Abstract
Purpose
To assess the shape of the dose response for various circulatory disease endpoints, and modifiers by age and time since exposure.
Methods and Materials
Analysis of the US peptic ulcer data testing for heterogeneity of radiogenic risk by circulatory disease endpoint (ischemic heart, cerebrovascular, other circulatory disease).
Results
There are significant excess risks for all circulatory disease, with an excess relative risk Gy−1 of 0.082 (95% CI 0.031, 0.140), and ischemic heart disease, with an excess relative risk Gy−1 of 0.102 (95% CI 0.039, 0.174) (both p<0.01), and indications of excess risk for stroke. There are no statistically significant (p>0.2) differences between risks by endpoint, and few indications of curvature in the dose response. There are significant modifications of relative risk by time since exposure, the magnitude of which does not vary between endpoints (p>0.2). Risk modifications are similar if analysis is restricted to those receiving radiation, although relative risks are slightly larger and the risk of stroke fails to be significant. The slopes of the dose response are generally consistent with those observed in the Japanese atomic bomb survivors and in occupationally and medically exposed groups.
Conclusions
There are excess risks for a variety of circulatory diseases in this dataset, with significant modification of risk by time since exposure. The consistency of the dose-response slopes with those observed in radiotherapeutically-treated groups at much higher dose, as well as in lower-dose exposed cohorts such as the Japanese atomic bomb survivors and nuclear workers implies that there may be little sparing effects of fractionation of dose or low dose-rate exposure.
Keywords: circulatory disease, ischemic heart disease, stroke, peptic ulcer, benign disease
Introduction
Based on observations in irradiated populations, the health risks of low-level exposure to ionizing radiation have been assumed to be related primarily to cancer. At high radiation doses a variety of other well-established effects are observed, in particular damage to the heart and to the coronary and other large arteries, both in patients receiving radiotherapy (RT) and in experimental animals 1. There are plausible, if not completely understood, mechanisms by which high doses of radiation affect the blood circulatory system 2;3.
Circulatory system effects have also been observed in populations exposed at somewhat lower doses, in particular in the Life Span Study (LSS) cohort of Japanese atomic bomb survivors 4, in a UK group treated with spinal X-rays for ankylosing spondylitis 5 and in a US cohort treated with X-rays for peptic ulcer 6. In the peptic ulcer cohort there is excess risk of ischemic heart disease (IHD) in patients receiving mean heart doses in the range 0–7.6 Gy, with excess IHD risk above 2.6 Gy, but no excess risk for other heart disease 6. Carr et al. described relative risks of IHD in various dose groups, but did not otherwise model the dose response, or consider modifying variables.
In the atomic bomb survivors, assessments have previously been made of heterogeneity between cancer types in the adjustments of the radiogenic excess risk for gender, time since exposure or age at exposure 7; similar analysis has not been attempted in relation to circulatory disease4;8.
In this paper we re-analyse the shape of the dose response for various circulatory disease endpoints in the US peptic ulcer dataset, and assess modifications of risk by age at exposure and time since exposure. Because of uncertainty as to the target for radiation-induced disease9, we consider risk in relation to dose to various target organs. Patients chosen for irradiation may have been less medically well (i.e., not fit for anesthetic/surgery) than those treated in other ways; assessing this potential bias requires that we analyze the full cohort (exposed+unexposed) as well as the exposed group only. Using the methods of Pierce and Preston 7 we formally evaluate heterogeneity of the shape of the dose-response and modifications by time and age in this dataset. The data used here are very similar to those used in previous analyses of this cohort 6;10.
Data and Methods
Data
The cohort consisted of 3719 persons, comprising 1860 unexposed persons and 1859 exposed patients. Removing 8 persons in the exposed group for whom the dosimetry was incomplete, and 111 who had received megavoltage or 60Co γ-therapy, and for whom phantom measurements were not done, led to an analysis cohort of 3600 persons. Follow-up started 5 years after radiation treatment in the exposed cohort, in contrast to the paper of Carr et al. 6 in which follow-up started 1 or 10 years after exposure. Follow-up finished with the earliest of date of death, date last known alive or December 31st 1997, yielding a total of 76,571.7 person-years of follow-up (Table 1). The distribution of persons and deaths from circulatory disease for various demographic parameters are given in Table 2. We concentrate on various circulatory disease endpoints, namely: (a) all circulatory disease; (b) IHD; (c) cerebrovascular disease; (d) all other circulatory disease.
Table 1.
