Abstract
Interleukin (IL)-21-producing CD4+ T cells are central to humoral immunity. Deciphering the signals that induce IL-21 production in CD4+ T cells and those triggered by IL-21 in B cells are, therefore, of importance for understanding the generation of antibody responses. Here, we show that IL-6 increased IL-21 production by human CD4+ T cells, particularly in those that express the transcriptional regulator B cell lymphoma (BCL)6, which is required in mice for the development of CXCR5+ IL-21-producing T follicular helper (TFH) cells. However, retroviral overexpression of BCL6 in total human CD4+ T cells, only transiently increased CXCR5, the canonical TFH–defining surface marker. We show here that IL-21 was required for the induction of antibody production by IL-6. In IL-21–treated B cells, signal transducer and activator of transcription (STAT)3 was required for optimal Ig production and upregulation of PRDM1, the master plasma cell factor. These results, therefore, demonstrate the critical importance of STAT3 activation in B cells during IL-21-driven humoral immunity and suggest that BCL6 expression, while not sufficient, may serve as a platform for the acquisition of a TFH–like phenotype by human CD4+ T cells.
Keywords: human, plasma cell differentiation, IL-6, IL-21, STAT3, TFH cells, BCL6
Introduction
During an adaptive immune response activated CD4+ T helper (TH) cells provide help to B cells by cell surface signals and by secreting cytokines to promote B cell activation, induction of Ig isotype switching, and terminal differentiation into Ig-producing plasma cells. There is considerable diversity in TH subsets that provide B cell help. Cytokines present during initial CD4+ T cell activation enact distinct transcriptional programs that control differentiation of TH subsets1. In particular, T follicular helper cells (TFH), have also been proposed as a distinct, dedicated B cell helper TH lineage2. TFH cells are defined by their presence in the B cell areas of germinal centers (GCs), high sustained expression of the GC–homing chemokine receptor CXCR5, and ability to promote differentiation of B cells to plasma cells3,4.
IL-21 is key cytokine produced TFH cells5,6. IL-21 is a common-γ chain cytokine with a critical function in promoting differentiation of activated B cells into plasma cells7,8. IL-21 activates several signaling pathways in lymphocytes such as Janus kinase (Jak)/ signal transducer and activator of transcription (STAT) pathway, mitogen activated protein kinases (MAPKs) and PI-3K9. In primary human B cells we10 and others11 have shown robust activation of STAT3 by IL-21. Indeed, forced activation " of STAT3 was sufficient to promote Ig secretion and plasma cell differentiation by activated B cells10.
Terminal differentiation of B cells into plasma cells during the production of an effective humoral immune response is controlled by the transcriptional repressors B cell lymphoma (BCL)6 and B lymphocyte induced maturation protein (BLIMP)-112. BCL6 represses PRDM1, the gene encoding BLIMP-1, and BLIMP1 represses the BCL6 gene13,14. In B cells, BCL6 expression appears to endow GC B cells with proliferative potential and the ability to endure the temporary genetic instability associated with class switch recombination and somatic hypermutation15. In contrast, BLIMP1 suppresses proliferation and enhances the protein production machinery of the endoplasmic reticulum necessary for high-level Ig secretion by plasma cells13,16. The BCL6-BLIMP1 axis also controls the development of TFH cells 2. BCL-6 is required for TFH cell differentiation17-19 and Blimp-1 counteracts this process17. Accordingly, human TFH cells also express high levels of BCL6 and low levels of PRDM120,21. Although it is not completely clear how BCL6 and BLIMP1 levels are controlled during TFH differentiation, there is evidence for cytokine and cellular help from antigen presenting cells (APCs) in this process21,22.
IL-6 has been recently shown to activate IL-21 production by murine CD4+ T cells23-25. Given that similar transcriptional mechanisms and cytokines (i.e. IL-6, IL-21, STAT3, BLIMP1, and BCL6) are involved in both T and B cells in the development of the antibody response, we assessed cell-specific responses and requirements for these factors during induction of Ig production and TFH development. First, we describe a role for IL-6 in promoting Ab production in complex cellular conditions through the enhanced production of IL-21 by IL-6-exposed CD4+ T cells. Second, we have determined a requirement for STAT3 expression in IL-21-mediated B cell differentiation at the level of Ig production and transcriptional activation of PRDM1 in normal human B cells. Lastly, in line with the ability of IL-6 to promote changes in CD4+ T cells consistent with TFH cells, (i.e. enhanced IL-21 production and expression of BCL6), we also observed that overexpression of BCL6 itself increased expression of the TFH–associated markers CXCR5 and CXCR4. Thus, IL-21 and STAT3 are required for plasma cell differentiation and antibody production by CD4+ T cells. Our results also reveal that BCL6 expression is involved in the early acquisition of the human CXCR5+ TFH phenotype.
Results
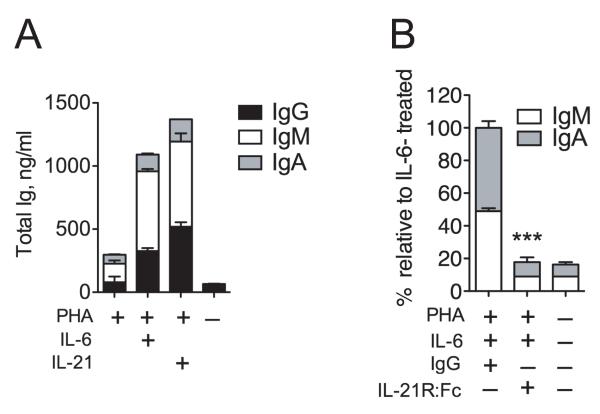
The cytokine environment during T cell-dependent activation of B cells can strongly influence antibody production. We, therefore, directly compared the B cell helper activity of T cell– and non-T cell–derived cytokines in response to T cell activation in a multicellular context. We found that in contrast to IL-2 or IL-4, both IL-6 and IL-21 significantly enhanced immunoglobulin (Ig) secretion in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) cultures (Fig. 1). B and T cell frequencies in the cultures (9 ± 2% and 70 ± 10%, respectively) were not affected by any cytokine during the culture (not shown). Similar results were obtained with anti-CD3/anti-CD28 stimulation (not shown). These results suggested that IL-6 or IL-21 were able to elicit Ig production by B cells in the presence of activated T cells.
Figure 1. Interleukin-6 induces Ig secretion in a multicellular context.
