Abstract
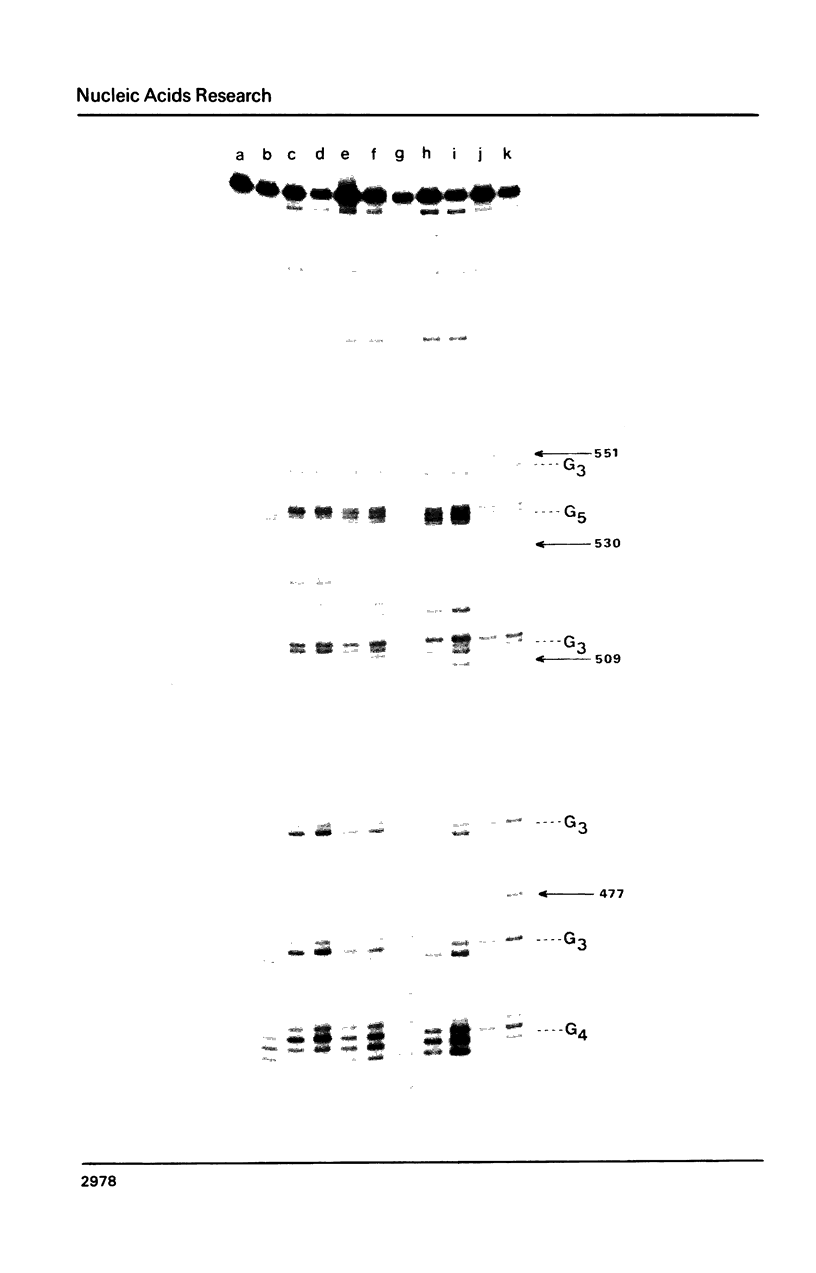
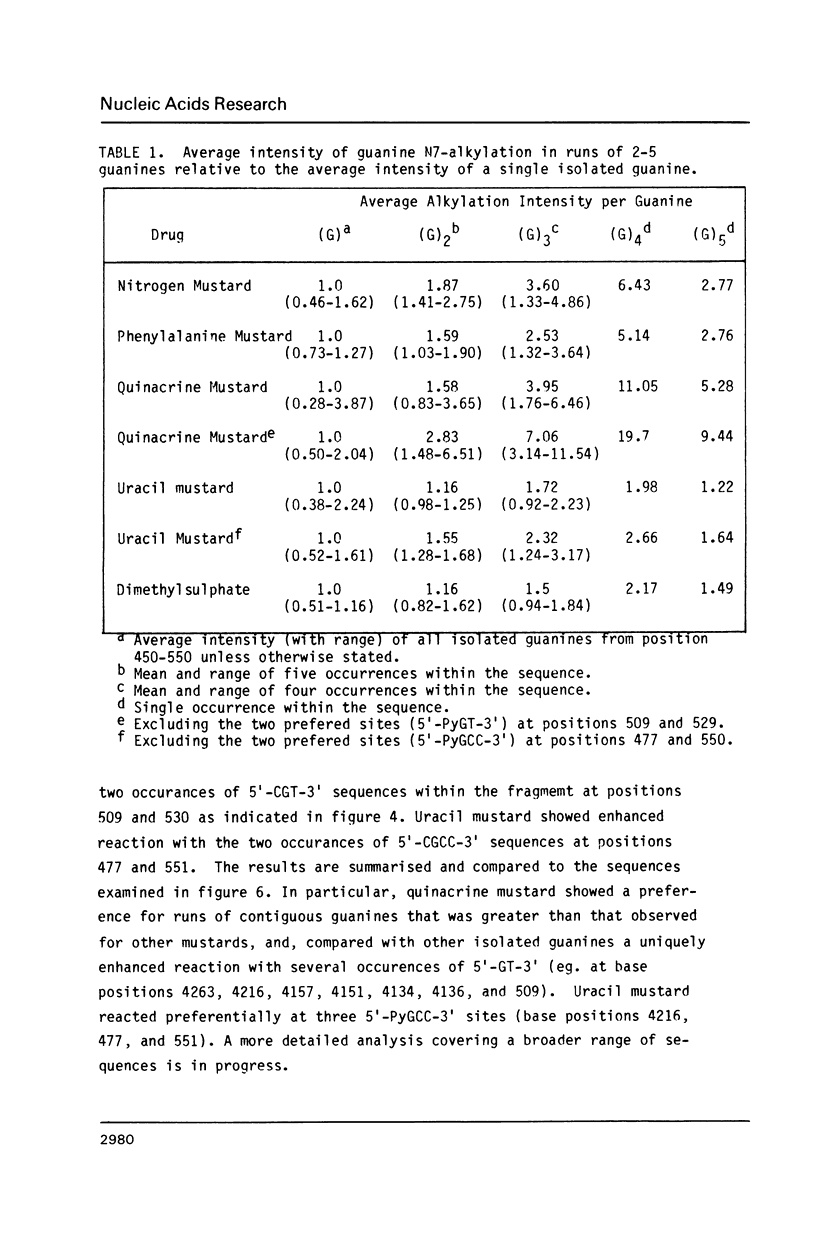
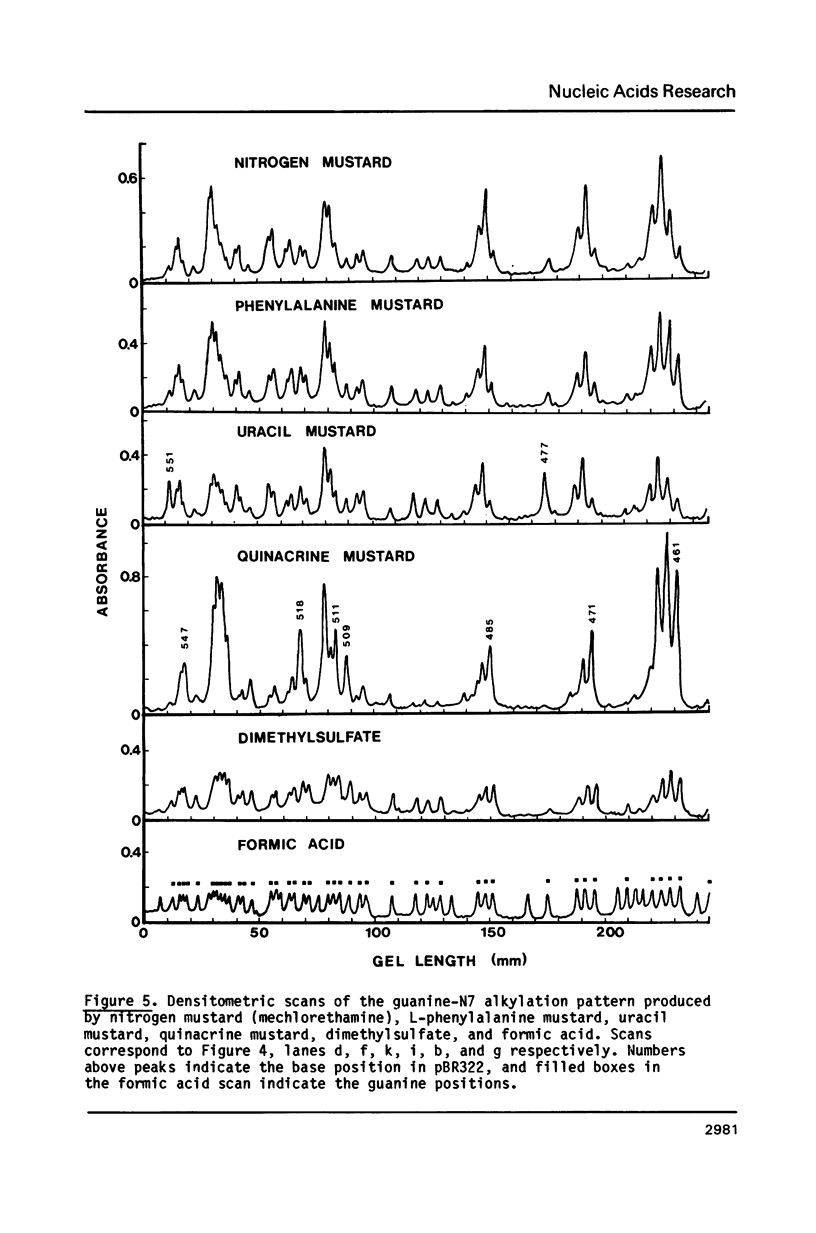
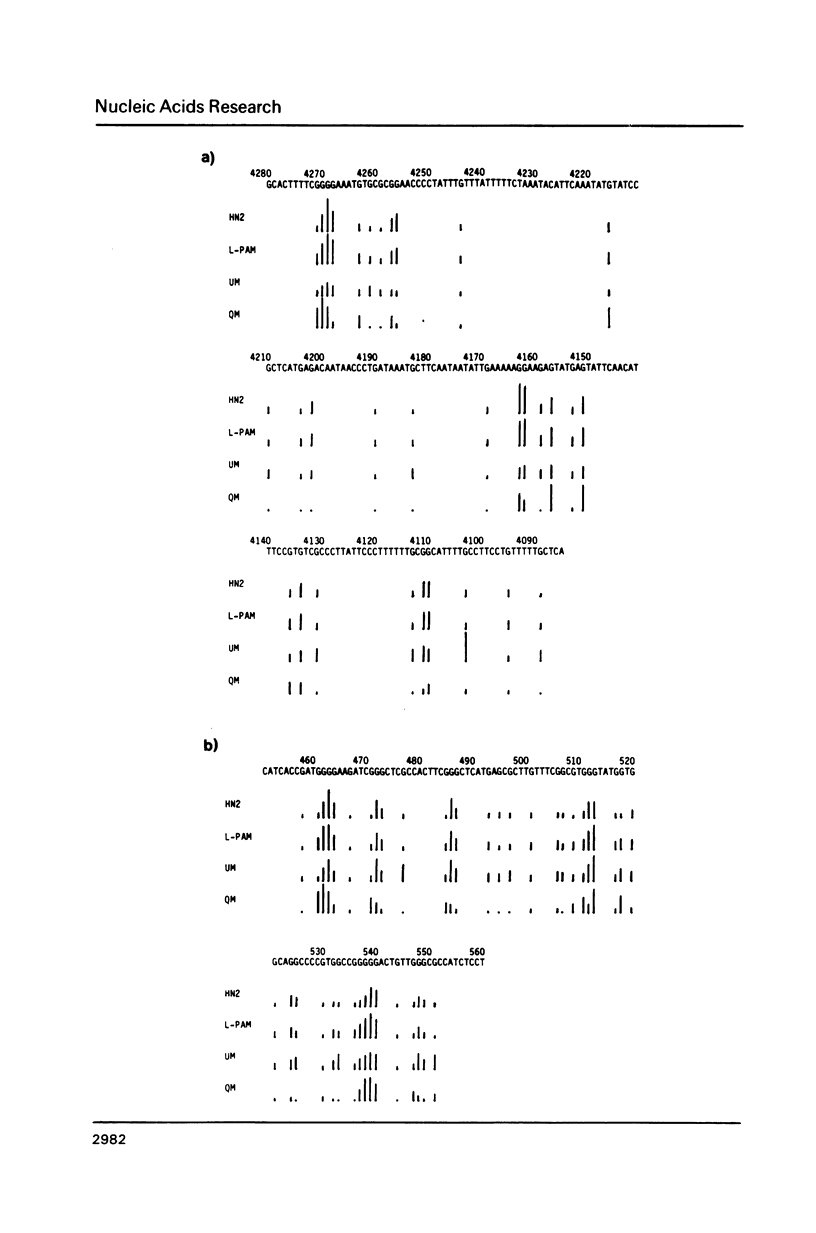
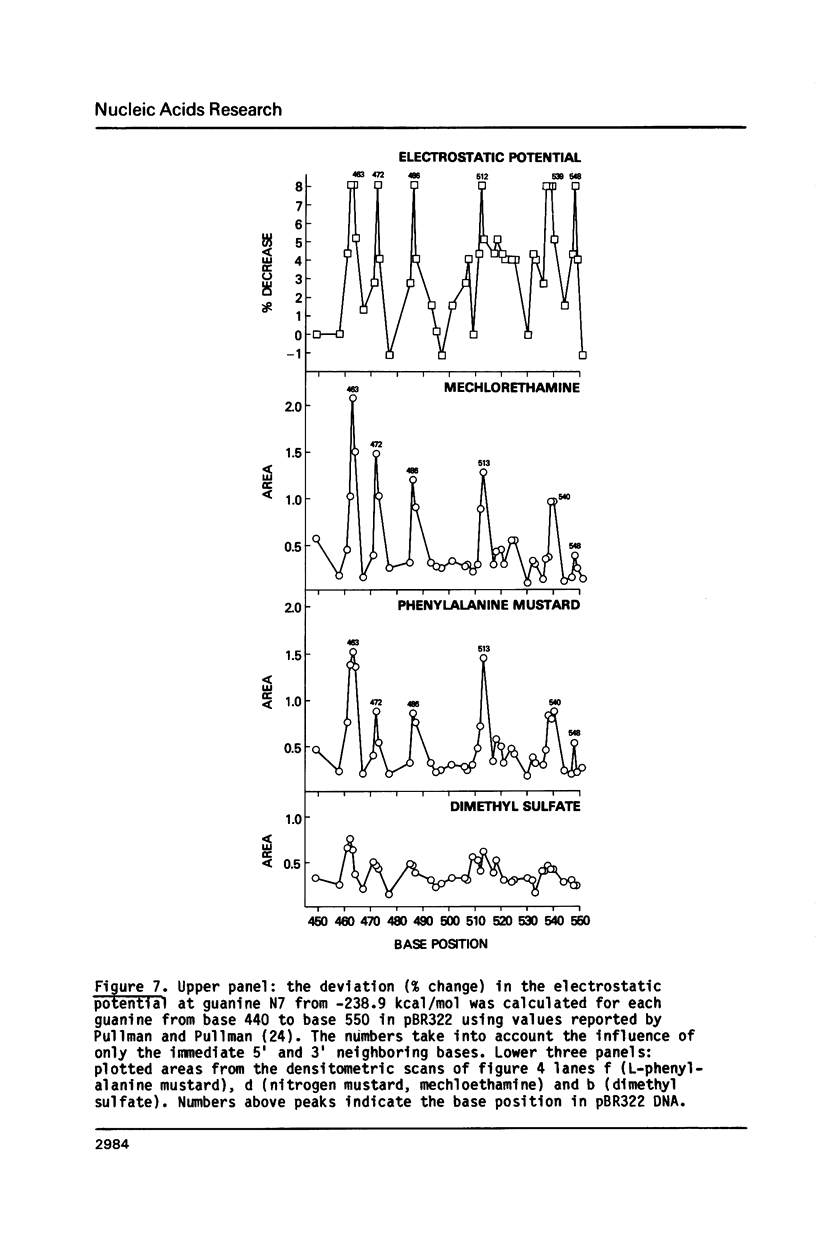
Nitrogen mustards alkylate DNA primarily at the N7 position of guanine. Using an approach analogous to that of the Maxam-Gilbert procedure for DNA sequence analysis, we have examined the relative frequencies of alkylation for a number of nitrogen mustards at different guanine-N7 sites on a DNA fragment of known sequence. Most nitrogen mustards were found to have similar patterns of alkylation, with the sites of greatest alkylation being runs