with similar results. ***P ≤ 0.001. (I) Schematic representation of the signaling pathways involved in cytokine production, which are controlled by p38γ and p38δ in LPS-stimulated macrophages.
TPL-2 and ABIN-2 are proteolysed by proteasome in macrophages after LPS stimulation, suggesting that the reduced levels of these proteins in p38γ/δ−/− BMDM could result from proteasome-mediated proteolysis (26, 27). This was investigated by testing the effect of proteasome inhibitor I (PSI) on TPL-2 and ABIN-2 protein levels in WT and p38γ/δ−/− BMDM. PSI increased the amount of TPL-2 and ABIN-2 proteins in both WT and p38γ/δ−/− BMDM (Fig. 4B). Earlier work has shown that ABIN-2 deficiency reduces steady-state levels of TPL-2 protein without affecting TPL-2 mRNA or p105 protein levels (24, 28). Therefore, it is possible that p38γ and p38δ control TPL-2 protein levels directly or via effects on ABIN-2 protein levels. To investigate this hypothesis, we performed rescue experiments in p38γ/δ−/− mouse embryonic fibroblasts (MEF), which express substantially lower amounts of TPL-2 and ABIN-2 than WT MEF, similar to BMDM (Fig. 4C). We transfected p38γ/δ−/− with plasmids encoding p38γ, p38δ, p38γ, and p38δ or ABIN-2 and examined the expression of TPL-2 and ABIN-2 protein. Overexpression of p38δ, alone or with p38γ, restored TPL-2 and ABIN-2 protein to levels similar to those in WT MEF, whereas p38γ transfection restored only the TPL-2 protein level (Fig. 4C). Overexpression of TPL-2 did not restore ABIN-2 levels (Fig. 4C). In addition, the overexpression of ABIN-2 restored TPL-2 protein in p38γ/δ−/− MEF to a level similar to that in WT cells (Fig. 4D), consistent with earlier experiments showing that ABIN-2 is required for TPL-2 protein stability (24, 28).
Together our data indicate that the impairment in LPS activation of ERK1/2 in p38γ/δ−/− BMDM was due to the reduced levels of TPL-2 protein. In support of this idea, TNFα activation of ERK1/2, which is TPL-2 dependent (29), was also blocked in p38γ/δ-null BMDM (Fig. 4E). In contrast, ERK1/2 activation by phorbol-12 myristate-13 acetate (PMA), which is mediated via Raf kinase (30), was unaffected by p38γ/δ−/− deficiency (Fig. 4F). To determine whether the decreased ERK1/2 activation in p38γ/δ−/− macrophages was the consequence of TPL-2 deficiency due to the lack of p38γ/δ, we reconstituted TPL-2 in p38γ/δ−/− macrophages by infection with recombinant retrovirus encoding TPL-2. Expression of TPL-2 increased ERK1/2 activation (Fig. 4G) and also TNFα production (Fig. 4G) in response to LPS.
Consistent with the hypothesis that p38γ/δ control cytokine production by regulating TPL-2/ERK1/2 pathway activation, pharmacological blockade of LPS-induced ERK1/2 activation using the MKK1 inhibitor PD184352 in WT BMDM mimicked the effects of genetic deletion of p38γ and p38δ on cytokine mRNA induction (SI Appendix, Fig. S8). Furthermore, comparative qRT-PCR analyses of WT and Tpl2−/− BMDM demonstrated that TPL-2 deficiency had similar effects on the mRNA levels of each of these cytokines (SI Appendix, Fig. S9), consistent with early studies (21).
p38γ/δ−/− Are More Resistant Than WT Mice to Endotoxic Shock.