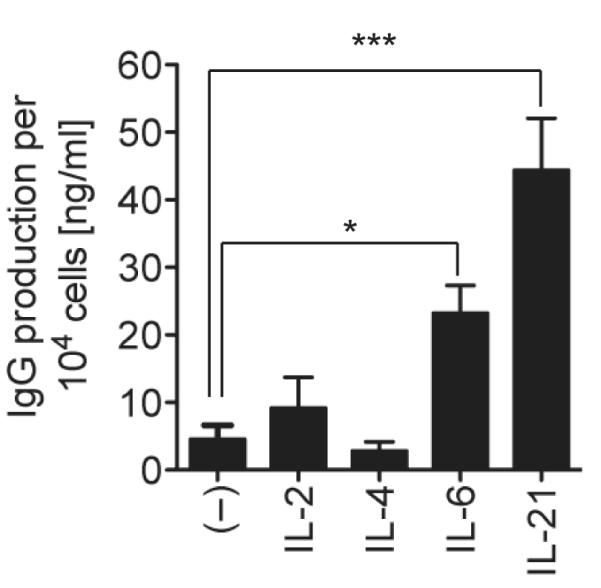
PBMCs (5 × 105/ml) were stimulated with (PHA (2 μg/ml) and the indicated cytokines: IL-2 (40 U/ml), IL-4 (10 ng/ml), IL-6 (100 ng/ml, or IL-21 (50 ng/ml) for 7 days. Cells were counted and IgG production relative to cell number was determined by ELISA. Results are means ± SD of two independent experiments using three different donors. Statistical significance (*, P < 0.05; ***, P < 0.001) was determined using one-way ANOVA with Dunnett’s multiple comparison test.
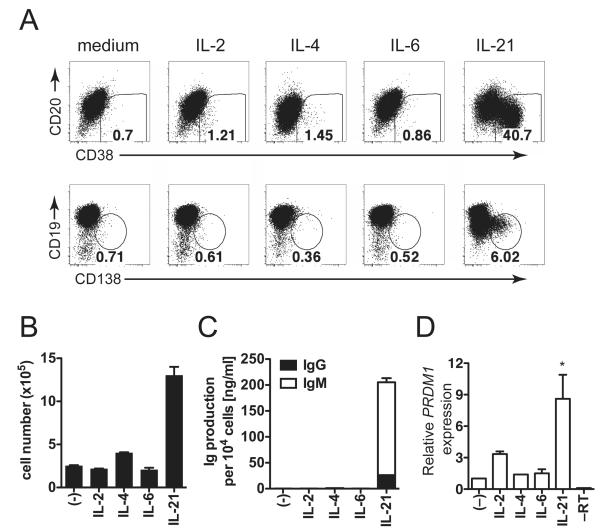
To determine B cell–specific effects of these cytokines, we cultured B cells with these cytokines and irradiated CD40L-expressing L cells (CD40L-L cells), to simulate T cell help in the GC IL-21 induced robust plasma cell differentiation as evidenced by the appearance of CD38+CD20lo cells (Fig. 2A, upper panels). Likewise, IL-21–treated B cells exhibited a CD138+CD19lo phenotype (Fig. 2A, lower panels). Neither IL-2 nor IL-4 increased plasma cell formation in CD40L-activated B cell cultures. IL-6 has been described to induce plasmablast survival, but despite IL-6Rα expression on CD40L-activated B cells, IL-6 did not induce plasma cell differentiation. We examined cell number in these cultures and found that while IL-4 did not induce differentiation, this cytokine modestly increased total cell number (Fig. 2B). IL-21 promoted robust B cell proliferation, while IL-6 did not (Fig. 2B). We then analyzed Ig production and, found that only IL-21 significantly augmented Ig secretion on a per cell basis, while neither IL-4 nor IL-6 had this effect (Fig. 2C). To further demonstrate that IL-21 specifically mediated plasma cell differentiation, we also examined the gene expression level of PRDM1, the gene encoding BLIMP126. IL-21 strongly upregulated PRDM1 expression in CD40L-activated human B cells (Fig. 2D). IL-4 and IL-6 did not promote PRDM1 expression and IL-2 had only a minor effect, though not statistically significant. These results are consistent with the known role for IL-21 in the initiation of human plasma cell differentiation10,27, but do not support a role for IL-6 acting directly on B cells to initiate plasma cell differentiation and promote Ig production.
Figure 2. IL-21, but not IL-6 directly initiates plasma cell differentiation.
Total CD19+ B cells were stimulated for 5 days with CD40L-L cells in the presence of the indicated cytokines (concentrations as in Fig. 1). (A) Staining for CD38, CD20, CD19, and CD138 in activated B cells. (B) Total cell numbers were determined. (C) IgG and IgM were measured by ELISA in supernatants from B and values are shown as production per 104 cells. (D) CD19+ B cells were stimulated with CD40L-L cells for 5 days in the presence of cytokines and PRDM1 mRNA levels were determined by qRT-PCR. ACTB levels were used for normalization. Results are means ± SD of three independent experiments using different donors. Statistical significance (*, P < 0.05) was determined by one-way ANOVA with Dunnett’s multiple comparison test.
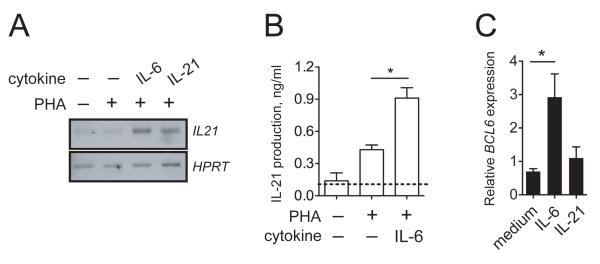
Since addition of IL-6 did not directly trigger Ig production by activated purified B cells, but did so in a multicellular context when activated T cells were present, we hypothesized that the effect of IL-6 was indirect, presumably mediated by T cells. In the GC, activated TFH cells produce large quantities of IL-215,6, the most potent known initiator of human plasma cell differentiation28,29. We, therefore, investigated whether IL-6 could induce the production of IL-21 by human CD4+ T cells. Total CD4+ T cells purified from peripheral blood of healthy donors were activated with PHA in the presence or absence of IL-6. Exogenous IL-6 as well as IL-21 induced IL21 gene expression (Fig. 3A). In addition, increased levels of secreted IL-21 protein were detected in supernatants of CD4+ T cells activated in the presence of IL-6 (Fig. 3B).
Figure 3. IL-6 induces IL-21 and BCL6 in human CD4+ T cells.
