Abstract
Testosterone (T) regulates many traits related to fitness, including aggression. However, individual variation in aggressiveness does not always relate to circulating T, suggesting that behavioural variation may be more closely related to neural sensitivity to steroids, though this issue remains unresolved. To assess the relative importance of circulating T and neural steroid sensitivity in predicting behaviour, we measured aggressiveness during staged intrusions in free-living male and female dark-eyed juncos (Junco hyemalis). We compared aggressiveness to plasma T levels and to the abundance of androgen receptor (AR), aromatase (AROM) and oestrogen receptor alpha (ORα) mRNA in behaviourally relevant brain areas (avian medial amygdala, hypothalamus and song control regions). We also asked whether patterns of covariation among behaviour and endocrine parameters differed in males and females, anticipating that circulating T may be a better predictor of behaviour in males than in females. We found that circulating T related to aggressiveness only in males, but that gene expression for ORα, AR and AROM covaried with individual differences in aggressiveness in both sexes. These findings are among the first to show that individual variation in neural gene expression for three major sex steroid-processing molecules predicts individual variation in aggressiveness in both sexes in nature. The results have broad implications for our understanding of the mechanisms by which aggressive behaviour may evolve.
Keywords: aggression, testosterone, aromatase, oestrogen receptor, androgen receptor, evolution
1. Introduction
In vertebrates, many complex phenotypes that affect survival or reproduction are mediated by the steroid hormone testosterone (T) [1–3]. T plays a central role in translating genotype to phenotype in both males and females [4–6], and this hormone is a likely target of selection in the evolution of hormone-mediated behaviours, such as intrasexual aggression [7,8]. However, individual variation in circulating T often does not relate to individual differences in aggression in both sexes [2,9] (see also [10–12]). Because individual variation is the raw material of evolution, these observations call into question the role of T in the evolution of aggressive behaviour. A hypothesis that may resolve this issue suggests that variability in the cellular and molecular properties of target tissues (i.e. measures of sensitivity to T and its metabolites) are critical to explaining functional variation in behaviour (e.g. gene expression and protein abundance for molecules that process hormones in the brain [13,14]).
Androgen receptor (AR), oestrogen receptors (ORs) and aromatase (AROM) are among the key molecules in initiating the genomic effects of T [15–17]: after binding with a steroid, AR and OR transcriptionally regulate other genes that ultimately affect physiology and behaviour, and AROM (the enzyme converting T to oestradiol) enables T to activate OR-dependent pathways. Variation in abundance of these sex-steroid processing molecules is therefore likely to affect the expression of T-mediated behaviours such as aggression. Measurement of the abundance of AR, OR or AROM transcript or protein may therefore estimate critical aspects of local steroid sensitivity, though other co-factors and repressors also play a role [13]. Many different experimental manipulations and ‘group comparisons’ have linked AR, AROM and OR in the brain to aggressive and social behaviour (e.g. by comparing males and females, more aggressive and less aggressive species, or receptor antagonist-treated individuals and controls [18–22]; see [2] for review). It has been widely hypothesized that these group differences can be extrapolated to individual differences [9,13,23,24]—for example, that more aggressive individuals will have a greater abundance of AR, AROM or OR alpha (ORα) in behaviourally relevant brain areas. However, most studies linking aggressive behaviour to individual variation in sensitivity to T have pooled control and experimentally manipulated animals, and efforts to relate these parameters among unmanipulated or free-living animals are particularly rare [25,26] (but see [27–29]).
To rectify this knowledge gap and better integrate evolutionary biology with neuroendocrinology, we employed an approach rooted in quantitative genetics [30,31]. We first considered the degree to which aggressive behaviour relates to individual variation in hormone signal, to measures of sensitivity to that signal in target tissues, or to some combination of the two. Because same-sex aggressive behaviour is an important predictor of reproductive success in females as well as males [32,33], we also asked whether the mechanisms underlying individual variation in aggression are similar or different in males and females. If selection acts on variation in signal strength, we might expect correlated responses to selection in both sexes, but if selection acts on variation in sensitivity, then the sexes may be more free to evolve independently [1,6,34].
Species comparisons have shown that male and female T levels are often positively correlated, which suggests that selection on T-mediated traits in one sex could lead to a correlated response in the other sex [6,35,36]. Experimentally or naturally elevated T can be costly [37], and thus we might expect selection to favour mechanisms of aggression that are somewhat independent of circulating T, particularly when circulating T is low or the costs of T are high (e.g. during the non-breeding season or in females [18,38,39]). Because the costs of T are thought to be particularly high for females [40,41], we predicted that mechanisms of aggression that do not depend upon T levels themselves may be particularly important for females, though experimentally elevated T has been shown to affect female aggression in this species [42].
We measured natural variation in aggressiveness towards a same-sex conspecific in male and female free-living dark-eyed juncos (Junco hyemalis) early in the breeding season. The dark-eyed junco is a North American sparrow that is well studied with respect to hormones, behaviour and sex differences [3,6,34]. We compared individual differences in aggressiveness with circulating levels of T and with neural gene expression for AR, AROM and ORα. We used real-time quantitative PCR to quantify these measures of sensitivity to T in three socially relevant brain regions [43,44]: the hypothalamus (hypo), ventromedial telencephalon (VmT, a dissection that is largely limited to the avian medial amygdala or nucleus taeniae) and the right posterior telencephalon (PTR, which includes song control nuclei). We asked whether individual differences in behaviour within each sex covaried with the relative abundance of AR, AROM or ORα gene expression, with the prediction that more aggressive individuals would express greater transcript abundance for these genes. Further, owing to greater potential costs of circulating T to females, we predicted that female behaviour would be less a function of circulating T compared with male behaviour. Because changes in gene expression are thought to be a major driver of evolutionary change in complex phenotypes, including aggression [45–47], we interpret the results in terms of their applicability to the mechanisms by which aggressive behaviour may evolve.
2. Material and methods
(a). Behavioural quantification and tissue collection
All subjects were captured during the breeding season (1 May–5 June 2010) in the area surrounding Mountain Lake Biological Station in Virginia, USA (37°22′ N, 80°32′ W), immediately after a short (6 min) simulated territorial intrusion (modified from [10,12]). All females were in the incubation stage. All males were in breeding condition as evidenced by date and enlarged gonads. Prior to the day of experimentation, territories were mapped using a combination of conspecific playback and observation of natural singing perches. During each aggression test, a live, caged same-sex decoy was placed in the approximate centre of the territory (for males) or 1 m from the nest (for females). To simulate natural male–male aggressive encounters, male aggression tests were accompanied with conspecific song broadcast at a natural rate and amplitude (six songs per minute; 85–90 dB at 1 m). Before each trial, a mist net was set up and furled. An observer retreated approximately 15 m before uncovering the live decoy and beginning the trial.
