Abstract
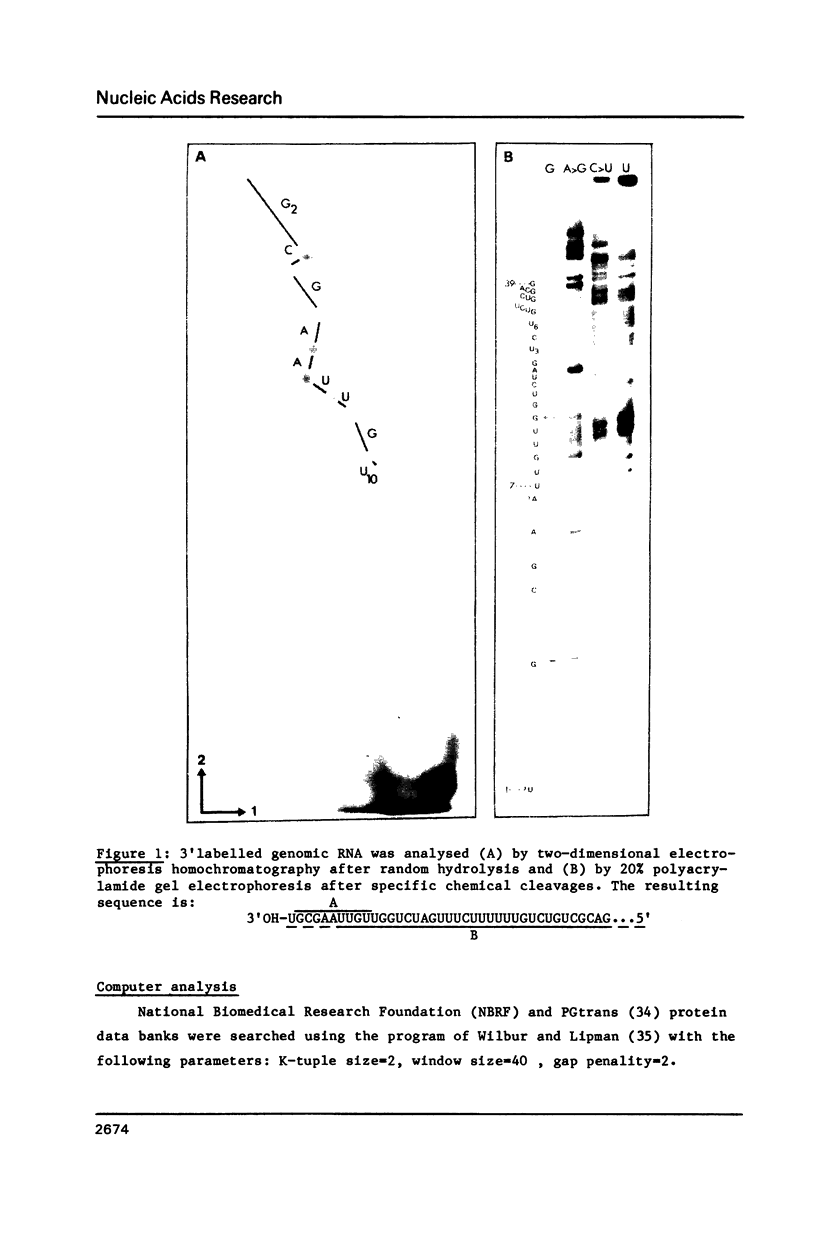
We have determined the nucleotide sequence of the 3'region of the rabies genome (PV strain). This work is a first step in a project aimed at establishing the complete primary structure. From the 3'nucleotide sequence of the RNA genome, an octadecanucleotide complementary to the 3'extremity was constructed and used to prime cDNA synthesis. Two overlapping recombinant cDNA clones hybridizing with the nucleoprotein mRNA (NmRNA) were isolated and sequenced. The 1500 first nucleotides of the rabies genome cover two transcriptional units: the leader RNA and the NmRNA which was shown to be initiated around residue 59 by S1 nuclease protection experiments. Comparison between rabies PV and CVS strains up to residue 180 suggests a rapid evolution in the leader region. Studies of the sequence relationships between the 3'regions of two Rhabdoviruses, rabies virus and Vesicular Stomatitis Virus (VSV), demonstrate that there is a segmented homology. Stretches of highly conserved amino acids possibly involved in the interaction with the RNA genome were observed in the N protein, despite a wide divergence in the remaining sequence. In addition, the high homology between the transcription start and stop signals reflects the conservation of a similar transcriptional mechanism in these two non segmented negative strand RNA viruses.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Wunner W. H., Curtis P. J. Structure of the glycoprotein gene in rabies virus. Nature. 1981 Nov 19;294(5838):275–278. doi: 10.1038/294275a0. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Davis N. L., Wertz G., Schubert M., Lazzarini R. A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985 May;41(1):259–267. doi: 10.1016/0092-8674(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Claverie J. M., Sauvaget I., Bougueleret L. Computer generation and statistical analysis of a data bank of protein sequences translated from GenBank. Biochimie. 1985 May;67(5):437–443. doi: 10.1016/s0300-9084(85)80261-3. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Coslett G. D., Holloway B. P., Obijeski J. F. The structural proteins of rabies virus and evidence for their synthesis from separate monocistronic RNA species. J Gen Virol. 1980 Jul;49(1):161–180. doi: 10.1099/0022-1317-49-1-161. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Cox J. H., Schneider L. G., Wiktor T. J., Koprowski H. Isolation and purification of a polymeric form of the glycoprotein of rabies virus. J Gen Virol. 1978 Jul;40(1):131–139. doi: 10.1099/0022-1317-40-1-131. [DOI] [PubMed] [Google Scholar]