(A) CD4+ T cells were activated for 2 days with PHA (2 μg/ml) in the presence of IL-6 (100 ng/ml) or IL-21 (50 ng/ml) and RT-PCR for IL21 was performed using HPRT as a control. (B) Human CD4+ T cells from donors were activated with PHA in the presence or absence of human IL-6 (100 ng/ml) for 2 days. IL-21 production was determined by ELISA. (C) CD4+ T cells were activated for 2 days with PHA in the presence of IL-6 (100 ng/ml) or IL-21 (50 ng/ml) and qRT-PCR for BCL6 and ACTB was performed. Values were normalized to ACTB and expressed relative to control. Results are means ± SD of duplicates of two independent donors. Statistical significance (*, P < 0.05) was determined using unpaired Student’s t-tests.
The GC-specific transcriptional repressor BCL6 is highly expressed in IL-21-secreting human tonsillar T cells20,21 FH and plays a critical role in TFH cell programming in mice5,17,18. Since IL-6 and " IL-21 increased IL-21 mRNA and protein in total CD4+ T cells, we analyzed whether IL-6 could influence BCL6 levels. In accordance with its positive effect on IL-21 production, IL-6 significantly (2.9 ± 0.6-fold) increased BCL6 transcript levels, while IL-21 did not affect BCL6 levels (Fig. 3C). Our results suggest that IL-6 promotes IL-21 production and upregulation of BCL6 in primary human CD4+ T cells.
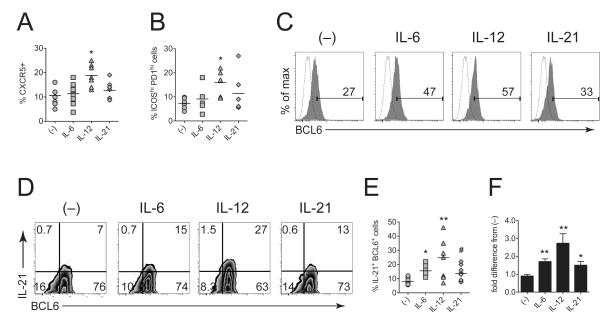
We next investigated whether IL-6 could promote the canonical CXCR5+ PD1hiICOShi TFH phenotype in vitro. IL-6 failed to induce a significant upregulation of these surface markers in activated CD4+ T cells (Fig. 4A, 4B, S1A, B). As a positive control, we confirmed that IL-12 significantly increased the proportion of cells with the TFH phenotype which is in line with other studies21,22, while lL-21, like IL-6, only marginally increased TFH surface marker expression (Fig. 4A, 4B). In confirmation of our mRNA data, however, IL-6 increased intracellular BCL6 expression in activated CXCR5+CD4+ T cells (Fig. 4C), but to a lesser extent than IL-12. We also sought to confirm and extend our ELISA data by intracellular flow cytometric analysis of IL-21 in CXCR5+ cells. Upon PMA/ionomycin treatment to elicit cytokine production, BCL6 expression in CXCR5+CD4+ T cells was uniformly increased as expected30, but IL-6, IL-12, and IL-21–stimulated cells exhibited a specific increase in the proportion of BCL6+IL-21+ cells (Fig. 4D, E). Normalization to no cytokine (–) control revealed significant effects on the proportion of BCL6+IL-21+ cells by culture with IL-6, IL-12, or IL-21 (Fig. 4F). In agreement with other studies21,31 these data show the potency of non-T cell–derived cytokines, namely IL-6 and IL-12, in the induction of IL-21 production by human CD4+ T cells.
Figure 4. IL-6 increases expression of IL-21 and BCL6, but not surface TFH markers.
Total CD4+ T cells were stimulated with CD3/CD28 beads for 5 days in the presence of no cytokine (–), IL-6, IL-21, or IL-21. Immediately after culture, CD4+ T cells were analyzed for surface CXCR5, ICOS, and PD1 expression by flow cytometry (see Fig S1 for representative FACS plots). Frequencies were determined from isotype control stainings using a sample pooled from the shown experiments). Quantitation of CXCR5+ cells within the CD3+CD4+–gated cells (A) and ICOS/PD1 (B) expression on CXCR5+CD4+–gated T cells is shown. (C) Intracellular BCL6 expression in CD3/CD28-activated CXCR5+CD4+–gated cells. Immediately after culture, cells were surface stained, fixed, permeablized and stained for intracellular BCL6. Number on graph indicates percent BCL6–positive cells. Dashed histogram is isotype control. (D) For intracellular IL-21 detection, CD3/CD28-stimulated cells were washed and restimulated with PMA and ionomycin in the presence of monensin for 6 hrs, surface stained, fixed, permeabilized, and stained for intracellular BCL6 and IL-21. Isotype control staining was used to determine frequencies. (E) Frequency of IL-21+BCL6+ cells (gated on CXCR5+CD4+ cells) and (F) and the fold difference in IL-21+BCL6+ cells as compared to no cytokine control. Graphical results show mean values and are derived from 7 donors in two separate experiments and FACS plots are representative. Statistical significance across donors (*, P < 0.05; **, P < 0.01; #, P = 0.06 vs no cytokine control) was determined by pairwise Mann-Whitney tests.
