Abstract
In general, there is a relationship between growth and reproduction, and gonads are known to be important organs for growth, but direct evidence for their role is lacking. Here, using a fish model, we report direct evidence that gonads are endocrine organs equal to the pituitary in controlling body growth. Gonadal loss of function, gain of function, and rescue of growth were investigated in tilapia. Gonadectomy experiments were carried out in juvenile males and females. Gonadectomy significantly retarded growth compared with controls; however, this retardation was rescued by the implantation of extirpated gonads. Because gonads express growth hormone, it is possible that gonads control body growth through the secretion of growth hormone and/or other endocrine factors. We propose that gonads are integral players in the dynamic regulation of growth in teleosts.
Keywords: gonadal function, gonadal implantation, Mozambique tilapia
Sexual dimorphism in body growth is a common phenomenon (1). In teleosts, for example, female flounder are larger than males (2, 3), whereas in tilapia, males are the larger sex (4). However, although these sexual differences in body size may be related to their reproductive organs, the mechanisms underlying sexually dimorphic body growth have not been determined.
In general, reproduction is closely related to body growth (5–8), and gonads are recognized as important organs for growth. However, few examples showing a clear relationship between the gonad and body growth have been published. To study the relationship between gonad and body growth, we selected Mozambique tilapia (Oreochromis mossambicus) as the experimental animal. This species displays sexually dimorphic growth, and males and females can be visually identified by appearance after hatching at 35 d. This species is also highly tolerant of a wide range of environmental conditions and of surgery, such as the gonadectomy procedure used in this study.
To clarify the direct relationship between gonads and growth, we used the simple approach of removing the gonads surgically. We autotransplanted the extirpated gonads to ectopic sites and investigated how the ectopic gonads affected growth. Here we report that the presence of gonads is necessary for normal body growth, leading to the conclusion that in terms of body growth, the gonad can be considered functionally as a “secondary pituitary.”
Results
Body Growth Is Delayed in Gonad-Deficient Fish.
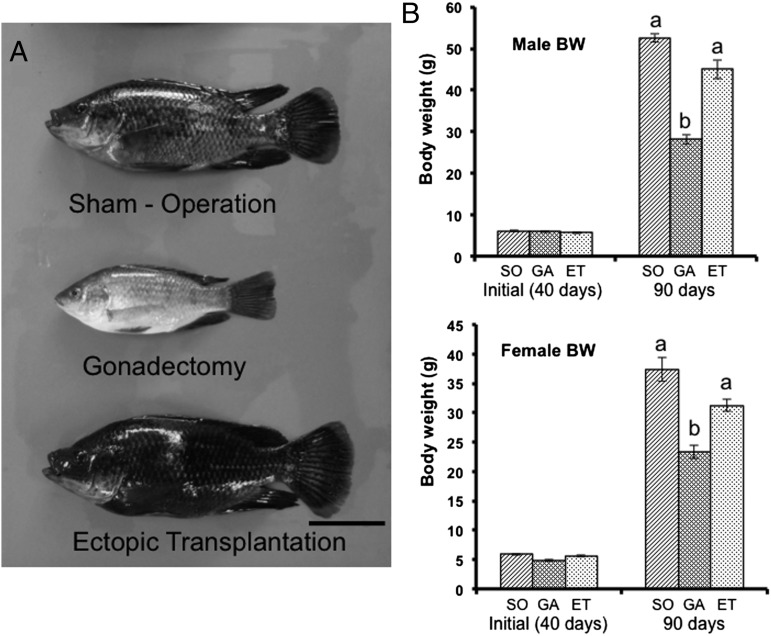
To investigate the relationship between body growth and the presence of gonads, we performed a loss-of-function experiment by surgically removing gonads from juvenile male and female tilapia at 40 d after hatching. At 50 d postsurgery, gonadectomized fish had significantly lower body weight than sham-operated fish of both sexes (Fig. 1 A and B). Tilapia reach sexual maturity by 90 d after hatching, marked by characteristic body color changes accompanied by advancement of spermatogenesis and oogenesis. The gonadectomized fish did not exhibit the changes in body color signifying sexual maturation; thus, prevention of sexual maturation by gonad removal was accompanied by retarded body growth.
Fig. 1.
Gonads promote the growth of tilapia. (A) Photographs of male tilapia at 50 d after sham operation, gonadectomy, or ectopic transplantation. (Scale bar: 2 cm.) (B) Comparison of body weight among the experimental groups. At 40 d (initial, before surgery), the average body weight was no different among groups. At 90 d (50 d after surgery), gonadectomized fish of both sexes (GA) exhibited growth retardation compared with sham-operated fish (SO). Fish that received the ectopic gonad transplant (ET) did not differ in weight compared with SO fish. Results are mean ± SEM calculated for each group. Values with different letters are significantly different (P < 0.05; n ≥14).
Body Growth Is Recovered in Ectopically Transplanted Fish.