We recorded the following behaviours: the number of times the focal bird attacked the decoy by swooping (‘flyovers’), distance to the decoy, number of songs (for males) and amount of time responding to the decoy (for females; i.e. time spent responding to the intruder instead of incubating). These behavioural parameters are measures of aggressive response or the likelihood of attack in this species and other songbirds [10,48,49].
At the conclusion of the 6-min trial, the mist net was unfurled and we quickly captured the bird (4.5 ± 0.5 min, range: 1.5–15 min after trial) and euthanized it immediately with an overdose of isoflurane followed by decapitation (2.2 ± 0.6 min, range: 1.5–3 min after capture). All subjects appeared within 2.25 min of beginning the trial, and we were successful in capturing all birds. Thus, the possibility that the sample is biased towards only the most aggressive individuals or those that were easy to capture is unlikely (latencies to appear = 0.25 ± 0.08 min). Brains were dissected from the skull using RNAse-free tools and frozen in powdered dry ice within 5 min. All tissues were stored at –80°C, until microdissection.
(b). Molecular methods
In the laboratory, brains were microdissected into functional regions, following Soma et al. [50,51]. After removing the optic tecta, optic chiasm and the hindbrain at the level of the mammillary bodies, we isolated the hypo to the depth of the anterior commissure (including the preoptic area and ventromedial hypothalamus). We collected the ventromedial telencelphalon (VmT) by removing ∼1 mm of the ventromedial portion of the caudal telencephalon, based on the position of nucleus taeniae in other songbirds [50], and we isolated tissues containing the song control nuclei by focusing on the PTR. We focused on these tissues because they include nuclei that are rich sites for steroid-mediated regulation of social, mating and aggressive behaviour [52–54]. We extracted RNA using the Trizol method (Invitrogen, Carlsbad, CA). After treating each RNA sample with DNAse (Promega, Madison, WI), we used Superscript III (Invitrogen) to synthesize cDNA from each brain area for each individual, from 1 µg of total RNA.
We quantified the expression of AR, AROM and ORα relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCt method [55] for each brain area, except that we did not quantify ORα PTR for females. All reactions were run in duplicate using SYBR green low ROX on a Stratagene MX3000P Real-Time PCR System (Agilent Technologies, Santa Clara, CA). The relative amount of transcript is reported as the fold difference relative to a pooled standard, normalized via the amount of GAPDH present in each sample. The pooled cDNA standard came from junco neural tissue collected during pilot work. Each qPCR reaction included 2.5 µl of cDNA diluted 1 : 10. Each reaction was optimized for high amplification efficiency (93.3–116.4%). We controlled for slightly unequal efficiencies using post-hoc corrections with MxPro software (v. 4.10, Agilent). Primer concentrations for each gene were 0.3 µM in a total volume of 25 µl. Primers were based on sequences from zebra finch (Taeniopygia guttata; see electronic supplementary material, table S1) and we confirmed high sequence identity in these regions (95–98%) using junco transcriptome sequences [56]. All reactions used the following thermal profile: 10 min at 95°C, followed by 40 cycles of 30 s at 95°C, 1 min at 60°C and 30 s at 70°C, with a final dissociation phase to confirm product specificity (1 min at 95°C, 30 s at 55°C and 30 s at 95°C).
(c). Hormone assays
We quantified circulating levels of T from trunk blood. Each blood sample was treated with 50 µl of water-based heparin sodium salt solution, stored on ice and centrifuged before plasma was drawn off the top (241 ± 16 µl, range: 90–410 µl). Plasma T concentrations were quantified using a commercially available enzyme immunoassay kit (Assay Design 901–065) that has already been validated in this species [41]. Briefly, we added tritiated T to each sample, and extracted plasma twice with diethyl ether. Extraction efficiencies were used to account for incomplete recoveries (efficiencies: 90.6 ± 0.5%). We used a logistic standard curve and curve-fitting program (Microplate Manager, Bio-Rad Laboratories) to obtain T concentrations, and we mathematically corrected for the additional volume from the water-based heparin solution. Samples were distributed over two plates, with intra-assay variability of 2.3 and 6.3 per cent, and inter-plate variability of 19.7 per cent. A plate correction factor was used to correct for inter-plate variation, based upon standards that were distributed over both plates multiple times [57].
(d). Statistical analyses
All statistical methods were performed using JMP v. 9.0.2 (SAS Institute, Cary, NC). We checked for normality using Shapiro–Wilk tests, and we transformed plasma T levels (natural log), flyovers (square-root) and transcript abundance (log2) to achieve normality. Log2-fold changes in gene expression include both positive and negative values, where negative values indicate that the focal sample expressed less transcript than the pooled standard. No transformation normalized the female response variable ‘time spent responding’, so all analyses with this behavioural measure used non-parametric Spearman correlations.
Because all individuals were rapidly captured following a 6-min intrusion and the time from initial disturbance to capture was short (10.5 ± 0.5 min), we assumed that measures of gene expression reflect individual differences that were present prior to the intrusion as opposed to plastic transcriptomic responses to the behavioural assay. However, to test this assumption, we used Pearson correlations to ask whether latency to sacrifice was related to transcript abundance in any gene or brain area.
Next, we tested for sex differences in behaviour and endocrine parameters (i.e. circulating T and transcript abundance in all brain areas) using unpaired t-tests, and we tested for correlations among the behavioural response variables (flyovers, latency to 5 and 1 m, songs and time spent responding). Other than latencies to 1 and 5 m, which are inherently correlated, we found no significant correlations among behavioural response measures (n = 32 individuals, p > 0.15), so we opted to treat each behavioural measure separately.
We also checked for significant relationships among T levels and measures of gene expression using Pearson correlations. Because many variables were strongly correlated, we used a two-step approach to constructing generalized linear models to measure the relationship between endocrine parameters and behavioural responses. First, we used partial least-squares regression as an exploratory tool to identify the salient variables predicting each behaviour. This approach examines variance explained by each dependent variable separately, and it is useful when multi-collinearity prevents the use of standard least-squares analyses [58]. We used cross-validation to determine the optimal number of latent variables based on the lowest r.m.s.e. We then eliminated variables with low scores for variable importance in the projection (VIP < 0.8) and low model coefficients (absolute value < 0.12) because these variables are unlikely to explain significant variance in the dependent variable [59]. These variables were then used in generalized linear models (GLMs, with normal error distribution and link function) to predict each behavioural output from each endocrine parameter on the narrowed list. For aggressive behaviours that were shared between the sexes (i.e. distance measures and flyovers), we included sex and a sex × neuroendocrine variable interaction to directly test whether mechanisms of aggression differed between the sexes. We then removed the main effect of sex and the interaction with sex if these variables were not significant on their own.
3. Results
We observed strong and significant relationships between several neuroendocrine variables and three common measures of aggression: territorial song (in males), time responding (in females) and flyovers (in both sexes, measured in terms of flights directly over the live decoy). In support of our assumption that the short behavioural assay did not cause appreciable changes in transcription prior to sacrifice, we did not observe any significant correlations between latency to sacrifice and transcript abundance for any gene in any brain area (all |r| < 0.18, all p > 0.36).