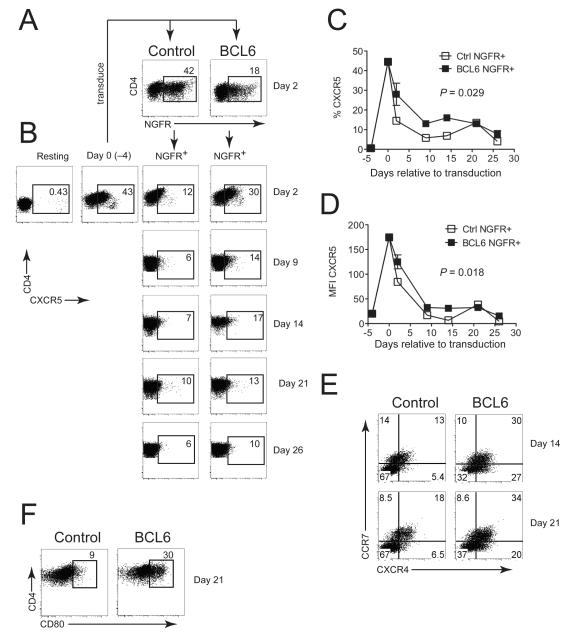
BCL6 has been implicated in the regulation of T differentiation17,18,20 FH and sustained CXCR5 is a key marker for TFH cells in both mouse and human1,2,20,21,32. Since IL-6 triggered upregulation of BCL6 and T-dependent Ig production by induction of IL-21, we hypothesized that BCL6 expression may enhance CXCR5 expression on human CD4+ T cells. To address this question, we retrovirally overexpressed BCL6 in total CD4+ T cells (Fig. 5A), cultured transduced cells on autologous irradiated feeder cells in the presence of PHA and IL-2, and monitored CXCR5 surface expression (Fig. 5B). Unlike its positive effect on long-term expansion in human B cells33, over-expression of BCL6 did not have this effect on transduced CD4+ T cells (Fig. S2A). In agreement with previous results34, CXCR5 expression rapidly increased after culture in vitro (Fig. 5B). In transduced (NGFR-gated) cells, BCL6 caused a transient, but statistically significant increase in the frequency (Fig. 5C) of CXCR5+ cells and CXCR5 mean fluorescence (Fig. 5D) compared with control transduced NGFR+ cells. As an additional control for bystander effects, CXCR5 levels were not affected in non-transduced NGFR− cells present in the same cultures (Fig. S2B, C). In addition, we assessed other markers involved in lymphoid/follicle homing. The B cell zone-localizing chemokine receptor CXCR4, which is expressed on TFH cells2, was stably increased in BCL6-transduced cells (Fig. 5E, Fig S2D). We also observed that CCR7 was upregulated on BCL6-transduced cells (Fig. 5E, Fig S2E). This was surprising considering that CCR7 is thought to be low on TFH cells2 due to its role in exclusion from B cell areas in the follicle, but a recent study identified CCR7+Bcl6+ central memory CD4+ T cell (TCM) precursors 35. As a marker for the heightened activation status of BCL6-expressing cells we examined CD80 expression36, which was significantly increased in BCL6-expressing CD4+ T cells compared with those transduced with control vector (Fig. 5F, Fig S2F). Taken together, these results suggest that enforced BCL6 expression in human CD4+ T cells transiently enhances surface CXCR5 expression on activated CD4+ T cells as well as stably increasing other markers associated with trafficking between B and T cell zones and T cell activation.
Figure 5. Enforced BCL6 expression enhances lymphoid/follicular markers, including CXCR5 in primary human CD4+ T cells.
Total CD4+ T cells from peripheral blood were stimulated with CD3/CD28 beads for 4 days and transduced (A) with retroviral vectors encoding either LZRS-IRES-ΔNGFR (Control) or LZRS-BCL6-IRES-ΔNGFR (BCL6). (B) CXCR5 surface expression on resting, activated (4 days), or Control and BCL6-transduced CD4+ T cells at the indicated timepoints after transduction. Transduced cells were cultured on irradiated allogeneic PBMC feeders with PHA (2 μg/ml) and IL-2 (20 U/ml) (methods). (C) Percent CXCR5+ and (D) CXCR5 mean fluorescence intensity (MFI) of CD4+NGFR+ cells over time. (E) CCR7/CXCR4 expression in CD4+NGFR+ cells at Days 14 and 21 after transduction. (F) CD80 expression on CD4+NGFR+ cells 21 days after transduction. For panels E and F, Gates were set using isotype control stainings and total tonsil cells to define positive populations as described for CXCR4 and CD8061. CCR7 gates in panel E were defined as described62. Graphical results are means ± SD of two independent experiments each containing two donors and FACS plots are representative. Two-way ANOVA was used to calculate statistical significance (P-values shown on graphs) in panels C and D. Quantitated data for CXCR4/CCR7 and CD80 are shown in Fig S2.
Both IL-21 and BCL6 expression in T cells help to facilitate antibody responses2. We then hypothesized that IL-6 endowed activated CD4+ T cells with B helper capacity. To test this, we analyzed the effect of IL-6 on Ig production in co-cultures with CD4+ T cells and B cells. We first treated total CD4+ T cells with mitomycin-c to induce cell cycle arrest and incubated these cells with autologous CD19+ B cells and PHA in the presence or absence of IL-6 or IL-21. Upon treatment with PHA and IL-6 or IL-21, the co-cultured total B cells (including both naïve and switched memory cells) were induced to produce IgG, IgM, and IgA (Fig. 6A). In the absence of either T cells or PHA, IL-6 did not induce Ig secretion. To establish that IL-6 mediated the B helper effect of CD4+ T cells via IL-21, we utilized an IL-21R:Fc fusion protein to block IL-21 function in vitro. Addition of IL-21R:Fc, but not control hIgG, to IL-6–treated co-cultures resulted in a block in the production of IgM and IgA (Fig. 6B). IgG was detected as a control to ensure addition of the hIgG or IL-21R:Fc (IgG subtype) (not shown). Taken together, these results show that production of IL-21 by activated CD4+ cells is required for IL-6-mediated antibody production in a multicellular context.
Figure 6. IL-21 is required for IL-6-induced Ig production during T-B cell collaboration.
(A) Mitomycin-c–treated CD4+ T cells (1 × 105) were cultured with 1 × 105 CD19+ B cells in 0.2 ml in the presence of PHA with medium, IL-6, or IL-21 and Ig production was determined after five days by ELISA. (B) IL-6–stimulated co-cultures of T and B cells were treated for five days with either control Ig (10 μg/ml) or IL-21R:Fc fusion protein (10 μg/ml). Results are means ± SD of three independent donors. Statistical significance (***, P < 0.001) was determined using unpaired Student’s t-tests.
