Abstract
Premarin (conjugated equine estrogens) is the most widely prescribed estrogen-only menopausal hormone therapy in the United States, and is comprised of over 50% estrone (E1) sulfate. Following CEE administration, E1 is the principal circulating estrogen. However, the cognitive and neurobiological effects of E1 in a middle-aged rodent model have not yet been evaluated. We assessed cognitive effects of continuous E1 treatment in middle-aged surgically menopausal rats using a maze battery. We also quantified number of choline acetyltransferase-immunoreactive (ChAT-IR) neurons in distinct basal forebrain regions known in earlier studies in to be impacted by the most potent naturally-circulating estrogen in rodents and women, 17β-estradiol (17β-E2), as well as CEE. On the spatial working memory delayed-match-to-sample water maze, the highest E1 dose impaired memory performance during acquisition and after delay challenge. E1 did not impact ChAT-IR neuron number in the medial septum (MS) or horizontal/vertical diagonal bands. In a comparison study, 17β-E2 increased MS ChAT-IR neuron number. Findings indicate that E1 negatively impacts spatial working memory and memory retention, but does not increase ChAT-IR neuron number in basal forebrain, as does 17β-E2. Thus, data from prior studies suggest that 17β-E2 and CEE can enhance cognition and increase number of ChAT-IR basal forebrain neurons, while here we show that E1 does not induce these effects. Findings from preclinical basic science studies can inform the design of specific combinations of estrogens that could be beneficial to the brain and cognition. Accumulating data suggest that E1 is not likely to be among these key beneficial estrogens.
Keywords: estrone, 17β-estradiol, estrogen, Premarin, working memory, spatial memory, basal forebrain, choline acetyltransferase
Introduction
Conjugated equine estrogens (CEE) have been given to menopausal women since 1942 (Stefanick, 2005), was the estrogenic component tested in the Women’s Health Initiative Memory study (Shumaker et al., 2004; Shumaker et al., 1998), and is the most widely prescribed estrogenic component of menopausal hormone therapy in the United States, even despite a decrease in use after the 2002 publication of clinical trial results (Hersh et al., 2004). CEE has been shown to have both positive and negative effects on cognition in menopausal women (for review see Hogervorst et al., 2000; Sherwin and Henry, 2008), and can enhance memory in the middle-aged Ovx rat, which is a dose-specific effect (Acosta et al., 2009b; Engler-Chiurazzi et al., 2011). CEE is a complex estrogen formulation comprised of 50% estrone (E1) sulfate, and it contains the sulfates of at least ten other estrogens (Kuhl, 2005), many of which have yet to be individually evaluated for cognition in women or rodent models. Determining effects of the specific estrogen components of this complex formulation could help determine why it sometimes enhances, and why it sometimes impairs, cognition. Furthermore, this strategy may identify a group of cognitively enhancing estrogens to be combined into optimal hormone therapy formulations for specific populations of women, as well as identify estrogens detrimental to the brain and cognition to be excluded from future formulations. In women, 17β-estradiol (17β-E2), present only in trace amounts in CEE, is the most potent naturally-circulating estrogen, followed by E1 and estriol, in order of receptor affinity (Kuhl, 2005). 17β-E2 and E1 are biologically interconvertible; in vivo, they readily get converted into one another (Kuhl, 2005; Prokai-Tatrai and Prokai, 2005). Circulating levels of E1 increase following treatment with CEE to menopausal and post-menopausal women (Yasui et al., 1999), and following administration of CEE to middle-aged ovariectomized (Ovx) rats (Acosta et al., 2009b; Engler-Chiurazzi et al., 2011). Although we have shown that the CEE components Δ8,9-dehydroestrone, and to a lesser extent, equilin, exert benefical cognitive effects in middle-aged, Ovx rats (Talboom et al., 2010), the cognitive impact of the principle circulating estogen following CEE administration, E1, is unclear. We hypothesize that E1 will impair cognition in middle-aged Ovx rats. Indeed, one paper in young rats has shown a single subcutaneous E1 injection impairs contextual fear conditioning memory when given 30 minutes before training (Barha et al., 2009). Furthermore, although not all in vitro studies report negative effects with E1 administration (Zhao and Brinton, 2006), for most measures in which other estrogenic CEE components (e.g., equilin and Δ8,9-dehydroestrone) were neuroprotective in vitro, E1 was ineffective (Zhao and Brinton, 2006).
The basal forebrain cholinergic system is important for learning and memory, issusceptible to age-related changes, and is impacted by ovarian hormone removal and 17β-E2 replacement (for review see Gibbs, 2010). For example, in aged female rats, less choline acetyltransferase (ChAT) protein activity was found in the vertical diagonal bands (vDB), relative to younger counterparts (Luine and Hearns, 1990). Also, in adult Ovx rats, 17β-E2 treatment increased ChAT protein activity in the horizontal diagonal bands (hDB; Luine, 1985), as well as ChAT-immunoreactive (ChAT-IR) neuron counts in the medial septum (MS; Gibbs, 1997). Evidence from Gibbs’ laboratory suggests that the effects of 17β-E2 on cognition require a functioning basal forebrain cholinergic system; for example, 17β-E2 was ineffective in animals with basal forebrain lesions, and enhanced memory only in non-lesion controls (Gibbs, 2002, 2007). Although it has been established that 17β-E2 impacts the basal forebrain cholinergic system, an effect which is likely related to cognitive enhancements (for review see Bimonte-Nelson et al., 2010; Gibbs, 2010), there has been no study evaluating whether E1 impacts basal forebrain cholinergic neurons.