Numbers of deaths people and person years in US peptic ulcer cohort
| Endpoint | Known radiation dose and >5 years of follow-up | Percentage of cardiovascular deaths |
|---|---|---|
| Ischemic heart disease (ICD9 410–414) | 1003 | 68.3 |
| Stroke (ICD9 430–438) | 226 | 15.4 |
| All other circulatory diseases | 240 | 16.3 |
| All circulatory disease (ICD9 390–459) | 1469 | 100.0 |
|
| ||
| Numbers of people | 3600 | |
| Person years | 76,571.7 | |
Table 2.
Numbers of circulatory disease deaths by radiotherapy status and distribution of other risk factors in US peptic ulcer cohort (all deaths 5 years after treatment)
| Radiotherapy | No radiotherapy | |||
|---|---|---|---|---|
|
|
|
|||
| Category | Circulatory disease deaths | Persons at risk | Circulatory disease deaths | Persons at risk |
| Age at treatment
| ||||
| <35 | 57 | 252 | 125 | 437 |
| 35–44 | 173 | 462 | 232 | 549 |
| 45–54 | 234 | 515 | 248 | 497 |
| ≥55 | 224 | 511 | 176 | 377 |
|
| ||||
| Year of treatment/entry
| ||||
| <1940 | 87 | 207 | 248 | 534 |
| 1940–44 | 147 | 373 | 199 | 459 |
| 1945–49 | 111 | 277 | 212 | 504 |
| 1950–59 | 296 | 738 | 122 | 363 |
| ≥1960 | 47 | 145 | - | - |
|
| ||||
| Gender
| ||||
| Male | 556 | 1389 | 585 | 1423 |
| Female | 132 | 351 | 196 | 437 |
|
| ||||
| Marital status
| ||||
| Not stated/unknown | 17 | 47 | 3 | 9 |
| Never married | 58 | 163 | 94 | 234 |
| Married | 546 | 1356 | 617 | 1471 |
| Divorced separated | 17 | 63 | 22 | 60 |
| Widowed | 50 | 111 | 45 | 86 |
|
| ||||
| Cigarette smoking status
| ||||
| Unknown | 120 | 301 | 140 | 369 |
| Never smoked | 156 | 418 | 239 | 499 |
| Smoked | 412 | 1021 | 402 | 992 |
|
| ||||
| Cigarette smoking quantity
| ||||
| Unknown | 291 | 749 | 395 | 903 |
| ≤1 pack/day | 286 | 694 | 295 | 726 |
| > 1 pack/day | 111 | 297 | 91 | 231 |
|
| ||||
| Alcohol drinking status
| ||||
| Unknown | 136 | 342 | 156 | 424 |
| Never drank | 218 | 574 | 308 | 642 |
| Drank | 334 | 824 | 317 | 794 |
|
| ||||
| Alcohol drinking quantity
| ||||
| Unknown | 397 | 1025 | 520 | 1214 |
| ≤5 drinks/week | 169 | 404 | 167 | 379 |
| 6–15 drinks/week | 59 | 143 | 42 | 123 |
| >15 drinks/week | 63 | 168 | 52 | 144 |
Dosimetry
The RT for this cohort was highly standardized, and has been described in detail elsewhere 10;11. Several machines were used, but, with rare exceptions, these were orthovoltage X-ray machines with 250 kVp X-ray beams and effective energies equivalent to 1.3–1.5 mm Cu half-value layers. Treatments were anterior and posterior parallel-opposed fields (typically 13 cm × 13 cm), centred on the stomach and under fluoroscopic control since 1949. Radiotherapy was delivered in daily fractions of 1.5 Gy at a dose rate of 0.3 Gy/min during one or two 6–14 day treatment courses; the goal, although it varied slightly over the years, was to provide a stomach dose of 16–17 Gy at each course. More than one treatment course was received by 182 (9.8%) of the 1859 irradiated patients.
Cardiac doses were estimated from measurements in an adult male Alderson phantom. The machine used to irradiate the phantom was one of the orthovoltage machines (General Electric Maxitron 250) used to treat the patients in the study. The beam was defined by the machine collimators; a 3 mm thick lead rubber cutout provided additional shielding of adjacent organs. Thermoluminescent dosimeters (TLD) were placed on a three-dimensional grid throughout the torso of the phantom.
A marked dose gradient occurred across the heart, decreasing with increasing distance from the edge of the treatment field. The physician who treated the patients estimated that about 5% (range 0–10%) of the heart (apex) was directly in the radiation field during all treatments 6,11. The remainder of the heart received scattered radiation.