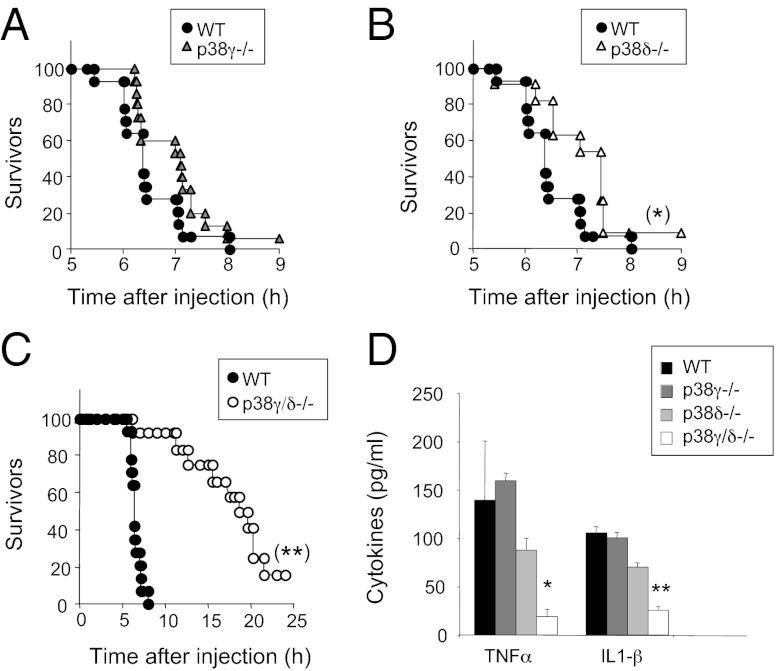
The reaction to bacterial LPS is a well-characterized innate immune response that leads to endotoxic or septic shock due primarily to TNFα overproduction. To examine the in vivo role of p38γ and p38δ in innate immunity, we administered i.p. injections of Escherichia coli-derived LPS plus the transcriptional inhibitor d-galactosamine (d-Gal) to age- and sex-matched WT, p38γ−/−, p38δ−/−, or p38γ/δ−/− mice (21). Both p38γ−/− (Fig. 5A) and p38δ−/− mice (Fig. 5B) were slightly more resistant to endotoxic shock induction and survived ∼1–2 h longer than WT mice whereas compound p38γ and p38δ deficiency had a more pronounced protective effect (Fig. 5C). And, whereas 100% of WT mice died within 8 h of LPS injection, ∼50% of p38γ/δ−/− mice survived for 20 h and ∼15% survived to 36 h, at which time they were killed (Fig. 5C).
Fig. 5.
Deletion of p38γ and p38δ decreases LPS/d-Gal–induced lethality. (A–C) Mice received injections of LPS (50 μg/kg) and d-Gal (1 g/kg), and death was monitored for up to 36 h. Wild type (n = 14) and (A) p38γ−/− (n = 15), (B) p38δ−/− (n = 11), or (C) p38γ/δ−/− (n = 12) are shown. (D) Serum from mice in A–C was collected 2 h after LPS and d-Gal challenge, and TNFα and IL-1β were measured in a luminex cytokine assay. Data show mean ± SD (n = 4–6 mice/group). *P ≤ 0.05 and **P ≤ 0.01 relative to WT mouse serum.
To determine whether p38γ and/or p38δ control cytokine release in response to LPS challenge in vivo, we measured TNFα and IL-1β levels in serum from WT, p38γ−/−, p38δ−/−, and p38γ/δ−/− mice at 2 h post LPS injection. Lack of p38γ or p38δ alone had little effect on TNFα and IL-1β production compared with WT mice (Fig. 5D). However, induction of both of these cytokines was substantially reduced in double mutant p38γ/δ−/− mice (Fig. 5D). IL-1α, IL-10, and IL-6 production was also diminished in LPS-treated p38γ/δ−/− mice compared with WT mice (SI Appendix, Fig. S10). These data suggest that the reduced susceptibility of p38γ/δ−/− mice to LPS-induced septic shock was due to an overall decrease in cytokine production and indicate that p38γ/δ−/− mice behave similarly to Tpl2−/− mice, which cannot up-regulate TNFα and IL-1β after LPS injection and are protected from LPS-induced toxic shock (21, 31).
