Abstract
BACKGROUND
We measured anti-Mullerian hormone (AMH), a marker of ovarian reserve, in women with lupus treated with cyclophosphamide (CYC) (Group I), CYC plus gonadotropin-releasing hormone agonist (GnRH-a) (Group II), or neither (Group III). We hypothesized that AMH would be diminished in women exposed to CYC vs women receiving adjunctive GnRH-a treatment or no CYC exposure.
METHODS
48 pre-menopausal lupus patients were retrospectively divided into three treatment groups: CYC alone (Group I, n=11), CYC + GnRH-a (Group II, n=10), neither (Group III, n=27). Serum AMH levels between groups were compared using a non-parametric test (Wilcoxon rank-sum). Multiple linear regression adjusting for age was performed.
RESULTS
AMH (ng/mL) levels at the last collection were significantly lower in Group I vs Group III (mean ± SD: 0.18 ± 0.20 Group I vs 1.33 ± 1.59 Group III; p=0.015), and vs Group II (mean ± SD: 0.86 ± 1.06; p=0.018). When centered on age 30 years, average AMH levels for Group I, Group II, and Group III were: 0.20, 0.44, and 1.00, respectively. When adjusted for age, AMH between all groups was significantly different (p<0.0001).
CONCLUSION
Post-treatment AMH levels were significantly higher among patients receiving CYC + GnRH-a compared to CYC alone, suggesting that GnRH-a co-administration mitigates CYC-induced ovarian injury.
Keywords: anti-Mullerian hormone (AMH), primary ovarian insufficiency, cyclophosphamide, systemic lupus erythematosus
Introduction
The use of cyclophosphamide (CYC) and other alkylating agents for treatment of severe manifestations of autoimmune diseases, including systemic lupus erythematosus (SLE), vasculitis and scleroderma, is associated with the onset of primary ovarian insufficiency (POI) resulting in irreversible amenorrhea and infertility in a large proportion of patients1-4. The risk of POI is related to cumulative CYC exposure and to a woman’s age at time of exposure 2-5. One non-invasive and relatively inexpensive proposed strategy for preserving ovarian function during CYC therapy is co-treatment with monthly gonadotropin releasing hormone agonist (GnRH-a), which has shown promise in some but not all studies of women receiving chemotherapy for malignancy or autoimmune disorders3,6-12. In an open label pilot study of 40 SLE patients, our group reported that GnRH-a co-treatment during CYC therapy was associated with significant protection against POI, after accounting for CYC dose and patient age6. However, the likelihood of success of GnRH-a therapy may depend on a number of variables, including age at treatment onset, cumulative dose of alkylating agents, and whether CYC is used as mono- or combination therapy with other gonadotoxic agents.
Anti-Mullerian hormone (AMH), a serum biomarker of ovarian function, is gaining interest as a predictor of time to natural menopause and for use in assisted reproductive therapy13-16. AMH is produced in ovarian granulosa cells after birth, with the highest production from preantral and small antral follicles possessing growth capacity, and minimal to no expression in final stages of follicular development17. AMH is more highly correlated to antral follicle count than other reproductive hormones, including follicle stimulating hormone and inhibin-B.13,18 Serum AMH declines to very low and non-detectable levels five years prior to the final menstrual period, 19 suggesting its utility as a surrogate marker of ovarian reserve and predictor of POI.
In the current study, we sought to characterize AMH levels among women with SLE exposed to CYC with versus without GnRH-a co-treatment for ovarian protection, as well as compared to SLE patients with no history of CYC exposure.
Methods
This research was approved by the University of Michigan Institutional Review Board, and written informed consent was obtained.
Study population
Pre-menopausal SLE patients enrolled in the Michigan Lupus Cohort (MLC), with data on treatment history (CYC doses and dates, GnRH-a co-treatment) and available frozen sera, were eligible. The MLC is comprised of patients meeting the American College of Rheumatology criteria for SLE 20,21.