We derived a volume-weighted average heart dose by summing the in-field dose received in the apex of the heart weighted by its proportion of the organ volume (0%, 5%, 10%) and the average of the dose received in the remainder of the heart weighted by its volume proportion (respectively 100%, 95%, 90%). The doses delivered to the stomach for individual patients were obtained from RT records. The measurements were renormalized on the basis of each patient’s stomach dose to obtain their volume-weighted average dose to the heart. The total average cumulative cardiac dose for each patient was obtained by summing the volume-average cardiac dose over all treatment courses. In addition to the average cardiac doses, we estimated the dose to the thyroid, kidney, pancreas and brain from TLD measurements; thyroid dose serves as a surrogate for the dose to carotid arteries.
Statistical methods
A Poisson relative risk model was fitted by Poisson maximum likelihood. Tests were based on the likelihood-ratio test 12. Confidence intervals are based on the profile likelihood 12. Tests of homogeneity of excess relative risk Gy−1 (ERR) and other parameters modifying the ERR across subtypes of circulatory disease are based on the methods of Pierce and Preston 7. All p-values are two-sided.
Results
The optimal background model is given in Table A1. As with Carr et al. 6, we used tobacco smoking and alcohol habit/quantity in the background model for circulatory disease because of their previously known effects on certain circulatory disease subtypes, although these variables were not statistically significant.
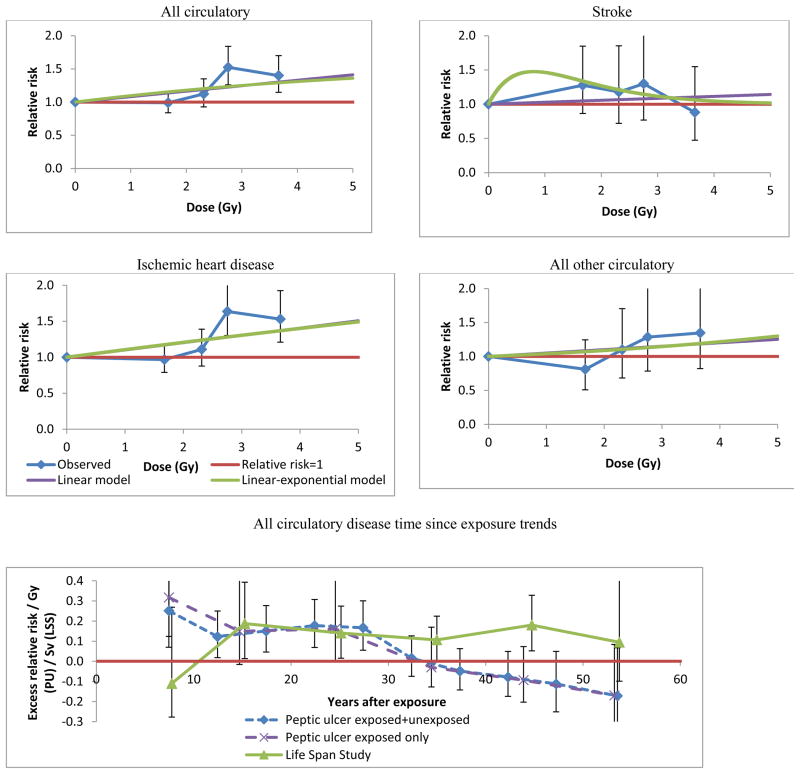
Table 3 (model 1) demonstrates that there are statistically significant increases in risk with dose for all circulatory diseases combined (p=0.001) and, more specifically, for IHD (p=0.001). For all other subtype endpoints there is no significant dose response (p>0.20). However, a 2 degrees of freedom (df) test of the dose response for stroke adjusted for time since exposure was highly statistically significant (p=0.009–0.011 for various organ doses) (Table 3 model 5, Table 4 model 6). Risk modifications are similar if analysis is restricted to those receiving radiation, although risks are slightly larger (Tables 3, 4, Figure 1) and the dose response for stroke fails to be significant using the 2 df test (p=0.101)(result not shown). When stroke is assessed in relation to heart dose there are no significant differences in the ERR between circulatory disease subtypes (p>0.20) (Table 5 panel A, Figure 1), but if thyroid dose is used for stroke there is significant (p=0.011) heterogeneity of ERR (Table 5 panel B). There are substantial differences in the magnitude of ERRs for IHD and stroke, which are relatively small for dose to the pancreas or kidney, more substantial for heart and thyroid dose, and very large for brain dose (Table 4).
Table 3. Modifiers of dose response for various categories of circulatory disease, among full cohort (exposed + unexposed) and among exposed group only. Parameter estimates (all Gy−1) with 95% profile likelihood CI, unless otherwise indicated.
