Introduction
Vitamin D, the “sunshine vitamin,” is back in the limelight.1 Reemergence of rickets among vulnerable infants (dark-skinned and/or breast-fed without vitamin D supplementation) and reports of excessive prevalence of vitamin D deficiency and insufficiency among children have reemphasized the importance of optimal intakes of vitamin D.2–5 The American Academy of Pediatrics (AAP) increased its recommended dietary allowance (RDA) of vitamin D for the prevention of vitamin D deficiency in infants and children from 200 IU to 400 IU in 2008.6–7 Recently, the Institute of Medicine (IOM) revised its RDA for vitamin D from 200 IU to 400 IU in infants (0 to 12 months) and from 200 IU to 600 IU in children (1 to 8 years).8 Without fortification, very few foods are rich in vitamin D. Humans meet their vitamin D needs from sunlight exposure, diet and/or supplements. Of these sources, photosynthesized vitamin D is the major contributor to human vitamin D status.9–11
Vitamin D-fortified milk and infant formula remains the main dietary source of vitamin D. An infant or a child must drink 4 servings (1 liter or 32 ounces) of vitamin D-fortified milk to meet the AAP recommended intake of vitamin D (400 IU/day), which seems to be a tall order for many. Therefore, vitamin D supplements become an important source of dietary vitamin D. In order to meet consumer desires to prevent deficiencies or optimize health benefits related to vitamin D, preparations of higher concentrations of over-the-counter vitamin D supplements have become readily available for the general public, with the potential for inadvertent overdosing in young infants and children.
Case Report
A 3-month-old Asian-American infant was evaluated by his primary care pediatrician for parental concerns of vitamin D overdosing. The infant was born at term after an uncomplicated pregnancy, labor, and delivery to a 43-year-old primiparous college-educated mother. As the infant was exclusively breastfed, oral vitamin D supplementation was started on day 5 (400 IU once daily). During subsequent visits, the parents had confirmed that they were administering 1 mL daily of D-Vi-Sol (400 IU/mL) infant vitamin D solution.
At 3 months, infant’s mother notified the pediatrician of a dosing error she had made in administering a new brand of infant vitamin D liquid preparation. The new vitamin D preparation was bought at a free-standing vitamin store. The concentration of vitamin D varied significantly between the 2 preparations: the new preparation provided 400 IU of vitamin D per drop, unlike D-Vi-Sol which had 400 IU of vitamin D per mL. Without realizing the variation in the concentrations between the two preparations, the parent had given the infant 1 mL daily of the new preparation for 20 days. Counting 30 drops in the 1 mL dropper, the mother correctly calculated that her infant had been receiving a 30-fold overdose of vitamin D (12,000 IU) daily. The infant was clinically stable with no symptoms and was feeding and acting well. Parents were advised to discontinue infant and maternal vitamin D supplementation and have the infant evaluated in the office the following day.
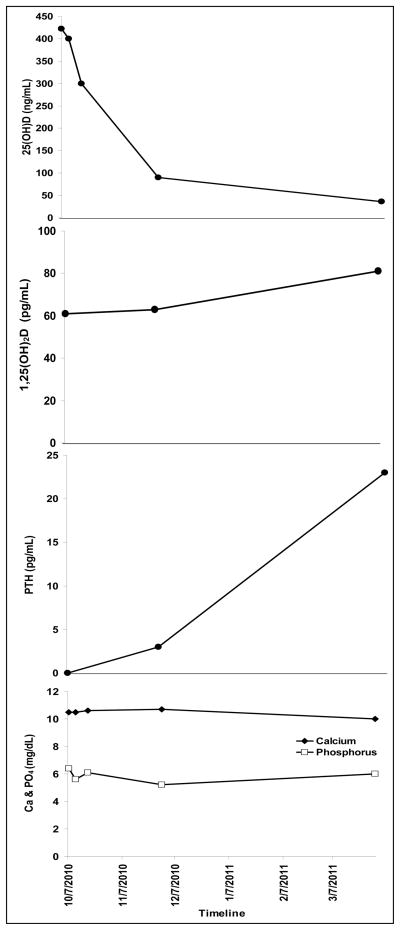
The infant had no history of irritability, constipation or abnormal movements. Examination revealed a calm, alert, well-appearing infant (wt 5.6 kg; 3rd–15th WHO percentile) with no focal findings except slightly increased patellar deep tendon reflexes; no clonus was present. Serum calcium (10.5 mg/dL, reference range 8.8 – 10.8 mg/dL), phosphorus (6.4 mg/dL, reference range 4.5 – 6.5 mg/dL), electrolyte panel, creatinine, and blood urea nitrogen were normal. Serum 25-hydroxyvitamin D [25(OH)D] was markedly elevated (422 ng/mL, reference range 30 – 100 ng/mL12), 1,25-dihydroxyvitamin D [1,25(OH)2D] was normal (61 pg/mL, reference range 27 – 71 pg/mL) and parathyroid hormone (PTH) was suppressed (<3 pg/mL, reference range 15 – 65 pg/mL), (Figure 1).