(a). Relationships among endocrine parameters
As expected, males showed significantly higher circulating T than females (t = 7.63, d.f. = 29, p < 0.0001); so we compared T levels with measures of sensitivity separately for each sex. T levels were related to only one measure of neural sensitivity to steroids, which was the amount of AROM mRNA hypo in males only (r = −0.72, n = 16, p = 0.016; for all other correlations, p > 0.16). The sexes did not differ in behavioural measures (t < 1.30, d.f. = 30, p > 0.20), with the exception of behaviours that are specific to one sex (e.g. songs), and the sexes did not differ in any measure of transcript abundance (t < 1.23, d.f.: 26–30, p > 0.22). Thus, we considered the sexes together when correlating transcript abundance within and among brain areas, though results are qualitatively similar if the sexes are treated separately. There were no significant relationships between brain areas in AR, AROM or ORα gene expression (all |r| < 0.25, d.f.: 26–30, p > 0.17). However, the abundance of AR, AROM and ORα were positively correlated within each brain area (AR v. AROM hypo: r = 0.64, p < 0.0001, PTR: r = 0.62, p < 0.0001, VmT: r = 0.31, p = 0.081, n = 32; AR v. ORα hypo: r = 0.69, p < 0.0001, n = 29, PTR: r = 0.54, p < 0.034, n = 15, VmT: r = 0.54, p = 0.0018, n = 31; AROM v. ORα hypo: r = 0.73, p < 0.0001, n = 29, PTR: r = 0.88, p < 0.0001, n = 15, VmT: r = 0.54, p = 0.0016, n = 31).
(b). Flyovers in males and females in relation to endocrine parameters
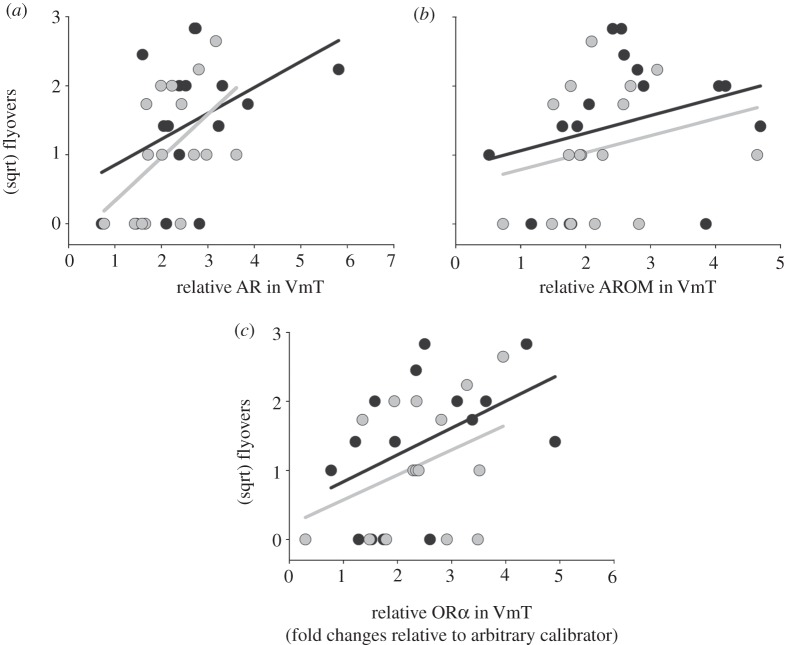
Partial least-squares analysis pointed to the relative abundance of AR, AROM and ORα transcript in VmT as potentially important predictors of the number of flyovers directed at the intruder; neither plasma T nor any other measures of transcript abundance were identified as explaining meaningful variance in the number of flyovers (see electronic supplementary material, table S2). GLMs revealed that more aggressive individuals expressed greater AR, AROM and ORα mRNA in VmT, and there were no sex differences in these patterns of covariation (AR GLM with sex: χ2 = 9.19, d.f. = 28, p = 0.027, AR: χ2 = 7.70, p = 0.0055, sex: χ2 = 0.30, p = 0.58, sex × AR: χ2 = 0.46, p = 0.46; AROM GLM with sex: χ2 = 5.10, d.f. = 28, p = 0.16, AROM: χ2 = 3.61, p = 0.057, sex: χ2 = 0.98, p = 0.32, sex × AR: χ2 = 0.02, p = 0.88; ORα GLM with sex: χ2 = 7.11, d.f. = 27, p = 0.069, ORα: χ2 = 5.80, p = 0.016, sex: χ2 = 0.99, p = 0.31, sex × ORα: χ2 = 0.01, p = 0.93; figure 1). Abundance of AR, AROM and OR transcript significantly predicted variance in the number of flyovers among individuals after removing the non-significant effect of sex (AR GLM: χ2 = 8.26, d.f. = 30, p = 0.004; AROM GLM: χ2 = 4.09, d.f. = 30, p = 0.043; ORα GLM: χ2 = 6.11, d.f. = 29, p = 0.0135.
Figure 1.
In both sexes, individuals performing more flights directly over the live intruder showed (a) more AR, (b) more AROM and (c) more ORα mRNA in ventromedial telencephalon (VmT). These relationships did not differ between the sexes. Abundance of each target gene is expressed as fold changes relative to an arbitrary calibrator (calibrator = 1), normalized by GAPDH. Black circles, females; grey circles, males.