Demographic and clinical data were collected, and patients were categorized into three groups according to CYC and GnRH-a exposure, as described below. Intravenous (IV) CYC was given at initial doses of 500-750 mg/m2 and titrated to achieve a nadir WBC count between 2-4,000 cells/m2 between days 10-14 after infusion, regardless of age or disease severity. A GnRH-a (3.75 mg depot leuprolide acetate; TAP Pharmaceuticals, Lake Forest, IL), was administered intramuscularly per our protocol6 monthly least 10 days prior to the subsequent monthly bolus of IVCYC to avoid CYC exposure during the initial surge of estrogen associated with this medication. For the purpose of this analysis, baseline was defined as the date of first AMH collection. Data prior to first AMH collection were not consistently available. Three treatment groups were defined as follows:
Group I - CYC alone
Inclusion criteria: Women of reproductive age with SLE who received CYC without GnRH-a co-treatment, and had disease activity requiring treatment with at least one course of CYC. Exclusion criteria: Age ≥ 35 or symptoms consistent with POI based on gynecologic evaluation at CYC initiation.
Group II - CYC + GnRH-a
Inclusion criteria: Women of reproductive age with SLE who received CYC plus GnRH-a co-treatment, and had disease activity requiring treatment with at least CYC course. Exclusion criteria: Age ≥ 35 or symptoms consistent with POI based on gynecologic evaluation at CYC initiation.
Group III - neither CYC nor GnRH-a
Inclusion criteria: Women with SLE age < 35, without history of CYC or GnRH-a exposure, were randomly selected from the Michigan Lupus Cohort.
AMH measurement
AMH levels were measured from banked serum specimens (stored at −70°C). Specimens were retrieved according to the following time points: 1) as close to initial CYC exposure as were retrospectively available; 2) follow-up after completion of CYC course. Assays were performed in the Central Ligand Assay Satellite Services laboratory at the University of Michigan School. A commercially available enzyme linked immunosorbent assay (ELISA) (Beckman Coulter; Marseille, France) was used for in vitro quantitative measurement of serum AMH. The Beckman Coulter MIS/AMH Coated Well ELISA kit features a two site sandwich type immunoassay with no sample extraction. Sample wells are coated with a primary monoclonal antibody. The detection system consists of a biotinylated secondary monoclonal antibody and strepavidinperoxidase. The biotinlyated antibody binds to the solid phase antibody-antigen complex and, in turn binds the conjugate. After incubation, the wells are washed and antigen complex bound to the well detected by addition of a chromatogenic substrate. The assay requires 50 uL of serum or plasma for duplicate analysis and measures analyte concentrations from a minimum detectable concentration of 1 pM (1 ng/mL corresponds to 7.14 pM) to 150 pM with an assay range (standard curve) of 3-150 pM. In the CLASS lab the inter- and intra-assay coefficients of variation (CVs) were 15.3% and 5.6%, respectively.
Statistical Analysis
Descriptive analyses and Kruskal-Wallis tests for nonparametric one way ANOVA were conducted to examine any group differences for baseline variables. The AMH levels at the last collection were compared between each two treatment groups in pair-wise fashion using Wilcoxon rank sum tests. The distribution of AMH level was observed to be skewed, so a log transformation was performed. A linear regression model was fit on the log AMH level from the last visit to evaluate the treatment group difference, adjusting for age at last visit, centered at age 30 years. Centering at age 30 allows for easier interpretation of the model coefficients and reduces the inter-correlations between the covariates in the model.
Results
Clinical characteristics and cumulative CYC doses are summarized in Table 1. The mean age of study patients was 33.1 years [standard deviation (SD) 7.9]. 43 (90%) of the patients were white and 5 (10%) were non-white. The cumulative CYC dose [median (interquartile range)] in those treated with CYC alone (Group I) was 10.8 (3.6, 15.9), and among those treated with CYC + GnRH-a (Group II) cumulative CYC dose was 8.6 (5.4, 12.3). All three groups were comparable in terms of age and race at the first AMH sample, and the cumulative CYC doses in Group I and Group II were likewise comparable.
Table 1. Baseline demographics of study subjects at first AMH collection.
| Mean (SD) for continuous and n (%) for categorical characteristics | |||||
|---|---|---|---|---|---|
| Characteristic | Overall | Treatment Group |
p-value† | ||
| Group I (CYC alone) |
Group II (CYC + GnRH-a) |
Group III (Neither) |
|||
|
| |||||
| n | 48 | 11 | 10 | 27 | |
| Age | 33.1 (7.9) |
34.2 (10.8) |
29.3 (5.9) |
34.0 (7.1) |
0.196 |
| Race: | 0.621 | ||||
| White | 43 (90%) |
9 (82%) | 9 (90%) | 25 (93%) | |
| Non-white | 5 (10%) | 2 (18%) | 1 (10%) | 2 (7%) | |
| Cumulative CYC dose |
-- | 18.1 (25.4) | 9.1 (6.3) | -- | 0.698 |
Kruskal-Wallis tests are used for three group comparison of AMH, Age, and Race, while Wilcoxon rank sum test used for pair-wise comparison of cumulative CYC dose
Among patients in Group I, the time span between sample collections ranged from 0.31 to 3.35 years (mean 1.37, SD 1.25), among patients in Group II, the time span ranged from 0.27 to 1.49 years (mean 0.77, SD 0.64), and among patients in Group III it ranged from 0.11 to 3.58 years (mean 2.07, SD 0.97).