Background model is quartic model in age, with adjustment for gender, smoking habit and quantity, alcohol habit and quantity, and calendar year period treated (year of entry).
| Model no. | Model/parameters | Exposed and unexposed
|
Exposed only
|
|||||
|---|---|---|---|---|---|---|---|---|
| All circulatory disease | IHD | Stroke | Other circulatory | All circulatory disease | IHD | |||
| 1 | α (Gy−1) | 0.082 (0.031, 0.140) | 0.102 (0.039, 0.174) | 0.028 (−0.085, 0.186) | 0.050 (−0.053, 0.194) | 0.194 (0.014, 0.648) | 0.376 (0.060, 1.735) | |
| Background[1+ α D] | p-valuesa | 0.001 | 0.001 | 0.665 | 0.384 | 0.028 | 0.007 | |
|
| ||||||||
| 2 | α (Gy−1) | 0.104 (0.026, 0.287) | 0.107 (0.025, 0.296) | 1.604 (−404.3, 807.5) | 0.035 (−0143b, 14.12) | 0.441 (0.042, 1.767b) | 1.043 (−0.657, 4.356b) | |
| Background[1+ α D exp[γ D]] | γ (Gy−1) | −0.074 (−0.468, 0.197) | −0.016 (−0.391, 0.262) | −1.242 (−157.1, 0.852b) | 0.105 (−0.987b, 1.196b) | −0.162 (−0.675, 0.140b) | −0.155 (−0.355, 0.086b) | |
| p-valuesc | 0.599 | 0.920 | 0.123 | 0.834 | 0.212 | 0.152 | ||
|
| ||||||||
| 3 | α (Gy−1) | 0.089 (0.034, 0.151) | 0.123 (0.054, 0.204) | 0.004 (−0.120b, 0.128b) | 0.051 (−0.056, 0.203) | 0.248 (0.035, 0.935) | 0.403 (0.060, 2.154) | |
| Background[1+ α D exp[κ1sex=female]] | κ | −0.625 (−54.32, 0.877) | −1124 (NA, NA) | 3.532 (<−104, 35.56b) | −0.123 (−5.647, 6.135b) | −8.049 (−3469b, 1.108) | −0.535 (−71.30, 3.233b) | |
| p-valuesc | 0.501 | 0.092 | 0.114 | 0.964 | 0.236 | 0.762 | ||
|
| ||||||||
| 4 | Background[1+ α D exp[τ (age at exposure – 41.32)]]d | α (Gy−1) | 0.062 (0.023, 0.113) | 0.078 (0.028, 0.143) | 0.016 (−0.050b, 0.137) | 0.046 (−0.063, 0.190) | 0.236 (0.067, 0.698) | 0.453 (0.118, 2.047) |
| τ (y−1) | 0.051 (0.025, 0.082) | 0.050 (0.023, 0.082) | 0.097 (−0.034b, 0.228b) | 0.021 (−0.115b, 0.157b) | 0.040 (0.023, 0.066) | 0.035 (0.020, 0.059) | ||
| p-valuesc | <0.001 | <0.001 | 0.064 | 0.677 | <0.001 | <0.001 | ||
|
| ||||||||
| 5 | α (Gy−1) | 0.115 (0.054, 0.181) | 0.140 (0.067, 0.223) | 0.107 (−0.085b, 0.299) | 0.004 (−0.048b, 0.195) | 0.318 (0.115, 0.794) | 0.596 (0.195, 2.250) | |
| Background[1+ α D exp[δ (time since exposure – 20.78)]]d | δ (y−1) | −0.075 (−0.126, −0.045) | −0.066 (−0.113, −0.036) | −0.118 (−0.395, −0.044) | −0.299 (−1.487b, 0.889b) | −0.048 (−0.086, −0.027) | −0.039 (−0.068, −0.021) | |
| p-valuesc | <0.001 | <0.001 | 0.003 | 0.270 | <0.001 | <0.001 | ||
| p-valuese | <0.001 | <0.001 | 0.009 | 0.372 | <0.001 | <0.001 | ||
|
| ||||||||
| 6 | Background[1+ α D exp[τ (age at exposure – 41.32) + δ (time since exposure – 20.78)]]d | α (Gy−1) | 0.115 (0.053, 0.193) | 0.133 (0.060, 0.229) | 0.150 (−0.125b, 0.554) | 0.001 (−0.020b, 0.022b) | 0.367 (0.112, 1.270) | 0.602 (0.150, 3.409) |
| τ (y−1) | −0.001 (−0.035, 0.033) | 0.006 (−0.031, 0.044) | −0.024 (−0.124, 0.079) | −0.044 (−0.217b, 0.128b) | −0.013 (−0.076, 0.044) | −0.001 (−0.074, 0.076) | ||
| δ (y−1) | −0.076 (−0.139, −0.036) | −0.061 (−0.119, −0.020) | −0.132 (−0.392, −0.035) | −0.434 (−1.723b, 0.856b) | −0.060 (−0.129, −0.000) | −0.040 (−0.112, 0.043) | ||
| p-valuesf | <0.001 | 0.004 | 0.014 | 0.111 | 0.050 | 0.286 | ||
| p-valuesg | 0.964 | 0.738 | 0.575 | 0.222 | 0.652 | 0.964 | ||
p-value for improvement of fit over model without linear term in dose
Wald-based confidence limit
p-value for improvement of fit over model with (unadjusted) linear term in dose
age at exposure and time since exposure are approximately centered by subtracting off their person-year weighted means (41.32 years, 20.78 years respectively) in the full cohort (exposed+unexposed), to stabilize parameter estimates.