In the present study, we evaluated the cognitive impact of subcutaneously administered continuous E1 treatment in middle-aged Ovx rats, utilizing several spatial memory mazes previously shown to be sensitive to the effects of aging (Frick et al., 1995; Talboom et al., 2008), and estrogen administration (Acosta et al., 2009b; Bimonte-Nelson et al., 2006; Engler-Chiurazzi et al., 2011; Walf et al., 2009), such that a potential pattern of E1’s effects on specific memory types could be revealed. Several classic peripheral markers of estrogenic action, including vaginal smears and uterine weights, were measured to confirm effects of Ovx and E1 treatment. Lastly, we evaluated the impact of E1 on the basal forebrain cholinergic system by quantifying the number of ChAT-IR neurons in the MS and the hDB/vDB of the basal forebrain in the cognitively tested animals. Because 17β-E2 has been shown to impact ChAT protein activity (Luine, 1985) and ChAT-IR neuron counts (Gibbs, 1997) in the basal forebrain, to aid in interpretation of potential E1 ChAT-IR effects, we performed a separate study evaluating ChAT-IR neuron numbers after treatment with 17β-E2 using the same quantification procedures as those used in the E1 study. Determining the impact of E1 on spatial memory and the cholinergic system will help to characterize the unique cognitive and neurobiological impacts of this estrogen, which is a primary circulating estrogen after administration of the commonly used hormone therapy, CEE.
Materials and Methods
Subjects
We used 32 middle-aged (13 months old at the beginning of the study) Fischer-344 female rats born and raised at the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN). Animals were pair-housed, acclimated for several weeks at Arizona State University, had exposure to food and water ad libitum, and were maintained on a 12-h light/dark cycle at 23C. Experimental procedures were approved by the Arizona State University Institutional Animal Care and Use Committee and adhered to Guidelines for the Care and Use of Laboratory Animals and NIH standards.
Hormone Treatments
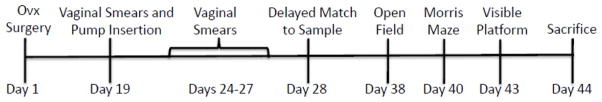
Figure 1 displays a timeline of the experimental protocol. Approximately 28 days before behavioral testing ensued, under isoflurane inhalant anesthesia, all rats underwent Ovx surgery to remove endogenous ovarian hormones. Dorsolateral incisions were made in the skin and peritoneum, and ovaries and tips of uterine horns were ligated and removed. Rats were then separated into the following groups: Ovx with vehicle only (polyethylene glycol, PEG)(Vehicle, n=9), Ovx plus 2.6μg/day of E1 (E1-Low, n=7), Ovx plus 4.0μg/day of E1 (E1-Med, n=8), and Ovx plus 8.0μg/day of E1 (E1-High, n=8). All hormones were purchased from Sigma (St. Louis, MO). The E1-Low dose was based on the most efficacious dose of 17β-E2 found in a dose response study conducted in our laboratory evaluating spatial working memory (unpublished observations). The E1-Med dose was based on findings from Beyer and colleagues (1976) in which 4.0 μg/day of E1 induced lordosis behavior and increased uterine weights. To assess the cognitive and physiological impact of a broad range of E1 doses, the E1-High dose was double the E1-Med dose. Corresponding to published studies evaluating E1 and other estrogens (Barha et al., 2009; Talboom et al., 2010) in which subjects were given approximately one to two weeks between Ovx and hormone treatment, in the current study, hormone treatment began 19±1 days after Ovx. In parallel with other studies (Engler-Chiurazzi et al., 2011; Talboom et al., 2010), Vehicle and E1 treatments were administered continuously using Alzet osmotic pumps (Model 2004; Durect Corporation, Cupertino, CA). Briefly, E1 was dissolved in PEG (Sigma, St. Louis, MO) and inserted into the pumps as per manufacturer’s instructions. For the Vehicle group, pumps were filled with PEG only. For pump insertion, under isoflurane anesthesia, a small incision was made in the dorsal scruff of the neck, and a subcutaneous pocket was created. One pump filled with Vehicle or the appropriate E1 dose was inserted into the pocket and the skin was stapled. Nine days after pump insertion surgery, cognitive testing began. All animals had Vehicle or E1 exposure until sacrifice.
Figure 1.
Study Timeline. A timeline summarizing the effects of Ovx surgery, pump insertion surgery for hormone administration, vaginal smears, and behavioral maze testing battery.
Markers of Peripheral Estrogenic Action
To confirm the effects of Ovx as well as E1 treatments, we assessed several peripheral physiological markers that routinely change with estrogen treatment. Notably, E1 has been found to impact peripheral tissues, including the uterus (Beyer et al., 1976). We therefore performed vaginal smears (Goldman et al., 2007) and measured uterine weights (Westerlind, 1998), the latter of which was done upon animal sacrifice. Smears were classified as proestrus, estrous, metestrus or diestrus (Acosta et al., 2009b; Engler-Chiurazzi et al., 2011; Goldman et al., 2007). Vaginal smears were first conducted to confirm the lack of uterine stimulation and complete Ovx 18 days after Ovx, which was one day before E1 administration via pump implantation. Vaginal smears were also conducted daily for four days beginning five days after pump implantation.
Delayed-Match-to-Sample Water Maze
The delayed-match-to-sample (DMS) water maze assessed spatial working memory. The maze had four arms (each 38.1 cm long and 12.7 cm wide) in a plus configuration, and was filled with room temperature water made opaque with black non-toxic paint. The maze had a hidden escape platform at the end of one arm. The platform location changed every day, but was fixed within a day. Rats received six consecutive trials within a daily session, for seven consecutive days. The first trial was an information trial, where the rat had to first locate the platform position for that day. Trials two through six were memory test trials, in which the location of the platform was repeatedly reinforced (Acosta et al., 2009b; Engler-Chiurazzi et al., 2011). For each trial (one-six), rats were dropped off in a semi-randomly chosen start arm location, and were given a maximum of 90 sec to swim to the platform. If an animal did not locate the platform within the allotted time limit, it was gently guided to the platform using a black plastic rod. Once on the platform, the rat remained on it for 15 sec, followed by placement into a heated cage for a 30 sec inter-trial interval (ITI). An arm entry was counted when the tip of a rat’s snout reached a mark delineated on the outside of the arm (11cm into the arm). Entry into an arm with no platform counted as an error, the dependent variable. The number of errors committed on trials two through six across all testing days was the dependent measure. As the rate of learning can change across the task and be impacted by treatment (Acosta et al., 2009a), and to gain insight into errors committed across different phases of task acquisition, we grouped the data into two three-day blocks (block one = days two to four; block two = days five to seven). As we have previously shown effects of CEE on six-hour delayed memory retention (Engler-Chiurazzi et al., 2011), to test effects of E1 on extended memory retention after only one exposure to the platform location, on day eight rats were tested with a delayed ITI of six hours. Within treatment group comparisons of performance on trial two of baseline versus trial two of the delay day revealed that no group was impacted by the six-hour delay. Thus, on day nine, rats were tested with a longer delayed ITI of eight hours. Delayed ITIs were instituted between trial one (information trial) and trial two. Thus, on these days, animals were given trial one, given the appropriate delayed ITI, and then given trial two.