Discussion
Here we used mice lacking p38γ and/or p38δ to analyze the effect of p38γ and p38δ on the inflammatory response. Our results show that these two kinases are central to regulation of the inflammatory response and that p38 MAPKs have a substantially more complex function in the immune response than previously thought. In both macrophages and DC, p38γ and p38δ deletion altered the response to LPS by reducing production of cytokines such as TNFα, IL-1β, or IL-10 and by increasing IL-12 and IFNβ synthesis. This was paralleled by specific blockade of the MKK1-ERK1/2 pathway activation. LPS induction of p38α and JNK activation, as well as IκB proteolysis, was unaffected by p38γ/δ deficiency. p38γ/δ−/− macrophages showed substantially lower levels of two MKK1-ERK1/2 upstream components, the proteins TPL-2 and ABIN-2. TPL-2 is a MKK kinase essential for LPS activation of MKK1-ERK1/2, but not of p38MAPK, JNK, or NF-κB, in macrophages and in DC (20–22). Optimal TPL-2 stability in vivo requires interaction with ABIN-2 as well as with NFκB1 p105, and ABIN-2 positively regulates the ERK1/2 signaling pathway by stabilizing TPL-2 (20, 26). Our data suggest that one function of p38γ and p38δ in the TLR4-signaling pathway is to regulate TPL-2 and ABIN-2 protein levels and, consequently, MKK1-ERK1/2 pathway activation. This idea is supported by our following findings: (i) expression of p38γ and p38δ in p38γ/δ-null cells restored ABIN-2 and TPL-2 protein levels, and (ii) expression of TPL-2 in p38γ/δ−/− macrophages not only increased ERK1/2 activation, but also rescued TPL-2–dependent TNFα production in response to LPS. In addition, ERK1/2 activation in p38γ/δ−/− macrophages was also impaired in response to TNFα, a ligand that also activates ERK1/2 via TPL-2 (29), whereas it was unimpaired in response to PMA, which activates ERK1/2 independently of TPL-2 (30). Our preliminary observations, based on transient transfection experiments in HEK293 cells, indicate that p38γ and p38δ regulate TPL-2 and ABIN-2 protein stability by phosphorylation and interaction with the TPL-2/ABIN-2/p105 complex. Further studies will be needed to determine the exact mechanism by which p38γ and p38δ regulate TPL-2 and ABIN-2 protein levels in macrophages.
Production of TNFα, IL-1β, and IL-10 was severely reduced in LPS-stimulated macrophages from p38γ/δ-null mice, whereas IL-12 and IFNβ production increased, and IL-6 synthesis was unaffected. p38γ/p38δ signaling thus has complex pro- and anti-inflammatory effects on cytokine production in innate immune responses. The effect of p38γ/δ deficiency on cytokine production resembled those described previously in macrophages generated from TPL-2 and ABIN-2 knockout mouse strains. Similar to p38γ and p38δ, TPL-2 and ABIN-2 are necessary for optimal TNFα and IL-1β production by LPS-stimulated macrophages, the major cell source of TNFα during inflammatory responses, whereas TNFα and IL-1β production by LPS-stimulated DC is partially TPL-2– and ABIN-2–dependent (24, 31). LPS-induced TNFα secretion, but not Tnfα mRNA or pre-TNFα synthesis, was abolished in p38γ/δ−/− macrophages and DC, showing the importance of p38γ and p38δ in the posttranslational control of TNFα production. These findings are again similar to those for TPL-2–deficient BMDM, in which TPL-2-ERK1/2 signaling is required for TNFα intracellular transport and maturation, but not induction of Tnfα mRNA (32, 33). IL-12 and IFNβ are other cytokines whose production was altered in p38γ/δ−/− BMDM, independently of any effects on IL-10 induction; we found increased production of IL-12p40, IL-12p35, and IFNβ mRNA as well as of IL-12p70 and IFNβ protein, as also was observed in TPL-2−/− BMDM (34). As for TPL-2 (34), p38γ and p38δ positively regulate IL-10 protein and mRNA induction in LPS-stimulated macrophages and DC.
In vivo analysis of p38γ/δ−/− mice also suggests critical p38γ and p38δ functions in the immune response, confirming the physiological relevance of our in vitro findings in macrophages and dendritic cells. Following LPS challenge, p38γ/δ−/− mice were less sensitive to endotoxic shock than WT mice, which was associated with a decrease in serum levels of proinflammatory (TNFα, IL-1β) and anti-inflammatory (IL-10) cytokines.