(c). Songs in males in relation to endocrine parameters
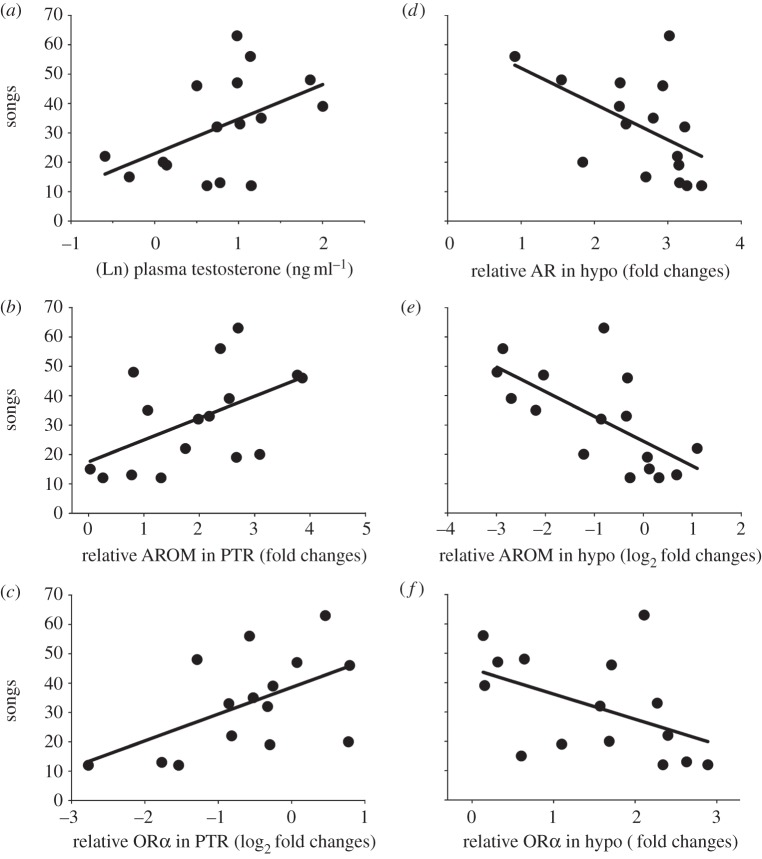
Partial least-squares analysis revealed several endocrine variables with potential relationships with the number of songs: circulating T, AR, AROM and ORα in hypo, and OR and AROM in PTR (see electronic supplementary material, table S3). GLMs demonstrated that males singing more songs expressed more AROM and ORα mRNA in PTR (AROM: χ2 = 5.09, d.f. = 14, p = 0.024, OR: χ2 = 5.06, d.f. = 13, p = 0.024), less AROM, AR and ORα in hypo (AROM: χ2 = 9.30, d.f. = 14, p = 0.0023, AR: χ2 = 4.98, d.f. = 14, p = 0.026, OR: χ2 = 5.09, d.f. = 13, p = 0.024), and have higher circulating T (χ2 = 4.75, d.f. = 13, p = 0.029, figure 2).
Figure 2.
Relationship between song and endocrine parameters: (a) plasma testosterone, (b) relative AROM mRNA in right posterior telencephalon (PTR), (c) relative ORα mRNA PTR, (d) relative AR mRNA in hypothalamus (hypo), (e) relative AROM mRNA hypo and (f) relative ORα mRNA hypo. Abundance of each target gene is expressed as fold changes relative to an arbitrary calibrator (calibrator = 1), or log2 fold changes (calibrator = 0), normalized by GAPDH.
(d). Time spent responding in females, in relation to endocrine parameters
Non-parametric correlations revealed no detectable relationships between the amount of time females spent responding to the intruder instead of incubating and any endocrine parameter, except for AR mRNA in hypo. Females that spent more time responding expressed marginally less AR mRNA (ρ = −0.47, n = 16, p = 0.066; figure 3).
Figure 3.
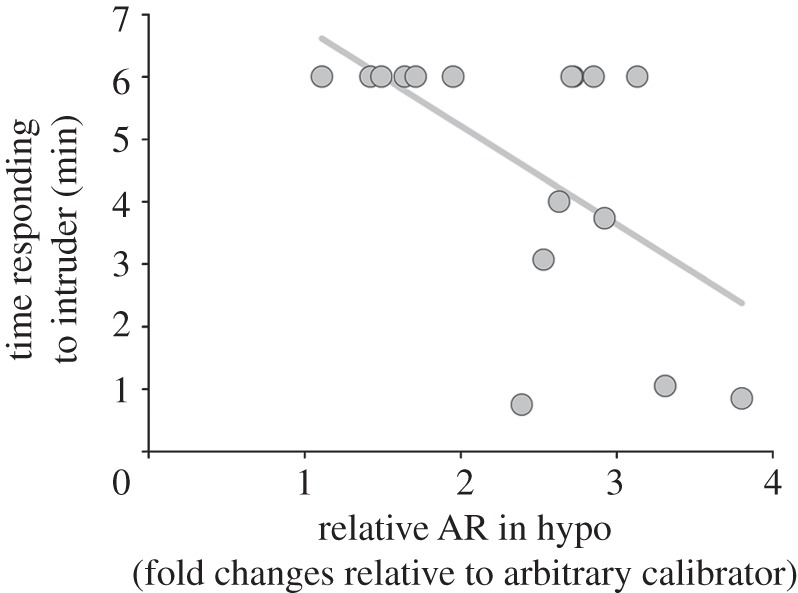
Females that spent more time responding aggressively to the intruder (i.e. instead of incubating) showed less AR mRNA in hypothalamus (hypo) than females that spent relatively more time incubating during the simulated territorial intrusion.
4. Discussion
Many fitness-relevant phenotypes are mediated by T [1–3], and it has been widely suggested that individual differences in hormone-mediated behaviour, particularly those that cannot be explained by circulating T, may instead relate to individual differences in sensitivity to hormones in the brain, such as the abundance of AR, AROM and ORα [9,13,14,23,24]. Despite the logical appeal of this explanation, few studies have demonstrated that individual differences in behaviour correlate with individual differences in measures of neural sensitivity to sex steroids [27–29]. We found that free-living songbirds demonstrate natural individual differences in the abundance of AR, AROM and ORα mRNA in behaviourally relevant brain areas, and that transcript abundance covaries with individual differences in aggressiveness in both males and females. While we cannot yet address whether these message-level patterns translate directly to protein abundance in the brain, our results provide strong correlational evidence that natural, individual differences in gene expression at three major neural targets of sex steroids account for natural variation in aggressive behaviour. Thus our results are consistent with the long-held assertion that behavioural differences may be the result of individual differences in sensitivity to hormones [60]. The observation that androgen- and oestrogen-dependent pathways show largely similar patterns of covariation with behaviour provides a unified sex-steroid-mediated link between aggression and hormone sensitivity in the brain.
Circulating T was also related to aggressive behaviour, but only in males, as revealed by the positive covariation between song and T. This finding confirms previous reports from this species showing that variation in hormone signal predicts variation in aggression [12]. Our observation that female T levels did not covary with any measure of female aggressiveness is consistent with the hypothesis that the regulation of aggression via differences in sensitivity to sex steroids, instead of variation in T levels themselves, may be an adaptive route to the expression of female aggressive behaviour while avoiding the costs of systemically high T [6,37]. These findings have important implications for basic mechanisms of behaviour and for behavioural evolution.
(a). Evolutionary implications of functional individual difference in neural sensitivity
Our approach links aggressive behaviour and three measures of neural sensitivity to sex steroids in naturally varying wild animals. This approach is complementary to the large body of research that seeks to identify the neural mechanisms of aggression by comparing groups of animals that differ in aggression or neural sensitivity to sex steroid [18,21,38,61,62], or by directly manipulating AR, AROM or ORα and aggression [17,19,20,63,64]. Because these studies typically did not quantify or assign functional significance to individual variability in the abundance of receptors or mRNA [9,23,65,66], the implications of these studies have not been fully integrated into an evolutionary framework. To draw inferences about potential sources of phenotypic variation and evolution in nature, a different approach, such as the one used here, is needed to estimate evolutionary parameters [30,31] (i.e. whether there is natural covariation between behaviour and physiology on which selection may act).