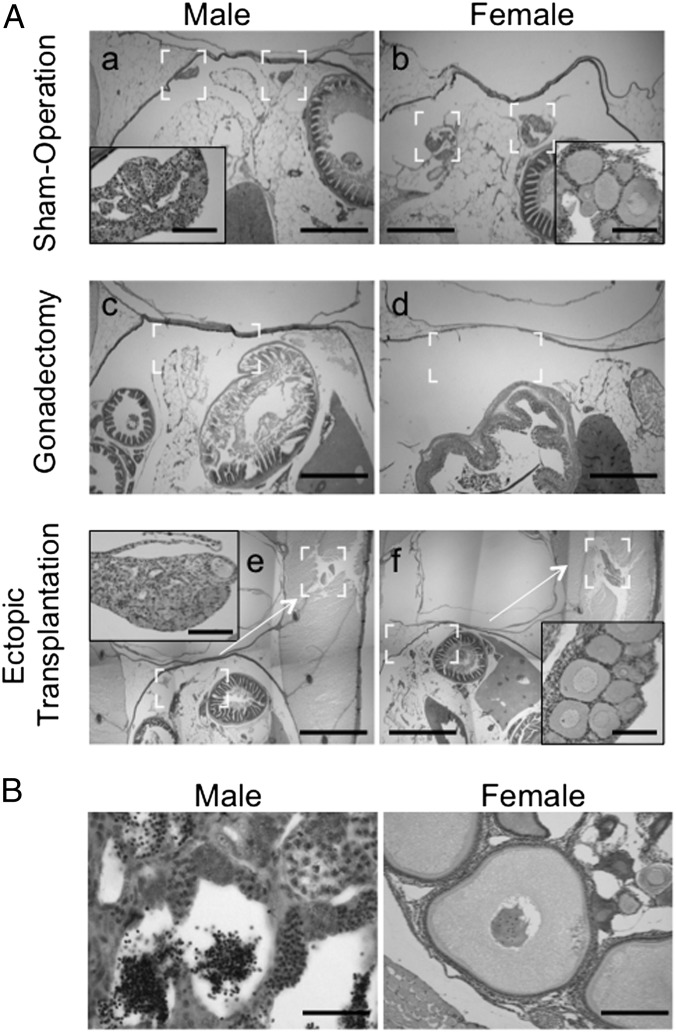
In a gain-of-function experiment, we autotransplanted gonads to an ectopic site between the skin and muscle. We confirmed that there was no gonad in the coelomic cavity of these transplanted fish (Fig. 2A). The transplanted fish were reared in the same way as sham-operated fish. At 90 d, transplanted testes developed with spermatozoa identified by histology (Fig. 2B). In females, mature eggs in the transplanted ovary were absent, but oocytes at the early vitellogenic stage were present (Fig. 2B). Under the same culture conditions, sham-operated females produced mature eggs at 90 d (Fig. S1). Furthermore, at 150 d, the ectopically transplanted ovaries contained mature eggs at 150 d. Thus, although oogenesis in the ovaries of ectopically transplanted females is delayed compared with sham-operated females, these ovaries have the capability to undergo normal oogenesis (Fig. S2). These results demonstrate that the ectopically transplanted gonads undergo further development, with the rate of progress dependent on sex. Body weights (Fig. 1B) and body length of males and females with transplanted gonads did not differ from those of sham-operated fish, showing that the recovery of weight occurred synchronously with the recovery of length. These data indicate that normal growth depends on the presence of gonads.
Fig. 2.
Histological examination of fish. (A) The complete fish was fixed at 2 d after surgery. All sections were stained with H&E. The insets in a, b, e, and f are enlarged in the brackets of figures. The sham-operated fish appeared normal with no damage (a and b). The gonads were completely removed in gonadectomized fish (c and d). Sections of ectopic-transplanted fish showed that the gonads were completely removed from the normal location, and the transplanted gonads were located in the muscle (e and f). (Scale bars: 200 μm in a, b, c, and d; 0.5 mm in e and f; 20 μm in Insets in a, b, e, and f.) (B) Ectopic gonads were fixed at 50 d after operation, and all sections were stained with H&E. Male gonads were completely mature, and spermatozoa were observed (male). Mature eggs were not observed in ectopic female gonads (female), but ovarian follicles at the early vitellogenic stage were present. (Scale bars: 20 μm for males, 50 μm for females.)
Steroid Hormone Level in the Experimental Fish.