Open Field Testing
The open field task evaluated activity and emotional reactivity in response to being placed in an empty open field (Denenberg, 1969). A black plexiglass box measuring 95.75 cm × 95.25 cm × 45.70 cm was utilized. The rat was placed in the apparatus facing the North wall. Each animal received a 10 min session whereby they were allowed to freely explore the box. Between each subject tested, the apparatus was thoroughly cleaned with 70% isopropyl alcohol. Each animal’s activity was recorded using Ethovision (XT 5.1, Noldus Information Technology, Wageningenm, Netherlands) and the dependent variable was distance moved (cm). Using the computer system, the open field arena (9120.19 cm2) was virtually divided into three concentric zones, including an outer (5790.19 cm2), middle (3078.78 cm2) and inner (251.63 cm2) zone. Overall activity (total distance travelled in the box), as well as movement in each zone, were the dependent variables.
Morris Water Maze Testing
The Morris water maze (MM) tested spatial reference memory and consisted of a round tub (188 cm in diameter) filled with room temperature water made opaque with black non-toxic paint. Briefly, the rat was placed in the maze from any of four locations (North, South, East, or West) and had 60 sec to locate the platform, which remained in a fixed location (Northeast quadrant; NE). If an animal did not locate the platform within the allotted time limit, it was gently guided to the platform. After 15 sec on the platform, the rat was placed into its heated cage until the next trial. Animals were tested in squads (eight or nine rats in each squad) so that the first trial was completed for each rat in the group, then the second, etc., as done previously (Engler-Chiurazzi et al., 2011; Stavnezer et al., 2002). The testing procedure was based on the work of Markham and colleagues (2002) wherein beneficial effects of 17β-E2 have been noted in rats. Rats received six trials/day for three days, with a 15 min delay instilled between trials three and four (Markham et al., 2002). There was approximately an eight to ten min ITI between all other trials. A video camera recorded each rat and a tracking system (Ethovision XT 5.1, Noldus) analyzed each rat’s path. The dependent measure was swim distance (cm). To assess platform localization, a probe trial was given on trial seven of the last day of testing, whereby the platform was removed from the maze. For the probe, percent of total swim distance (cm) travelled in the target NE quadrant (i.e., quadrant that contained the platform) as compared to the opposite Southwest (SW) quadrant was the dependent measure (Stavnezer et al., 2002). Additional probe trial dependent variables included the frequency of crossing into the platform zone, the NE quadrant, and the SW quadrant.
Visible Platform Testing
The visible platform task confirms that animals can perform the procedural components of a water escape task, including visual and motor competence. A rectangular tub (99 cm × 58.5 cm) was filled with clear water. A black platform (10 cm wide) was positioned approximately 3.75 cm above the water surface (Hunter et al., 2003). Opaque curtains covered obvious extramaze cues. Animals were given six trials in one day. The drop off location remained the same across trials, and the platform location for each trial varied semi-randomly. Each rat had 90 sec to locate the platform, where the rat then remained for 15 sec before being placed back into its heated cage. If an animal did not locate the platform within the allotted time limit, it was gently guided to the platform. The ITI was approximately eight min. Latency (sec) to reach the platform was the dependent measure.
Tissue Collection, Hormone Assays, and Uterine Weights
The day following the completion of maze testing, all animals (15 months old at this time) were sacrificed on the same day, with researchers blinded to treatment group assignment. Rats were anesthetized with isoflurane, and rats were decapitated. Brains were rapidly removed and the anterior portion of the brain containing the basal forebrain was separated from the posterior portion of the brain. Uterine tissues were collected, trimmed of fat and connective tissue, and weighed as per previous methods (Acosta et al., 2009b). Wet uterine weight (g) was the dependent measure.
Basal Forebrain ChAT-IR Neuron Counts
Each brain was post fixed in 4% paraformaldehyde in phosphate-buffer solution (PB, pH 7.4) for 48 hours, and then the tissues were transferred to PB until sectioning. The basal forebrain region was sectioned (plates 1–25; Paxinos and Watson, 2005) on a Vibratome 3000 (Vibratome) in phosphate-buffered saline (PBS; pH 7.4) at 40 microns for immunohistochemistry (Granholm et al., 2002). Every fourth section through the basal forebrain was selected for the ChAT immunohistochemistry and incubated for 15 min in a 0.03% Triton (Triton 100X) in PBS (PBST) to permeabilize the tissue. As done previously (Acosta et al., 2009b), the tissue was then blocked, by incubating tissues at room temperature for 30 min in a blocking solution (BKS) containing 0.03% PBST and 0.03% heat inactivated horse serum (Fischer Scientific, Pittsburg, PA). Three PBS washes (3 min each) were then done. The primary polyclonal antibody, goat Anti-Rat-ChAT (1:1000, Millipore, Billerica, MA), was added to each well, and sections were incubated overnight at 4°C on a Rocker II (Boekel Scientific, Feasterville, PA). Next, sections were washed in PBS three times (3 min each) followed by immersion in the secondary antibody solution (1: 200 biotinylated Donkey anti-Goat IgG, Vector) and BKS for 45 min on a Titer Plate Shaker (Barnstead International, Dubuque, IO) at RT. Sections were washed three times in PBS (3 min each), and then placed into an 11% methanol and 1% H202 (Fischer) in PBS solution for 30 min on a Titer Plate Shaker to quench endogenous peroxidase activity. After three washes in PBS (3 min each), ABC reagent (Vector Laboratories, Burlingame, CA) was added to each well and incubated for 45 min at RT on a Titer Plate Shaker. Sections were washed three times in PBS (3 min each), and were then incubated with DAB Peroxidase Substrate (Vector). After the desired color was achieved (dark purple), brain sections were washed three times in PBS (3 min each), mounted on subbed slides, air dried, dehydrated and cover slipped with Permount (VWR, Randor, PA). Each group was equally represented in each round of staining, to avoid group inter-variability in staining. Further, control procedures were run excluding primary and secondary antibodies. Exclusion of the primary antibody resulted in no cell staining, and exclusion of secondary antibody resulted in a lack of DAB Peroxidase Substrate color development.