of contiguous guanines, and relatively weak alkylation at isolated guanines. Uracil mustard and quinacrine mustard, however, were found to have uniquely enhanced reaction with at least some 5'-PyGCC-3' and 5'-GT-3' sequences, respectively. In addition, quinacrine mustard showed a greater reaction at runs of contiguous guanines than did other nitrogen mustards, whereas uracil mustard showed little preference for these sequences. A comparison of the sequence-dependent variations of molecular electrostatic potential at the N7-position of guanine with the sequence dependent variations of alkylation intensity for mechlorethamine and L-phenylalanine mustard showed a good correlation in some regions of the DNA, but not others. It is concluded that electrostatic interactions may contribute strongly to the reaction rates of cationic compounds such as the reactive aziridinium species of nitrogen mustards, but that other sequence selectivities can be introduced in different nitrogen mustard derivatives.
Full text
PDF

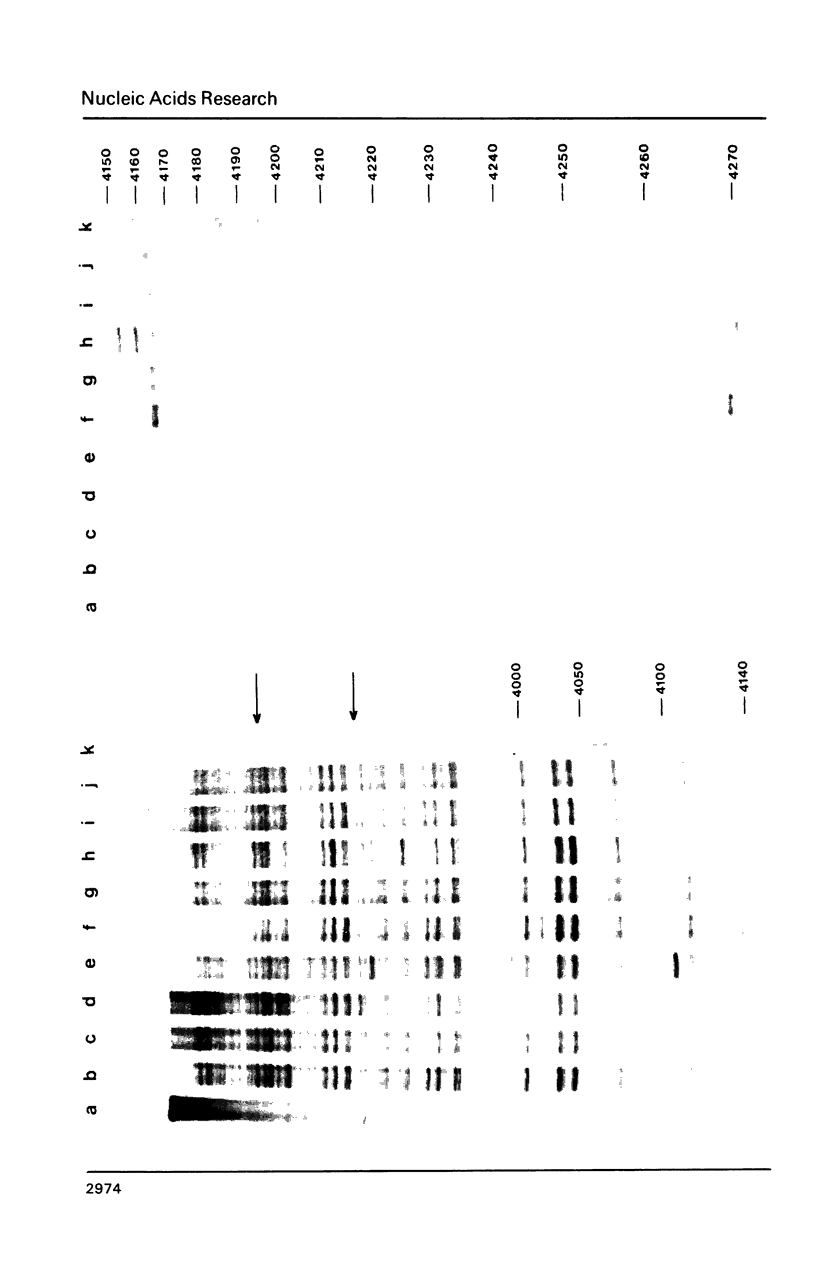

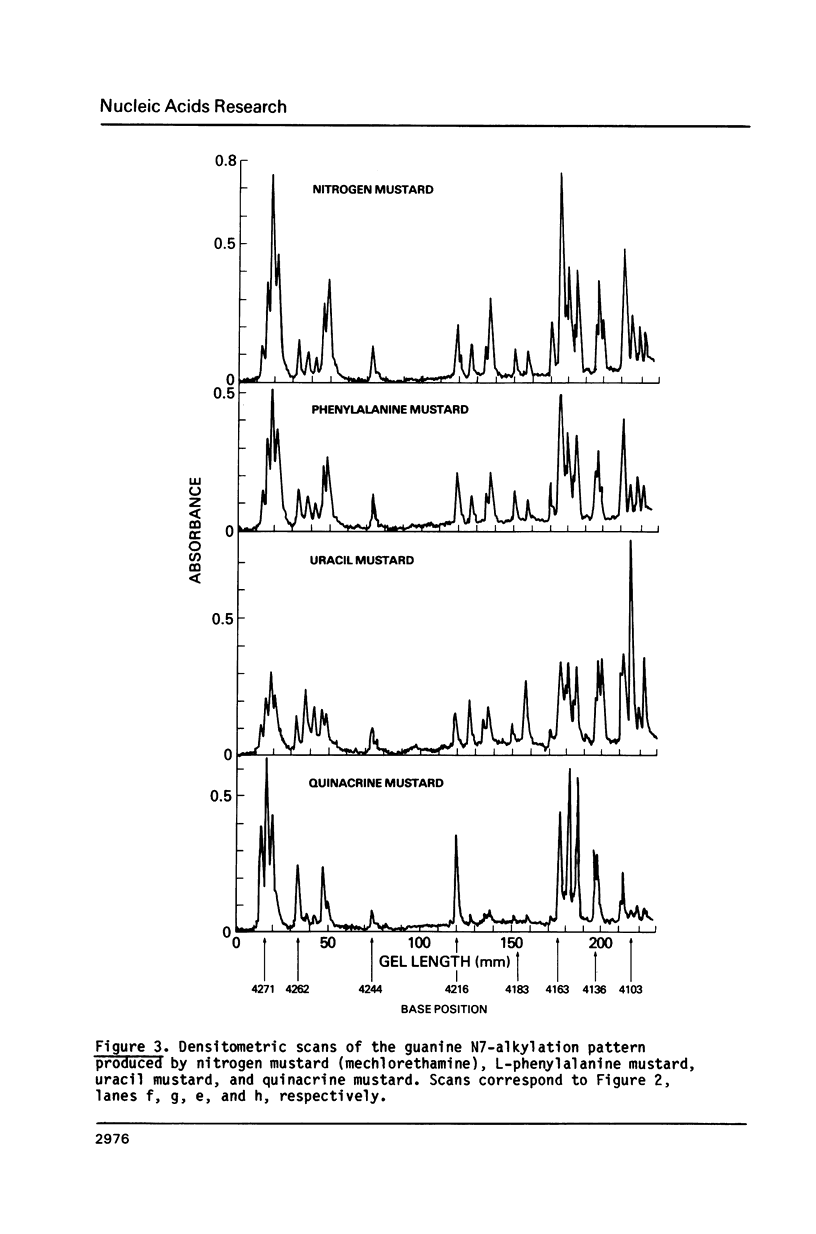






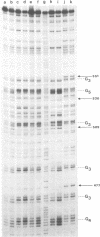
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boles T. C., Hogan M. E. Site-specific carcinogen binding to DNA. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5623–5627. doi: 10.1073/pnas.81.18.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Modification of DNA by aflatoxin B1 creates alkali-labile lesions in DNA at positions of guanine and adenine. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4120–4124. doi: 10.1073/pnas.75.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A., Philips F. S. The Biological Actions and Therapeutic Applications of the B-Chloroethyl Amines and Sulfides. Science. 1946 Apr 5;103(2675):409–436. doi: 10.1126/science.103.2675.409. [DOI] [PubMed] [Google Scholar]
- Grunberg S. M., Haseltine W. A. Use of an indicator sequence of human DNA to study DNA damage by methylbis(2-chloroethyl)amine. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6546–6550. doi: 10.1073/pnas.77.11.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K., Ludlum D. B. Covalent modification of DNA by antineoplastic agents. J Natl Cancer Inst. 1984 Nov;73(5):1021–1028. [PubMed] [Google Scholar]
- Kohn K. W., Spears C. L. Alkylated DNA: buoyant density changes and modes of decomposition. Biochim Biophys Acta. 1967;145(3):720–733. doi: 10.1016/0005-2787(67)90131-1. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Spears C. L., Doty P. Inter-strand crosslinking of DNA by nitrogen mustard. J Mol Biol. 1966 Aug;19(2):266–288. doi: 10.1016/s0022-2836(66)80004-9. [DOI] [PubMed] [Google Scholar]
