Abstract
Infection with the protozoan parasite Toxoplasma gondii is characterized by asymptomatic latent infection in the central nervous system and skeletal muscle tissue in the majority of immunocompentent individuals. Life-threatening reactivation of the infection in immunocompromized patients originates from rupture of Toxoplasma cysts in the brain. While major progress has been made in our understanding of the immunopathogenesis of infection the mechanism(s) of neuroinvasion of the parasite remains poorly understood. The present review presents the current understanding of blood-brain barrier (patho)physiology and the interaction of Toxoplasma gondii with cells of the blood-brain barrier.
Keywords: Toxoplasma gondii, astrocytes, blood-brain barrier, cytokines, endothelial cells
The protozoan parasite Toxoplasma gondii can infect a variety of warm-blooded hosts including humans although the sexual life cycle only occurs in members of the feline family.1 The infection is mostly acquired through the oral route by ingestion of Toxoplasma tissue cysts or oocysts from undercooked or raw food or water.2-4 Within a short period of time the tachyzoite form of the parasite actively crosses the gastrointestinal barrier by penetrating enterocytic cells in the small intestine and entering submucosal tissue.5,6 Intracellular tachyzoites form a parasitophorous vacuole that ruptures following multiple cycles of replication. From there tachyzoites disseminate throughout the body and reach immunologically protected sites including brain, retina and fetus.7-9 In vitro studies revealed that tachyzoites can invade astrocytes, microglia and neurons of the mouse brain with subsequent formation of tissue cysts within these cells.10 Latent infection with T. gondii involves an elaborate interplay between the parasite and the host in which the parasite ensures its survival and proliferation but avoids fatal damage to the host at the same time.11 It has been hypothesized that during the latent phase of infection tissue cysts containing bradyzoites are controlled by the intact immune system, and only in the case of immune suppression, i.e., AIDS, bradyzoites released convert to tachyzoites and reactivated toxoplasmosis takes a lethal course if left untreated.12 Alternatively, cyst rupture and re-formation of cysts may be a constant process even in immunocompetent individuals, and the immune system’s role may be limited to the control of the tachyzoite form of the parasite.
After passage of the blood-brain barrier (BBB) bradyzoite-filled tissue cysts develop which are predominantly found in neuronal cells in the cerebral cortex, the hippocampus, basal ganglia, and amygdala.13,14 Latent infection is thought to be asymptomatic but latent infection has been associated with manipulation of the hosts’ behavior and development of mental disorders including depression and schizophrenia.15-18
While major progress has been made in our understanding of the interplay between the parasite and the host immune system our knowledge regarding the fascinating ability of the parasite to cross biological barriers, i.e., the BBB, remains surprisingly poor. Importantly, the most severe forms of the disease occur as a result of the parasite accessing sites protected by barriers, including congenital toxoplasmosis,19 retinochoroiditis20 and encephalitis in immunocompromised individuals.21 A detailed understanding of the mechanisms of BBB passage and establishment of latency in the brain however may allow to develop innovative strategies to prevent invasion of the central-nervous system by the parasite and subsequent disease. While the passage of biological barriers driven by the motility of the parasite has recently been reviewed,5 this review focuses on the interaction of the parasite with the BBB.
T. gondii Strain-Specific Differences in Virulence
Differences in susceptibility to infection with T. gondii of different hosts have been attributed primarily to the route of infection, host genetic background, and Toxoplasma strain type. The T. gondii population structure consists of three major clonal lineages (types I–III), which differ in their virulence and their geographical occurrence.22-24 As few as one parasite of a type I strain may cause lethal infection in mice but does not cause lethal infection in rats; type II and III strains are mildly virulent and establish latent or chronic-progressive infections in the mouse.25,26 In humans type II strains of T. gondii were found in about 80% of patient samples.27,28 Recent reports support the association of atypical strains of T. gondii with more severe disease presentation in humans.24 In this regard the development and recurrence of ocular toxoplasmosis appear to be dependent on the Toxoplasma genotype in patient cohorts in Europe (Shobab et al., manuscript in preparation) and the US (M. Grigg, personal communication).
The differences in virulence of T. gondii strains are mainly caused by the expression of polymorphic rhoptry (ROP) kinases, i.e., ROP16, ROP18 and the ROP5 pseudokinases that the parasite secretes into the host cell.29-34 ROP16 is a secreted protein kinase that leads to activation of the transcription factors STAT3 and STAT6 in host cells that in turn downregulate the host innate immune responses.32,35 Type II strains of T. gondii show a defect in the kinase ROP16 and therefore fail to suppress immune responses.36 The downregulation of STAT3 and STAT6 activation after type II strain infection enhances the hosts’ ability to mount a protective Th1 immune response characterized by the production of IL12 and effective control of parasite replication.37,38 Type I ROP18 inactivates host GTPases of the IRG family that accumulate on the parasitophorous vacuole membrane in infected cells and contribute to rupture of the vacuolar membrane.39,40 Recently, the host endoplasmic reticulum-bound transcription factor ATF6β was identified as the host cell target for ROP18. ROP18-induced degradation of ATF6β in dendritic cells resulted in defective CD8+-T cell defenses against the parasites.41
While the contribution of individual factors of virulence in the parasite and the host have been investigated in detail in mice as outlined above, limited information is available from other rodent species and in particularly from humans. Differences in the presence of absence of specific host factors (i.e., IRG family members, NO), in addition to the mostly asymptomatic nature of human infection are limiting the access to controlled human studies and add to the complexity in translating results from mouse models to the human patient.