p-value for 2 degrees of freedom test of improvement of fit over model with no dose-response terms
p-value for improvement of fit over linear model in dose with adjustment for age at exposure.
p-value for improvement of fit over linear model in dose with adjustment for time since exposure
Table 4. Modifiers of dose response for ischemic heart disease and stroke using dose to various organs (heart, thyroid, kidney, pancreas, brain), among full cohort (exposed + unexposed) and among exposed group only. Parameter estimates (all Gy−1) with 95% profile likelihood CI, unless otherwise indicated.
Background model is quartic model in age, with adjustment for gender, smoking habit and quantity, alcohol habit and quantity, and calendar year period treated (year of entry).
| Model no. | Model/parameters | Heart dose | Thyroid dose | Kidney dose | Pancreas dose | Brain dose | |
|---|---|---|---|---|---|---|---|
| Ischemic heart disease (exposed and unexposed)
| |||||||
| 1 | α (Gy−1) | 0.102 (0.039, 0.174) | 1.696 (0.651, 2.907) | 0.033 (0.012, 0.056) | 0.020 (0.008, 0.035) | 10.36 (3.925, 17.82) | |
| Background[1+ α D] | p-valuesa | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
|
| |||||||
| 2 | α (Gy−1) | 0.140 (0.067, 0.223) | 2.338 (1.114, 3.719) | 0.045 (0.021, 0.072) | 0.028 (0.013, 0.044) | 14.34 (6.800, 22.85) | |
| δ (y−1) | −0.066 (−0.113, −0.036) | −0.066 (−0.113, −0.036) | −0.066 (−0.114, −0.036) | −0.066 (−0.113, −0.036) | −0.066 (−0.114, −0.036) | ||
| Background[1+ α D exp[δ (time since exposure – 20.78)]]c | p-valuesb | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| p-valuesd | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||
| Ischemic heart disease (exposed only)
| |||||||
| 3 | α (Gy−1) | 0.376 (0.060, 1.735) | 6.371 (1.041, 29.59) | 0.114 (0.016, 0.514) | 0.078 (0.013, 0.368) | 37.54 (5.681, 173.9) | |
| Background[1+ α D] | p-valuesa | 0.007 | 0.006 | 0.010 | 0.006 | 0.008 | |
|
| |||||||
| 4 | α (Gy−1) | 0.596 (0.195, 2.250) | 9.991 (3.277, 38.42) | 0.186 (0.061, 0.683) | 0.122 (0.040, 0.478) | 60.31 (19.60, 229.7) | |
| δ (y−1) | −0.039 (−0.068, −0.021) | −0.039 (−0.068, −0.021) | −0.039 (−0.070, −0.021) | −0.038 (−0.067, −0.021) | −0.039 (−0.069, −0.021) | ||
| Background[1+ α D exp[δ (time since exposure – 20.78)]]c | p-valuesb | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| p-valuesd | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||
| Stroke (exposed and unexposed)
| |||||||
| 5 | α (Gy−1) | 0.028 (−0.085, 0.186) | 0.422 (−1.455, 3.039) | 0.009 (−0.028, 0.060) | 0.005 (−0.017, 0.036) | 2.649 (−8.912, 18.740) | |
| Background[1+ α D] | p-valuesa | 0.665 | 0.698 | 0.678 | 0.704 | 0.692 | |
|
| |||||||
| 6 | α (Gy−1) | 0.107 (−0.085e, 0.299) | 1.734 (−1.437e, 4.923) | 0.034 (−0.028e, 0.097) | 0.020 (−0.017e, 0.058) | 10.53 (−8.940e, 30.35) | |
| δ (y−1) | −0.118 (−0.395, −0.044) | −0.118 (−0.411, −0.044) | −0.118 (−0.396, −0.044) | −0.119 (−0.411, −0.044) | −0.120 (−0.413, −0.044) | ||
| Background[1+ α D exp[δ (time since exposure – 20.78)]]c | p-valuesb | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | |
| p-valuesd | 0.009 | 0.011 | 0.010 | 0.010 | 0.010 | ||
p-value for improvement of fit over model without linear term in dose
p-value for improvement of fit over model with (unadjusted) linear term in dose
time since exposure is approximately centered by subtracting off its person-year weighted mean (20.78 years) in the full cohort (exposed+unexposed), to stabilize parameter estimates.