- Lawley P. D. Effects of some chemical mutagens and carcinogens on nucleic acids. Prog Nucleic Acid Res Mol Biol. 1966;5:89–131. doi: 10.1016/s0079-6603(08)60232-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Muench K. F., Misra R. P., Humayun M. Z. Sequence specificity in aflatoxin B1--DNA interactions. Proc Natl Acad Sci U S A. 1983 Jan;80(1):6–10. doi: 10.1073/pnas.80.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman A., Pullman B. Molecular electrostatic potential of the nucleic acids. Q Rev Biophys. 1981 Aug;14(3):289–380. doi: 10.1017/s0033583500002341. [DOI] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Takeshita M., Grollman A. P., Ohtsubo E., Ohtsubo H. Interaction of bleomycin with DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5983–5987. doi: 10.1073/pnas.75.12.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Morita J., Komano T. Sequence specificity of heat-labile sites in DNA induced by mitomycin C. Biochemistry. 1984 Apr 10;23(8):1634–1640. doi: 10.1021/bi00303a008. [DOI] [PubMed] [Google Scholar]
- Wilkins R. J. Sequence specificities in the interactions of chemicals and radiations with DNA. Mol Cell Biochem. 1984 Sep;64(2):111–126. doi: 10.1007/BF00224768. [DOI] [PubMed] [Google Scholar]