Blood-Brain Barrier
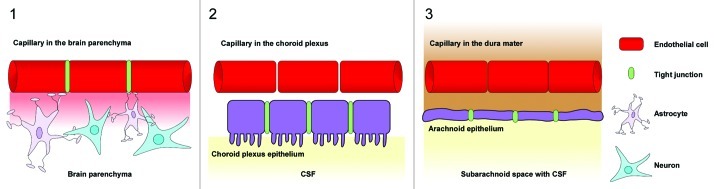
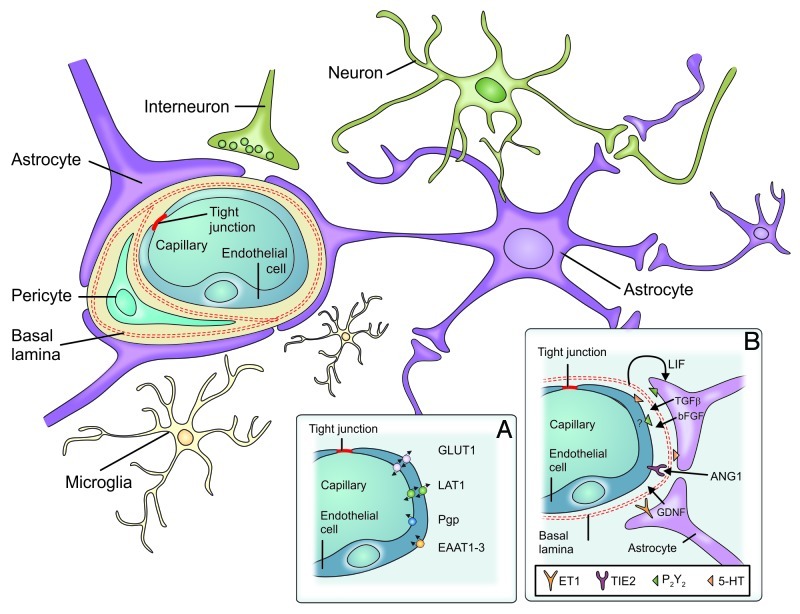
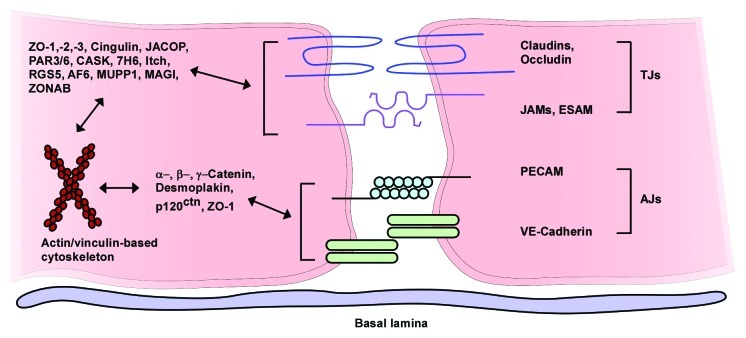
The central nervous system (CNS) contains three main barrier sites, the arachnoid epithelium, the epithelium of the choroid plexus, and the blood-brain barrier (Fig. 1).42 The arachnoid epithelium separates the subarachnoid cerebrospinal fluid from potentially harmful blood-born molecules that might pass from bloods vessels in the dura mater. Leukocytes or pathogens may also enter the CNS at the choroid plexus where the cerebrospinal fluid (CSF) is produced. But in contrary to the fenestrated blood capillaries of the choroid plexus the choroid plexus epithelium possesses tight junctions. The blood-CSF barriers protect the meningeal spaces as several meningitis eliciting pathogens including Neisseria meningitidis, Streptococcus pneumoniae or Streptococcus agalactiae use the blood-CSF interface as an entry site.43 The third barrier in the CNS is the blood-brain barrier (BBB). Highly specialized microvascular endothelial cells build a functional and structural barrier that is characterized by low permeability, low pinocytotic activity, lacking fenestrations, and high transendothelial resistance (Fig. 2). Surrounding cells like pericytes, microglia and astrocytes interact with endothelial cells of the BBB and support the maintenance of barrier functions through the release of soluble agents including transforming growth factor-β (TGFβ), glial-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF) and angiopoetin 1.42,44-50 Additionally, hydrocortisone and intracellular cAMP elevating agents like adrenomedullin decrease paracellular permeability and strengthen the endothelial barrier.46,48,51 In contrast to these factors impairment of BBB function may be caused by inflammation or CNS injuries mediated by cytokines (IL-1, IL-6, TNF), reactive oxygen species (ROS), nitric oxide (NO), vasoactive mediators (bradykinin, histamine, serotonin), phospholipase A2, arachidonic acid, prostaglandins or leukotrienes.42,46,52 Interendothelial tight junction and adherence junction proteins [members of the claudin family, occludin, junctional adhesion molecules (JAMs), platelet-endothelial cell adhesion molecule (PECAM) and vascular endothelial cadherin (VE-cadherin)] together with several intracellular components seal the spaces between endothelial cells and separate the apical from the basolateral site44,53-55 (Fig. 3). The presence of tight junctions and adherence junctions restricts the paracellular flux of hydrophilic molecules and prevents the migration of cells through the endothelial barrier. Nevertheless, there are several pathways for molecular trafficking through the BBB. The lipid membranes of the endothelium for example allow the diffusion of lipid-soluble agents, while specific receptors (insulin receptor, transferrin receptor) carry their ligands across the endothelium through receptor-mediated endocytosis and transcytosis.42 Transport proteins (including carriers for glucose, amino acids, purine bases and nucleosides) in turn provide the brain with nutrients and other substances while the transport protein P-glycoprotein acts as an efflux pump that can actively transport lipophilic drugs out of endothelial cells.42,56
Figure 1. Barrier sites in the CNS. The CNS contains three main barrier sites: (1) The blood-brain barrier which is formed by specialized brain capillary endothelial cells, (2) the barrier between the blood and the cerebrospinal fluid that exists at the choroid plexus epithelial cells and (3) the arachnoid epithelium presenting the middle layer of the meninges. While the endothelial cells of the BBB restrict the migration of potentially harmful blood-born agents to the central-nervous tissue, the choroid plexus epithelium and the arachnoid epithelium protect the cerebrospinal fluid. Tight junctions between endothelial and epithelial cells seal the intercellular spaces and minimize paracellular pathways.
Figure 2. Components of the blood-brain barrier. The blood-brain barrier consists of specialized capillary endothelial cells that are lined by the basal lamina, astrocytic endfeet, pericytes and microglial cells. (A) Among several other transporters and receptors brain endothelial cells express excitatory amino acid transporters (EAAT1–3), glucose transporter 1 (GLUT1), L-system for large neutral amino acids (LAT1) and P-glycoprotein (Pgp). (B) Surrounding cells intensely interact with endothelial cells and release soluble agents in order to support the maintenance of BBB functions [5-HT (5-hydroxytryptamine [serotonin]), angiopoetin 1 (ANG1), basic fibroblast growth factor (bFGF), endothelin 1 (ET1), glial cell line-derived neurotrophic factor (GDNF), leukemia inhibitory factor (LIF), purinergic receptor (P2Y2), transforming growth factor-β, endothelium-specific receptor tyrosine kinase 2 (TIE2)] (from ref. 42, with permission).
Figure 3. Assembly of endothelial tight junctions. Transmembranous molecules like claudins, occludin, junctional adhesion molecules (JAMs) and endothelial selective adhesion molecule (ESAM) are important tight junction components. On the cytoplasmic site these proteins are connected to adaptor and regulatory/signaling proteins [zonula occludens-1, -2 and -3 (ZO-1–3), cingulin, junction-associated coiled-coil protein (JACOP), the partitioning defective proteins 3 and 6 (PAR3/6), Ca2+-dependent serine protein kinase (CASK), tight junction-associated protein 7H6, Itch (E3 ubiquitin protein ligase), regulator of G-protein signaling 5 (RGS5), afadin (AF6), multi-PDZ-protein 1 (MUPP1), MAGI (membrane-associated guanylate kinase with inverted orientation of protein-protein interaction domains), ZO-1-associated nucleic acid-binding protein (ZONAB)], which link the membranous components to the actin/vinculin-based cytoskeleton. Vascular endothelial cadherin (VE-cadherin) and the platelet-endothelial cell adhesion molecule (PECAM) are components of endothelial adherens junctions and interact via homophilic bindings. Catenins, desmoplakin and p120 catenin (p120ctn) connect the adherence junction proteins with the cytoskeleton (modified from ref. 55, with permission).