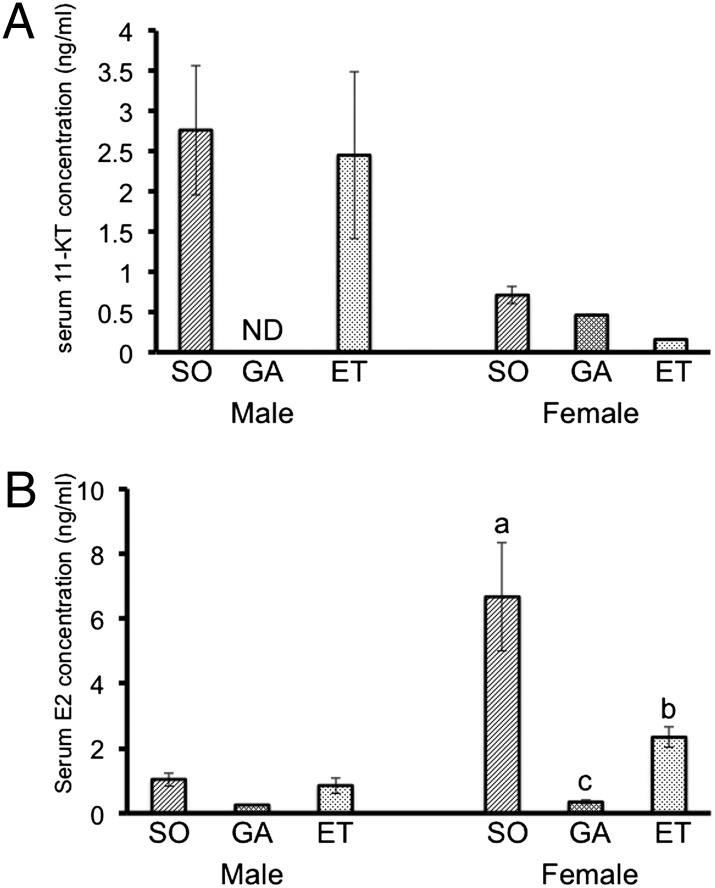
The endocrine control of growth and sexual maturation differs significantly between males and females. Androgens have been shown to experimentally promote growth in male fish (9, 10). Estrogens also act at the level of the pituitary to regulate GH secretion (11). Measurement of sex steroid hormones in the blood of fish in this experiment yielded the expected results; levels of sex steroid hormones 11-ketotestosterone (11-KT) in males and estradiol-17β (E2) in females) decreased significantly in gonadectomized fish, and concentrations in fish with ectopically transplanted gonads recovered to the same level as those in sham-operated male fish (Fig. 3). In females with ectopic ovaries, E2 levels recovered only partially, to approximately 35% of control values (Fig. 3). At 90 d, the ovaries of ectopically transplanted female fish contained only early vitellogenic stage oocytes, and sham-operated females already had mature eggs (Fig. 2B and Fig. S1). This finding indicates that serum E2 level is dependent on the developmental stage of the ovary; females with ectopic ovaries exhibited delayed oogenesis and had lower levels of E2 compared with the sham-operated female fish. Although this finding does not provide direct evidence that the decrease in growth is due to decreased sex steroid hormone levels, it does suggest a relationship between growth and sex steroid hormones. Thus, suppression of growth in gonadectomized fish and recovery of growth in fish receiving ectopic gonads might be relayed to the absence and presence of sex steroids, respectively.
Fig. 3.
Measurement of steroid hormone levels. (A) Serum 11-KT levels. Levels were similar in sham-operated (SO) and ectopically transplanted (ET) male fish. 11-KT was not detected in serum of male gonadectomized fish (GA). In females, serum 11-KT levels were very low compared those detected in male fish. (B) Serum E2 levels. ET fish had higher levels of E2 compared with GA fish, but unlike 11-KT in males, E2 levels did not recover to the same level in ET fish as in SO fish. This result was in agreement with the findings of histological analysis. Given that E2 level is dependent on the developmental stage of the ovary, SO and ET female fish have differing E2 levels. Transplanted male gonads developed into mature testis containing spermatozoa, but female gonads were not completely mature and contained early vitellogenic ovarian follicles. Results are given as mean ± SEM. Value with different letters are significantly different (P < 0.05; n ≥ 6).