Figure 1.
Timeline of changes in serum 25-hydroxyvitamin D [25(OH)D]; 1,25-dihydroxyvitamin D [1,25(OH)2D]; parathyroid hormone (PTH); calcium (Ca), (-◆-); and phosphorus (PO4) (-□-).
In addition, the vitamin bottle was sent to the lab of one of the authors (MFH) to determine the concentration of vitamin D. The average of three samples from the bottle was 374.3 IU per drop, confirming the concentration stated on the bottle label.
The infant was frequently seen for clinical and laboratory follow-up over the next six months. The baby has continued with normal growth and development, and no apparent ill effects of the vitamin D overdose. There was a steady decline in serum 25(OH)D and return of PTH to normal, and calcium and phosphorus levels remained normal throughout the follow-up period (Figure 1).
The infant’s pediatrician (ECR) contacted the Food and Drug Administration (via a MedWatch Report) and the vitamin distributor, and alerted the network of local pediatricians about the potential risk for hypervitaminosis D arising from dosing errors with high-concentration over-the-counter vitamin D preparations. One day after notifying the vitamin store, the company decided to pull the product from its shelves and online store.
Discussion
Our case vignette highlights the potential risk for hypervitaminosis D, a prelude to vitamin D intoxication, from accidental over-dosing with over-the-counter high concentration liquid vitamin D preparations. Data regarding hypervitaminosis D (defined as above the reference range of 25(OH)D >100 ng/mL) in infants are limited. In this infant, ingestion of 240,000 IU of vitamin D3 (12,000 IU daily × 20 days) resulted in hypervitaminosis D with concentrations of serum 25(OH)D greater than 400 ng/mL and was tolerated without evidence of vitamin D toxicity, i.e. hypercalcemia and hyperphosphatemia.
The current IOM-set tolerable upper limit for vitamin D intake for infants aged 0 to 6 months is 1000 IU/day and for infants aged 6 to 12 months is 1500 IU/day.8 Pooled data from vitamin D trials in adults have shown that intakes of vitamin D up to 10000 IU/day are safe13, however such data are not available for infants and children. Although vitamin D has a significant safety profile, excessive intakes of vitamin D over prolonged period of time poses the risk for hypervitaminosis D and subsequent vitamin D toxicity.
Vitamin D (cholecalciferol [vitamin D3] and ergocalciferol [vitamin D2]) are fat-soluble vitamins with a long whole-body half-life (≈2 months), and when absorbed from the gut, they are stored in body-fat depot and metabolized slowly.14 Therefore, when ingested in excess, its intoxication effects can last for several months. Hypercalcemia is the primary complication of vitamin D intoxication and is associated with increased circulating concentrations of 25(OH)D.8,14 Symptoms of vitamin D intoxication are non-specific and are due to hypercalcemia, and include reduced appetite, nausea, vomiting, fatigue, weakness, weight loss, constipation, abdominal pain, polydypsia, polyuria, headache, depression or apathy, confusion and disorientation.8, 14–16 Hypercalcemia associated with vitamin D intoxication stems from increased absorption of calcium from the intestinal tract and increased bone resorption.8, 14, 17 Hypercalcemia, when prolonged, can cause metastatic vascular calcifications, nephrocalcinosis, nephrolithiasis, renal failure, and cardiac failure and death.