The results shown here imply that p38γ and p38δ have largely redundant functions. The absence of p38γ or p38δ did not affect TPL-2 or ABIN2 levels, LPS-induced ERK1/2 activation, or, in most cases, cytokine production. However, we found that lack of either p38γ or p38δ has a similar effect on the induction of IL-1β mRNA or IL-1β protein in BMDM (SI Appendix, Fig. S3). IL-1β gene transcription is a complex, precisely regulated process that involves many transcription factors (35). Both p38γ- and p38δ-null BMDM have TPL-2 protein levels similar to those of WT macrophages, indicating that p38γ and/or p38δ might control IL-1β transcription at one or various steps; further studies are needed to determine their specific roles in this process and also to establish the molecular mechanism by which both p38γ and p38δ are necessary to keep TPL-2 or ABIN2 protein levels in BMDM and BMDC.
Our results show a previously unreported, essential function for p38γ and p38δ in the immune response and identify two possible targets for treatment of inflammatory conditions such as septic shock and rheumatoid arthritis. We propose that p38γ and p38δ act simultaneously at different levels to control inflammatory cytokine expression by (i) regulating expression of other signaling pathway components essential for cytokine production during the immune response to endotoxic shock and/or (ii) by directly modulating transcription of cytokines such as IL-1β (Fig. 4I). In contrast, p38αMAPK controls cytokine production mainly by regulating transcriptional activation and mRNA stability (3). Use of p38γ and p38δ as therapeutic targets might obviate the pleiotropic and adverse side effects of the numerous p38α inhibitors currently being tested for sepsis and rheumatoid arthritis treatment (36, 37). TPL-2 has recently become an attractive target for anti-inflammatory drugs, although further study is needed to develop sufficiently potent compounds with physicochemical properties appropriate for in vivo use (36). Our results demonstrate that p38γ and p38δ are players in the inflammatory response and indicate that their suitability as targets for the treatment of certain inflammatory disorders should be evaluated.
Materials and Methods
Cell Culture, Stimulation, and Protein Overexpression.
BMDM were isolated from adult mouse femurs (38). Bone marrow cells were allowed to differentiate on bacteria-grade plastic dishes in DMEM with 20% (vol/vol) FBS and 30% (vol/vol) L929 cell-conditioned media (CSF-1 source). After 7 d, adherent cells were removed, counted, and replated at a constant density (106 cells/mL). At 4–8 h after replating in DMEM with 10% (vol/vol) FBS, cells were stimulated for various times with LPS, TNFα, or PMA (all from Sigma). Cells were lysed in buffer A [50 mM Tris⋅HCl (pH 7.5), 1 mM EGTA, 1 mM EDTA, 0.15 M NaCl, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 50 mM sodium β-glycerophosphate, 5 mM pyrophosphate, 0.27 M sucrose, 0.1 mM phenylmethylsulfonyl fluoride, 1% (vol/vol) Triton X-100] plus 0.1% (vol/vol) 2-mercaptoethanol and complete proteinase inhibitor mixture (Roche). In experiments using PSI, the cells were lysed in buffer A supplemented with 0.5% (wt/vol) SDS to obtain soluble and insoluble proteins. Lysates were centrifuged (13,000 × g, 15 min, 4 °C), and supernatants were removed, quick-frozen in liquid nitrogen, and stored at −80 °C.
In IL-10 blockade experiments, BMDM were stimulated with LPS alone or in the presence of neutralizing antibody to IL-10 or of an isotype control antibody (both at 1 μg/mL); after 2 and 12 h, culture medium was collected (and stored at −80 °C), cells were lysed, and RNA was extracted using the Micro RNeasy kit (Qiagen; 74004).
Protein transfection in MEF and retroviral expression of macrophages were carried out as described (15, 24).
LPS/d-Gal–Induced Endotoxic Shock.
A combination of LPS (50 μg/kg body weight) and d-Gal (1 g/kg body weight) was simultaneously injected intraperitoneally in 12-wk-old p38γ−/−, p38δ−/−, p38γ/δ−/− (38) and control mice of both sexes. Plasma samples were collected for cytokine analysis 2 h after LPS injection, and mice were killed 36 h post injection.
Supplementary Material
Acknowledgments
We thank Dr. A. O’Garra for the anti–IL-10 antibody; Dr. S. Alemany and D. Alessi for helpful discussions; L. Almonacid for help with the qPCR; and C. Mark for editorial assistance. A.C. was supported by grants from the Comunidad de Madrid-Consejo Superior de Investigaciones Cientificas (CSIC) (CCG07-CSIC/SAL-2215, CCG08-CSIC/SAL-3780) and Ministry of Science and Innovation (MICINN) of Spain (BFU2007-67577; BFU2010-19734).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207290109/-/DCSupplemental.