In the course of inflammation circulating leukocytes leave the blood stream and migrate across the endothelial barrier into inflamed tissue. Transendothelial migration of leukocytes follows a defined sequence of adhesion and extravasation steps which result in the crossing of the endothelial barrier, the basal lamina and the extracellular matrix.57-59 Circulating leukocytes interact with endothelial cells mediated by members of the selectin family and their corresponding ligands on both endothelial cells and leukocytes. Endothelial cells express selectins such as E- and P- selectin, while P-selectin glycoprotein ligand 1 (PSGL-1) is one of the corresponding ligands on leukocytes.57,60,61 Chemokines on the luminal surface of activated endothelial cells induce changes in affinity and valency of leukocyte integrins. Activated integrins (VLA-4, LFA-1, Mac-1 and α4β7) then bind to endothelial adhesion molecules (VCAM-1, ICAM-1, ICAM-2 and MAdCAM-1) that function as integrin ligands and induce a stronger adherence to the endothelium.57,62 Leukocyte extravasation can involve a paracellular or a transcellular route, possibly due to variable cell signaling.57,63
The immunologically privileged state of the CNS paraphrases the fact that certain foreign antigens circumvent the systemic immunological recognition in order to avoid impairment of neuronal tissue by cytotoxic cells.64 Nevertheless, microglia, macrophages and other perivascularly located cells may circulate from the blood to the brain parenchyma to fulfill routine surveillance.65,66
Immunopathogenesis of Cerebral Toxoplasmosis
Immunosuppression of the host as in the case of immunosuppression caused by AIDS and transplantation may lead to the uncontrolled release of parasites during rupture of tissue cysts in the brain of latently infected individuals. Subsequently, released bradyzoites converting into rapidly proliferating tachyzoites may cause reactivated toxoplasmosis and lethal encephalitis if left untreated.12,13 In seropositive AIDS patients cerebral toxoplasmosis is among the most frequent CNS pathologies and as many as one third of all T. gondii-infected HIV-positive patients not treated with antiretroviral therapy may develop toxoplasmic encephalitis (TE).67,68 A CD4 T-cell count of < 200/µl renders a seropositive patient susceptible to reactivation and the onset of TE.69
As a primary response to infection with T. gondii, macrophages, granulocytes and dendritic cells secret proinflammatory cytokines, i.e., IL-12, the most important inducer of IFN-γ synthesis.70 A proper IFN-γ production in turn is inevitable for successful host resistance against infection with the parasite.71 Activated antigen-presenting cells together with IFN-γ support the proliferation of CD4+- and CD8+-T cells that are subsequently recruited to the brain.72 CD8+-T cells are essential in resistance due to their cytotoxic action as they lyse Toxoplasma infected cells during the active phase of infection.73 CD4+-T cells and astrocytes also contribute to resistance and activate CD8+-T cells by secretion of cytokines.74,75 During acute TE, monocytes, CD4+- and CD8+-T cells migrate into the CNS and activate resident microglia cells.76-78 Nevertheless, glial cell activation might be observed before parasite invasion of the CNS due to systemic levels of pro-inflammatory cytokines during acute infection.
The movement of infiltrating cells was associated with an infection-induced reticular system of fibers.79 Thus, the inflamed brain appears to induce specialized structures that guide the migration of T cells in this immuno-privileged environment whereas pre-existing scaffolds for guidance of lymphocyte migration exist in other tissues. Astrocytes and microglial cells become activated by IFN-γ and are major effector cells in the control of parasite replication.78 Upon infection, astroglia and microglia secrete IL-1, IL-6, GM-CSF or IL-10 and TNF, respectively.10 During TE a microglial upregulation of adhesion molecules like LFA-1 and Mac-1 was observed.80 As there is also a prominent upregulation of the cell adhesion molecule ICAM-1 on cerebral endothelia and choroid plexus epithelium during acute and chronic TE, this may support the infiltration of circulating leukocytes.80 Although the cell adhesion molecule VCAM-1 has been shown to mediate control of infection with T. gondii, other adhesion molecules may compensate for the leukocytic homing functions of VCAM-1.81 The production of IL-10 in T. gondii-infected brains favors parasite survival and therefore rather aids chronicity of the infection.82,83
While DCs cannot be detected in the brain parenchyma of healthy hosts, brains of chronically infected mice show a 50- to 100-fold expansion of DCs upon brain infection. The marked increase might be explained by the development of DCs from infiltrating blood monocytes, the recruitment of meningeal DCs, the proliferation and differentiation of perivascular macrophages or the development of brain DCs from intracerebral progenitors or resident microglia. These brain-derived DCs resemble a myeloid subset of mouse DCs and are related to macrophages/microglia.84 The recruitment of DCs to the CNS seems to be dependent on the signaling through multiple chemokine receptors and possible changes in the affinity of the leukocyte integrin LFA-1.85 Based on their strong expression of costimulatory molecules and the ability to process and present antigen to naive T cells in vitro brain DCs are supposed to be important inducers of T cell responses in TE.85 As brain DCs also show a high level of IL-12 production ex vivo they might be important for maintaining IFN-γ production by T cells in the brain.84 In low doses brain DCs induced significantly higher T cell proliferative responses compared with normal spleen DCs although this effect was reversed in experiments where higher doses of DCs were used.84 The presence of IFN-γ is an important requirement for the immune system to control acute and chronic infections with T. gondii. IFN-γ mediates a variety of host anti-Toxoplasma immune mechanisms. Along with neurons, astrocytes are the most frequent host cells harboring cysts in the brain.10,14,86 IFN-γ stimulation of astrocytes inhibits parasite growth through the production of reactive oxygen intermediates, the activation of indolamin-2,3-dioxygenase and the action of small GTPases.87-90 Small GTPases of the IRG family are found in mice but not in humans. Mice lacking nitric oxide (NO) production develop severe necrotizing lesions and uncontrolled tachyzoite replication in the CNS during chronic infection.91,92 Experiments with TNF-, lymphotoxin-α- and TNF/lymphotoxin-α-deficient mice revealed that TNF receptor type I-mediated immune reactions influence NO production and are crucial for the survival of mice with TE.93
It was shown in mice with TE that astrocytes are the main producers of the chemotactic cytokines IP-10 and MCP-1 while activated microglia and leukocytes infiltrating across the BBB also secrete chemokines.94 Chemokine secretion is in turn responsible for an enhanced neuroinvasion of leukocytes as astrocyte derived MCP-1 can mediate the migration of monocytes across an in vitro model of the BBB.95 In mice which lack the signal-transducing receptor gp130, astrocytes possess a crucial role in maintaining immunoregulatory functions during CNS infections with T. gondii by containing inflammatory lesions, supporting parasite control and preventing lethal necrotizing TE.96 Microglial cell activation is dependent on the interaction of CD200 on blood vessel endothelial cells and CD200R on microglia during infection with T. gondii.97
In summary, T. gondii interferes with multiple arms of the innate immune system to ensure an environment suitable for sustained parasite growth in the absence of severe pathology. T. gondii is remarkably able to control its own fate via modulation of many of the intricate pathways described above that the host uses to try to kill it.98
Neuroinvasion by Pathogenic Microorganisms
Several human pathogens gain entry to the CNS by crossing the endothelium of cerebral microvessels or by crossing the epithelium of the choroid plexus. Additionally, the uptake and transport of pathogens may occur via unprotected axon endings in the periphery or along olfactory neurons that allow pathogens to reach the CNS.99,100 Selected important pathogens are listed in Table 1.