p-value for 2 degrees of freedom test of improvement of fit over model with no dose-response terms
Wald-based confidence limit
Figure 1. Relative risk vs dose (with 95% CI) for various circulatory disease endpoints in the peptic ulcer study, with fit of optimal linear and linear-exponential models, and time after exposure trends (adjusted for age at exposure) in aggregate and in the Life Span Study 4. All use average heart dose (5% of heart in beam).
Background model is as in Tables 3+4.
Table 5. Test of heterogeneity in dose response and its modification by time since exposure and age at exposure in various categories of circulatory disease and cancer.
The background model for each circulatory disease subtype is a quartic model in age, with adjustment for gender, smoking habit and quantity, alcohol habit and quantity, and calendar year period treated (year of entry). Unless otherwise stated heart dose is used for all circulatory disease endpoints.
| Panel | Model | p-value |
|---|---|---|
| A | Test of heterogeneity across four circulatory disease endpoints (using heart dose throughout), linear dose response | |
|
| ||
| Background[1+ a D exp[δ (time since exposure - 20.78)]]a | <0.001b | |
| Background[1+ ai D exp[δ (time since exposure - 20.78)]]a | 0.283c | |
| Background[1+ ai D exp[δi (time since exposure - 20.78)]]a | 0.372d | |
|
| ||
| B | Test of heterogeneity across four circulatory disease endpoints (using thyroid dose for stroke, heart dose otherwise), linear dose response | |
|
| ||
| Background[1+ a D exp[δ (time since exposure - 20.78)]]a | <0.001b | |
| Background[1+ ai D exp[δ (time since exposure - 20.78)]]a | 0.011c | |
| Background[1+ ai D exp[δi (time since exposure - 20.78)]]a | 0.370d | |
time since exposure is approximately centered by subtracting off its person-year weighted mean (20.78 years) in the full cohort (exposed+unexposed), to stabilize parameter estimates.
p-value for improvement of fit over model without radiation dose response term.
p-value for improvement of fit over model with the same excess relative risk coefficient (α) for each endpoint.
p-value for improvement of fit over model with the same time trend in excess relative risk (δ) for each endpoint.
Table 3 (model 5) and Figure 1 demonstrate that for all circulatory disease, IHD, and stroke there are highly statistically significant (p<0.005) decreasing trends of ERR with time after exposure, which changes by 100(exp(−0.075) −1) =−7.3% (95% CI −11.8, −4.4) per year after exposure; once adjustment is made for time, there are no significant modifications in risk by age at exposure (p>0.2) (model 6). There are no statistically significant (p>0.2) differences between the three endpoints in the trends with time since exposure of ERR (Table 5 panels A, B).
Discussion
The present analysis shows that radiation of the stomach for peptic ulcer increased the risk of circulatory disease as a function of dose received. There are significant excess risks for a variety of circulatory disease endpoints, in particular IHD, stroke, and all circulatory disease. There are significant reductions of ERR with increasing time since exposure. Risk modifications are similar if analysis is restricted to those receiving radiation, although relative risks are slightly larger and the risk of stroke fails to be significant; this does not suggest any marked difference in underlying health status between those selected for radiotherapy and those given surgery.
The reduction in relative risk with increasing time since exposure is somewhat novel. The LSS is the only group in which time- or age-at-exposure-modifications of the radiogenic excess circulatory disease risk has been modeled 4;8. Although no time trends (or increasing ones) in aggregate SIR have been observed in a few other cohorts 13–15, the absence of individual dosimetry combined with the substantial temporal changes in mean heart dose for treatment of breast and other cancers 16 mean that these studies are largely uninformative on this issue. There are borderline significant (p=0.04) variations in ERR with attained age for stroke in the LSS, with indications of greater ERR for those under age 60, but no variations with exposure age or gender (as here) 4. As here, Shimizu et al. documented no significant modifications of ERR for IHD by age, exposure age or gender in the LSS4, although further analyses using underlying and contributing causes of death (rather than just underlying cause of Shimizu et al.4) demonstrate significant reduction in ERR with increasing exposure age (Table A2). Because of the limited exposure-age spread in the peptic ulcer cohort there is little power to detect variations of risk by this variable. Although there are no time trends in the LSS data, the trends are statistically consistent with those in the peptic ulcer cohort (Tables 3, A2, Figure 1).