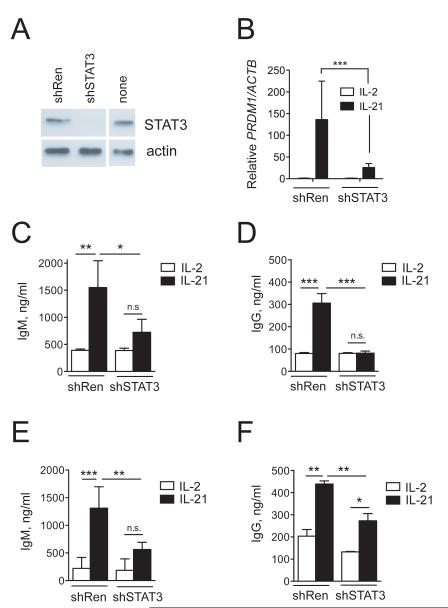
IL-21 potently activates STAT3 in human B cells10,11. B cell-specific loss of Stat3 in mice leads to impaired antibody responses37 and mutation of STAT3 in humans leads to dysregulated humoral immunity11,38. We therefore directly tested whether upregulation of PRDM1 and Ig production by IL-21 in B cells were dependent upon STAT3. To address this we utilized stable retroviral overexpression of shRNA targeting STAT3. We first tested the STAT3 shRNA efficacy in Raji B cells expressing endogenous STAT3 (Fig. 7A). We transduced cells with control shRNA targeting firefly Renilla luciferase (shRen) or STAT3 shRNA, purified transduced GFP+ cells by flow cytometry, and determined STAT3 protein levels. STAT3 shRNA reduced STAT3 protein levels by ~90% (Fig. 7A). We next assessed the requirement for STAT3 in the induction of PRDM1 gene expression by IL-21 in normal primary human B cells. Total PB CD19+ cells were transduced with either shRen or shSTAT3, GFP-sorted, and cultured in the presence of IL-2 or IL-21 for three days. PRDM1 gene expression was upregulated by IL-21 in cells expressing control shRNA, while this effect was severely blunted in primary B cells expressing shSTAT3.
Figure 7. STAT3 is required for IL-21-induced plasma cell differentiation.
(A) Raji B cells were transduced with retrovirus encoding control shRNA targeting firefly Renilla luciferase (shRen) or with STAT3i (shSTAT3). Whole cell extracts from GFP-sorted cells were analyzed for STAT3 protein expression by immunoblot. Non-transduced (none) cells are shown as control. (B) Total CD19+ B cells were activated with CD40L, transduced with shRen or shSTAT3, and after 2 days GFP sorted, cultured with CD40L-cells and IL-2 or IL-21 (or: for 3 days). After three additional days, total RNA was isolated and PRDM1 and ACTB mRNA expression were analyzed by quantitative RT-PCR. PRDM1 expression was normalized to ACTB expression within each sample and the means +/− SD of triplicate measurements in two different donors is shown. (C to F) Naïve IgM+CD27− B cells (C,D) or total CD19 B cells (E,F) from peripheral blood were transduced with the indicated retrovirus, GFP-sorted and cultured on CD40L-L cells in the presence of IL-2 or IL-21 for three days. IgM (C,E) and IgG (D,F) were measured in the supernatant by ELISA. Results are means ± SD of two independent experiments each containing two donors. Statistical significance (*, P < 0.05; **, P < 0.01, ***, P < 0.001) was determined by one-way ANOVA with Tukey’s post-test for differences.
We then tested the requirement for STAT3 in IL-21-induced IgM and IgG production by both naïve (IgD+CD27−) and total CD19+ B cells from peripheral blood. IL-21 promoted IgM (Fig. 7C) and IgG (Fig. 7D) production by GFP+ control-transduced naïve B cells. In contrast, the ability of IL-21 to promote IgM (Fig. 7C) and IgG (Fig. 7D) production by naïve B cells was sharply reduced in cells transduced with shSTAT3. In total CD19+ cells (containing 71 ± 5% IgD+CD27− naïve B cells and 7 ± 3% IgD−CD27+ memory B cells), IL-21 also induced IgM (Fig. 7E) and IgG (Fig. 7F) production in control-transduced B cells. Similar to the effect seen in naïve B cells, shSTAT3-transduced total B cells also exhibited reduced IgM production in response to IL-21 (Fig. 7E). Although IL-21 induced modest IgG production in shSTAT3-transduced total CD19+ B cells (Fig. 7F), these levels were still significantly reduced compared to those made by IL-21-treated shRen-transduced total CD19+ B cells (Fig. 7F). Together, these results demonstrate that IL-21 requires STAT3 to promote plasma cell differentiation and optimal Ig production in human B cells.
Discussion
Here we have investigated the molecular requirements for human T cell: B cell collaboration in the induction of antibody production. Non-T cell-derived cytokines such as IL-6 and IL-12 stimulated IL-21 production by CD4+ T cells, with or without acquisition of a canonical surface TFH phenotype. IL-12 promoted expression of CXCR5, ICOS, PD-1, BCL6 and IL-21 in activated CD4+ T cells. Although IL-6 failed to affect CXCR5, ICOS, or PD-1 expression, this cytokine significantly increased BCL6 and IL-21 production and perpetuated Ig production by B cells. Nonetheless, retroviral overexpression of BCL6 itself triggered only a brief upregulation of CXCR5, rather than the stable reprogramming observed in murine CD4+ T cells17. While multiple stimuli induce IL-21 production and contribute to the TFH phenotype, it is clear from our results using stable genetic reduction of STAT3 expression in normal B cells that this transcription factor is required for IL-21-induced B cell differentiation. These results, therefore, provide an important confirmation of previous results obtained in individuals containing rare STAT3 mutations11.
From our results and those from others it is becoming clear that non-T cell-derived cytokines are important in the induction of IL-21-mediated B cell helper activity by CD4+ T cells. In vitro, IL-12 has been shown to increase IL-21 production by naïve human CD4+ T cells and provide B cell help in an IL-21-dependent manner21,22. In the mouse IL-12 does not exert this effect23. Compared to IL-12, others have shown a less potent effect of IL-6 on IL-21 production in naïve human CD4+ T cells21. Nonetheless, our results show that the positive effect of IL-6 on IL-21 production by total CD4+ T cells and provision of B cell help is also dependent on IL-21. As to the in vivo source of IL-21-inducing cytokines, IL-6 and IL-12 are likely to be important since, in the GC these cytokines are produced by dendritic cells22,39,40 and IL-6 is produced by follicular dendritic cells41. Thus it is possible that these cytokines may synergize in vivo to induce IL-21 production and promote effective humoral immunity.
IL-21 itself has also been shown as a differentiation factor for TFH cells in the murine system5. We confirmed that IL-21 can autoregulate itself42 in total CD4+ T cells. Since IL-21 is produced predominantly by T cells, it is possible that IL-21 may reinforce TFH differentiation upon activation of naïve CD4+ T cells by APCs. Since our studies were performed in total CD4+ T cells, it is also possible that memory or differentiating effector CD4+ T cells selectively respond to IL-21 and upregulate its own expression. This issue may require further study for two reasons: (1) CXCR5+ TFH cells are not the only CD4+ T cells capable of producing IL-21 and (2) although CD45RO+ CXCR5+ TFH memory-like cells have been described, their ultimate contribution to long-lived humoral immunity has not been clarified2.