It is recognized that 25(OH)D is the vitamin D metabolite that mediates the toxic effects of hypervitaminosis D.8, 14 This is confirmed by our observation that serum 1,25(OH)2D was normal when 25(OH)D was markedly elevated. At the 6 month follow-up, 1,25(OH)2D was slightly elevated (81 pg/mL, reference range 27 – 71 pg/mL), which is most likely due to the fact that PTH levels returned to normal. PTH is a recognized stimulator of 1-α hydroxylase18–20, the enzyme that converts 25(OH)D to 1,25(OH)2D. The threshold levels of 25(OH)D associated with vitamin D toxicity are not well characterized, and the concentrations of 25(OH)D and the dose of vitamin D associated with toxic manifestations have wide variability between subjects.21 Toxic effects of hypercalcemia secondary to hypervitaminosis D is usually evident when intakes of vitamin D exceed 25,000 IU/day and the concomitant serum 25(OH)D level is at least 200 ng/mL.8 However the toxicity thresholds for vitamin D intake have varied from 10,000 IU to 40,000 IU/day, and have been associated with concentrations of serum 25(OH)D up to 200 to 240 ng/mL, with a tendency for frank toxicity at concentrations of 25(OH)D above 300 ng/mL.8
Hypervitaminosis D has been attributed to a variety of factors including over-fortification of milk21–22, infant formula23, and over-the-counter vitamin D supplements16, 24–25, contaminated foods26–28, and dosing errors.15, 29 Parents were recently warned about the risk of infant overdose of liquid vitamin D due to inappropriately large droppers in the FDA MedWatch Safety Alert.26 Such a packaging flaw [including a dropper marked to 1.0 mL with a bottle containing high concentration vitamin D solution (400 IU/drop)] was precisely the cause of the parent dosing error in this case.
Recognizing vitamin D intoxication can be difficult as the initial symptoms of toxicity are non-specific. Clinicians should remain aware of this entity and elicit history regarding use, brand and dosing of over-the-counter supplements in order to make a timely diagnosis and initiate treatment. Pertinent treatment of hypervitaminosis D/vitamin D intoxication associated with hypercalcemia would include preventing further ingestion of vitamin D and reducing calcium intake. Various modalities of treatment of hypercalcemia associated with vitamin D intoxication include intravenous hydration and administration of loop-diuretics, calcitonin, glucocorticoids, and bisphosphonates.17, 29
Our vignette also highlights the advocacy role pediatricians can play in preventing potentially harmful dosing errors from occurring. In this case, one phone call to the distributor prompted a complete recall of the product. However, other high concentration vitamin D preparations remain on the market. Accordingly, pediatricians should continue to educate caregivers about proper dosing of vitamin D supplements, specific to the product concentration.
Conclusion
This case report illustrates the consequence of parental dosing error in the use of a high concentration over-the-counter vitamin D preparation. Pediatricians should be cognizant that over-the-counter high concentration vitamin D preparations pose the potential risk for hypervitaminosis D if they are inappropriately dosed. Awareness of this possibility would help in early recognition of hypervitaminosis D as its symptoms are often non-specific.
Acknowledgments
Dr. Rajakumar is supported by a K23HD052550 award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD).
