Concerns about public education, specifically in the area of teaching literacy, are growing. The Los Angeles Times recently reported that up to 60% of local children may fail their third-grade reading proficiency test. Millions of American students who are not learning disabled but enter school with weak English skills are not learning to read and are ending up in special education classes with lowered expectations and dim prospects. It is not surprising that language learning disabilities are emerging as one of the greatest social issues of our times. A recent briefing on learning disabilities for the Congressional Biomedical Research Caucus* included the following statistics: It costs public schools twice as much to provide special education services to a child. Despite these extra services, twice as many students with learning disabilities drop out of high school. This leads to lower employment rates and higher adjudication rates (85% of juvenile delinquents are learning disabled as are 60% of prison inmates). It is noteworthy that 75–80% of students classified as learning disabled have their basic deficits in oral and written language (1).
Finding out “why Johnny can't read,” in addition to being of increasing social concern, has become a focus of scientific research. Although initially the domain of educational research, more recently a growing interest in the neurobiological basis of higher cortical functions, especially language and reading (and the sensory, perceptual, and cognitive systems that subserve these functions), has captured the attention of neuroscientists.
The majority of scientific studies of reading have focused on developmental reading deficits of unknown origin (dyslexia). Early research studies focused mostly on the visual (orthographic) components of reading. However, in this issue of PNAS Talcott et al. (2) point out that learning to read a language depends on acquiring an understanding of both its spoken properties (phonology) and its written form (orthography). In alphabetic languages such as English, printed characters (graphemes) correspond to phonemes, the smallest meaningful unit of sound that amalgamates to constitute a word. A large body of research now has demonstrated that proficiency in phonological analysis of words (i.e., decoding words into their phonetic segments), significantly differentiates dyslexics from controls (3, 4), as well as predicts future literacy skills (5).
Although it is widely accepted that dyslexia is characterized by both orthographic and phonological deficits, the precise etiology of these deficits remains the focus of research. Other central research issues focus on whether (i) dyslexia constitutes a distinct disability or rather represents the disadvantageous end of individual differences within a normal distribution, (ii) phonological deficits related to reading failure are speech specific or derive from more basic auditory processing deficits, (iii) dyslexia is a deficit specific to written language or rather the manifestation of a more pervasive delay in language development, and (iv) dyslexia results from genetic, neurological, social, and/or educational causes.
A considerable body of research on individuals with oral and/or written language learning impairments (LLI) has shown that, in addition to their weak language and literacy skills, they are less sensitive to dynamic (brief, rapidly changing) sensory stimuli, both in the auditory and visual modality (6, 7). Deficits in detecting rapidly presented or rapidly changing acoustic stimuli have been hypothesized to play a direct role in phonological development and disorders. Specifically, many of the acoustic temporospectral changes that are critical for identifying and discriminating phonemic segments within speech occur within tens of milliseconds, requiring a rate of acoustic processing that has been shown to be impaired in many individuals with LLI (8). However, the extent to which nonverbal acoustic processing rate constraints are related directly to subsequent oral and/or written language development and disorders has been hotly debated in the scientific literature, specifically by scientists who support a modular theory of language specificity in the brain (9, 10).
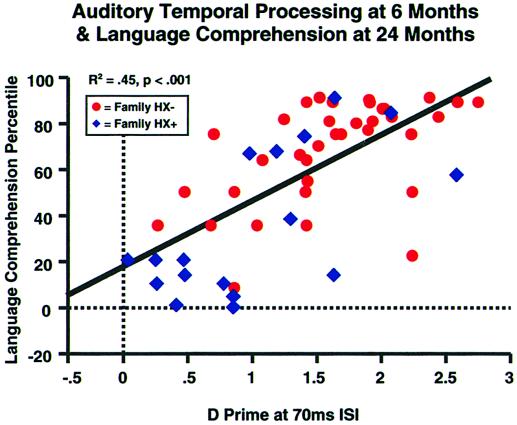
The majority of data supporting language modularity, however, derive from studies with older children or adults, after they already have developed (or failed to develop) language learning skills normally. Thus, they may miss underlying developmental mechanisms that exerted significant effects on the calibration of sensory neural maps, as well as the learning progression for developing phonological categorization and representation. However, recent studies with infants born into families that already have one or more relatives with LLI offer a unique opportunity to observe the processes influencing language, prospectively, as it develops. These studies are consistent in showing that, within the first weeks of life, infants with a positive family history for LLI, process temporospectral changes within both nonverbal and verbal acoustic stimuli significantly more slowly than family history-negative infants (11, 12). Furthermore, an infant's nonverbal auditory temporal processing threshold significantly predicts subsequent receptive and expressive language development, with infants demonstrating the slowest processing rates being most likely to be slower in language development (12, 13). As can be seen in Fig. 1, not only does nonverbal acoustic processing rate predict rate of language development in family history-positive infants, it is equally robust as a predictor of language development in infants with no risk factors.† Taken together, these studies demonstrate population-wide that individual differences along a dimension of dynamic acoustic processing sensitivity, specifically within the time range that is critical for speech perception, affects the course of language learning beginning in early infancy.