Basal Forebrain ChAT-IR Image Analysis
ChAT-IR neurons were quantified in the basal forebrain. Images were acquired using PictureFrame software (MicrobrightField, Burlington, VT) from a CX9000 camera (MicrobrightField) coupled to an Olympus BX51 microscope. A 4x objective was used to capture images (Olympus, Center Valley, PA). Captured images for each section were then manually counted using the “Point Picker” plugin from NIH ImageJ software(Rasband, 1997–2004). Three sections per animal within the range of plates 23–28 from Paxinos and Watson (2005) were quantified similar to prior publications (Gibbs, 1997). ChAT-IR neurons were counted in the MS, and the hDB/vDB, and counts from the three sections were averaged to yield one value per basal forebrain region per animal.
Since 17β-E2 impacts ChAT protein activity (Luine, 1985) and ChAT-IR neuron counts in the basal forebrain (Gibbs, 1997), a group of rats that had been administered continuous subcutaneous Vehicle (propylene glycol) or 17β-E2 were analyzed separately for basal forebrain quantifications to aid in interpretation of potential E1 effects found in the current study. These 15–16 month old rats were given Ovx, and 19 days later, administered an Alzet osmotic pump containing either propylene glycol or 4.0μg/day 17β-E2. The Ovx and pump insertion surgical procedures were similar to those used in the current study, and these animals were behaviorally tested on a cognitive maze battery (unpublished). 17β-E2 treatment was initiated 19±1 days after Ovx in the comparison study, which corresponds exactly to the E1 study whereby E1 treatment was administered 19±1 days after Ovx. For the 17β-E2 treated rats, treatment continued for approximately 50 days, until animals were sacrificed, brains removed, and sections processed via immunohistochemistry for ChAT identical to the methods described for the E1 study.
Dependent Variables and Statistical Analyses
Data were analyzed for each maze separately, using ANOVA with Hormone Treatment as the between factor with all four treatment groups included, and Days and/or Trials as the repeated measure, depending on the maze. Before we performed the experiment, we laid out two-group comparisons to be performed upon study completion (Denenberg, 1976). Since our a priori interest was to determine the impact that each dose of E1 had on maze performance, we planned to compare each dose of E1 to the vehicle control. Thus, to test the effects of each E1 dose, following the omnibus ANOVA we used Dunnett’s correction for multiple comparisons, comparing the Vehicle group to each E1-dosed group (E1-Low, E1-Med, and E1-High). To determine whether each treatment group showed learning of DMS and MM, we also assessed overall performance within each treatment group, with Days and/or Trials as the repeated measure.
For uterine weights, data were analyzed first using ANOVA with all groups, followed by the Dunnett’s test for two group comparisons. In each distinct basal forebrain region, ChAT-IR analyses were performed using an omnibus ANOVA for the animals in the E1 study, and a t-test for the two-group comparison study testing Vehicle versus 17β-E2. The comparison study using 17β-E2 was analyzed separately from the E1 study. Only marginal (p <. 10) or significant interactions or main effects are reported.
Results
Delayed-Match-to-Sample Water Maze
Testing with a 30 sec ITI: Acquisition Effects
To evaluate learning, we analyzed performance within each treatment group with days one to seven, and trials two to six, as repeated measures. This analysis revealed a main effect of Day for each group [Vehicle: F(6,48)= 4.81; p<0.001, E1-Low: F(6,36)= 2.63; p<0.05, E1-Med: F(6,42)= 3.68; p<0.005, E1-High: F(6,42)= 4.74; p<0.001], with errors decreasing as days progressed, indicating learning for each group (data not shown). There was also a main effect of Trial for each group [Vehicle: F(4,32)= 7.57; p<0.0005, E1-Low: F(4,24)= 8.70; p<0.0005, E1-Med: F(4,28)= 5.97; p<0.005, E1-High: F(4,28)= 6.27; p<0.001], with errors decreasing as trials progressed, indicating learning of the new platform location within a day for each group (data not shown).
Testing with a 30 sec ITI
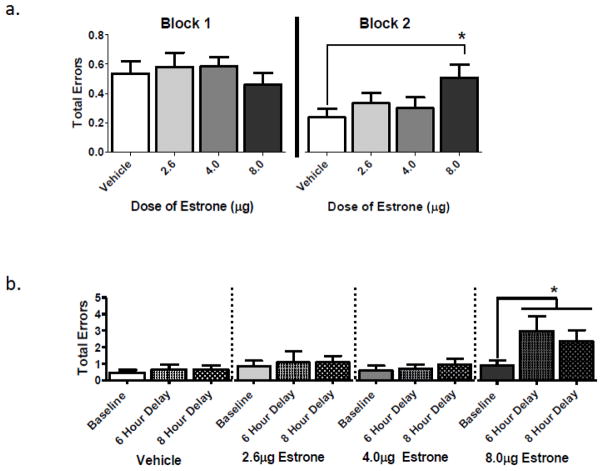
Treatment Effects: Since we have shown ovarian-hormone effects on this task that are specific to testing phase (Acosta et al., 2009a), we collapsed the data into two three-day blocks. The Treatment main effect for the omnibus ANOVA during the first testing block (days two to four) was not significant. During the second testing block (days five to seven), there was a marginal Treatment main effect for the omnibus ANOVA with all treatment groups included [F(3,28)=2.586; p=0.073]. Noting that the omnibus analysis was marginal, that it takes into account all possible group comparisons, and that our a priori interest was to compare each E1-treated group to Vehicle only, we moved forward with these select two-group evaluations. To be conservative, we employed Dunnett’s correction. During the second testing block, the highest E1 dose impaired performance (Figure 2a), with the E1-High group making more errors relative to the Vehicle group [Vehicle vs. E1-High: Dunnett’s p=0.03].
Figure 2.