Table 1. Selected important pathogens that cross the BBB.
| Bacteria | Viruses | Helminths | Protozoa | Fungi |
|---|---|---|---|---|
|
Neisseria meningitidis |
human immunodeficiency virus |
Schistosoma mansoni |
Toxoplasma gondii |
Candida albicans |
|
Escherichia coli |
tick-borne encephalitis virus |
Taenia solium |
Trypanosoma brucei |
Cryptococcus neoformans |
|
Streptococcus pneumoniae |
enteroviruses |
Echinococcus granulosus |
Entamoeba histolytica |
Aspergillus spp |
|
Listeria monocytogenes |
herpes viruses |
Toxocara canis |
Balamuthia mandrillaris |
|
|
Mycobacterium tuberculosis |
rabies virus |
Trichinella spiralis |
(Plasmodium falciparum)* |
|
|
Treponema pallidum |
JC virus |
|
|
|
|
Bacillus anthracis |
West Nile virus |
|
|
|
|
Staphylococcus aureus |
measles virus |
|
|
|
| Borrelia burgdorferi |
P. falciparum does not cross the BBB and remains physiologically outside the BBB but symptoms are present in the CNS
Whereas extracellular bacteria typically cause severe acute meningitis (i.e., S. pneumoniae and N. meningitidis), encephalitis is often less severe and typically caused by intracellular pathogens (i.e., viruses and protozoa). Another group of organisms causes brain abscesses (i.e., E. histolytica, Candida spp and Aspergillus spp). It is tempting to speculate that the route of entry and the immune response elicited by these pathogens impacts the clinical outcome of CNS disease.
Beside the anatomical localization of CNS entry, the mechanisms of neuroinvasion differ among pathogens. Crossing the cells of the blood-brain barrier can occur paracellularly, transcellularly or inside infected leukocytes (Trojan horse mechanism). In paracellular migration, pathogens must pass the tight junction proteins connecting neighboring cells while transcellular migration is characterized by uptake of microorganisms by endothelial cells of the BBB or direct infection by pathogens to invade the CNS.99 In vitro experiments with Trypanosoma brucei101 and Borrelia burgdorferi102 point to a paracellular migration across cells in a human BBB model while Cryptococcus neoformans,103 Candida albicans,104 Escherichia coli,105 S. pneumoniae106 and West Nile virus107 cross the endothelial barrier in a transcellular way. For N. meningitidis both ways of migration seem to be possible.108,109
In addition to paracellular and transcellular neuroinvasion there exists a way of intracellular pathogen trafficking in which host leukocytes are used as vehicles for transport purposes. Infection with certain pathogens subverts host signaling cascades and affects proinflammatory responses of infected host cells.38,110,111 Host cells may present a save shelter for intracellular pathogens. Through intracellular migration the pathogen can evade microbicidal effectors and clearance by the host immune response, while the downregulation of proinflammatory responses in the host supports survival of host and pathogen.112-114 In case of Listeria monocytogenes several ways of bacterial migration have been described. Ly6C-positive monocyte populations are exploited by L. monocytogenes as “Trojan horses” for their passage across the BBB; in addition, an axonal uptake and transport of bacteria via the trigeminal nerve and transcellular ways of migration through an endothelial barrier have been shown.115-118 The infection of endothelial BBB cells with human immunodeficiency virus in turn impairs BBB functions and facilitates the migration of potentially infected monocytes into the brain.119,120
Dissemination and Neuroinvasion of T. gondii
Reactivated toxoplasmosis in mice is typically localized in the frontal and parietal cortex, and sites of reactivation are found perivascularly thereby supporting the idea of parasite dissemination from the bloodstream into the CNS.13 Tachyzoites of T. gondii are sensitive to several arms of the humoral immune responses of the host. First, sera containing antibodies directed against Toxoplasma mediate parasite lysis by activation of the complement system while T. gondii-specific IgM blocks host cell invasion by tachyzoites. For this reason the intracellular habitat of the parasite poses important survival and dissemination advantages.121-123
Parasite- and host cell-specific factors of invasion and dissemination
After the ingestion of cyst-containing material the epithelium of the small intestine is the first cellular barrier the parasite crosses before disseminating in the host. Extracellular tachyzoites were shown to actively overcome cell monolayers and migrate into deeper cell layers.5,124 As T. gondii lacks cilia or flagella it relies on a type of migration called gliding motility.125,126 The invasion of host cells is an active process and no contribution of the host cell is required. The penetration itself is mediated by the secretion of distinct parasite proteins into the host cells.127,128 Upon infection micronemal proteins (MICs) are exocytosed from the apical site of the parasite and are secreted into the host cell.129 A micronemal protein called MIC2 is displayed on the apical surface of the parasite and appears to bind the host cell adhesion molecule ICAM-1. Before paracellular migration across cell layers parasites seem to localize near intercellular junctions while maintaining cell barrier integrity.128,130 Toxoplasma infection can induce modulations in the expression of different cell adhesion molecules and cytokines in host cells. Upon infection with T. gondii tachyzoites, brain endothelial cells upregulate ICAM-1 expression as soon as 2 h post-infection while the secretion of the chemokine MCP-1 occurrs by 12 h post-infection.131 The upregulation of CD44 and ICAM-1 on the surface of T. gondii-infected human monocytic cells leads to a better adherence of infected cells to immobilized hyaluron compared with uninfected cells. Thereby T. gondii-infected monocytes of the circulating blood might improve their capacities to bind to extracellular matrix and favor their extravasation into deeper tissues.132 When monolayers of human HeLa epithelial cells and fibroblasts were infected with T. gondii an increase in the secretion of the proinflammatory and chemoattractant cytokines IL-8, GRO-α and MCP-1 was observed.133 Human retinal pigment epithelial cells respond to infection with T. gondii with the secretion of IL-1, IL-6, GM-CSF and ICAM-1 while infected BUVEC cells upregulate the expression of the chemokines GRO-α, IL-8, IP-10, MCP-1, RANTES and GM-CSF.134,135
Thus, proinflammatory chemokine secretion and upregulation of adhesion molecules or immunomodulatory molecules appears to be an important host cell response to infection with T. gondii and could favor the entry of Toxoplasma infected leukocytes into the CNS.