Figure 1.
Language comprehension percentile (preschool language scale-3) for matched groups [family history positive (HX+) and family history negative (HX−)] at 24 months, as a function of D prime (difference between hit and false alarm rates) at 6 months, in a psychophysical task assessing discrimination of two 75-ms duration complex tones separated by an interstimulus-interval of 70 ms. Poor discrimination is represented by D primes below 0.9. Figure used with permission from A. A. Benasich.†
In this issue of PNAS Talcott et al. (2) extend these findings to the domain of written language. They investigate the hypothesis that sensitivity to dynamic visual and auditory stimuli, both of which previously have been shown to be impaired in individuals with LLI (6, 7), also influence the development of literacy skills in normal readers. Specifically, they hypothesize that sensitivity to dynamic auditory stimuli will significantly covary with phonological decoding abilities, whereas sensitivity to dynamic visual stimuli will covary with orthographic abilities, across the full range of reading development.
Inspection of Talcott et al.'s results (2) shows that sensitivity to nonverbal dynamic frequency-modulated acoustic stimuli, specifically those that are modulated at rates important for speech perception (2 Hz), was as significantly correlated with literacy measures (reading, spelling, and phonological decoding) as these literacy measure were correlated with each other. Sensitivity to dynamic visual stimuli (coherent motion) was significantly correlated primarily with measures reflecting orthographic aspects of single-word reading and spelling. These results demonstrate for the first time that sensitivity to dynamic visual and auditory stimuli vary along a continuum, with individual differences being highly correlated with the development of literacy skills.
Physiological and neuroimaging data from dyslexics provide converging evidence supporting a neurobiological substrate linking dynamic sensory processing mechanisms with mechanisms subserving language and literacy skills (14–16). Postmortem analysis of dyslexic brains has revealed neocortical malformations, aberrant left-right asymmetries in temporal speech regions, and significant differences in cell size in thalamic sensory nuclei (17–20). These findings have given rise to a hypothesis that the magnocellular divisions of the thalamus are selectively impaired in dyslexics, which may result in the disruption of thalamocortical transmission. As magno (large) cells putatively transmit information more quickly than parvo (small) cells, these anatomical findings have been interpreted to be the neurobiological substrate for the dynamic sensory processing deficits (also referred to as temporal or rate processing deficits) that have been shown to characterize many individuals with LLI.
Postmortem electron microscopy studies of the language areas of normal brains have shown that axons of the left posterior superior temporal lobe are more thickly myelinated (21). As axons with thicker myelin sheaths conduct faster and require greater volume, these results support previous behavioral studies that suggest that left hemisphere dominance for rapid sensory signal processing may underlie functional asymmetry for language in the left hemisphere (22).
These hypotheses are now amenable to investigation in vivo by using neuroimaging procedures in humans. The microstructural integrity of white matter in adults, with or without a history of developmental reading problems, recently was investigated by using diffusion tensor magnetic resonance imaging. White matter diffusion anisotropy in the temporo-parietal region of the left hemisphere was found to be significantly correlated with reading scores for both reading impaired and control readers (23). These findings demonstrate for the first time a consistent structural correlate of reading skills in normal as well as impaired readers. Several histological characteristics could affect the anisotropy of white matter including number of axons, thickness of axons or the amount, and integrity of myelin. Intact myelination is important for rapid conduction of axon potentials, and thus the integrity of myelination is important for accurate coding and transmission of rapidly changing, dynamic stimuli. The finding of significant correlations between white diffusion anisotropy in the temporo-parietal region of the left hemisphere and reading, across the range of normal as well as abnormal readers is consistent with the behavioral data reported by Talcott et al. (2). These data provide a plausible neurobiological substrate for linking individual behavioral and anatomical differences pertaining to both left hemisphere specialization for dynamic sensory processing and the development of language and literacy skills.