Expression of BCL6 in T cells has been shown to be essential for optimal IL-21 production and TFH development in vivo17-19. We show here that in accordance with the ability to promote IL-21 secretion, non-T cell-derived cytokines like IL-6 and IL-12 also induce BCL6 in CD4+ T cells. IL-21 itself, however, did not induce significant BCL6 expression although we observed autoregulation of IL-21. This is in contrast to B cells, where IL-21 induces BCL610,43,44. Perhaps regulation of BCL6 by IL-21 in CD4+ T cells is delayed compared to the effect of IL-6. Moreover, regulation of BCL6 is complex. Cytokine-mediated gene regulation, antigen receptor signaling and CD40L ligation all modulate BCL6 protein stability and its molecular interactions in B cells45-47, but whether similar mechanisms are at play in CD4+ T cells is unknown. We propose that non-T cell-derived cytokines like IL-6 and IL-12 are strong initial inducers of BCL6 and IL-21 expression and that IL-21 reinforces its own production in a mechanism that may or may not require BCL6. Consistent with this notion is the fact that many different GC-resident cells produce these initiating cytokines in vivo, while IL-21 production is restricted mainly to activated T cells48.
Our data here depict a complex mechanism regulating human TFH development. On the one hand, our data show that, in line with the ability of IL-6 to trigger IL-21 production in CD4+ T cells, IL-6 also increased BCL6 mRNA expression. Sustained CXCR5 expression is a key defining TFH cell marker1,2. BCL6 overexpression led to a transient upregulation of CXCR5 and a stable increase in other lymphoid/ follicle chemokine receptors (CCR7 and CXCR4, respectively) in CD4+ T cells. CCR7 expression is present on a proportion of CD4+CXCR5+CD200+ TFH cells in the tonsil20. Recently the development of CCR7+CD4+ TCM precursors was shown to require expression of Bcl635. We also note the increased expression of CD80 on BCL6-transduced CD4+ T cells. On the contrary, in GC B cells, BCL6 has been shown to downregulate CD80 expression49. CD80 expression on mouse T cells has been proposed to have an immunoregulatory role50, but high CD80 expression on murine TFH cells has also been reported51. CD80 expression on human CD4+ T cells is thought to be immunostimulatory36. Given that CD80 is a ligand in the PD-1/PD-L152 pathway and that high PD-1 expression correlates with high human T function53 FH it is possible that CD80 expression may help support the function of PD-1hi TFH cells. Taken together, acquisition of these markers under conditions of high BCL6 expression may, therefore, enable a subset of functional memory precursor CD4+ T cells to transit rapidly between T and B cell zones in follicles to promote the humoral response. Indeed expression of BCL6 has been previously linked to T cell memory 54,55.
Our data suggest that sustained BCL6 expression transiently increases CXCR5 expression in CD4+ T cells. However, the CXCR5 levels achieved are not as high as those found on bona-fide tonsil TFH cells (not shown). Thus, BCL6 expression may support, but is not solely sufficient for, the acquisition of a CXCR5hi TFH phenotype in human CD4+ T cells in vitro. Ex vivo sorted tonsillar CXCR5hi cells are BCL6+, IL-21+, and are efficient B cell helpers20,21. Since our culture conditions involved robust T cell stimulation, our results suggest that factors in addition to high BCL6 expression, TCR stimulation, and endogenously produced T-cell-derived cytokines are necessary to produce the sustained CXCR5hi TFH phenotype in human CD4+ T cells in vitro. Further study of CXCR5 gene regulation56 may reveal insights into factors necessary to convert human BCL6hi CD4+ T cells into CXCR5hi cells.
Irrespective of which factors may trigger IL-21 production, the presence of this cytokine strongly promotes B cell differentiation29. We showed that IL-21 was required for the indirect promotion of Ig production by IL-6 in CD4 T cell: B cell co-cultures. A similar dependence on IL-21 for IL-12-induced Ig production was also shown21,22. Activated human primary B cells upregulate PRDM1 and secrete Ig in response to IL-2110,27. We previously found that specific activation of STAT3 induced plasma cell differentiation and Ig secretion to levels similar to those induced by IL-2110. These results suggested, but did not demonstrate, a requirement for STAT3 in the induction of plasma cell differentiation and Ab secretion. Here we now show that the induction of PRDM1 by IL-21 requires activation of STAT3. Downregulation of STAT3 levels also led to a significantly blunted ability of B cells to produce IgM or IgG in response to IL-21. Our data indicate that both naïve and switched memory B cells require STAT3 for optimal Ab production in response to IL-21. Our results using normal cells from healthy donors are in concordance with findings in patients with inactivating STAT3 mutations wherein B cell responses to IL-21 are severely diminished11 Together these data demonstrate the critical importance of STAT3 activation for efficient generation of high-level Ab secretion by and its role as a critical molecular hub in the integration of plasma cell differentiation and production of both switched and non-switched Ig.
In summary, our data provide mechanistic insight into how human CD4+ T cells may respond to non-T cell-derived cytokines in order to promote humoral immunity via the IL-21/STAT3 axis. IL-6 and IL-12 triggered IL-21 production by CD4+ T cells, and IL-21 in turn induced PRDM1 gene expression and Ig production by B cells in a STAT3-dependent manner. IL-6 and IL-12 also upregulated expression of BCL6 in activated CD4+ T cells, which is associated with production of IL-21 and a TFH phenotype21. These results also posit that non-T cell-derived cytokines may synergize to drive IL-21 production in vivo. Our overexpression studies indicate that high BCL6 levels may serve as a platform for the acquisition of the TFH phenotype in humans, and suggest that additional cellular factors such as costimulation57,58 may be required to fully drive the TFH phenotype. Deciphering the signals required to manifest and maintain the TFH phenotype will be important for understanding of humoral immunity and for vaccine development.
Materials and Methods
Human cell isolation
Peripheral blood mononuclear cells (PBMCs) were obtained from leukofilters prepared from adult peripheral blood (Sanguin Blood bank, Amsterdam, Netherlands) or from venipuncture and separation by Ficoll-Paque gradients (GE Heatlthcare). Tissue use was approved by the AMC and UVM institutional review boards and was contingent upon informed consent. CD4+ T cells and CD19+ B cells were isolated with MACS®microbeads (Miltenyi Biotech). Purity was ≥97% as determined by flow cytometry. For isolation of naïve B cells, CD19+ MACS-selected B cells were sorted to 99.9% purity for CD19+CD3−IgM+CD27− phenotype on a FACSAria® (Becton Dickinson Immunocytometry Systems).