This study demonstrates behavioural relevance to natural individual variation in gene expression for AR, AROM and ORα in areas of the brain known to mediate social behaviour (song control nuclei, avian medial amygdala and hypothalamic nuclei), a key step in the cohesive synthesis of evolutionary biology and behavioural neuroendocrinology [13]. This observed covariation between behaviour and physiology constitutes a natural performance gradient, linking behaviour and physiology [31]. The next step in the synthesis will be to relate performance to selection, by asking whether there is covariation among neural sensitivity to sex steroids, behaviour and Darwinian fitness. Together, performance and selection gradients may reveal how selection leads to behavioural evolution via underlying physiology. In males, it is well established that greater same-sex aggression can directly determine access to territories or mates, and females also may benefit from increased aggressiveness in the context of female–female competition for mates, territories or other resources [32,33]. Our results suggest that selection could shape the evolution of aggression via changes in the expression of AR, AROM or ORα in both males and females, to some degree independently of circulating levels of T.
Past research comparing groups of animals that vary in aggression would have predicted a positive association between aggression and the abundance of AR, AROM or ORα among individuals (summarized above), which is similar to the patterns we found in tissues containing the song control nuclei and medial amygdala. The positive correlations between male song and gene expression along oestrogenic pathways in telencephalic tissues (figure 2b,c) and the positive correlations between flyovers and sex steroid sensitivity in amygdalar tissues in both sexes (figure 1a–c) are entirely consistent with research linking these nuclei with the expression of aggression in birds and mammals [43,51,67]. However, previous findings would not have predicted the surprising results found in the hypothalamus, where greater song output was associated with less AR, AROM and ORα transcript (figure 2d–f).
The negative correlation between circulating T levels and the abundance of AROM mRNA in the hypothalamus in males suggests the possibility of auto-regulatory processes. The hypothalamus includes many heterogeneous cell populations, which collectively integrate various internal and external stimuli, while mediating many social and reproductive behaviours, homeostatic functions and biological rhythms [2]. High T can have a suppressive effect on AR mRNA in the brain [68], and these hypothalamus-wide patterns of covariation may reflect a large-scale buffering of this brain area from the effects of sex steroids for reasons that may be correlated with, though not directly related to, aggressive behaviour.
Previous findings also would have not predicted the observation that females with more AR mRNA in the hypothalamus spent more time incubating instead of responding to an intruder (figure 3), but this observation is also consistent with the view that greater sensitivity to T in the hypothalamus may relate to the effect of sex steroids on other aspects of behaviour or physiology, such as parental care or reproduction. While additional experiments at a finer spatial scale are needed to pursue these details, the patterns we observed provide a critical link between behavioural variation and individual differences in gene expression in the brain.
(b). Does variation reflect plasticity or stable individual differences?
If individual differences in gene expression relate to behaviour, a natural question is whether these differences reflect an immediate response to the experimental conditions or whether they instead reflect variation that was present prior to the simulated intrusion. Because we found no relationship between latency to sacrifice and any measures of gene expression, our results suggest that individual differences in transcript abundance for AR, AROM and ORα were not directly attributable to rapid changes in transcription in the minutes preceding sacrifice. While the non-genomic actions of sex steroids can occur quite rapidly (e.g. less than 15 min [69]), the time frame in which these animals were collected is unlikely to involve socially induced changes in the expression of these genes because appreciable transcription of all but immediate early genes probably requires more time (i.e. after neuronal activation and the assembly of upstream transcription factors) [70]. Our findings are also consistent with a study that captured wild birds after roughly 30 min of aggressive interactions, which also reports no rapid effect on some of these same genes [71].
It is currently difficult to speculate on the degree to which the individual differences in gene expression reported here relate to heritable individual differences. On the one hand, levels of gene expression may have a heritable component [21,46,72,73]. On the other hand, environmental and social conditions can affect transcription as well [25]. An exciting possibility for future research is to determine whether the genes that are up- or downregulated in response to environmental stimuli may be the same genes that contribute to phenotypic evolution (e.g. genetic assimilation [74]). Given the integral role that hormones play in translating environmental stimuli into a phenotypic effect, hormone-metabolizing enzymes and hormone receptors may be good candidates for this process.
(c). Sex similarities and differences in mechanisms of aggression
One of the most intriguing patterns revealed by this study is that neural gene expression for AR, AROM and ORα similarly predict aggression in both sexes, but circulating T levels predict aggressiveness only in males. Comparative studies demonstrate that circulating T levels may reflect a compromise between the sexes [6,35], where selection favours higher T in males than in females. Based on genetic correlations and coevolution between the sexes, we thus might expect to see T-dependent aggressive behaviour in both sexes. Sexually biased or hormone-induced gene expression is thought to provide a solution to the problem posed by different selective pressures on the two sexes [75], and our results suggest a related solution in which aggression in both sexes depends upon gene expression for the molecules that initiate the genomic effects of sex steroids in the brain. Data presented here indicate that some measures of aggression can vary among individuals independently of variation in circulating T in both sexes (though singing in males does correlate with T). Behavioural independence from circulating T may permit adaptive expression of aggression that can side-step the costs of high systemic T to some degree [1,34,37]. Similar patterns appear to apply where high levels of aggression persist in the non-breeding season, despite low circulating T [38,39], and these mechanisms of aggression that relate to neural sensitivity to sex steroids may allow individuals of both sexes to express aggression somewhat independently of circulating T.
5. Conclusion
Natural variation in aggressive behaviour in males and females mapped onto individual differences in gene expression for three key molecules that facilitate the effects of T in neural target tissues, and these patterns of covariation appear to be largely shared between androgen- and oestrogen-mediated pathways. Furthermore, male aggressive behaviour, as reflected in song rate, also related to individual variation in circulating T levels, demonstrating that individual differences in hormone signal and sensitivity to signal may both account for the individually variable behaviours observed in nature. Hormones and gene expression are thought to lie at the intersection of the proximate and ultimate [76,77], and our results reveal that there is ample variation in hormone signal and in gene expression on which selection may act to affect aggressiveness. These data therefore establish a prerequisite for the evolution of T-mediated phenotypes via changes in localized gene expression for the key molecules that process sex steroids, and they suggest that phenotypic evolution can occur with some degree of independence from circulating T levels [1,34].
Acknowledgements
All procedures used in this study were approved by the Bloomington Institutional Animal Care and Use Committee. Many thanks to the Mountain Lake Biological Station for access to field sites; to D. G. Reichard for playback audio files; to N. Tonge, A. Mirzatoni, R. Stewart, M. P. Peterson and the Center for the Integrative Study for Animal Behaviour for assistance in the field and/or laboratory; and to members of the Ketterson and Goodson labs for feedback on earlier versions of the manuscript. This research was supported by an NIH NRSA fellowship to K.A.R. (T32HD049336 and F32HD068222), Indiana Academy of Sciences grant to K.A.R. and E.D.K., NSF IOS-0820055 to E.D.K., NIMH 061994 and NSF IBN-0646459 to B.A.S., and NSF DDIG (IOS-0909834) to C.M.B.B.