Body Growth Regulation by Growth Hormone and Insulin-Like Growth Factor I in Gonads.
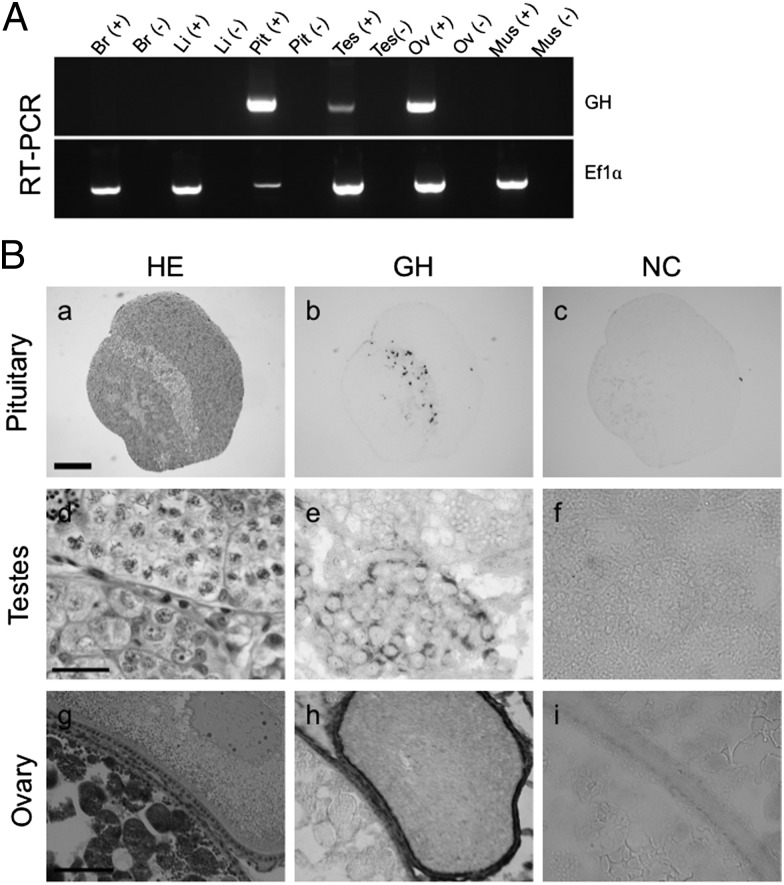
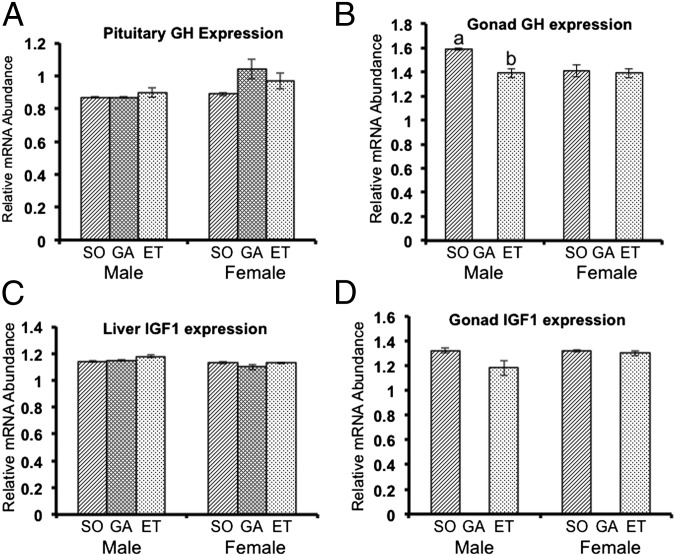
In this study, we focused on growth hormone (GH) and insulin-like growth factor I (IGF-I) as factors involved in this interesting phenomenon. These two hormones are known to directly stimulate growth. Although GH is classically associated with the pituitary, and the major source of IGF-I is the liver, recent studies have demonstrated that these hormones are expressed in other tissues as well (12–18), where their functions are unclear. In tilapia, both hormones are expressed as mRNA and protein in the testis and the ovary (Fig. 4) (19). RT-PCR analysis detected mRNA of GH expressed in pituitary, testis, and ovary (Fig. 4A). In organs other than pituitary, GH expression was observed in both male and female gonads. Using immunohistochemical analysis, GH-producing cells were detected in the organs expressing GH mRNA (Fig. 4B). In pituitary, GH was expressed in GH cells, a localization identical to that reported previously (20). GH was expressed in the Sertoli cells of testis and in the granulosa and fibroblast (thecal) cells of ovary, and GH expression in the gonads appeared to be in somatic cells.
Fig. 4.
Expression of GH in tilapia. (A) GH mRNA expression in tilapia as detected by RT-PCR analysis of various tissues, including brain (Br), liver (Li), pituitary (Pit), testis (Tes), ovary (Ov), and muscle (Mus). (−) indicates a reverse-transcription reaction without reverse transcriptase. EF1α served as the internal control. (B) Immunohistochemical analysis of GH in tilapia pituitary, testis, and ovary. Each adjacent section of the tissues was stained with H&E (HE; a, d, and g), immunostained with anti-GH antibody and AP-conjugated secondary antibody (GH; b, e, and h), or immunostained without primary antibody (NC; c, f, and i). Strong signals (black) were detected in GH cells of pituitary (b). No signal was detected without primary antibody (c). Signals were detected in the Sertoli cells around the spermatogonia of testis (e) and in the granulosa and fibroblast (thecal) cells in the ovary (h). (Scale bars: 200 μm in a, 20 μm in d, 50 μm in g.)