Cell culture
PBMC cultures
5 × 105 cells were stimulated with PHA (HA16, 2 μg/ml, Sigma-Aldrich) in a 1 ml culture in 24-well plates (Costar). Cells were cultured in Iscoves Modified Dulbecco’s Medium (IMDM, Invitrogen) supplemented with 8% FCS (Hyclone) and penicillin/streptomycin (Roche Applied Science).
B cell cultures
5 × 105 B cells were co-cultured on 105 γ-irradiated (50 Gy) mouse L cell fibroblasts stably expressing CD40L (CD40L-L cells) in IMDM 8% FCS. Cytokines used were IL-2 (40 U/ml), IL-4 (10 ng/ml, R&D Systems), IL-6 (100 ng/ml, Miltenyi), or rmIL-21 (50 ng/ml; R&D).
CD4+ T cell cultures
2–3 × 105 cells were stimulated with PHA (2 μg/ml) or with Dynabeads® CD3/CD28 T cell expander (Invitrogen/Dynal) and IL-6 (100 ng/ml, Miltenyi), rhIL-12 (20 ng/ml, R&D), or rhIL-21 (20 ng/ml, Peprotech).
CD4+ T cell
B cell co-cultures
MACS-selected CD4+ T cells were treated with mitomycin c (40 μg/ml, Sigma-Aldrich) for 1 hr at room temperature and washed 3 times in complete medium. Autologous T and B cells (each 105 cells per well) were cultured in 0.2 ml IMDM 8% FCS and stimulated with PHA (2 μg/ml), IL-6 (100 ng/ml, Miltenyi), control hIgG (10 μg/ml, Sigma Aldrich), hIL-21R:Fc (10 μg/ml, R&D), and rmIL-21 (50 ng/ml, R&D).
Flow cytometry analysis
The following mAbs against the human molecules CD3 (SK7), CD4 (RPA-T4), CD19 (4G7, SJ25C1, ID3, or HIB19), CD20 (2H7), CD27 (L128) (CD38 (HB7), IgM (G20-127), CXCR4, CCR7, or CD80 were directly conjugated to FITC, PE, PerCP-Cy5.5, PE-Cy7, APC, APC-Cy7, Horizon V450, or Horizon V500 and were purchased from BD-Pharmingen (BD Biosciences). PE-labeled anti-CD138 (MI15) was from DAKO. Anti-LNGFR (CD271) was from Miltenyi Biotech. For CXCR5 detection, PerCP-Cy5.5-labeled anti-CXCR5 (TG2, BioLegend) or a biotinylated anti-CXCR5 mAb (BD) plus PE-Cy-7–labeled streptavidin (BD) were used. Biotinylated anti-ICOS (D10.G4.1, BioLegend), FITC-conjugated anti-PD-1 (MIH4, BD Pharmingen), eFlour660–conjugated anti-IL-21 (3A3-N2, eBioscience), and PE-conjugated anti-BCL6 (K112-91, BD Pharmingen) were also used. For analysis of CXCR5, ICOS, PD-1, and BCL6, cells were first surface stained, fixed and permeabilized using Cytofix/Cytoperm (BD) and then stained for BCL6. For intracellular IL-21 analysis, 5 day-stimulated cells were washed and treated with phorbol-12-myristate-13-acetate (PMA, 100 ng/ml, Sigma) and ionomycin (750 ng/ml, Calbiochem) for 6 hours at 37°C with Monensin (2 μM, Sigma) for the last 4 hours. Cells were then surface stained, fixed and permeabilized, and then stained for intracellular IL-21 and BCL6. For surface stainings, DAPI-negative stained cells were analyzed. Single color controls and fluorescence minus one (FMO) staining59 was used to determine voltages and compensation. Data was collected on an LSR II (BD) and flow cytometry data were processed using FlowJo (TreeStar). Isotype control stainings and analysis of tonsil samples were used to determine gating. Histograms and bi-exponential dot plots represent log10 fluorescence intervals.
Retroviral constructs and virus production
DNA sequences encoding shRNA targeting STAT3 (350–gagtcgaatgttctctatc–369)38 or firefly Renilla luciferase (119-aaaacatgcagaaaatgctgt-139) were cloned into the pRETROSUPER (pRS) construct co-expressing GFP (pRS-pgk-GFP). The LZRS-IRES-ΔNGFR and LZRS-IRES-BCL6-ΔNGFR retroviral vectors have been described33. GALV-pseudotyped retroviruses were produced using the Phoenix GalV packaging cell line (kind gift of Garry Nolan, Stanford University, Stanford, CA, USA).
Transductions
T cell transductions
106 MACS-isolated CD4+ T cells were activated for 4 days in 1 ml medium with CD3/CD28 beads in the presence of 20 U/ml rhIL-2. Cells were resuspended in 1:1 mix of virus:medium, plated on Retronectin® (Takara)-coated nontreated 24-well tissue culture plates (30 μg/ml in PBS for 2 hr at room temperature followed by 30 min of blocking with 2% human serum albumin in PBS), spun at 360 × g for 60 min at 30°C, and cultured overnight at 37°C. Transduced cells (106) were then cultured in 1 ml medium with 2 × 106 irradiated (20Gy) allogeneic PBMC feeders and 2 × 105 irradiated (20Gy) JY cells plus PHA (2 μg/ml) and IL-2. IL-2 was replenished weekly and feeders replenished bi-weekly.
B cell transductions
5 × 105 B cells were activated with CD40L-L cells and rmIL-21 for 36-48 hours and were spin-transduced 1:1 with virus:medium on Retronectin®-coated plates as described above. Transduced cells were then cultured for 4 days with IL-2 + IL-4, sorted for GFP by FACS, and plated on fresh CD40L-L cells for experiments.
RT-PCR
Total RNA was isolated with TRIzol (Invitrogen) and reverse transcribed using oligo (dT) (Promega) and SuperscriptIII reverse transcriptase (Invitrogen). Primer sequences for BCL6, ACTB, and HPRT are published33. Quantitative RT-PCR was carried out with SYBR Green mastermix (Abgene) on an iCycler (BioRad) and relative mRNA expression levels were normalized to ACTB and calculated using the 2−(ΔΔCT) method. IL21 was detected by RT-PCR using described primers60.