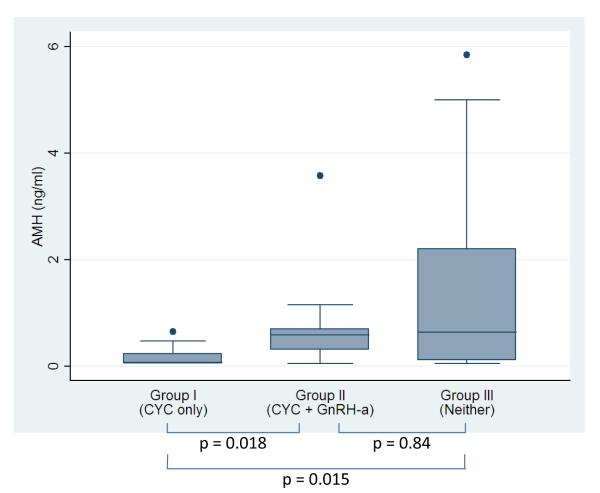
Figure 1 shows the comparison of AMH levels unadjusted for other covariates, at last collection among the three groups. According to the Wilcoxon rank sum test, Group I had the lowest mean AMH levels (0.18, SD 0.20), and it was found to be statistically significantly different from Group II (0.86, SD 1.06; p=0.018), and from Group III (1.33, SD 1.59 Group III; p=0.015). There was no significant difference found between the Group II and Group III (p=0.842).
Figure 1.
AMH levels at last collection. CYC, cyclophosphamide; GnRH-a, gonadotropin-releasing hormone agonist.
As expected, age was inversely related to AMH level, with older age corresponding to lower AMH levels (Spearman’s rho −0.57, p<0.0001). Based on linear regression modeling on the log AMH of the last visit, when adjusted for patient age at visit (centered on age 30), treatment with CYC alone (Group I) compared with neither (Group III) was associated with decreased AMH level, with the AMH level for Group III 5.1 times higher than Group I [5.1; 95% CI: (2.4, 11.0); p=0.0001]. Exposure to CYC+GnRH-a (Group II) was also marginally associated with decreased AMH versus with neither treatment, with the AMH level for Group III 2.3 times higher than Group II [OR 2.3; 95% CI: (1.0, 5.5); p=0.061]. The magnitude of decrease was attenuated as compared to Group I, who received CYC alone. The AMH level in Group II was higher compared to Group I (with Group II 2.2 times higher than Group I), though there was inadequate power to detect this as significant (p=0.11) (see Table 2).
Table 2. Linear regression estimates on the log AMH level at the last visit, controlled for the patient’s age centered at 30.
| Treatment Group† | Ratio of mean AMH level |
p-value | 95% CI |
|---|---|---|---|
| Group II vs Group I | 2.22 | 0.112 | 0.82, 6.00 |
| Group III vs Group I | 5.10 | 0.0001 | 2.37, 10.96 |
| Group III vs Group II | 2.29 | 0.061 | 0.96, 5.48 |
| Age (centered at 30) | 0.89 | <0.0001 | 0.86, 0.93 |
Group I (CYC only), Group II (CYC +GnRH-a), Group III (Neither)
Discussion
The use of AMH is gaining support as a sensitive and non-invasive measure of ovarian function12. Because it is measured on a continuous scale, AMH better approximates gradations in ovarian reserve, and at earlier points in the natural history, thus providing insight beyond conventional endpoints like POI.
In the current study, we compared AMH levels from SLE patients who had previously received gonadotoxic medication with those from SLE patients who never received CYC and therefore had less severe disease, and from those with GnRH-a co-treatment during CYC therapy. As expected, AMH levels were significantly lower in the women who received CYC alone compared to those without CYC exposure, which may also reflect an association between heightened SLE activity and disease duration with ovarian insufficiency 22. While AMH levels were also lower among SLE patients who received CYC + GnRH-a compared to SLE patients with no CYC exposure, the difference was of lower magnitude than for women treated with CYC alone. Taken together, our results suggest that GnRH-a co-treatment may offer partial protection against ovarian damage in women receiving CYC for severe SLE.