References
- 1.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 3.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8:480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 5.Adams RH, et al. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6(1):109–116. [PubMed] [Google Scholar]
- 6.Mudgett JS, et al. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci USA. 2000;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura K, et al. Requirement for p38alpha in erythropoietin expression: A role for stress kinases in erythropoiesis. Cell. 2000;102:221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 8.Kang YJ, et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 9.Kim C, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsuka M, et al. Distinct effects of p38alpha deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology. 2010;138(4):1255–1265. doi: 10.1053/j.gastro.2010.01.005. 1265.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beardmore VA, et al. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25:10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumara G, et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie MA, et al. p38-gamma-dependent gene silencing restricts entry into the myogenic differentiation program. J Cell Biol. 2009;187:991–1005. doi: 10.1083/jcb.200907037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindler EM, et al. p38delta mitogen-activated protein kinase is essential for skin tumor development in mice. Cancer Res. 2009;69:4648–4655. doi: 10.1158/0008-5472.CAN-08-4455. [DOI] [PubMed] [Google Scholar]
- 15.Cerezo-Guisado MI, et al. Evidence of p38γ and p38δ involvement in cell transformation processes. Carcinogenesis. 2011;32:1093–1099. doi: 10.1093/carcin/bgr079. [DOI] [PubMed] [Google Scholar]
- 16.Fearns C, et al. Coordinate activation of endogenous p38alpha, beta, gamma, and delta by inflammatory stimuli. J Leukoc Biol. 2000;67:705–711. doi: 10.1002/jlb.67.5.705. [DOI] [PubMed] [Google Scholar]
- 17.Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999;162:4246–4252. [PubMed] [Google Scholar]
- 18.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 19.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 20.Gantke T, Sriskantharajah S, Ley SC. Regulation and function of TPL-2, an IκB kinase-regulated MAP kinase kinase kinase. Cell Res. 2011;21(1):131–145. doi: 10.1038/cr.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumitru CD, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 22.Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beinke S, et al. NF-kappaB1 p105 negatively regulates TPL-2 MEK kinase activity. Mol Cell Biol. 2003;23:4739–4752. doi: 10.1128/MCB.23.14.4739-4752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papoutsopoulou S, et al. ABIN-2 is required for optimal activation of Erk MAP kinase in innate immune responses. Nat Immunol. 2006;7:606–615. doi: 10.1038/ni1334. [DOI] [PubMed] [Google Scholar]
- 25.Waterfield MR, Zhang M, Norman LP, Sun SC. NF-kappaB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol Cell. 2003;11:685–694. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 26.Beinke S, Robinson MJ, Hugunin M, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol. 2004;24:9658–9667. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterfield M, Jin W, Reiley W, Zhang M, Sun SC. IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol Cell Biol. 2004;24:6040–6048. doi: 10.1128/MCB.24.13.6040-6048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang V, et al. ABIN-2 forms a ternary complex with TPL-2 and NF-kappa B1 p105 and is essential for TPL-2 protein stability. Mol Cell Biol. 2004;24:5235–5248. doi: 10.1128/MCB.24.12.5235-5248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das S, et al. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280:23748–23757. doi: 10.1074/jbc.M412837200. [DOI] [PubMed] [Google Scholar]
- 30.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 31.Mielke LA, et al. Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1 beta production. J Immunol. 2009;183:7984–7993. doi: 10.4049/jimmunol.0901336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau S, et al. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci. 2008;121(2):149–154. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- 33.Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–2380. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser F, et al. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J Exp Med. 2009;206:1863–1871. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Saccani S, Shin H, Nikolajczyk BS. Dynamic protein associations define two phases of IL-1beta transcriptional activation. J Immunol. 2008;181:503–512. doi: 10.4049/jimmunol.181.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Genovese MC. Inhibition of p38: Has the fat lady sung? Arthritis Rheum. 2009;60:317–320. doi: 10.1002/art.24264. [DOI] [PubMed] [Google Scholar]
- 38.Hume DA, et al. Preparation and characterization of human bone marrow-derived macrophages. J Leukoc Biol. 1985;38:541–552. doi: 10.1002/jlb.38.4.541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.