References
- 1.Hau M. 2007. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29, 133–144 10.1002/bies.20524 (doi:10.1002/bies.20524) [DOI] [PubMed] [Google Scholar]
- 2.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Ketterson E. D., Nolan V., Wolf L., Ziegenfus C. 1992. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am. Nat. 140, 980–999 10.1086/285451 (doi:10.1086/285451) [DOI] [Google Scholar]
- 4.Sandell M. I. 2007. Exogenous testosterone increases female aggression in the European starling (Sturnus vulgaris). Behav. Ecol. Sociobiol. 62, 255–262 10.1007/s00265-007-0460-9 (doi:10.1007/s00265-007-0460-9) [DOI] [Google Scholar]
- 5.Staub N. L., DeBeer M. 1997. The role of androgens in female vertebrates. Gen. Comp. Endocrinol. 108, 1–24 10.1006/gcen.1997.6962 (doi:10.1006/gcen.1997.6962) [DOI] [PubMed] [Google Scholar]
- 6.Ketterson E. D., Nolan V., Sandell M. 2005. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 166, S85–S98 10.1086/444602 (doi:10.1086/444602) [DOI] [PubMed] [Google Scholar]
- 7.McGlothlin J. W., Whittaker D. J., Schrock S. E., Gerlach N. M., Jawor J. M., Snajdr E. A., Ketterson E. D. 2010. Natural selection on testosterone production in a wild songbird population. Am. Nat. 175, 687–701 10.1086/652469 (doi:10.1086/652469) [DOI] [PubMed] [Google Scholar]
- 8.Veiga J. P., Polo V. 2008. Fitness consequences of increased testosterone levels in female spotless starlings. Am. Nat. 172, 42–53 10.1086/587850 (doi:10.1086/587850) [DOI] [PubMed] [Google Scholar]
- 9.Koolhaas J. M., de Boer S. F., Coppens C. M., Buwalda B. 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol. 31, 307–321 10.1016/j.yfrne.2010.04.001 (doi:10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 10.Cain K. E., Ketterson E. D. 2012. Competitive females are successful females: phenotype, mechanism, and selection in a common songbird. Behav. Ecol. Sociobiol. 66, 241–252 10.1007/s00265-011-1272-5 (doi:10.1007/s00265-011-1272-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.While G. M., Isaksson C., McEvoy J., Sinn D. L., Komdeur J., Wapstra E., Groothuis T. G. G. 2010. Repeatable intra-individual variation in plasma testosterone concentration and its sex-specific link to aggression in a social lizard. Horm. Behav. 58, 208–213 10.1016/j.yhbeh.2010.03.016 (doi:10.1016/j.yhbeh.2010.03.016) [DOI] [PubMed] [Google Scholar]
- 12.McGlothlin J. W., Jawor J. M., Greives T. J., Casto J. M., Phillips J. L., Ketterson E. D. 2008. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J. Evol. Biol. 21, 39–48 [DOI] [PubMed] [Google Scholar]
- 13.Ball G. F., Balthazart J. 2008. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Phil. Trans. R. Soc. B 363, 1699–1710 10.1098/rstb.2007.0010 (doi:10.1098/rstb.2007.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlinger B. A., Callard G. V. 1991. Brain–steroid interactions and the control of aggressive behavior in birds. In Neuroendocrine perspectives (eds MacLeod R. M., Muller E.), pp. 1–43 New York, NY: Springer [Google Scholar]
- 15.Cornil C. A., Ball G. F., Balthazart J. 2006. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 1126, 2–26 10.1016/j.brainres.2006.07.098 (doi:10.1016/j.brainres.2006.07.098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang C. S., Saltzman A., Yeh S. Y., Young W. J., Keller E., Lee H. J., Wang C. H., Mizokami A. 1995. Androgen receptor: an overview. Crit. Rev. Eukaryot. Gene Expr. 5, 97–125 [DOI] [PubMed] [Google Scholar]
- 17.Forlano P. M., Schlinger B. A., Bass A. H. 2006. Brain aromatase: new lessons from non-mammalian model systems. Front. Neuroendocrinol. 27, 247–274 10.1016/j.yfrne.2006.05.002 (doi:10.1016/j.yfrne.2006.05.002) [DOI] [PubMed] [Google Scholar]
- 18.Voigt C., Goymann W. 2007. Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii). Dev. Neurobiol. 67, 1560–1573 10.1002/dneu.20528 (doi:10.1002/dneu.20528) [DOI] [PubMed] [Google Scholar]
- 19.Sperry T. S., Wacker D. W., Wingfield J. C. 2010. The role of androgen receptors in regulating territorial aggression in male song sparrows. Horm. Behav. 57, 86–95 10.1016/j.yhbeh.2009.09.015 (doi:10.1016/j.yhbeh.2009.09.015) [DOI] [PubMed] [Google Scholar]
- 20.Soma K. K., Sullivan K., Wingfield J. 1999. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen. Comp. Endocrinol. 115, 442–453 10.1006/gcen.1999.7334 (doi:10.1006/gcen.1999.7334) [DOI] [PubMed] [Google Scholar]
- 21.Goncalves D., Saraiva J., Teles M., Teodosio R., Canario A. V. M., Oliveira R. F. 2010. Brain aromatase mRNA expression in two populations of the peacock blenny Salaria pavo with divergent mating systems. Horm. Behav. 57, 155–161 10.1016/j.yhbeh.2009.10.007 (doi:10.1016/j.yhbeh.2009.10.007) [DOI] [PubMed] [Google Scholar]
- 22.Riters L. V., Baillien M., Eens M., Pinxten R., Foidart A., Ball G. F., Balthazart J. 2001. Seasonal variation in androgen-metabolizing enzymes in the diencephalon and telencephalon of the male European starling (Sturnus vulgaris). J. Neuroendocrinol. 13, 985–997 10.1046/j.1365-2826.2001.00723.x (doi:10.1046/j.1365-2826.2001.00723.x) [DOI] [PubMed] [Google Scholar]
- 23.Williams T. D. 2008. Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Phil. Trans. R. Soc. B 363, 1687–1698 10.1098/rstb.2007.0003 (doi:10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crews D. 1998. On the organization of individual differences in sexual behavior. Am. Zool. 38, 118–132 10.1093/icb/38.1.118 (doi:10.1093/icb/38.1.118) [DOI] [Google Scholar]
- 25.Fuxjager M. J., Forbes-Lorman R. M., Coss D. J., Auger C. J., Auger A. P., Marler C. A. 2010. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc. Natl Acad. Sci. 107, 12 393–12 398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dessi-Fulgheri F., Lucarini N., Lupo di prisco C. 1976. Relationships between testosterone-metabolism in brain, other endocrine variables and intermale aggression in mice. Aggress. Behav. 2, 223–231 (doi:10.1002/1098-2337(1976)2:3<223::AID-AB2480020307>3.0.CO;2-0) [DOI] [Google Scholar]