Immunoblotting
Whole cell extracts were isolated using Triton Lysis Buffer (20 mM Tris [pH 7.4], 137 mM NaCl, 25 mM β-glycerolphosphate, 2 mM EDTA [pH 7.4], 1% Triton X-100, 10% Glycerol) supplemented with HALT® protease inhibitor cocktail (Roche) and 1 mM Na3VO4. 15-30 μg of lysate was separated on acrylamide gels and transferred to nitrocellulose (Schleicher-Schuell). Monoclonal antibodies to STAT3 (C-20) and actin (I-19) were purchased from Santa Cruz Biotechnology. HRP-conjugated anti-rabbit and anti-goat HRP secondary Abs were from Pierce. Blots were developed by enhanced chemiluminescence (Pierce) and exposed to x-ray film (GE Health Sciences).
Enzyme-linked Immunosorbent Assay
Plates were coated with capture Abs anti-human IgG, IgM, or IgA (Dako) at 5 μg/ml in 0.1 M NaHCO3 pH 9.6 for 2 hr at 37°C and washed in ELISA wash buffer (PBS, 0.5% Tween-20). 4% nonfat milk in PBS was used as blocking agent and diluent for cell supernatants and for enzyme-conjugated detection Abs (Dilutions: 1:2500 for horseradish peroxidase (HRP)-conjugated anti-IgG, 1:5000 for HRP-anti-IgM, and 1:2500 for HRP-anti-IgA (all from Jackson ImmunoResearch Europe). TMB substrate/stop solutions (Biosource) were used for development. Detection of IL-21 was performed by sandwich ELISA using a capture mAb and a biotin-conjugated detection mAb from BD-Pharmingen.
Statistical analysis
All analyses are indicated in the Figure legends and were calculated with Prism 5.
Supplementary Material
Acknowledgements
Funding: This work was supported by NIH grants AI063846 (to S.A.D.) and by the National Center for Research Resources and the National Institute of General Medical Sciences of the NIH through Grant Numbers P20RR021905 and P30GM103532.
We thank Berend Hooibrink and Colette Charland of the Academic Medical Center and University of Vermont flow cytometry core facilities, respectively, for cell sorting and equipment maintenance. We thank Dr. Oliver Dienz for critical reading of the manuscript.
Footnotes
Author contributions: SAD, H Schmidlin, and MN performed experiments. BB and H Spits analyzed data and provided valuable intellectual input. SAD conceived and directed the project and wrote the paper (with input from the other authors).
Disclosures: The authors declare that there are no conflicts of interest, financial or otherwise, concerning this work.
References
- 1.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 2.Crotty S. Follicular Helper CD4 T Cells (T(FH)) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 3.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 8.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 9.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007 doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E, et al. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidlin H, Diehl SA, Blom B. New insights into the regulation of human B-cell differentiation. Trends Immunol. 2009;30:277–285. doi: 10.1016/j.it.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 15.Melnick A. Reprogramming specific gene expression pathways in B-cell lymphomas. Cell Cycle. 2005;4:239–241. [PubMed] [Google Scholar]
- 16.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee A-N, Qian S-B, Zhao H, et al. XBP1, downstream of Blimp-1, Expands the secretory apparatus and other organelles, and increases protein sysnthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 21.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426–2433. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS one. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 27.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 29.Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, et al. IL-21 is the primary common gamma chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118:6824–6835. doi: 10.1182/blood-2011-06-362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda T, Miki T, Yoshida T, Hatano M, Ohashi K, Hirosawa S, et al. The murine BCL6 gene is induced in activated lymphocytes as an immediate early gene. Oncogene. 1995;11:1657–1663. [PubMed] [Google Scholar]
- 31.Wong MT, Ye JJ, Alonso MN, Landrigan A, Cheung RK, Engleman E, et al. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol Cell Biol. 2010;88:624–631. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 33.Scheeren FA, Naspetti M, Diehl S, Schotte R, Nagasawa M, Wijnands E, et al. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol. 2005;6:303–313. doi: 10.1038/ni1172. [DOI] [PubMed] [Google Scholar]
- 34.Moser B, Schaerli P, Loetscher P. CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002;23:250–254. doi: 10.1016/s1471-4906(02)02218-4. [DOI] [PubMed] [Google Scholar]
- 35.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. J Exp Med. 1993;177:845–850. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fornek JL, Tygrett LT, Waldschmidt TJ, Poli V, Rickert RC, Kansas GS. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 2006;107:1085–1091. doi: 10.1182/blood-2005-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 39.Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, et al. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- 40.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, El Shikh ME, El Sayed RM, Best AM, Szakal AK, Tew JG. IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation. Int Immunol. 2009;21:745–756. doi: 10.1093/intimm/dxp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caprioli F, Sarra M, Caruso R, Stolfi C, Fina D, Sica G, et al. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol. 2008;180:1800–1807. doi: 10.4049/jimmunol.180.3.1800. [DOI] [PubMed] [Google Scholar]
- 43.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, et al. BCL-6 expression during B-cell activation. Blood. 1996;87:5257–5268. [PubMed] [Google Scholar]
- 46.Niu H, Ye BH, Dalla-Favera R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 1998;12:1953–1961. doi: 10.1101/gad.12.13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polo JM, Ci W, Licht JD, Melnick A. Reversible disruption of BCL6 repression complexes by CD40 signaling in normal and malignant B cells. Blood. 2008;112:644–651. doi: 10.1182/blood-2008-01-131813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 49.Niu H, Cattoretti G, Dalla-Favera R. BCL6 controls the expression of the B7-1/CD80 costimulatory receptor in germinal center B cells. J Exp Med. 2003;198:211–221. doi: 10.1084/jem.20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections] J Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 51.Hams E, McCarron MJ, Amu S, Yagita H, Azuma M, Chen L, et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011;186:5648–5655. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 52.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC immunology. 2011;12:53. doi: 10.1186/1471-2172-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 55.Manders PM, Hunter PJ, Telaranta AI, Carr JM, Marshall JL, Carrasco M, et al. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:7418–7425. doi: 10.1073/pnas.0501585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf I, Pevzner V, Kaiser E, Bernhardt G, Claudio E, Siebenlist U, et al. Downstream activation of a TATA-less promoter by Oct-2, Bob1, and NF-kappaB directs expression of the homing receptor BLR1 to mature B cells. J Biol Chem. 1998;273:28831–28836. doi: 10.1074/jbc.273.44.28831. [DOI] [PubMed] [Google Scholar]
- 57.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 59.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nat Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 60.Scheeren FA, Diehl SA, Smit LA, Beaumont T, Naspetti M, Bende RJ, et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood. 2008;111:4706–4715. doi: 10.1182/blood-2007-08-105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CM, Lukens MV, et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.