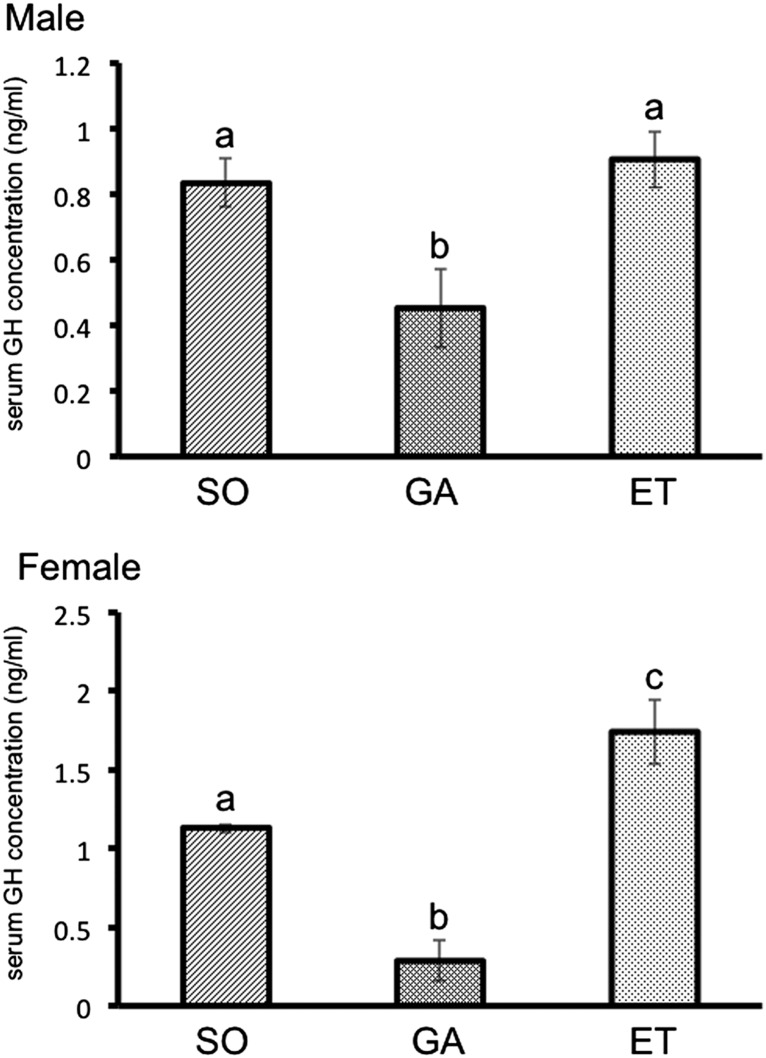
To assess whether GH and IGF-I expression changes after application of the experimental treatments used in this study, we measured transcripts encoding these hormones in various tissues by quantitative real-time PCR. We found no difference in GH expression in the ovaries of the sham-operated fish and ectopically transplanted fish. GH expression in testes was moderately but significantly higher in the sham-operated males than in the ectopically transplanted males (Fig. 5). We predicted that circulating GH levels would decrease after the gonads were removed. Removal of the gonads resulted in a significant decrease in blood GH levels, and subsequent ectopic gonad transplantation led to recovery in males and levels exceeding those in controls in females (Fig. 6), a result paralleling the data from real-time RT-PCR. On the other hand, there were no changes in blood IGF-I level among all experimental groups (Fig. S3). In female goldfish, E2 stimulates GH production at posttranscriptional or translational level in the pituitary (21). This relationship between serum GH and E2 levels is not observed in female tilapia, however (Figs. 3B and 6). Control of GH synthesis and secretion may differ between species. Our data demonstrate that the gonads are a significant source of circulating GH, and that the presence of GH in both pituitary and gonad is needed for the normal control of body growth.
Fig. 5.
Quantitative real-time PCR analysis of expression of GH and IGF-I mRNAs in various tissues, using the EF1α gene as an internal control. (A) GH expression in the pituitary. (B) GH expression in the gonads. (C) IGF-I expression in the liver. (D) IGF-I expression in the gonads. No significant differences were seen among treatments. Results are given as mean ± SEM. Values with different letters are significantly different (P < 0.05; n ≥6). ET, ectopically transplanted; GA, gonadectomized; SO, sham-operated.
Fig. 6.
Measurement of serum GH levels by ELISA. At 90 d, serum levels were significantly lower in male gonadectomized (GA) fish compared with the other two male groups. In females, serum levels were higher in ectopically transplanted (ET) fish compared with sham-operated (SO) fish. Results are given as mean ± SEM. Values with different letters are significantly different (P < 0.05; n ≥ 6).
Discussion
Fish Growth Requires the Gonads.
In this study, we have shown a direct relationship between gonads and growth in male and female tilapia. Gonadectomized fish exhibited decreased growth, and fish with ectopically transplanted gonads exhibited statistically complete recovery of growth compared with gonadectomized fish. These findings indicate that in addition to the pituitary, the gonads are major participants in the regulation of body growth in tilapia and possibly other fish species as well. The pituitary is a critical component of the neuroendocrine system that is present in all vertebrates. It is essential for the maintenance of homeostasis, metabolism, reproduction, growth, lactation, immunity, and cellular differentiation (22–25). GH is produced primarily in the pituitary by the somatotrophic cells of the mesoadenohypophysis, although it is well established that some extrapituitary tissues express the GH gene, and that its product is not released into the systematic circulation but acts locally in an autocrine or paracrine manner (26). Although GH is expressed in the gonads of mammals (13, 14), birds (15–18), and fish (27, 28), the function of gonadal GH in regulating growth has not been determined. Our results indicate that the gonads play an important role in the regulation of body growth in both male and female tilapia.
Role of Gonads in Growth.
The relationship between sexual maturation and growth has been investigated in other animals, and numerous reports on the effects of castration on growth in domestic animals have been published (29–31). The present results differ from those reports, which reached conflicting conclusions. For example, whereas one study reported that growth was promoted by castration, others found that castration inhibited growth (29–31). These results imply that the effects of castration on growth differ among species. In this study, we focused on the GH/IGF-I system because the rescue of growth was observed in male and female fish with ectopically transplanted gonads. If the steroid hormones were in fact involved in gonadal regulation of growth, then the sex differences in the type of steroid hormone released from the gonad would be expected to result in different effects in males and females. However, both male and female fish exhibited growth retardation after gonadectomy and growth recovery after ectopic transplantation of gonads. GH mRNA expression in the pituitary of all experimental fish did not differ significantly among the treatment arms, but serum GH levels were lower in the gonadectomized fish. In Mozambique tilapia, pituitary GH mRNA level is a better indicator of growth than serum GH level (32). Ectopic gonads expressed GH at a level similar to that of normal gonads in sham-operated males and females. These results suggest that the release of GH from ectopically transplanted gonads stimulates growth. GH and IGF-I are expressed in the testis of human and chicken (17–21), and the gonads might produce both GH and IGF-I, although there might be differences between species. The roles of these hormones produced from the gonads in growth are not clearly understood, however.