These studies support the hypothesis that reading disorders (including developmental dyslexia) lie at the disadvantageous end of a normal distribution, related to a common neurobiological substrate, rather than representing a discrete syndrome. Furthermore, similar sensory processing mechanisms appear to play an important role in the development of both oral and written language. Learning to read a language is constrained by the oral language skills an individual has developed in that language. Specifically, learning to read a language depends on acquiring an understanding of both its spoken properties and its written form. Biological as well as environmental factors that affect the efficiency of neural processing, specifically the neural processing of rapidly presented or rapidly changing dynamic sensory stimuli, appear to significantly affect the development of both the orthographic and phonological components of reading and spelling.
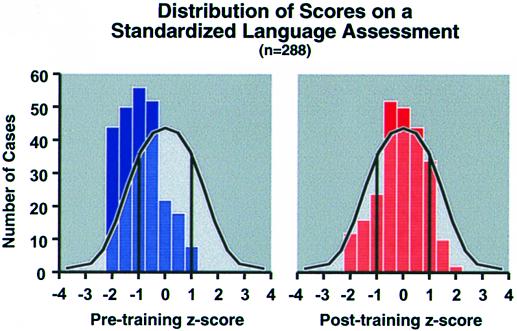
Such convergent neurobiological and behavioral studies also support results emerging from remediation research with LLI children (24, 25). Electrophysiological studies of sensory fields in the cortex of primates have shown that the underlying neural circuitry is altered after specific, temporally cohesive training regimens, leading to “remapping,” demonstrating the dynamic plasticity of the brain (26). Based on these studies, temporally cohesive training programs (disguised as computer games) were developed for children with LLI. These remediation studies incorporated for the first time explicit dynamic auditory training, coupled with language training using speech that had been computer modified to enhance and extend dynamic changes within the ongoing acoustic waveform. These studies demonstrated that explicit training aimed at enhancing dynamic auditory sensitivity results in highly significant improvement in temporal processing thresholds, speech discrimination and listening comprehension (24, 25). In field trials using these new methods, comparable outcomes have been found not only for children with LLI, but also for a broader range of children who are struggling to learn language (27). Fig. 2 shows that training with these methods also resulted in a significant shift along the normal distribution of language comprehension scores for “academically at risk” children.‡
Figure 2.
Pretraining and posttraining frequency histograms using Z scores (test of auditory comprehension of language-revised) superimposed onto shaded bell curves representing normal distribution of scores for this test. Z scores of −1 equal 1 SD below mean, +1 equals 1 SD above mean. Number of cases scoring in each Z score bin is plotted before and after 4–8 weeks of FastForWord training. The number of children performing at or above the median improved significantly from 11% pretraining to 39% posttraining (F = 26.3, P < 0.0001). Figure used with permission of Miller et al. (Miller, S. L., DeVivo, K., LaRossa, K., Pycha, A., Peterson, B. E., Tallal, P., Merzenich, M. M. & Jenkins, W. M., Society for Neuroscience Meeting, November 7–12, 1998, Los Angeles, CA).
In this issue Talcott et al. (2) demonstrate that individual differences in dynamic sensory sensitivity is highly correlated with children's individual differences in developing phonological, orthographic, reading, and spelling proficiency. The finding that this is the case for normally developing children, as well as for those experiencing significant difficulty developing literacy skills, has broad implications for developing more efficacious methods for teaching reading. Explicit training in dynamic sensory sensitivity, combined with phonological, orthographic, and comprehension skill teaching, are likely to be the most effective method for improving literacy skills.
If students fall behind in reading proficiency, they will find it difficult to benefit from all aspects of the curriculum. Poor readers also will find it difficult to participate in an economy requiring increasingly sophisticated communication skills. There is a growing public interest in, and increasing demand for, scientific inquiry into the causes and determinants of reading failure. Current research has the potential to significantly improve the efficacy of the methods chosen to teach our children to read.
Acknowledgments
P.T. is a cofounder of Scientific Learning Corporation, the company that produces the Fast ForWord training programs (www.scientific-learning.com).
Footnotes
See companion article on page 2952.
Tallal, P., Briefing on Learning Disabilities presented to the Biomedical Research Congressional Caucus, April 21, 1999, Washington, DC.