Delayed-Match-to-Sample Water Maze Performance. a) Mean (±SE) total errors during DMS testing. During the second three-day testing block, the highest dose of E1 impaired performance, with E1-High rats making more total errors than Vehicle rats (* p<0.05). b) Mean (±SE) total errors during DMS delay testing. Within subjects comparisons revealed that E1-High treated rats made more errors on the post-delay trial (trial two) of the combined delay measure, as compared to trial two of baseline (* p<0.05).
Testing with a Delayed ITI
No group showed a difference in errors committed on the post-delay test trial on day eight (the six-hour delay) versus that on day nine (the eight-hour delay); thus, we averaged the error scores across the two delays into one overall delayed ITI measure. There was a significant Treatment effect for the omnibus ANOVA [F(3,28)=4.44; p<0.02]. E1-High treatment impaired performance as compared to Vehicle [Dunnett’s p=0.009], indicating that the E1-High group was impaired on the post-delay trial. As we found group differences between the Vehicle and the E1-High groups during the last three-day block of the DMS task, we then assessed performance on the last three-day block for trial two (baseline) as compared to the combined days of the delay challenge for trial two (the post-delay trial). For this assessment, a repeated measures ANOVA was used for each within group comparison, with Day as the repeated measure. The E1-High treated rats were impaired on the combined delay measure (Figure 2b), making more errors relative to their baseline performance on the post-delay trial during the last testing block [F(1,7)=5.78; p<0.05]. Neither the Vehicle, E1-Low, or E1-Med groups were impaired by the delay as interpreted relative to their own baseline score (ps>0.50).
Open Field
To determine the impact of E1 on locomotor activity, we assessed distance moved (cm) in the whole open field arena, as well as each of the zones, with Zone as a repeated measure (inner, middle, and outer zones). There were no Hormone Treatment main effects or interactions for locomotor activity in any analysis (data not shown).
Morris Water Maze
Learning Effects
To evaluate learning, we analyzed performance across all days (days one to three), collapsed across all test trials (trials one to six). There was a main effect of Day for each treatment group [Vehicle: F(2,16)= 33.55; p<0.0001, E1-Low: F(2, 12)= 27.33; p<0.0001, E1-Med: F(2,14)= 47.15; p<0.0001, E1-High: F(2,14)= 29.69; p<0.0001], with swim distance decreasing as days progressed, indicating learning for each treatment group (data not shown).
Treatment Effects
There was no Hormone Treatment effect for the omnibus ANOVA. As we and others have shown that 17β-E2 (Markham et al., 2002; Talboom et al., 2008) and CEE (Acosta et al., 2009b) can enhance retention of the platform location overnight, we assessed overnight retention here by comparing swim distance from the last trial on the first day (trial six on day one) to the first trial the next day (trial one on day two), as well as from day two to three (trial six on day two, to trial one on day three). The Hormone Treatment effect for the omnibus ANOVA was not significant for either interval. When we collapsed the data across the two overnight intervals to increase power, still, the Hormone Treatment effect for the omnibus ANOVA was not significant.
Probe Trial Effects
For the probe trial, there was a Quadrant main effect, with a greater percent swim distance in the NE target, versus the SW opposite, quadrant [F(1,28)=181.62; p<0.0001], and no Hormone Treatment x Quadrant interactions. Thus, all groups localized to the target quadrant (data not shown). There were no Hormone Treatment effects in the frequency of crossings in the NE target quadrant, or in the SW opposite quadrant, again suggesting that E1 did not affect spatial localization on this task.
Visible Platform
Table 1 displays the Mean (±SE) latency (s) to reach the platform for each group on each trial. All animals located the platform within 20 seconds during trial six confirming that all animals had the visual and motor competence to solve a swimming maze task.
Table 1.
Mean (±SE) latency (s) to reach the platform for each group on each trial of the Visible Platform task.
| Treatment | Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | Trial 6 |
|---|---|---|---|---|---|---|
| Vehicle | 10.04 ± 4.02 | 7.17 ± 1.10 | 10. 60 ± 3.27 | 7.59 ± 2.91 | 6.12 ± 1.72 | 6.55 ± 1.71 |
| E1-Low (2.6μg E1) | 14.40 ± 5.21 | 9.65 ± 2.61 | 19.88± 11.21 | 6.72 ± 0. 83 | 5.41 ± 0.74 | 6.62 ±1.75 |
| E1-Med (4.0μg E1) | 8.42 ±1.87 | 10.44 ± 1.66 | 15.29 ± 4.94 | 10.03 ±1.98 | 7.38 ± 1.25 | 5.25 ± 0.65 |
| E1-High (8.0 μg E1) | 21.65 ± 5.28 | 16.35 ± 5.59 | 5.35 ± 1.30 | 6.93 ± 1.27 | 8.66 ±2.52 | 8.29 ± 1.43 |
Markers of Peripheral Stimulation
After Ovx (before E1 administration), vaginal smears revealed that all animals were in diestrous, indicating a lack of uterine stimulation, as expected (Goldman et al., 2007). Five days after pump implantation, all Vehicle rats continued to exhibit diestrous smears, while E1-treated animals (all doses) alternated between estrous and metestrous smears, with each smear showing numerous cornified cells, indicating uterine stimulation (Goldman et al., 2007). At sacrifice, pump inspection revealed that all pumps were intact, and no pumps were cracked.
For uterine wet weights, as previously reported for E1 (Beyer et al., 1976), there was a significant effect of Hormone Treatment with E1 [omnibus ANOVA F(3,28)=50.78; p<0.001]. Each dose of E1 increased uterine weights compared to the Vehicle group [Vehicle vs. E1-Low: Dunnett’s p=0.00000002; Vehicle vs. E1-Med: Dunnett’s p=0.00000002; Vehicle vs. E1-High: Dunnett’s p=0.00000002] (Figure 3).
Figure 3.