Our results clearly demonstrate that GH in gonads plays a significant role in body growth; however, growth is also controlled by a multiple mechanisms and factors, including as steroid hormones, other endocrine factors, and also regulation of appetite. In this study, through loss-of-function and gain-of-function experiments, we have shown that the gonads are necessary for normal growth and thus, from the perspective of regulation of body growth, can be considered a “secondary pituitary.”
Materials and Methods
Fish and Operations.
This study was carried out in accordance with Guideline for the Animal Care and Use of Laboratory Animals at Ehime University. Tilapia (Oreochromis mossambicus) were maintained in a recirculating fresh water system at 28 °C with UV filtration under a 14-h light:10-h dark photoperiod, and were fed to satiation (∼3–5% of body weight). For gonadectomy of early developing immature (40 daf) fish, the very soft and fragile gonadal tissue, which is firmly attached into the dorsal celomic cavity behind the digestive organs, was carefully removed. The fish were briefly anesthetized, immediately after which surgical incisions were made in the lower abdomen dorsoventrally, between the pelvic and anal fins. Sham-operated fish received only the incision, whereas the paired gonads were completely removed from gonadectomized fish. These immature fish had either testes with only type A spermatogonia or ovaries with perinucleolus stage ovarian follicles. Either gonads were discarded or gonadal tissue was transplanted into the same animal at an ectopic site on the back between the skin and muscles. In sham-operated fish, an incision was made at the ectopic site. Incisions were immediately sutured with Aron Alpha (Daiichi Sankyo). Fish were healed completely by 5–10 d after the surgery. Each experimental group was maintained separately. The food intake of the experimental fish was the same as that of the nonoperated fish (normal fish) at the same developmental stage during the first 5 d after surgery, and the food intake of all experimental groups was kept equal by weighing the food.
Histological Examination.
Ovaries and testes of all treatment groups were examined histologically. Organs were excised and fixed with Bouin’s solution at room temperature for 1 wk. Standard methods of sectioning and H&E staining were used. The developmental stages of gonads were determined as described previously (33).
RT-PCR.
Total RNA was isolated from adult tilapia using Sepasol RNA I Super (Nacalai Tesque). For this, 5 μg of RNA of various organs was reverse-transcribed to single-strand cDNA using SuperScript II reverse transcriptase (Invitrogen) and oligo (dT) primers according to the manufacturer’s instructions. RT-PCR was performed using specific primer pairs for tilapia: GH forward, 5′-AGAGACTCTTCTCGGACTTTGAGAG-3′; reverse, 5′-ATTTAGCTACCGTCAGGTAGGTCTC-3′; EF1α forward, 5′-GCGGAGGAATCGACAAGAGAA-3′; reverse, 5′-GTACCGGGCTTCAGGATACCA-3′).
Immunohistochemistry.
Detection of immunoreactive GH was performed as follows. The samples were fixed in Davidson’s solution at 4 °C overnight, embedded in paraffin wax, and cut into 6- to 10-μm serial sections. Sections were deparaffinized in xylene and hydrated in a graded ethanol series. Immunohistochemical analysis was performed using the Histofine SAB-AP Kit (Nichirei). Anti-bream GH (GroPep) was used at a dilution of 1/1,000 in Can Get Signal Immunostain Immunoreaction Enhancer Solution (Toyobo).
Measurement of Sex Steroids.
Sera were extracted twice with 5× volumes of diethyl ether. The 11-KT and E2 levels in these extracts were measured by time-resolved fluoroimmunoassay as described previously (34).
Measurement of GH.
Serum GH levels were measured using a standard sandwich ELISA method. The antibody for capture was anti-yellowtail GH antibody, which was used for elution of sera from the DE52 column. The detection antibody was F(ab′)2 fragmented anti-yellowtail GH antibody, labeled with biotin.
Quantitative Real-Time RT-PCR.