Intracellular vs. extracellular invasion of the CNS
Courret et al.6 showed that during the early phase of infection and dissemination T. gondii tachyzoites are frequently associated with CD11c+ and CD11b+ leukocytes of the lamina propria, Peyer’s patches, and lymph nodes. A few days later blood leukocytes harboring T. gondii are mainly CD11c−/CD11b+. Seven days after intragastrical delivery of Toxoplasma cysts into mice an increased number of CD11b+ and CD11c+ cells could be detected in the brain so that these two leukocyte subsets accounted for about 60% of the total cells that had migrated from the blood to the brain by this time point. Fifteen days after oral infection parasites were found in the brain in both CD11c+/CD11b+/− and CD11c−/CD11b+ leukocyte populations. In other experiments it was shown that in the brains of 7- or 9-d parasitized mice the majority of parasites could be found in leukocytes that must have infiltrated the brain earlier before.6 We used an in vitro co-culture model of the BBB to investigate the migratory capacities of infected and uninfected antigen presenting cells. Blood mononuclear cells from rats were infected with T. gondii tachyzoites. After migration through an in vitro model of the BBB the percentage of infected CD45+/CD11bc+ cells in the migrated fraction was 13-fold higher compared with the percentage in the starting population. Also, infection rates in the migrated CD45+/CD11bc+ fraction showed a 5-fold increase compared with the same population before migration; infection rates of infected CD45+/CD11bc+ cells were 18-fold higher than those of CD45+/CD11bc− cells. Interestingly, not only infected but also uninfected CD45+/CD11bc+ cells showed enhanced migratory capacities through the BBB model.131 CD11b+/CD11c+ mouse dendritic cells were preferably infected among all CD11b+, CD11c+ and double positive cells. Nevertheless, infected CD11b+/CD11c− cells—most likely monocytic cells—dominated the infected antigen presenting cell population after migration through an in vitro BBB model. Interestingly, CD11b+/CD11c− cells showed an increased overall migration potential compared with other PBMC subpopulations.131
Parasite-Dependent Effects on Host Cell Motility and Parasite Dissemination
Interestingly type I and type II strains differ in their ability to recruit cells to the site of infections. Type I strains preferentially attract neutrophils (Gr-1+/CD68−) while type II strains are more often associated with activated macrophages (CD68+/Gr-1+).136 T. gondii also seems to alter host cell motility to promote its own dissemination. DCs were challenged with live parasites and allowed to migrate in a transwell system. In response to active invasion by T. gondii tachyzoites, dendritic cells adopted a state of hypermotility that enabled them to cross biological barriers at higher frequencies than uninfected DCs. According to the dose dependent modulation of cell motility the absolute number of migrating cells also increased with higher MOI.137 While infected macrophages increased their migration rate 6–20-fold, other leukocytes did not show a modified migratory phenotype upon infection.138 The subversion of host cell properties is furthermore a genotype specific matter as infections with type II strains lead to higher host cell migration rates than infections with type I or type III strains do.139 Type I strain tachyzoites in turn are capable of migrating longer distances on host-cell monolayers as extracellular parasites.5 This strain dependent characteristic is possibly one feature that allows tachyzoites of the type I strain a quicker proliferation in the infected host and thereby mediates the pronounced virulence in mice.140 Despite the fact that type II strains only show a moderate expansion and less pronounced extracellular migration type II tachyzoites are nevertheless successful in establishing latent infections; thus, these parasite may exploit alternative (intracellular) habitats for effective dissemination.121 Although both strains show bias to infection of CD11c+ compared with CD11c− cells, type II strains appear more often in intracellular compartments than type I strains do. When mice were co-inoculated with free type I and type II tachyzoites this resulted in a strong domination of type I parasites referring to the total parasite load in the spleen. While in case of type I strain parasites were found mainly extracellular the type II population showed an even distribution between intracellular and extracellular compartments.139 Unno et al. used green and red fluorescent parasites of the type II PLK strain to establish an intracellular and an extracellular form of the parasite. Following injection into mice at the same time the majority of tachyzoites found in tissues corresponded to the tachyzoite form that had been administered inside host cells. The dissemination of intracellularly located parasites appeared to outperform the capacities of extracellular parasites in reaching peripheral sites of the host and thereby mediated a more widespread distribution.141
In conclusion, monocytes and dendritic cells are the most important candidates for the transport of T. gondii from the periphery to the immunologically privileged sites of the brain. Intracellular neuroinvasion represents the starting point for establishment of latent infection characterized by cyst formation.
Genetic Tools and In Vivo Imaging to Study the Immunopathogenesis of Infection with T. gondii
While the key host molecules involved in the immune response to T. gondii have been identified and analyzed with the help of gene knockout mice, knowledge on critical parasite molecules that specifically modulate the host’s immune system is still embryonic. This might be in part because of a lack of communication between researchers focusing on basic parasite cell biology, who perform most of their assays in vitro and researchers, who work in vivo, focusing on the host’s immune response.
In recent years the adaptation of several reverse and forward genetic tools established T. gondii as an attractive model system for apicomplexan parasites and general molecular biology.142 However, these techniques are not frequently employed for the study of host-pathogen interactions and most of the times, the only in vivo experiments performed to study the function of a gene of interest are virulence studies (survival experiments), without a detailed analysis of the immune response.143,144 Only in some cases knockout parasites have been analyzed in more detail to study for example tissue dissemination.145 Parasite mutants with specific effects during their asexual life cycle could be useful tools to study the immunopathogenesis, i.e., if combined with in vivo imaging. Over the years several conditional parasite mutants have been generated with specific defects during invasion,143-147 replication148,149 or egress;150,151 in most cases the underlying mechanism has been well described. While these mutants allow the dissection of the respective molecular pathway in vitro, they can also be used for studies of immunopathogenesis in vivo.