Benasich, A. A., Seventh Annual Meeting of the International Society for Behavioral Neuroscience, June 30-July 4, 1999, Santorini, Greece.
Miller, S. L., DeVivo, K., LaRossa, K., Pycha, A., Peterson, B. E., Tallal, P., Merzenich, M. M. & Jenkins, W. M., Society for Neuroscience Meeting, November 7–12, 1998, Los Angeles, CA.
References
- 1.Hall S L, Moats L. Straight Talk about Reading: How Parents Can Make a Difference During the Early Years. Chicago, IL: Contemporary Books; 1999. [Google Scholar]
- 2.Talcott J B, Witton C, McClean M F, Hansen P C, Rees A, Green G G R, Stein J F. Proc Natl Acad Sci USA. 2000;97:2952–2957. doi: 10.1073/pnas.040546597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennington B, Van Orden G, Smith S, Green P, Haith M. Child Dev. 1990;61:1753–1778. [PubMed] [Google Scholar]
- 4.Stanovich K. J Learn Disabil. 1988;21:590–612. doi: 10.1177/002221948802101003. [DOI] [PubMed] [Google Scholar]
- 5.Bradley L, Bryant P. Nature (London) 1983;301:419. [Google Scholar]
- 6.Tallal P, Galaburda A, Von Euler C, Llinas R. Temporal Information Processing in the Nervous System. New York: N.Y. Acad. Sci.; 1993. [Google Scholar]
- 7.Farmer M, Klein R. Psychol Bull Rev. 1995;2:460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- 8.Tallal P, Merzenich M, Miller S, Jenkins W. Exp Brain Res. 1998;123:210–219. doi: 10.1007/s002210050563. [DOI] [PubMed] [Google Scholar]
- 9.Mody M, Studdert-Kennedy M, Brady S. J Exp Child Psychol. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- 10.Denenberg V H. J Learn Disabil. 1999;32:379–383. doi: 10.1177/002221949903200502. [DOI] [PubMed] [Google Scholar]
- 11.Leppänen P H, Pihko E, Eklund K M, Lyytinen H. NeuroReport. 1999;10:969–973. doi: 10.1097/00001756-199904060-00014. [DOI] [PubMed] [Google Scholar]
- 12.Benasich A A, Tallal P. Infant Behav Dev. 1996;19:339–357. [Google Scholar]
- 13.Trehub S E, Henderson J L. J Speech Hear Res. 1996;39:1315–1320. doi: 10.1044/jshr.3906.1315. [DOI] [PubMed] [Google Scholar]
- 14.Leppänen P H, Lyytinen H. Audiol Neurootol. 1997;2:308–340. doi: 10.1159/000259254. [DOI] [PubMed] [Google Scholar]
- 15.Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich M M. Proc Natl Acad Sci USA. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eden G, Van Meter J, Rumsey J, Maisog J, Woods R, Zeffiro T. Nature (London) 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- 17.Galaburda A M, Kemper T L. Ann Neurol. 1979;6:94–100. doi: 10.1002/ana.410060203. [DOI] [PubMed] [Google Scholar]
- 18.Galaburda A M, Sherman G F, Rosen G D, Aboitiz F, Geschwind N. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 19.Galabuda A M, Menard M T, Rosen G D. Proc Natl Acad Sci USA. 1994;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingstone M S, Rosen G D, Drislane F W, Galaburda A M. Proc Natl Acad Sci USA. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson B, Brian D, Powers R E. Neuropsychiatry Neuropsychol. 1999;12:247–254. [PubMed] [Google Scholar]
- 22.Schwartz J, Tallal P. Science. 1980;207:1380–1381. doi: 10.1126/science.7355297. [DOI] [PubMed] [Google Scholar]
- 23.Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli J D E, Moseley M E, Poldrack R A. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 24.Tallal P, Miller S L, Bedi G, Byma G, Wang X, Nagarajan S S, Schreiner C, Jenkins W M, Merzenich M M. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 25.Merzenich M, Jenkins W, Johnston P S, Schreiner C, Miller S L, Tallal P. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins W M, Merzenich M M, Ochs M T, Allard T, Guie R E. J Neurophys. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Merzenich, M. M., Miller, S., Jenkins, W. M., Protopapas, A., Saunders, G., Peterson, B., Ahissar, M. & Tallal, P. (2000) J. Learn. Disabil., in press.