GH and IGF-I mRNA expression levels in the pituitary, liver, and gonads were measured by quantitative real-time PCR (Step One Plus; Applied Biosystems). Total RNA was isolated from pituitary, liver, and gonads using Sepasol RNA I Super (Nacalai Tesque). To avoid contamination with genomic DNA, extracted total RNA was treated with 2 U of DNase (TURBO DNase-Free TM Kit; Ambion). cDNAs were produced with M-MLV transcriptase (Promega, 200 U/μL), and quantitative real-time RT-PCR was preformed with SYBR Premix Ex Taq TM II (TaKaRa). The primer sequences were as follows: GH reverse, 5′TTGGTGAAATCTGGTTCCTCA-3′; forward, 5′-AGCTCGGTCCTGAAGCTG-3′; IGF-I reverse, 5′-CCATAGCCTGTTGGTTTATTGAA-3′; forward, 5′-TCACTACTGCTGTGCGTCCTC-3′; Ef1α reverse, 5′-GATACCGCCAATCTTGTAGACG-3′; forward, 5′-TGAGCTGGTTCAAGGGATG-3′. Temperature conditions were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 15 s, and 72 °C for 30 s. The specificity of the amplified products was verified systematically by the amplification reaction. Standard curves were generated using serially diluted series prepared from the cDNA products. Tissue expression was normalized using Ef1α as an internal control. Quantitative values were calculated from the threshold PCR cycle number (Ct) at which the increase in signal associated with an exponential growth of PCR products was detected, and verified using a melting curve run after the PCR. Relative transcript quantification was done after dilution of the RT product, and a real-time PCR assay was conducted in the duplicate for each sample.
Statistical Analysis.
All results presented in this study are expressed as mean ± SEM. All values were analyzed by one-way ANOVA followed by a Bonferroni multicomparison test using KaleidaGraph statistical software (Synergy Software). Significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Prof. Graham Young, University of Washington, for critical reading and discussion and Prof. Tsuyoshi Ogasawara, Kanagawa University, for providing Mozambique tilapia. This work was supported by a grant from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118704109/-/DCSupplemental.
References
- 1.Fairbair D-J. Allometry for sexual size dimorphism: Pattern and process in the coevolution of body size in males and females. Annu Rev Ecol Syst. 1997;28:659–687. [Google Scholar]
- 2.Norman J-R. A Systematic Monograph of the Flatfishes (Heterosomata), 1: Psettodidae, Bothidae, Pleuronectidae. London: British Museum; 1934. [Google Scholar]
- 3.Kobelkowsky A. Sexual dimorphism of the flounder Bothus robinsi (Pisces: Bothidae) J Morphol. 2004;260:165–171. doi: 10.1002/jmor.10218. [DOI] [PubMed] [Google Scholar]
- 4.Lowe-McConnel R-H. Ecological Studies in Tropical Fish Communities. Cambridge, UK: Cambridge Univ Press; 1987. [Google Scholar]
- 5.Zakes Z, Demska-Zakes K. Effect of diets on growth and reproductive development of juvenile pikeperch, Stizostedion lucioperca (L.), reared under intensive culture conditions. Aquacult Res. 1996;27:841–845. [Google Scholar]
- 6.Gomez J-M, et al. Growth hormone (GH) and gonadotropin subunit gene expression and pituitary and plasma changes during spermatogenesis and oogenesis in rainbow trout (Oncorhynchus mykiss) Gen Comp Endocrinol. 1999;113:413–428. doi: 10.1006/gcen.1998.7222. [DOI] [PubMed] [Google Scholar]
- 7.Papadaki M, et al. Growth, sex differentiation, and gonadal and plasma levels of sex steroids in male- and female-dominant populations of Dicentrarchus labrax L. obtained through repeated size grading. J Fish Biol. 2005;66:938–956. [Google Scholar]
- 8.Campbell B, et al. Previtellogenic oocyte growth in salmon: Relationships among body growth, plasma insulin-like growth factor-1, estradiol-17beta, follicle-stimulating hormone and expression of ovarian genes for insulin-like growth factors, steroidogenic-acute regulatory protein and receptors for gonadotropins, growth hormone, and somatolactin. Biol Reprod. 2006;75:34–44. doi: 10.1095/biolreprod.105.049494. [DOI] [PubMed] [Google Scholar]
- 9.Charkraborty S-B, Mazumdar D, Chatterji U, Banerjee S. Growth of mixed-sex and monosex Nile tilapia in different culture systems. Turk J Fish Aquatic Sci. 2011;11:131–138. [Google Scholar]
- 10.Green B-W, Teichert-Coddington D-R. Growth of control and androgen-treated Nile tilapia, Oreochromis niloticus (L.), during treatment, nursery and grow-out phases in tropical fish ponds. Aquacult Res. 1994;25:613–621. [Google Scholar]
- 11.Cardenas R, et al. Estradiol reduces pituitary responsiveness to somatostatin (SRIF-14) and down-regulates the expression of somatostatin sst2 receptors in female goldfish pituitary. Gen Comp Endocrinol. 2003;132:119–124. doi: 10.1016/s0016-6480(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 12.Reinecke M, et al. Growth hormone and insulin-like growth factors in fish: Where we are and where to go. Gen Comp Endocrinol. 2005;142:20–24. doi: 10.1016/j.ygcen.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Untergasser G, et al. Organ-specific expression pattern of the human growth hormone/placental lactogen gene-cluster in the testis. Mol Cell Endocrinol. 1997;130:53–60. doi: 10.1016/s0303-7207(97)00073-7. [DOI] [PubMed] [Google Scholar]