Consensus among experts regarding potential benefit of GnRH-a therapy for ovarian protection among women receiving chemotherapy is lacking. 23-26 Clinical trials of GnRH-a in populations undergoing heterogeneous chemotherapeutic protocols report encouraging results3,8,10,11,27, as has a recent meta-analysis, inclusive of 320 patients from seven controlled studies, which found GnRH-a use during chemotherapy was significantly associated with ovarian function preservation (RR 1.7; 95% CI 1.4-2.2)28. However, GnRH-a failed to significantly impact the rate of ovarian insufficiency during chemotherapy in other studies12,29, and a recent clinical review found insufficient evidence of any protective effect of GnRH-a therapy24.
Results from our retrospective study add to supportive evidence for GnRH-a therapy by showing that AMH, arguably the most reliable biomarker of ovarian reserve, is more preserved with GnRH-a therapy after CYC, and that GnRH-a provides at least partial ovarian protection during alkylating agent monochemotherapy in a cohort of lupus patients. These results may not be applicable to other patient populations receiving aggressive anti-neoplastic treatment regimens with more than one alkylating agent.
Study limitations include small sample size and retrospective nature. Additionally, average baseline AMH was lower among patients receiving CYC alone compared to CYC plus GnRH-a, raising the possibility that unknown factors were involved that we were unable to measure. However, neither age nor cumulative CYC dose at baseline were statistically different between the two groups. We did not adjust for baseline AMH measures due to the observational nature of this study; in non-randomized studies, adjustment for baseline measures could result in erroneous conclusions when the assumption of equal population distributions of the baseline predictor is not met 30, as was the case for AMH between the three treatment groups in this study. Adjustment for baseline AMH would address a different scientific question that would be related to the group differences given that the individual has the same baseline AMH measure. A randomized trial would more adequately ensure baseline comparability between groups. A final limitation is that sera were not uniformly available prior to the initial time of CYC exposure, which would be important for demonstrating rate of decline of AMH after CYC exposure. The rate may be similar to the rapid and consistent decline of AMH levels from pretreatment levels observed in a study of breast cancer patients, as early as three months after initiation of polychemotherapy31.
Data from our study add support to the hypothesis that GnRH-a co-therapy mitigates chemotherapy-induced gonadotoxicity. Prospective, randomized, controlled trials are indicated to confirm these results, and to enable detailed investigation of longitudinal trends in AMH, cumulative dose effects of CYC, and interaction between dose and age.
Acknowledgements
None
Footnotes
Declaration of Interest
There are no conflicting interests among any authors to declare. This work was supported by The Pilot and Collaborative Grant Program, Michigan Institute for Clinical and Health Research (MICHR) UL1RR024986 (to WM). WM was supported by K12HD001438 from National Institutes of Health and the Elizabeth Caroline Crosby Research Fund. ECS was supported by UL1RR024986 from the National Center for Research Resources. This work was also supported in part by the Herbert and Carol and Amster Lupus Research Fund, and the Michael and Marcia Klein Lupus Research Fund.