- 27.Schlinger B. A., Callard G. V. 1989. Aromatase-activity in quail brain: correlation with aggressiveness. Endocrinology 124, 437–443 10.1210/endo-124-1-437 (doi:10.1210/endo-124-1-437) [DOI] [PubMed] [Google Scholar]
- 28.Silverin B., Baillien M., Balthazart J. 2004. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm. Behav. 45, 225–234 10.1016/j.yhbeh.2003.10.002 (doi:10.1016/j.yhbeh.2003.10.002) [DOI] [PubMed] [Google Scholar]
- 29.Trainor B. C., Greiwe K. M., Nelson R. J. 2006. Individual differences in estrogen receptor alpha in select brain nuclei are associated with individual differences in aggression. Horm. Behav. 50, 338–345 10.1016/j.yhbeh.2006.04.002 (doi:10.1016/j.yhbeh.2006.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGlothlin J. W., Ketterson E. D. 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 10.1098/rstb.2007.0002 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold S. J. 1983. Morphology, performance, and fitness. Am. Zool. 23, 347–361 10.1093/icb/23.2.347 (doi:10.1093/icb/23.2.347) [DOI] [Google Scholar]
- 32.Stockley P., Bro-Jørgensen J. 2011. Female competition and its evolutionary consequences in mammals. Biol. Rev. 86, 341–366 10.1111/j.1469-185X.2010.00149.x (doi:10.1111/j.1469-185X.2010.00149.x) [DOI] [PubMed] [Google Scholar]
- 33.Rosvall K. A. 2011. Intrasexual competition in females: evidence for sexual selection? Behav. Ecol. 22, 1131–1140 10.1093/beheco/arr106 (doi:10.1093/beheco/arr106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ketterson E. D., Atwell J. W., McGlothlin J. W. 2009. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr. Comp. Biol. 49, 365–379 10.1093/icb/icp057 (doi:10.1093/icb/icp057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Møller A. P., Garamszegi L. Z., Gil D., Hurtrez-Bousses S., Eens M. 2005. Correlated evolution of male and female testosterone profiles in birds and its consequences. Behav. Ecol. Sociobiol. 58, 534–544 10.1007/s00265-005-0962-2 (doi:10.1007/s00265-005-0962-2) [DOI] [Google Scholar]
- 36.Mank J. E. 2007. The evolution of sexually selected traits and antagonistic androgen expression in actinopterygiian fishes. Am. Nat. 169, 142–149 10.1086/510103 (doi:10.1086/510103) [DOI] [PubMed] [Google Scholar]
- 37.Wingfield J. C., Lynn S. E., Soma K. K. 2001. Avoiding the ‘costs’ of testosterone: ecological bases of hormone–behavior interactions. Brain Behav. Evol. 57, 239–251 10.1159/000047243 (doi:10.1159/000047243) [DOI] [PubMed] [Google Scholar]
- 38.Canoine V., Fusani L., Schlinger B., Hau M. 2007. Low sex steroids, high steroid receptors: increasing the sensitivity of the nonreproductive brain. Dev. Neurobiol. 67, 57–67 10.1002/dneu.20296 (doi:10.1002/dneu.20296) [DOI] [PubMed] [Google Scholar]
- 39.Pradhan D. S., Newman A. E. M., Wacker D. W., Wingfield J. C., Schlinger B. A., Soma K. K. 2010. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm. Behav. 57, 381–389 10.1016/j.yhbeh.2010.01.008 (doi:10.1016/j.yhbeh.2010.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGlothlin J. W., Neudorf D. L. H., Casto J. M., Nolan V., Ketterson E. D. 2004. Elevated testosterone reduces choosiness in female dark-eyed juncos (Junco hyemalis): evidence for a hormonal constraint on sexual selection? Proc. R. Soc. Lond. B 271, 1377–1384 10.1098/rspb.2004.2741 (doi:10.1098/rspb.2004.2741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clotfelter E. D., O'Neal D. M., Gaudioso J. M., Casto J. M., Parker-Renga I. M., Snajdr E. A., Duffy D. L., Nolan V., Ketterson E. D. 2004. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm. Behav. 46, 171–178 10.1016/j.yhbeh.2004.03.003 (doi:10.1016/j.yhbeh.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 42.Zysling D. A., Greives T. J., Breuner C. W., Casto J. M., Demas G. E., Ketterson E. D. 2006. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis). Horm. Behav. 50, 200–207 10.1016/j.yhbeh.2006.03.004 (doi:10.1016/j.yhbeh.2006.03.004) [DOI] [PubMed] [Google Scholar]
- 43.Goodson J. L. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22 10.1016/j.yhbeh.2005.02.003 (doi:10.1016/j.yhbeh.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeigler H. P., Marler P. 2004. Behavioral neurobiology of bird song. New York, NY: Annals of the New York Academy of Sciences; [DOI] [PubMed] [Google Scholar]
- 45.Carroll S. B. 2005. Evolution at two levels: on genes and form. PLoS Biol. 3, e245 10.1371/journal.pbio.0030245 (doi:10.1371/journal.pbio.0030245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards A. C., Ayroles J. F., Stone E. A., Carbone M. A., Lyman R. F., Mackay T. F. C. 2009. A transcriptional network associated with natural variation in Drosophila aggressive behavior. Genome Biol. 10, R76 10.1186/gb-2009-10-7-r76 (doi:10.1186/gb-2009-10-7-r76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alaux C., et al. 2009. Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl Acad. Sci. USA 106, 15 400–15 405 10.1073/pnas.0907043106 (doi:10.1073/pnas.0907043106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGlothlin J. W., Jawor J. M., Ketterson E. D. 2007. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am. Nat. 170, 864–875 10.1086/522838 (doi:10.1086/522838) [DOI] [PubMed] [Google Scholar]
- 49.Searcy W. A., Beecher M. D. 2009. Song as an aggressive signal in songbirds. Anim. Behav. 78, 1281–1292 10.1016/j.anbehav.2009.08.011 (doi:10.1016/j.anbehav.2009.08.011) [DOI] [Google Scholar]
- 50.Soma K. K., Bindra R. K., Gee J., Wingfield J. C., Schlinger B. A. 1999. Androgen-metabolizing enzymes show region-specific changes across the breeding season in the brain of a wild songbird. J. Neurobiol. 41, 176–188 (doi:10.1002/(SICI)1097-4695(19991105)41:2<176::AID-NEU2>3.0.CO;2-2) [DOI] [PubMed] [Google Scholar]
- 51.Soma K. K., Schlinger B. A., Wingfield J. C., Saldanha C. J. 2003. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J. Neurobiol. 56, 209–221 10.1002/neu.10225 (doi:10.1002/neu.10225) [DOI] [PubMed] [Google Scholar]
- 52.Pfaff D. W. 1968. Autoradiographic localization of radioactivity in rat brain after injection of tritiated sex hormones. Science 161, 1355–1356 10.1126/science.161.3848.1355 (doi:10.1126/science.161.3848.1355) [DOI] [PubMed] [Google Scholar]