The suggestions of homogeneity of ERR and speed of variation of ERR over time by different circulatory disease subtypes is also a novel finding. Possibly because there were only relatively weak indications in the LSS of modifications of ERR by gender, age and exposure age 4, no formal analysis of heterogeneity of modification of risk was performed there. However, not too much should be made of this, since the only endpoints in the present study with significant dose response were IHD and stroke.
One limitation of the study is that the radiation dosimetry, although of high quality in many respects, fails to account for variability in patient anatomy, e.g., the heart size/shape/position and its relation to the diaphragm and stomach. The dose received by the heart for the “same” radiation technique (as recreated on the phantom) may vary markedly between patients. However, the treatments were set up using fluoroscopy, in order to ensure stomach exposure and reduce exposure to other organs.
The magnitude of radiation-induced circulatory disease ERR, 0.082 – 0.194 Gy−1, is consistent with the value of 0.11 – 0.15 Sv−1 for this endpoint in the LSS 4. [The contrast between the present data, in which the excess is largely of IHD, and the LSS 4, in which this endpoint is not significantly elevated should be noted; however, doubts as to the accuracy of death certificate coding in both cohorts suggest that one should not over-emphasise this possible discrepancy.] The ERRs are also consistent with those predicted by a recent meta-analysis of occupational and environmentally exposed groups, of 0.19 Sv−1 (95% CI 0.14, 0.23) 17. The risks of stroke are somewhat uncertain; nevertheless, the indications of much larger ERR (2.649 – 10.53 Gy−1) in relation to brain dose (Table 4) than those observed in the LSS 4 (0.12 Sv−1) or in a number of groups exposed to moderate and low radiation doses 17 (0.27 Sv−1), suggest (albeit weakly) that dose to the heart, thyroid, pancreas or kidney, or something strongly correlated with these, may be the more appropriate dose measure than brain dose for this endpoint, at least in the present dataset. This is also supported by indications of excess stroke risk in groups treated with RT for Hodgkin’s disease and breast cancer 18;19; the brain doses in such patients would be expected to be much lower than those to the heart, coronary and carotid arteries. Possibly it is the dose to some combination of the heart (or other nearby target in the circulatory system) and carotid that may be relevant for this and IHD. Radiation-induced renal damage is well documented20, and can result in hypertension21 and very likely subsequent circulatory disease. There is increased risk of diabetes mellitus in this cohort22, likely due to radiation damage to the pancreas, which is located partly in the radiation treatment field. Overt or subclinical diabetes is an added factor in increasing the risk of circulatory disease.
The relevance of this study to radiation therapy regimes is intriguing. For a substantial part of the cohort the cumulative mean heart doses exceed 2 Gy (Figure 1), larger than the vast majority of those in the LSS cohort. The mean doses are more comparable with those received therapeutically, if not quite as large as those received by some groups, e.g., childhood cancer survivors 23;24. Mulrooney et al.23 and Tukenova et al.24 demonstrate that there is significant excess risk of various types of circulatory disease in two groups of childhood cancer survivors receiving ≥15 Gy mean heart dose. Tukenova et al.24 estimated an ERR of 0.6 Gy−1 (95% CI 0.2, 2.5), much higher than the risk we estimate, 0.082 Gy−1 (Table 3). The discrepancy likely reflects the younger exposure age in the cancer-survivor group, although as above, the present dataset has little power to detect such a variation. The calculations that we performed with the latest LSS data4 imply a 2.8% (95% CI 0.8, 4.9) reduction in circulatory disease ERR per year of exposure age (Table A2). Applying the central estimate (2.8%) to the ERR of Tukenova et al.24 adjusted for the exposure-age difference between the two cohorts (5.9 vs 41.3 years) yields an ERR of 0.22 Gy−1 (95% CI 0.07, 0.91), still higher than the estimate we obtain, 0.082 Gy−1, but statistically compatible with it. The ERR in the LSS 4 in a subset with similar exposure-age range (30–59 years) as the present study is 0.127 Sv−1 (95% CI 0.068, 0.188) (Table A2), again compatible with the present study.