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- 1.Rajakumar K, Greenspan SL, Thomas SB, Holick MF. SOLAR ultraviolet radiation and vitamin D: a historical perspective. Am J Public Health. 2007;97(10):1746–1754. doi: 10.2105/AJPH.2006.091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreiter SR, Schwartz RP, Kirkman HN, Jr, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137(2):153–157. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 3.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124(3):e362–370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404–1410. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajakumar K, Thomas SB. Reemerging nutritional rickets: a historical perspective. Arch Pediatr Adolesc Med. 2005;159(4):335–341. doi: 10.1001/archpedi.159.4.335. [DOI] [PubMed] [Google Scholar]
- 6.Gartner LM, Greer FR. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111(4 Pt 1):908–910. doi: 10.1542/peds.111.4.908. [DOI] [PubMed] [Google Scholar]
- 7.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 9.Haddad JG, Jr, Hahn TJ. Natural and synthetic sources of circulating 25-hydroxyvitamin D in man. Nature. 1973;244(5417):515–517. doi: 10.1038/244515a0. [DOI] [PubMed] [Google Scholar]
- 10.Lawson DE, Paul AA, Black AE, Cole TJ, Mandal AR, Davie M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br Med J. 1979;2(6185):303–305. doi: 10.1136/bmj.2.6185.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poskitt EM, Cole TJ, Lawson DE. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. Br Med J. 1979;1(6158):221–223. doi: 10.1136/bmj.1.6158.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 14.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 15.Barrueto F, Jr, Wang-Flores HH, Howland MA, Hoffman RS, Nelson LS. Acute vitamin D intoxication in a child. Pediatrics. 2005;116(3):e453–456. doi: 10.1542/peds.2004-2580. [DOI] [PubMed] [Google Scholar]
- 16.Kaptein S, Risselada AJ, Boerma EC, Egbers PH, Nieboer P. Life-threatening complications of vitamin D intoxication due to over-the-counter supplements. Clin Toxicol (Phila) 2010;48(5):460–462. doi: 10.3109/15563650.2010.486382. [DOI] [PubMed] [Google Scholar]
- 17.Rizzoli R, Stoermann C, Ammann P, Bonjour JP. Hypercalcemia and hyperosteolysis in vitamin D intoxication: effects of clodronate therapy. Bone. 1994;15(2):193–198. doi: 10.1016/8756-3282(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 18.Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;381(1):143–152. doi: 10.1006/abbi.2000.1970. [DOI] [PubMed] [Google Scholar]
- 19.Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, et al. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci U S A. 1998;95(4):1387–1391. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garabedian M, Holick MF, Deluca HF, Boyle IT. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobus CH, Holick MF, Shao Q, Chen TC, Holm IA, Kolodny JM, et al. Hypervitaminosis D associated with drinking milk. N Engl J Med. 1992;326(18):1173–1177. doi: 10.1056/NEJM199204303261801. [DOI] [PubMed] [Google Scholar]
- 22.Blank S, Scanlon KS, Sinks TH, Lett S, Falk H. An outbreak of hypervitaminosis D associated with the overfortification of milk from a home-delivery dairy. Am J Public Health. 1995;85(5):656–659. doi: 10.2105/ajph.85.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nako Y, Tomomasa T, Morikawa A. Risk of hypervitaminosis D from prolonged feeding of high vitamin D premature infant formula. Pediatr Int. 2004;46(4):439–443. doi: 10.1111/j.1442-200x.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 24.Klontz KC, Acheson DW. Dietary supplement-induced vitamin D intoxication. N Engl J Med. 2007;357(3):308–309. doi: 10.1056/NEJMc063341. [DOI] [PubMed] [Google Scholar]
- 25.Koutkia P, Chen TC, Holick MF. Vitamin D intoxication associated with an over-the-counter supplement. N Engl J Med. 2001;345(1):66–67. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 26.Down PF, Polak A, Regan RJ. A family with massive acute vitamin D intoxication. Postgrad Med J. 1979;55(654):897–902. doi: 10.1136/pgmj.55.650.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettifor JM, Bikle DD, Cavaleros M, Zachen D, Kamdar MC, Ross FP. Serum levels of free 1,25-dihydroxyvitamin D in vitamin D toxicity. Ann Intern Med. 1995;122(7):511–513. doi: 10.7326/0003-4819-122-7-199504010-00006. [DOI] [PubMed] [Google Scholar]
- 28.Vieth R, Pinto TR, Reen BS, Wong MM. Vitamin D poisoning by table sugar. Lancet. 2002;359(9307):672. doi: 10.1016/S0140-6736(02)07814-5. [DOI] [PubMed] [Google Scholar]
- 29.Bereket A, Erdogan T. Oral bisphosphonate therapy for vitamin D intoxication of the infant. Pediatrics. 2003;111(4 Pt 1):899–901. doi: 10.1542/peds.111.4.899. [DOI] [PubMed] [Google Scholar]