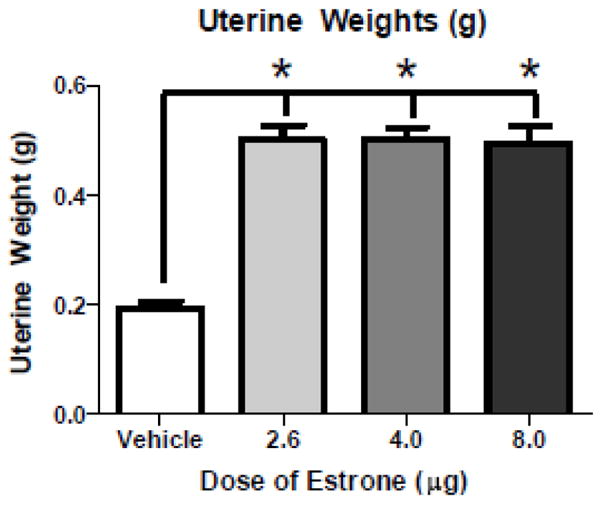
Markers of Peripheral Estrogenic Action. Mean (±SE) uterine weights (g). All doses of E1 increased uterine weights relative to Vehicle rats (* p<0.0001).
Basal Forebrain ChAT-IR Neuron Counts
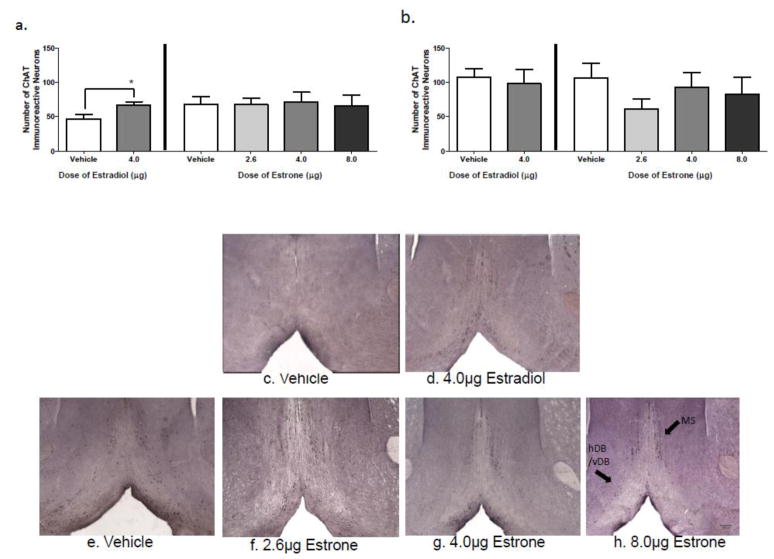
Figures 4a and 4b show mean ±SE MS and hDB/vDB ChAT-IR neuron counts, and Figures 4c-h are photomicrographs of the basal forebrain for each treatment group. For the MS, in the current study, the Hormone Treatment effect for the omnibus ANOVA was not significant. However, the comparison study showed that, replicating findings of others (Gibbs, 1997), 17β-E2 increased the number of ChAT-IR neurons in the MS relative to Vehicle treatment [t(8)=2.34; p<0.05]. For the hDB/vDB, neither 17β-E2 nor E1 impacted the number of ChAT-IR neurons.
Figure 4.
Basal Forebrain ChAT-IR Neuron Counts. a) Mean (±SE) ChAT-IR neurons in the MS. In a separate comparison study, a group treated with 17β-E2, and corresponding Vehicle group, was used. Treatment with 4.0 g/day 17β-E2, but no dose of E1, increased ChAT-IR neurons (* p<0.05). b) Mean (±SE) ChAT-IR neurons in the hDB/vDB. Neither 17β-E2 nor any dose of E1 impacted the number of ChAT-IR neurons compared to Vehicle rats in this region. Representative basal forebrain photomicrograph of the: c) Vehicle comparison group, d) 4.0 g/day 17β-E2 comparison group, e) Vehicle group, f) E1-Low group, g) E1-Med group, and h) of E1-High group.
Discussion
In the current study, all E1 doses evaluated resulted in the peripheral estrogenic actions expected if estrogenic stimulation did indeed occur. These included increases in uterine weights (Westerlind, 1998) and cornified vaginal smears (Goldman et al., 2007). This confirms that E1 stimulated peripheral tissues, and was present in the E1-treated animals until the end of the experiment. No such effects were seen in Ovx animals given vehicle treatment, as expected. These findings concur with work showing peripheral stimulation with CEE treatment in middle-aged Ovx rats (Acosta et al., 2009b; Engler-Chiurazzi et al., 2011). Here, we found that the low and medium doses of E1 did not differ from Vehicle treatment in their impact on spatial memory performance. Yet, the high dose of E1 impaired both acquisition and retention on the spatial working memory DMS task. The observed impairments of E1 therefore do not appear to be generalized to all memory types, as we found specific detriments on spatial working memory and delayed memory retention, with no impact on spatial reference memory, at least as measured on the MM. Further, in the current report while 17β-E2 increased basal forebrain ChAT-IR neuron counts, as expected based on prior studies (for review see Gibbs, 2010), no dose of E1 impacted this measure when compared to Vehicle treatment. Taken together, these findings extend those of previous studies and indicate that E1, a primary circulating estrogen present after CEE administration, impairs spatial working memory and delayed memory retention, and does not alter the number of cholinergic positive neurons in a brain region known to modulate memory, the basal forebrain, as does 17β-E2.
Behavioral findings regarding the impact of E1 on cognition are mixed. Administration of E1 directly into the hippocampus of adult mice post-training improved performance by decreasing the number of retention test trials needed to reach criterion on a T-maze footshock avoidance task (Farr et al., 2000). Behavioral effects may be dose specific, as Barha and colleagues (2009) noted that in adult rats, a subcutaneous E1 injection of 1.0 μg impaired contextual fear conditioning, while 0.3 μg or 10.0 μg had no impact. It is important to note these outcomes reported previously may be mediated by a number of factors including the route of administration (intrahippocampal vs. subcutaneous), the timing of administration (post-training vs. before conditioning), the species (mouse vs. rat), or the specific paradigm wherein E1 is being studied. Further, since in our current study animals were tested on a cognitive maze battery, it is possible that the experience of being tested on multiple mazes influenced performance outcomes, especially on the latter cognitive tests. As such, further evaluation elucidating the potentially interactive effects of previous maze experience and estrogen treatments on memory is an interesting future research direction. Systematic evaluations of each of these factors are important future directions in characterizing the potential cognitive impact of E1.