- 53.Guerriero G. 2009. Vertebrate sex steroid receptors: evolution, ligands, and neurodistribution. Ann N Y Acad. Sci. 1163, 154–168 10.1111/j.1749-6632.2009.04460.x (doi:10.1111/j.1749-6632.2009.04460.x) [DOI] [PubMed] [Google Scholar]
- 54.Metzdorf R., Gahr M., Fusani L. 1999. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 407, 115–129 (doi:10.1002/(SICI)1096-9861(19990428)407:1<115::AID-CNE9>3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 55.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408 10.1006/meth.2001.1262 (doi:10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 56.Peterson M. P., Whittaker D. J., Ambreth S., Sureshchandra S., Mockatis K., Buechlein A., Podicheti R., Choi J. H., Lai Z., Colbourne J. K., Tang H., Ketterson E. D. In press De novo transcriptome sequencing in a songbird, the dark-eyed junco (Junco hyemalis): genomic tools for an ecological model system. BMC Genomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jawor J. M., McGlothlin J. W., Casto J. M., Greives T. J., Snajdr E. A., Bentley G. E., Ketterson E. D. 2007. Testosterone response to GnRH in a female songbird varies with stage of reproduction: implications for adult behaviour and maternal effects. Funct. Ecol. 21, 767–775 10.1111/j.1365-2435.2007.01280.x (doi:10.1111/j.1365-2435.2007.01280.x) [DOI] [Google Scholar]
- 58.Wold S., Ruhe A., Wold H., Dunn W. J., III 1984. The collinearity problem in linear regression: the partial least squares (PLS) approach to generalized inverses. SIAM. J. Sci. Stat. Comput. 5, 735–743 10.1137/0905052 (doi:10.1137/0905052) [DOI] [Google Scholar]
- 59.Wold S. 1994. PLS for multivariate linear modeling. In QSAR: chemometric methods in molecular design (ed. H. van de Waterbeemd), pp. 195–218. Weinheim, Germany: VerlagChemie [Google Scholar]
- 60.Grunt J. A., Young W. C. 1952. Differential reactivity of individuals and the response of the male guinea pig to testosterone propionate. Endocrinology 51, 237–248 10.1210/endo-51-3-237 (doi:10.1210/endo-51-3-237) [DOI] [PubMed] [Google Scholar]
- 61.Wacker D. W., Wingfield J. C., Davis J. E., Meddle S. L. 2010. Seasonal changes in aromatase and androgen receptor, but not estrogen receptor mRNA expression in the brain of the free-living male song sparrow, Melospiza melodia morphna. J. Comp. Neurol. 518, 3819–3835 10.1002/cne.22426 (doi:10.1002/cne.22426) [DOI] [PubMed] [Google Scholar]
- 62.Goodson J. L., Wilson L. C., Schrock S. E. In press. To flock or fight: neurochemical signatures of divergent life histories in sparrows. Proc. Natl Acad. Sci. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsumoto T., Honda S., Harada N. 2003. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology 77, 416–424 10.1159/000071313 (doi:10.1159/000071313) [DOI] [PubMed] [Google Scholar]
- 64.Ogawa S., Lubahn D. B., Korach K. S., Pfaff D. W. 1997. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl Acad. Sci. USA 94, 1476–1481 10.1073/pnas.94.4.1476 (doi:10.1073/pnas.94.4.1476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikinmaa M., Waser W. 2007. Molecular and cellular studies in evolutionary physiology of natural vertebrate populations: influences of individual variation and genetic components on sampling and measurements. J. Exp. Biol. 210, 1847–1857 10.1242/jeb.002717 (doi:10.1242/jeb.002717) [DOI] [PubMed] [Google Scholar]
- 66.Bennett A. F. 1987. Interindividual variability: an underutilized resource, pp. 147–169 Cambridge, UK: Cambridge University Press [Google Scholar]
- 67.Catchpole C. K., Slater P. J. B. 1995. Bird song: biological themes and variations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 68.Bagamasbad P., Denver R. J. 2011. Mechanisms and significance of nuclear receptor auto- and cross-regulation. Gen. Comp. Endocrinol. 170, 3–17 10.1016/j.ygcen.2010.03.013 (doi:10.1016/j.ygcen.2010.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.London S. E., Remage-Healey L., Schlinger B. A. 2009. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front. Neuroendocrinol. 30, 302–314 10.1016/j.yfrne.2009.05.001 (doi:10.1016/j.yfrne.2009.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herdegen T., Leah J. D. 1998. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 28, 370–490 10.1016/S0165-0173(98)00018-6 (doi:10.1016/S0165-0173(98)00018-6) [DOI] [PubMed] [Google Scholar]
- 71.Mukai M., Replogle K., Drnevich J., Wang G., Wacker D., Band M., Clayton D. F., Wingfield J. C. 2009. Seasonal differences of gene expression profiles in song sparrow (Melospiza melodia) hypothalamus in relation to territorial aggression. PLoS ONE 4, e8182 10.1371/journal.pone.0008182 (doi:10.1371/journal.pone.0008182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamatoyannopoulos J. A. 2004. The genomics of gene expression. Genomics 84, 449–457 10.1016/j.ygeno.2004.05.002 (doi:10.1016/j.ygeno.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 73.Whitehead A., Crawford D. L. 2006. Variation within and among species in gene expression: raw material for evolution. Mol. Ecol. 15, 1197–1211 10.1111/j.1365-294X.2006.02868.x (doi:10.1111/j.1365-294X.2006.02868.x) [DOI] [PubMed] [Google Scholar]
- 74.Bell A. M., Robinson G. E. 2011. Behavior and the dynamic genome. Science 332, 1161–1162 10.1126/science.1203295 (doi:10.1126/science.1203295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ellegren H., Parsch J. 2007. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 8, 689–698 10.1038/nrg2167 (doi:10.1038/nrg2167) [DOI] [PubMed] [Google Scholar]
- 76.Fitzpatrick M. J., Ben-Shahar Y., Smid H. M., Vet L. E. M., Robinson G. E., Sokolowski M. B. 2005. Candidate genes for behavioural ecology. Trends Ecol. Evol. 20, 96–104 10.1016/j.tree.2004.11.017 (doi:10.1016/j.tree.2004.11.017) [DOI] [PubMed] [Google Scholar]
- 77.Robinson G. E. 1999. Integrative animal behaviour and sociogenomics. Trends Ecol. Evol. 14, 202–205 10.1016/S0169-5347(98)01536-5 (doi:10.1016/S0169-5347(98)01536-5) [DOI] [PubMed] [Google Scholar]