Various imaging techniques, including bioluminescent imaging, confocal and multiphoton microscopy have been applied to study the immunopathogenesis of infection with T. gondii. The ability to manipulate parasites to express fluorescent/bioluminescent markers or model antigens/enzymes combined with the development of reporter mice that allow the detection of distinct immune populations have been crucial to the success of many of these studies. These approaches have permitted the visualization of parasites and immune cells in real-time and provided new insights into the nature of host-pathogen interactions. In this regard, fluorescent tagged parasites and host cells allowed dynamic imaging of T. gondii interactions with—among others—T cells, neutrophils and astrocytes.79,152-154 The use of the combinations of conditional knockout parasites with in vivo imaging will certainly increase to give novel insights into host-parasite interactions.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/19004
References
- 1.Dubey JP, Miller NL, Frenkel JK. Toxoplasma gondii life cycle in cats. J Am Vet Med Assoc. 1970;157:1767–70. [PubMed] [Google Scholar]
- 2.Tenter AM. Toxoplasma gondii in animals used for human consumption. Mem Inst Oswaldo Cruz. 2009;104:364–9. doi: 10.1590/S0074-02762009000200033. [DOI] [PubMed] [Google Scholar]
- 3.Jones JL, Dubey JP. Waterborne toxoplasmosis–recent developments. Exp Parasitol. 2010;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Dabritz HA, Miller MA, Atwill ER, Gardner IA, Leutenegger CM, Melli AC, et al. Detection of Toxoplasma gondii-like oocysts in cat feces and estimates of the environmental oocyst burden. J Am Vet Med Assoc. 2007;231:1676–84. doi: 10.2460/javma.231.11.1676. [DOI] [PubMed] [Google Scholar]
- 5.Barragan A, Sibley LD. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 2003;11:426–30. doi: 10.1016/S0966-842X(03)00205-1. [DOI] [PubMed] [Google Scholar]
- 6.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–16. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter CA, Remington JS. Immunopathogenesis of toxoplasmic encephalitis. J Infect Dis. 1994;170:1057–67. doi: 10.1093/infdis/170.5.1057. [DOI] [PubMed] [Google Scholar]
- 8.Pavesio CE, Lightman S. Toxoplasma gondii and ocular toxoplasmosis: pathogenesis. Br J Ophthalmol. 1996;80:1099–107. doi: 10.1136/bjo.80.12.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008;47:554–66. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 10.Fischer HG, Nitzgen B, Reichmann G, Gross U, Hadding U. Host cells of Toxoplasma gondii encystation in infected primary culture from mouse brain. Parasitol Res. 1997;83:637–41. doi: 10.1007/s004360050311. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33:745–51. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 13.Dellacasa-Lindberg I, Hitziger N, Barragan A. Localized recrudescence of Toxoplasma infections in the central nervous system of immunocompromised mice assessed by in vivo bioluminescence imaging. Microbes Infect. 2007;9:1291–8. doi: 10.1016/j.micinf.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Melzer TC, Cranston HJ, Weiss LM, Halonen SK. Host cell preference of Toxoplasma gondii cysts in murine brain: A confocal study. J Neuroparasitology 2010; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33:757–60. doi: 10.1093/schbul/sbl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocazeybek B, Oner YA, Turksoy R, Babur C, Cakan H, Sahip N, et al. Higher prevalence of toxoplasmosis in victims of traffic accidents suggest increased risk of traffic accident in Toxoplasma-infected inhabitants of Istanbul and its suburbs. Forensic Sci Int. 2009;187:103–8. doi: 10.1016/j.forsciint.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Kar N, Misra B. Toxoplasma seropositivity and depression: a case report. BMC Psychiatry. 2004;4:1. doi: 10.1186/1471-244X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg Infect Dis. 2003;9:1375–80. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kravetz JD, Federman DG. Toxoplasmosis in pregnancy. Am J Med. 2005;118:212–6. doi: 10.1016/j.amjmed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Klaren VN, Kijlstra A. Toxoplasmosis, an overview with emphasis on ocular involvement. Ocul Immunol Inflamm. 2002;10:1–26. doi: 10.1076/ocii.10.1.1.10330. [DOI] [PubMed] [Google Scholar]
- 21.Luft BJ, Hafner R, Korzun AH, Leport C, Antoniskis D, Bosler EM, et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N Engl J Med. 1993;329:995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- 22.Saeij JP, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–81. doi: 10.1016/j.pt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP. Globalization and the population structure of Toxoplasma gondii. Proc Natl Acad Sci USA. 2006;103:11423–8. doi: 10.1073/pnas.0601438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dardé ML. Toxoplasma gondii, “new” genotypes and virulence. Parasite. 2008;15:366–71. doi: 10.1051/parasite/2008153366. [DOI] [PubMed] [Google Scholar]
- 25.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–6. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 26.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–42. doi: 10.1016/S1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 27.Howe DK, Honore S, Derouin F, Sibley LD. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol. 1997;35:1411–4. doi: 10.1128/jcm.35.6.1411-1414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honoré S, Couvelard A, Garin YJ, Bedel C, Henin D, Darde ML, et al. [Genotyping of Toxoplasma gondii strains from immunocompromised patients] Pathol Biol (Paris) 2000;48:541–7. [PubMed] [Google Scholar]
- 29.Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6:79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 30.Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol. 2007;10:582–7. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinfeldt T, Konen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, et al. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010;8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–3. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–7. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci USA. 2011;108:9625–30. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong YC, Reese ML, Boothroyd JC. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem. 2010;285:28731–40. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, et al. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med. 2009;206:2747–60. doi: 10.1084/jem.20091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172:3686–94. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 38.Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J Immunol. 2005;174:3148–52. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- 39.Hunn JP, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, et al. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J. 2008;27:2495–509. doi: 10.1038/emboj.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, et al. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E, et al. ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. 2011;208:1533–46. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 43.Join-Lambert O, Morand PC, Carbonnelle E, Coureuil M, Bille E, Bourdoulous S, et al. Mechanisms of meningeal invasion by a bacterial extracellular pathogen, the example of Neisseria meningitidis. Prog Neurobiol. 2010;91:130–9. doi: 10.1016/j.pneurobio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 46.Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kis B, Snipes JA, Deli MA, Abraham CS, Yamashita H, Ueta Y, et al. Chronic adrenomedullin treatment improves blood-brain barrier function but has no effects on expression of tight junction proteins. Acta Neurochir Suppl. 2003;86:565–8. doi: 10.1007/978-3-7091-0651-8_115. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–63. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–15. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 51.Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, et al. Hydrocortisone reinforces the blood-brain barrier properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;244:312–6. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- 52.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 53.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 54.Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolburg H. Blood-Brain Barriers - From Ontogeny to Artificial Interfaces. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2006. [Google Scholar]
- 56.Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 58.Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82:521–33. doi: 10.1038/labinvest.3780446. [DOI] [PubMed] [Google Scholar]
- 59.Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–39. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87. [PubMed] [Google Scholar]