References
- 1.Raptopoulou A, Sidiropoulos P, Boumpas D. Ovarian failure and strategies for fertility preservation in patients with systemic lupus erythematosus. Lupus. 2004;13:887–90. doi: 10.1191/0961203304lu2029ed. [DOI] [PubMed] [Google Scholar]
- 2.Boumpas DT, Austin HA, 3rd, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993;119:366–9. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld Z, Shapiro D, Shteinberg M, Avivi I, Nahir M. Preservation of fertility and ovarian function and minimizing gonadotoxicity in young women with systemic lupus erythematosus treated by chemotherapy. Lupus. 2000;9:401–5. doi: 10.1191/096120300678828596. [DOI] [PubMed] [Google Scholar]
- 4.Warne GL, Fairley KF, Hobbs JB, Martin FI. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289:1159–62. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- 5.Mok CC, Lau CS, Wong RW. Risk factors for ovarian failure in patients with systemic lupus erythematosus receiving cyclophosphamide therapy. Arthritis Rheum. 1998;41:831–7. doi: 10.1002/1529-0131(199805)41:5<831::AID-ART9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Somers EC, Marder W, Christman GM, Ognenovski V, McCune WJ. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52:2761–7. doi: 10.1002/art.21263. [DOI] [PubMed] [Google Scholar]
- 7.Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117:561–7. doi: 10.1007/s10549-009-0313-5. [DOI] [PubMed] [Google Scholar]
- 8.Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694–7. doi: 10.1016/j.fertnstert.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 9.Blumenfeld Z, Eckman A. Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. J Natl Cancer Inst Monogr. 2005:40–3. doi: 10.1093/jncimonographs/lgi015. [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996;11:1620–6. doi: 10.1093/oxfordjournals.humrep.a019457. [DOI] [PubMed] [Google Scholar]
- 11.Blumenfeld Z, Avivi I, Eckman A, Epelbaum R, Rowe JM, Dann EJ. Gonadotropin-releasing hormone agonist decreases chemotherapy-induced gonadotoxicity and premature ovarian failure in young female patients with Hodgkin lymphoma. Fertil Steril. 2008;89:166–73. doi: 10.1016/j.fertnstert.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Behringer K, Wildt L, Mueller H, et al. Final results of a phase II trial from the German Hodgkin Study Group No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated withc escalated BEACOPP for advanced-stage Hodgkin lymphoma. Ann Oncol. 2010 doi: 10.1093/annonc/mdq066. [DOI] [PubMed] [Google Scholar]
- 13.Majumder K, Gelbaya TA, Laing I, Nardo LG. The use of anti-Mullerian hormone and antral follicle count to predict the potential of oocytes and embryos. Eur J Obstet Gynecol Reprod Biol. 2010;150:166–70. doi: 10.1016/j.ejogrb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Steiner AZ. Clinical implications of ovarian reserve testing. Obstet Gynecol Surv. 2009;64:120–8. doi: 10.1097/OGX.0b013e3181932e3f. [DOI] [PubMed] [Google Scholar]
- 15.Robertson DM, Hale GE, Fraser IS, Hughes CL, Burger HG. A proposed classification system for menstrual cycles in the menopause transition based on changes in serum hormone profiles. Menopause. 2008;15:1139–44. doi: 10.1097/gme.0b013e3181735687. [DOI] [PubMed] [Google Scholar]
- 16.Sowers M, McConnell D, Gast K, et al. Anti-Mullerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94:1482–6. doi: 10.1016/j.fertnstert.2009.07.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 18.Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–7. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 19.Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros MM, Silveira VA, Menezes AP, Carvalho RC. Risk factors for ovarian failure in patients with systemic lupus erythematosus. Braz J Med Biol Res. 2001;34:1561–8. doi: 10.1590/s0100-879x2001001200008. [DOI] [PubMed] [Google Scholar]
- 23.Somers EC, Christman GM, Fisseha S, Marder W, McCune WJ. Response to “Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2008;13:613–4. doi: 10.1634/theoncologist.2008-0021. author reply 5-7. [DOI] [PubMed] [Google Scholar]
- 24.Beck-Fruchter R, Weiss A, Shalev E. GnRH agonist therapy as ovarian protectants in female patients undergoing chemotherapy: a review of the clinical data. Hum Reprod Update. 2008;14:553–61. doi: 10.1093/humupd/dmn041. [DOI] [PubMed] [Google Scholar]
- 25.Oktay K, Sonmezer M, Oktem O. Ovarian cryopreservation versus ovarian suppression by GnRH analogues: primum non nocere’: reply. Hum Reprod. 2004;19:1681–3. doi: 10.1093/humrep/deh300. [DOI] [PubMed] [Google Scholar]
- 26.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–34. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- 27.Blumenfeld Z. Ovarian rescue/protection from chemotherapeutic agents. J Soc Gynecol Investig. 2001;8:S60–4. doi: 10.1016/s1071-5576(00)00112-x. [DOI] [PubMed] [Google Scholar]
- 28.Clowse ME, Behera MA, Anders CK, et al. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. J Womens Health (Larchmt) 2009;18:311–9. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waxman JH, Ahmed R, Smith D, et al. Failure to preserve fertility in patients with Hodgkin’s disease. Cancer Chemother Pharmacol. 1987;19:159–62. doi: 10.1007/BF00254570. [DOI] [PubMed] [Google Scholar]
- 30.Fitzmaurice G, Laird N, Ware J. Hoboken. Vol. 2004. Wiley-Interscience; NJ: 2004. Applied longitudinal analysis; pp. 122–32. [Google Scholar]
- 31.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–92. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]