In women, CEE can have beneficial effects on some memory measures; yet, some studies have found that CEE has null or detrimental effects on memory (for review see Hogervorst et al., 2000; Sherwin and Henry, 2008). Several factors, including socioeconomic status and age at time of hormone therapy treatment, as well as the temporal window of treatment after ovarian hormone loss, can impact the potential cognitive benefits of CEE in human clinical studies (for review see Hogervorst et al., 2000; Rocca et al., 2010). Studies using animal models, in which such issues are obviated or can be controlled, have reported beneficial cognitive effects of CEE. For example, a one-time subcutaneous CEE injection to middle-aged, Ovx rats benefitted memory retention for objects (Walf and Frye, 2008). We have shown that chronic subcutaneous CEE injections enhanced spatial working memory, prevented scopolamine-induced amnesia, and prevented overnight retention on the MM in middle-aged Ovx rats (Acosta et al., 2009b). We have also found that CEE administered continuously via subcutaneous Alzet osmotic pumps to middle-aged, Ovx rats at medium (24 μg daily CEE) and high (36 μg daily CEE) doses enhanced working memory retention (Engler-Chiurazzi et al., 2011). However, the lowest dose (12 μg daily CEE) impaired learning on the DMS and MM tasks. We have previously hypothesized that this dose-dependent effect of CEE was related to the resulting circulating relative levels of E1 and 17β-E2. Indeed, in the same study, the lowest CEE dose, which impaired memory scores, increased serum E1 but not 17β-E2 levels; whereas, the two higher doses each enhanced performance and concomitantly increased both E1 and 17β-E2. This suggested that elevated levels of E1 in the absence of sufficient 17β-E2, similar to the hormone profile of some postmenopausal woman (Gruber et al., 2002), impairs memory. In line with this, although the previously reported cognitive impacts of E1 are mixed (Barha et al., 2009; Farr et al., 2000), many studies report enhanced spatial working memory (Bimonte and Denenberg, 1999; Daniel et al., 1997; Fader et al., 1999; Gibbs, 1999; Hruska and Dohanich, 2007) and reference memory (Bimonte-Nelson et al., 2006; Feng et al., 2004; Frick et al., 2004; Markham et al., 2002; Talboom et al., 2008) with subcutaneous 17β-E2 administration to Ovx rats, although this effect appears to depend on task, dose, age, and timing after surgical menopause (for review see Bimonte-Nelson et al., 2010; Daniel and Bohacek, 2010; Frick, 2009; Gibbs, 2010). The current findings build on this previous work, supporting the tenet that E1 can impair spatial memory.
CEE is a complex mixture of at least 10 different estrogen moieties (Kuhl, 2005). It is therefore possible that some estrogens contained in CEE, such as Δ8,9-dehydroestrone (Kuhl, 2005), are increased with higher CEE doses, and, thus, contribute to CEE’s beneficial cognitive effects. Along these lines, we recently showed that middle-aged, Ovx rats treated with Δ8,9-dehydroestrone, but not the CEE component equilin, enhanced learning on spatial working and reference memory (Talboom et al., 2010). As such, it appears that the issue is quite complex, and likely involves ratios of other steroid hormones. Indeed, E1 can be derived from the androgen precursor, androstenedione (Martini et al., 1993), which we have recently shown to correlate with memory impairment in the rodent at higher physiological levels (Acosta et al., 2010). Collectively, this suggests that cognitive benefits can be realized with estrogen-containing hormone therapies given the “proper” parameters, including a hormone preparation with an optimum ratio of the various estrogens.
It has been established that estrogen and the basal forebrain cholinergic system are each intimately involved in learning and memory (for review see Gibbs, 2010). Basal forebrain cholinergic neurons project to the hippocampus and surrounding cortical areas (Woolf, 1991), and basal forebrain lesion results in significant spatial memory impairments (Gibbs, 2002). Additionally, basal forebrain ChAT may be related to memory scores, as 2 month-old female rats that were significantly impaired on the spatial working memory land radial-arm maze had less ChAT protein activity in the basal forebrain (Luine and Hearns, 1990). CEE treatment in Ovx rats increased basal forebrain ChAT-IR neuron counts in the vDB, and concomitantly aided spatial working memory and MM overnight retention (Acosta et al., 2009b). Similarly, 17β-E2 treatment in Ovx rats increases ChAT-IR neuron counts (Gibbs, 1997) and ChAT protein activity (Luine, 1985) in the basal forebrain. Here, in the 17β-E2 comparison evaluation, continuous 17β-E2 treatment increased ChAT-IR cell counts in the MS, as expected. Yet, using the same quantifying procedures, E1 did not impact ChAT-IR cell counts in either the MS or the hDB/vDB regions, at least at the E1 doses tested. Although the duration of hormone treatment in the 17β-E2 comparison study was not identical to the hormone treatment used in the E1 study (treatment was 3 weeks longer in the 17β-E2 comparison study), for both the 17β-E2 and E1 analyses, the sections were counted by the same experimenter who was blind to treatment group assignment, using the same counting protocol. Thus, the finding that 17β-E2 increased ChAT-IR cell counts in the MS suggests that our counting procedure is effective in detecting significant treatment group differences and adds an important interpretative value for the lack of effects of E1 on this basal forebrain cholinergic measurement. Taken together, these findings suggest that E1 does not impact the basal forebrain cholinergic system as does 17β-E2 or CEE. Thus, the negative impact of E1 on cognition may involve other estrogen sensitive neural systems, such as monoamines (Luine, 1998) and/or neurotrophins (Granholm, 2000).
In conclusion, this study demonstrates that the principal estrogen moiety E1, a primary circulating estrogen present after CEE administration, can impair specific memory domains of spatial memory in middle-aged, surgically menopausal rats. E1 treatment at the doses tested in this study did not impact the number of ChAT-IR neurons in the MS or the hDB/vDB regions, whereas in a comparison study using the same quantification procedures, 17β-E2 increased the number of ChAT-IR neurons in the MS. That we have previously shown CEE can enhance cognition and increase ChAT-IR basal forebrain neuron number, and now find that the primary circulating estrogen after CEE treatment, E1, does not have these effects, suggests that previously observed beneficial effects of CEE on these variables are not likely due to the E1 component alone. Findings from preclinical, interdisciplinary basic science studies can inform the design of specific combinations of estrogens that could be beneficial to the brain and cognition. The results shown here build on the findings of others and suggest that, for cognitive and brain health measures, E1 is not likely one of these key beneficial Estrogens
Highlights.