- 61.Zarbock A, Muller H, Kuwano Y, Ley K. PSGL-1-dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86:1119–24. doi: 10.1189/jlb.0209117. [DOI] [PubMed] [Google Scholar]
- 62.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 63.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–97. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–8. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Kleine TO, Benes L. Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A. 2006;69:147–51. doi: 10.1002/cyto.a.20225. [DOI] [PubMed] [Google Scholar]
- 66.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–24. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 67.Mamidi A, DeSimone JA, Pomerantz RJ. Central nervous system infections in individuals with HIV-1 infection. J Neurovirol. 2002;8:158–67. doi: 10.1080/13550280290049723. [DOI] [PubMed] [Google Scholar]
- 68.Grant IH, Gold JW, Rosenblum M, Niedzwiecki D, Armstrong D. Toxoplasma gondii serology in HIV-infected patients: the development of central nervous system toxoplasmosis in AIDS. AIDS. 1990;4:519–21. doi: 10.1097/00002030-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Nascimento LV, Stollar F, Tavares LB, Cavasini CE, Maia IL, Cordeiro JA, et al. Risk factors for toxoplasmic encephalitis in HIV-infected patients: a case-control study in Brazil. Ann Trop Med Parasitol. 2001;95:587–93. doi: 10.1080/00034980120073931. [DOI] [PubMed] [Google Scholar]
- 70.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, et al. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–43. [PubMed] [Google Scholar]
- 71.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen TA, et al. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun. 1997;65:2339–45. doi: 10.1128/iai.65.6.2339-2345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filisetti D, Candolfi E. Immune response to Toxoplasma gondii. Ann Ist Super Sanita. 2004;40:71–80. [PubMed] [Google Scholar]
- 73.Subauste CS, Koniaris AH, Remington JS. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J Immunol. 1991;147:3955–9. [PubMed] [Google Scholar]
- 74.Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunol Rev. 2011;240:269–85. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 75.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–88. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlüter D, Lohler J, Deckert M, Hof H, Schwendemann G. Toxoplasma encephalitis of immunocompetent and nude mice: immunohistochemical characterisation of Toxoplasma antigen, infiltrates and major histocompatibility complex gene products. J Neuroimmunol. 1991;31:185–98. doi: 10.1016/0165-5728(91)90040-E. [DOI] [PubMed] [Google Scholar]
- 77.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–80. [PubMed] [Google Scholar]
- 78.Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002;185(Suppl 1):S58–65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- 79.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–11. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deckert-Schlüter M, Schluter D, Hof H, Wiestler OD, Lassmann H. Differential expression of ICAM-1, VCAM-1 and their ligands LFA-1, Mac-1, CD43, VLA-4, and MHC class II antigens in murine Toxoplasma encephalitis: a light microscopic and ultrastructural immunohistochemical study. J Neuropathol Exp Neurol. 1994;53:457–68. doi: 10.1097/00005072-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Deckert M, Lutjen S, Leuker CE, Kwok LY, Strack A, Muller W, et al. Mice with neonatally induced inactivation of the vascular cell adhesion molecule-1 fail to control the parasite in Toxoplasma encephalitis. Eur J Immunol. 2003;33:1418–28. doi: 10.1002/eji.200322826. [DOI] [PubMed] [Google Scholar]
- 82.Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol. 2005;5:162–70. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 83.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 84.Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–34. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 85.John B, Ricart B, Tait Wojno ED, Harris TH, Randall LM, Christian DA, et al. Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog. 2011;7:e1002246. doi: 10.1371/journal.ppat.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halonen SK, Lyman WD, Chiu FC. Growth and development of Toxoplasma gondii in human neurons and astrocytes. J Neuropathol Exp Neurol. 1996;55:1150–6. doi: 10.1097/00005072-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Peterson PK, Gekker G, Hu S, Chao CC. Human astrocytes inhibit intracellular multiplication of Toxoplasma gondii by a nitric oxide-mediated mechanism. J Infect Dis. 1995;171:516–8. doi: 10.1093/infdis/171.2.516. [DOI] [PubMed] [Google Scholar]
- 88.Oberdörfer C, Adams O, MacKenzie CR, De Groot CJ, Daubener W. Role of IDO activation in anti-microbial defense in human native astrocytes. Adv Exp Med Biol. 2003;527:15–26. doi: 10.1007/978-1-4615-0135-0_2. [DOI] [PubMed] [Google Scholar]
- 89.Melzer T, Duffy A, Weiss LM, Halonen SK. The gamma interferon (IFN-gamma)-inducible GTP-binding protein IGTP is necessary for toxoplasma vacuolar disruption and induces parasite egression in IFN-gamma-stimulated astrocytes. Infect Immun. 2008;76:4883–94. doi: 10.1128/IAI.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liesenfeld O, Parvanova I, Zerrahn J, Han SJ, Heinrich F, Munoz M, et al. The IFN-gamma-inducible GTPase, Irga6, protects mice against Toxoplasma gondii but not against Plasmodium berghei and some other intracellular pathogens. PLoS ONE. 2011;6:e20568. doi: 10.1371/journal.pone.0020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–73. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khan IA, Schwartzman JD, Matsuura T, Kasper LH. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci USA. 1997;94:13955–60. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schlüter D, Kwok LY, Lutjen S, Soltek S, Hoffmann S, Korner H, et al. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol. 2003;170:6172–82. doi: 10.4049/jimmunol.170.12.6172. [DOI] [PubMed] [Google Scholar]
- 94.Strack A, Asensio VC, Campbell IL, Schluter D, Deckert M. Chemokines are differentially expressed by astrocytes, microglia and inflammatory leukocytes in Toxoplasma encephalitis and critically regulated by interferon-gamma. Acta Neuropathol. 2002;103:458–68. doi: 10.1007/s00401-001-0491-7. [DOI] [PubMed] [Google Scholar]
- 95.Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–903. [PubMed] [Google Scholar]
- 96.Drögemüller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, et al. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol. 2008;181:2683–93. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- 97.Deckert M, Sedgwick JD, Fischer E, Schluter D. Regulation of microglial cell responses in murine Toxoplasma encephalitis by CD200/CD200 receptor interaction. Acta Neuropathol. 2006;111:548–58. doi: 10.1007/s00401-006-0062-z. [DOI] [PubMed] [Google Scholar]
- 98.Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 2009;39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol. 2008;6:625–34. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kristensson K. Microbes' roadmap to neurons. Nat Rev Neurosci. 2011;12:345–57. doi: 10.1038/nrn3029. [DOI] [PubMed] [Google Scholar]
- 101.Grab DJ, Nikolskaia O, Kim YV, Lonsdale-Eccles JD, Ito S, Hara T, et al. African trypanosome interactions with an in vitro model of the human blood-brain barrier. J Parasitol. 2004;90:970–9. doi: 10.1645/GE-287R. [DOI] [PubMed] [Google Scholar]
- 102.Grab DJ, Perides G, Dumler JS, Kim KJ, Park J, Kim YV, et al. Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier. Infect Immun. 2005;73:1014–22. doi: 10.1128/IAI.73.2.1014-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 2004;72:4985–95. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jong AY, Stins MF, Huang SH, Chen SH, Kim KS. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect Immun. 2001;69:4536–44. doi: 10.1128/IAI.69.7.4536-4544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim KS. Strategy of Escherichia coli for crossing the blood-brain barrier. J Infect Dis. 2002;186(Suppl 2):S220–4. doi: 10.1086/344284. [DOI] [PubMed] [Google Scholar]
- 106.Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102:347–60. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, et al. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier. Virology. 2009;385:425–33. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nikulin J, Panzner U, Frosch M, Schubert-Unkmeir A. Intracellular survival and replication of Neisseria meningitidis in human brain microvascular endothelial cells. Int J Med Microbiol. 2006;296:553–8. doi: 10.1016/j.ijmm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–7. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butcher BA, Kim L, Johnson PF, Denkers EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J Immunol. 2001;167:2193–201. doi: 10.4049/jimmunol.167.4.2193. [DOI] [PubMed] [Google Scholar]