Excess circulatory disease risk associated with high dose RT have been established for some time 1, although in the absence of individual radiation dosimetry, quantitative evaluation risks in most RT data is problematic – some recent high quality studies remedy this defect 23;24. At high radiation doses, such as would be received by patients treated with RT, a variety of so-called deterministic or tissue-reaction effects are observed 1;2. Among such effects are damage to the structures of the heart – including marked diffuse fibrotic damage, especially of the pericardium and myocardium, pericardial adhesions, microvascular damage and stenosis of the valves – and to the coronary, carotid and other large arteries; these sorts of damage occur frequently both in patients receiving RT and in experimental animals 1;25. An association between lower doses (< 0.5 Gy) and late circulatory disease has only recently been suspected and remains controversial. Recent reviews present evidence suggesting an excess radiation-induced risk for IHD and stroke at occupational and environmental dose levels 9;17. A review of much biological data suggested that many inflammatory endpoints potentially relevant to circulatory disease may be differentially regulated below and above about 0.5 Gy 9, emphasizing the importance of assessing risks in biological and epidemiological data below this value. Although uncertainties in dosimetry in the present cohort suggest a measure of caution, there is perhaps remarkable consistency between the risks in this data, in which a highly heterogeneous dose was delivered acutely to the heart in about 10 daily fractions with those in the LSS, in which a uniform whole body dose was also delivered acutely but in a single fraction, and with the risks in occupational series in which whole body dose was delivered at low dose rate in small daily fractions; this implies that there may be little sparing effect of low dose rate exposure or dose fractionation, and that similar circulatory disease mechanisms operate over this wide range of doses and dose rates.
Acknowledgments
The authors are grateful for the detailed and helpful comments of the three referees. This work was supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Footnotes
Conflicts of interest notification
No authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 2.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 3.Little MP, Gola A, Tzoulaki I. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure. PLoS Comput Biol. 2009;5:e1000539. doi: 10.1371/journal.pcbi.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darby SC, Doll R, Gill SK, Smith PG. Long term mortality after a single treatment course with X-rays in patients treated for ankylosing spondylitis. Br J Cancer. 1987;55:179–190. doi: 10.1038/bjc.1987.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr ZA, Land CE, Kleinerman RA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 7.Pierce DA, Preston DL. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiat Res. 1993;134:134–142. [PubMed] [Google Scholar]
- 8.Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 9.Advisory Group on Ionising Radiation. Report of the independent Advisory Group on Ionising Radiation. RCE-16. Health Protection Agency; Holborn Gate, 330 High Holborn, London: Jan 10, 2010. Circulatory disease risk; pp. 1–116. [Google Scholar]
- 10.Griem ML, Kleinerman RA, Boice JD, Jr, et al. Cancer following radiotherapy for peptic ulcer. J Natl Cancer Inst. 1994;86:842–849. doi: 10.1093/jnci/86.11.842. [DOI] [PubMed] [Google Scholar]
- 11.Griem ML. External irradiation at the University of Chicago. In: Palmer WL, editor. Gastric irradiation in peptic ulcer. Chicago, London: The University of Chicago Press; 1974. pp. 39–51. [Google Scholar]
- 12.McCullagh P, Nelder JA. Monographs on statistics and applied probability. 2. Vol. 37. Boca Raton, FA: Chapman and Hall/CRC; 1989. Generalized linear models; pp. 1–526. [Google Scholar]
- 13.Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–288. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 14.Aleman BM, van den Belt-Dusebout AW, de Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 15.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 16.Taylor CW, Nisbet A, McGale P, et al. Cardiac doses from Swedish breast cancer radiotherapy since the 1950s. Radiother Oncol. 2009;90:127–135. doi: 10.1016/j.radonc.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Little MP, Tawn EJ, Tzoulaki I, et al. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat Environ Biophys. 2010;49:139–153. doi: 10.1007/s00411-009-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–937. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson G, Holmberg L, Garmo H, et al. Radiation to supraclavicular and internal mammary lymph nodes in breast cancer increases the risk of stroke. Br J Cancer. 2009;100:811–816. doi: 10.1038/sj.bjc.6604902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fajardo LF. Is the pathology of radiation injury different in small vs large blood vessels? Cardiovasc Radiat Med. 1999;1:108–110. doi: 10.1016/s1522-1865(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 21.Verheij M, Dewit LG, Valdes Olmos RA, Arisz L. Evidence for a renovascular component in hypertensive patients with late radiation nephropathy. Int J Radiat Oncol Biol Phys. 1994;30:677–683. doi: 10.1016/0360-3016(92)90955-h. [DOI] [PubMed] [Google Scholar]
- 22.Kleinerman RA, Weinstock RM, Mabuchi K. High-dose abdominal radiotherapy and risk of diabetes mellitus. Arch Intern Med. 2010;170:1506–1507. doi: 10.1001/archinternmed.2010.285. [DOI] [PubMed] [Google Scholar]
- 23.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 25.Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation-associated valvular disease. Chest. 1991;99:538–545. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]