- Emerson S. U. Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell. 1982 Dec;31(3 Pt 2):635–642. doi: 10.1016/0092-8674(82)90319-1. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Ermine A., Flamand A. Rna syntheses in BHK21 cells infected by rabies virus. Ann Microbiol (Paris) 1977 May-Jun;128A(4):477–488. [PubMed] [Google Scholar]
- Flamand A., Delagneau J. F. Transcriptional mapping of rabies virus in vivo. J Virol. 1978 Nov;28(2):518–523. doi: 10.1128/jvi.28.2.518-523.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B., Kolakofsky D. Sequence determination of the (+) leader RNA regions of the vesicular stomatitis virus Chandipura, Cocal, and Piry serotype genomes. J Virol. 1983 Apr;46(1):125–130. doi: 10.1128/jvi.46.1.125-130.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Holloway B. P., Obijeski J. F. Rabies virus-induced RNA synthesis in BHK21 cells. J Gen Virol. 1980 Jul;49(1):181–195. doi: 10.1099/0022-1317-49-1-181. [DOI] [PubMed] [Google Scholar]
- Kawai A. Transcriptase activity associated with rabies virion. J Virol. 1977 Dec;24(3):826–835. doi: 10.1128/jvi.24.3.826-835.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith G., Pixa G., Fix C., Dirheimer G. Primary structure of three tRNAs from brewer's yeast: tRNAPro2, tRNAHis1 and tRNAHis2. Biochimie. 1983 Nov-Dec;65(11-12):661–672. doi: 10.1016/s0300-9084(84)80030-9. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Cabradilla C. D., Holloway B. P., Keene J. D. Nucleotide sequence and host La protein interactions of rabies virus leader RNA. J Virol. 1984 Jun;50(3):773–778. doi: 10.1128/jvi.50.3.773-778.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthier J. J., Dreyfus M., Roux T. L., Rougeon F. Mouse kidney and submaxillary gland renin genes differ in their 5' putative regulatory sequences. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5489–5493. doi: 10.1073/pnas.81.17.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Doolittle R. F., Anilionis A., Curtis P. J., Wunner W. H. Homology between the glycoproteins of vesicular stomatitis virus and rabies virus. J Virol. 1982 Jul;43(1):361–364. doi: 10.1128/jvi.43.1.361-364.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of rabbit alpha- and beta-globin gene sequences into Escherichia coli plasmids. J Biol Chem. 1977 Apr 10;252(7):2209–2217. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Sprague J., Condra C. S., Lazzarini R. A. In vitro transcription of vesicular stomatitis virus: initiation with GTP at a specific site within the N cistron. J Virol. 1982 Jul;43(1):166–173. doi: 10.1128/jvi.43.1.166-173.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelverton E., Norton S., Obijeski J. F., Goeddel D. V. Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli. Science. 1983 Feb 11;219(4585):614–620. doi: 10.1126/science.6297004. [DOI] [PubMed] [Google Scholar]