- 111.Denkers EY, Kim L, Butcher BA. In the belly of the beast: subversion of macrophage proinflammatory signalling cascades during Toxoplasma gondii infection. Cell Microbiol. 2003;5:75–83. doi: 10.1046/j.1462-5822.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 112.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol. 2002;3:1041–7. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 113.Drevets DA, Jelinek TA, Freitag NE. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect Immun. 2001;69:1344–50. doi: 10.1128/IAI.69.3.1344-1350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Persson CM, Lambert H, Vutova PP, Dellacasa-Lindberg I, Nederby J, Yagita H, et al. Transmission of Toxoplasma gondii from infected dendritic cells to natural killer cells. Infect Immun. 2009;77:970–6. doi: 10.1128/IAI.00833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drevets DA, Bronze MS. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol. 2008;53:151–65. doi: 10.1111/j.1574-695X.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 116.Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, Sunderkotter C, et al. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004;172:4418–24. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- 117.Greiffenberg L, Goebel W, Kim KS, Weiglein I, Bubert A, Engelbrecht F, et al. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–7. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Join-Lambert OF, Ezine S, Le Monnier A, Jaubert F, Okabe M, Berche P, et al. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell Microbiol. 2005;7:167–80. doi: 10.1111/j.1462-5822.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- 119.Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–34. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, et al. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–99. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lambert H, Barragan A. Modelling parasite dissemination: host cell subversion and immune evasion by Toxoplasma gondii. Cell Microbiol. 2010;12:292–300. doi: 10.1111/j.1462-5822.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 122.Fuhrman SA, Joiner KA. Toxoplasma gondii: mechanism of resistance to complement-mediated killing. J Immunol. 1989;142:940–7. [PubMed] [Google Scholar]
- 123.Couper KN, Roberts CW, Brombacher F, Alexander J, Johnson LL. Toxoplasma gondii-specific immunoglobulin M limits parasite dissemination by preventing host cell invasion. Infect Immun. 2005;73:8060–8. doi: 10.1128/IAI.73.12.8060-8068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002;195:1625–33. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soldati D, Meissner M. Toxoplasma as a novel system for motility. Curr Opin Cell Biol. 2004;16:32–40. doi: 10.1016/j.ceb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 126.Wetzel DM, Hakansson S, Hu K, Roos D, Sibley LD. Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol Biol Cell. 2003;14:396–406. doi: 10.1091/mbc.E02-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morisaki JH, Heuser JE, Sibley LD. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995;108:2457–64. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- 128.Carruthers VB, Giddings OK, Sibley LD. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell Microbiol. 1999;1:225–35. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 129.Dowse T, Soldati D. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr Opin Microbiol. 2004;7:388–96. doi: 10.1016/j.mib.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 130.Barragan A, Brossier F, Sibley LD. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005;7:561–8. doi: 10.1111/j.1462-5822.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- 131.Lachenmaier SM, Deli MA, Meissner M, Liesenfeld O. Intracellular transport of Toxoplasma gondii through the blood-brain barrier. J Neuroimmunol. 2011;232:119–30. doi: 10.1016/j.jneuroim.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Unno A, Kitoh K, Takashima Y. Up-regulation of hyaluronan receptors in Toxoplasma gondii-infected monocytic cells. Biochem Biophys Res Commun. 2010;391:477–80. doi: 10.1016/j.bbrc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 133.Denney CF, Eckmann L, Reed SL. Chemokine secretion of human cells in response to Toxoplasma gondii infection. Infect Immun. 1999;67:1547–52. doi: 10.1128/iai.67.4.1547-1552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nagineni CN, Detrick B, Hooks JJ. Toxoplasma gondii infection induces gene expression and secretion of interleukin 1 (IL-1), IL-6, granulocyte-macrophage colony-stimulating factor, and intercellular adhesion molecule 1 by human retinal pigment epithelial cells. Infect Immun. 2000;68:407–10. doi: 10.1128/IAI.68.1.407-410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taubert A, Zahner H, Hermosilla C. Dynamics of transcription of immunomodulatory genes in endothelial cells infected with different coccidian parasites. Vet Parasitol. 2006;142:214–22. doi: 10.1016/j.vetpar.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 136.Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J Leukoc Biol. 2003;74:1015–25. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 137.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8:1611–23. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 138.Lambert H, Dellacasa-Lindberg I, Barragan A. Migratory responses of leukocytes infected with Toxoplasma gondii. Microbes Infect. 2011;13:96–102. doi: 10.1016/j.micinf.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 139.Lambert H, Vutova PP, Adams WC, Lore K, Barragan A. The Toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype. Infect Immun. 2009;77:1679–88. doi: 10.1128/IAI.01289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hitziger N, Dellacasa I, Albiger B, Barragan A. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell Microbiol. 2005;7:837–48. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 141.Unno A, Suzuki K, Xuan X, Nishikawa Y, Kitoh K, Takashima Y. Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow. Parasitol Int. 2008;57:515–8. doi: 10.1016/j.parint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 142.Meissner M, Klaus K. What new cell biology findings could bring to therapeutics: is it time for a phenome-project in Toxoplasma gondii? Mem Inst Oswaldo Cruz. 2009;104:185–9. doi: 10.1590/S0074-02762009000200010. [DOI] [PubMed] [Google Scholar]
- 143.Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–40. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- 144.Mital J, Meissner M, Soldati D, Ward GE. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol Biol Cell. 2005;16:4341–9. doi: 10.1091/mbc.E05-04-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huynh MH, Carruthers VB. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2006;2:e84. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kessler H, Herm-Gotz A, Hegge S, Rauch M, Soldati-Favre D, Frischknecht F, et al. Microneme protein 8–a new essential invasion factor in Toxoplasma gondii. J Cell Sci. 2008;121:947–56. doi: 10.1242/jcs.022350. [DOI] [PubMed] [Google Scholar]
- 147.Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–62. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Agop-Nersesian C, Egarter S, Langsley G, Foth BJ, Ferguson DJ, Meissner M. Biogenesis of the inner membrane complex is dependent on vesicular transport by the alveolate specific GTPase Rab11B. PLoS Pathog. 2010;6:e1001029. doi: 10.1371/journal.ppat.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Agop-Nersesian C, Naissant B, Ben Rached F, Rauch M, Kretzschmar A, Thiberge S, et al. Rab11A-controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis. PLoS Pathog. 2009;5:e1000270. doi: 10.1371/journal.ppat.1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Breinich MS, Ferguson DJ, Foth BJ, van Dooren GG, Lebrun M, Quon DV, et al. A dynamin is required for the biogenesis of secretory organelles in Toxoplasma gondii. Curr Biol. 2009;19:277–86. doi: 10.1016/j.cub.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 152.John B, Harris TH, Tait ED, Wilson EH, Gregg B, Ng LG, et al. Dynamic Imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 2009;5:e1000505. doi: 10.1371/journal.ppat.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schaeffer M, Han SJ, Chtanova T, van Dooren GG, Herzmark P, Chen Y, et al. Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J Immunol. 2009;182:6379–93. doi: 10.4049/jimmunol.0804307. [DOI] [PubMed] [Google Scholar]
- 154.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–96. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]