- 14.Hull K-L, Harvey S. Growth hormone: Roles in female reproduction. J Endocrinol. 2001;168:1–23. doi: 10.1677/joe.0.1680001. [DOI] [PubMed] [Google Scholar]
- 15.Harvey S, et al. Testicular growth hormone (GH): GH expression in spermatogonia and primary spermatocytes. Gen Comp Endocrinol. 2004;139:158–167. doi: 10.1016/j.ygcen.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Moreno C-G, et al. Cellular and intracellular distribution of growth hormone in the adult chicken testis. Gen Comp Endocrinol. 2011;172:344–357. doi: 10.1016/j.ygcen.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Hrabia A, Sechman A, Gertler A, Rząsa J. Effect of growth hormone on steroid content, proliferation and apoptosis in the chicken ovary during sexual maturation. Cell Tissue Res. 2011;345:191–202. doi: 10.1007/s00441-011-1187-5. [DOI] [PubMed] [Google Scholar]
- 18.Ahumada-Solórzano S-M, et al. Local expression and distribution of growth hormone and growth hormone receptor in the chicken ovary: Effects of GH on steroidogenesis in cultured follicular granulosa cells. Gen Comp Endocrinol. 2012;175:297–310. doi: 10.1016/j.ygcen.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Reinecke M, Schmid A, Ermatinger R, Loffing-Cueni D. Insulin-like growth factor I in the teleost Oreochromis mossambicus, the tilapia: Gene sequence, tissue expression, and cellular localization. Endocrinology. 1997;138:3613–3619. doi: 10.1210/endo.138.9.5375. [DOI] [PubMed] [Google Scholar]
- 20.Melamed P, et al. Hypothalamic and thyroidal regulation of growth hormone in tilapia. Gen Comp Endocrinol. 1995;97:13–30. doi: 10.1006/gcen.1995.1002. [DOI] [PubMed] [Google Scholar]
- 21.Zou J-J, et al. Estradiol stimulates growth hormone production in female goldfish. Gen Comp Endocrinol. 1997;106:102–112. doi: 10.1006/gcen.1996.6857. [DOI] [PubMed] [Google Scholar]
- 22.Scully K-M, Rosenfeld M-G. Pituitary development: Regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- 23.Mingarro M, et al. Endocrine mediators of seasonal growth in gilthead sea bream (Sparus aurata): The growth hormone and somatolactin paradigm. Gen Comp Endocrinol. 2002;128:102–111. doi: 10.1016/s0016-6480(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Sánchez J, Le Bail P-Y. Growth hormone axis as marker of nutritional status and growth performance in fish. Aquaculture. 1999;177:117–128. [Google Scholar]
- 25.Le Gac F, et al. Growth hormone (GH) and reproduction: A review. Fish Physiol Biochem. 1993;11:219–232. doi: 10.1007/BF00004569. [DOI] [PubMed] [Google Scholar]
- 26.Harvey S, Hull K-L. Growth hormone: A paracrine growth factor? Endocrine. 1997;7:267–279. doi: 10.1007/BF02801319. [DOI] [PubMed] [Google Scholar]
- 27.Miura C, et al. Gh is produced by the testis of Japanese eel and stimulates proliferation of spermatogonia. Reproduction. 2011;142:869–877. doi: 10.1530/REP-11-0203. [DOI] [PubMed] [Google Scholar]
- 28.Van der Kraak G, Rosenblum P-M, Peter R-E. Growth hormone-dependent potentiation of gonadotropin-stimulated steroid production by ovarian follicles of the goldfish. Gen Comp Endocrinol. 1990;79:233–239. doi: 10.1016/0016-6480(90)90108-x. [DOI] [PubMed] [Google Scholar]
- 29.Fullar M-F. Sex differences in the nutrition and growth of pigs. In: Haresighn L, editor. Recent Advances in Animal Nutrition. Oxford, UK: Butterworth-Heinemann; 1980. pp. 169–174. [Google Scholar]
- 30.Newell J-A, Bowland J-P. Performance, carcass composition, and fat composition of boars, gilts, and barrows fed two levels of protein. Can J Anim Sci. 1972;52:543–551. [Google Scholar]
- 31.Cronin G-M, et al. The effects of immuno- and surgical-castration on the behaviour and consequently growth of group-housed, male finisher pigs. Appl Anim Behav Sci. 2003;81:111–126. [Google Scholar]
- 32.Riley L-G, Richman N-H, 3rd, Hirano T, Gordon Grau E. Activation of the growth hormone/insulin-like growth factor axis by treatment with 17 α-methyltestosterone and seawater rearing in the tilapia, Oreochromis mossambicus. Gen Comp Endocrinol. 2002;127:285–292. doi: 10.1016/s0016-6480(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 33.Hatikakoty G, Biswas S-P. Studies on certain aspects of the reproductive biology of mouth-brooding tilapia, Oreochromis mossambicus (Peters) from Assam, India. 2004. Available from: http://ag.arizona.edu/azaqua/ista/ista6/ista6web/pdf/112.pdf. Accessed September 16, 2004.
- 34.Yamada H, Satoh R, Yamashita T, Kambegawa A, Iwata M. Development of a time-resolved fluoroimmunoassay (TR-FIA) for testosterone: Measurement of serum testosterone concentrations after testosterone treatment in the rainbow trout (Oncorhynchus mykiss) Gen Comp Endocrinol. 1997;106:181–188. doi: 10.1006/gcen.1996.6861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.