Estrone treatment given to middle-aged, surgically menopausal rats was evaluated.
Estrone increased uterine weights and induced positive vaginal smears.
Estrone impaired spatial working memory during acquisition and after a delay.
Estrone did not impact basal forebrain cholinergic neuron number.
Acknowledgments
This research was funded by a grant awarded to HAB-N from the National Institute of Aging (AG028084). We sincerely thank Cynthia Zay for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth B. Engler-Chiurazzi, Email: eengler@asu.edu.
Joshua S. Talboom, Email: joshua.talboom@asu.edu.
B. Blair Braden, Email: bbbraden@asu.edu.
Candy W.S. Tsang, Email: Candy.Tsang@asu.edu.
Sarah Mennenga, Email: Sarah.Mennenga@asu.edu.
Madeline Andrews, Email: Madeline.gail.andrews@gmail.com.
Laurence M. Demers, Email: lmd4@psu.edu.
Heather A. Bimonte-Nelson, Email: bimonte.nelson@asu.edu.
References
- Acosta JI, Mayer L, Talboom JS, Tsang CWS, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional Versus Surgical Menopause in a Rodent Model: Etiology of Ovarian Hormone Loss Impacts Memory and the Acetylcholine System. Endocrinology. 2009a;150:4248–4259. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009b;55:454–464. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The Cognitive Effects of Conjugated Equine Estrogens Depend on Whether Menopause Etiology Is Transitional or Surgical. Endocrinology. 2010;151:3795–3804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LAM. Low Doses of 17[alpha]-Estradiol and 17[beta]-Estradiol Facilitate, Whereas Higher Doses of Estrone and 17[alpha]- and 17[beta]-Estradiol Impair, Contextual Fear Conditioning in Adult Female Rats. Neuropsychopharmacology. 2009;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Morali G, Larsson K, Södersten P. Steroid regulation of sexual behavior. Journal of Steroid Biochemistry. 1976;7:1171–1176. doi: 10.1016/0022-4731(76)90051-0. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as Cartographers: Mapping the Crossroads of Gonadal Hormones, Memory and Age Using Animal Models. Molecules. 2010;15:6050–6105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochimica et Biophysica Acta (BBA) - General Subjects. 2010;1800:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Open-field Behavior in the Rat: What Does it Mean? Ann N Y Acad Sci. 1969;159:852–859. doi: 10.1111/j.1749-6632.1969.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Statistics and Experimental Design for Behavioral and Biological Researchers. Washington, DC: Hemisphere Publishing; 1976. [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiology of Aging. 2011;32:680–697. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Estradiol potentiates acetylcholine and glutamate-mediated post-trial memory processing in the hippocampus. Brain Research. 2000;864:263–269. doi: 10.1016/s0006-8993(00)02184-3. [DOI] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang JT. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Hormones and Behavior. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. European Journal of Neuroscience. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen Therapy and Cognition: A Review of the Cholinergic Hypothesis. Endocrine Reviews. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997;757:10–16. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Granholm AC. Oestrogen and nerve growth factor - neuroprotection and repair in Alzheimer’s disease. Expert Opin Investig Drugs. 2000;9:685–694. doi: 10.1517/13543784.9.4.685. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol Behav. 2002;77:371–385. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Hruska Z, Dohanich GP. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1–42) and ibotenic acid. Horm Behav. 2007;52:297–306. doi: 10.1016/j.yhbeh.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. 4. Pearson Prentice Hall; Upper Saddle River, N.J: 2004. [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Luine V, Hearns M. Spatial memory deficits in aged rats: contributions of the cholinergic system assessed by ChAT. Brain Research. 1990;523:321–324. doi: 10.1016/0006-8993(90)91507-d. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. The Journal of Steroid Biochemistry and Molecular Biology. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Academic Press; New York: 2005. [Google Scholar]
- Prokai-Tatrai K, Prokai L. Impact of Metabolism on the Safety of Estrogen Therapy. Annals of the New York Academy of Sciences. 2005;1052:243–257. doi: 10.1196/annals.1347.018. [DOI] [PubMed] [Google Scholar]
- Rasband W. ImageJ. National Institute of Health; Bethesda, Maryland: 1997–2004. http://www.info.nih/gov/ij/ [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Research. 2010 doi: 10.1016/j.brainres.2010.10.031. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, Bowen D, Terrell T, Jones BN. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Singhal R, Shankar K, Badger TM, Ronis MJ. Hepatic gene expression following consumption of soy protein isolate in female Sprague ÄìDawley rats differs from that produced by 17β-estradiol treatment. Journal of Endocrinology. 2009;202:141–152. doi: 10.1677/JOE-09-0059. [DOI] [PubMed] [Google Scholar]
- Stavnezer AJ, Hyde LA, Bimonte HA, Armstrong CM, Denenberg VH. Differential learning strategies in spatial and nonspatial versions of the Morris water maze in the C57BL/6J inbred mouse strain. Behav Brain Res. 2002;133:261–270. doi: 10.1016/s0166-4328(02)00021-9. [DOI] [PubMed] [Google Scholar]
- Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(Suppl 12B):64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Engler-Chiurazzi EB, Whiteaker P, Simard AR, Lukas R, Acosta JI, Prokai L, Bimonte-Nelson HA. A component of Premarin® enhances multiple cognitive functions and influences nicotinic receptor expression. Hormones and Behavior. 2010;58:917–928. doi: 10.1016/j.yhbeh.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008 doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Conjugated equine estrogen enhances rats’ cognitive, anxiety, and social behavior. Neuroreport. 2008;19:789–792. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlind K, Gibson CKJ, Malone P, Evans GL, Turner RT. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. Journal of Bone and Mineral Research. 1998;13:1023–1031. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Yasui T, Yamada M, Kinoshita H, Uemura H, Yoneda N, Irahara M, Aono T, Sunahara S, Mito Y, Kurimoto F, Hata K. Combination of automatic HPLC-RIA method for determination of estrone and estradiol in serum. J Clinical Laboratory Analysis. 1999;13:266–272. doi: 10.1002/(SICI)1098-2825(1999)13:6<266::AID-JCLA3>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]