Abstract
Two protein families that represent major components of essential amino acid transport in insects have been identified. They are annotated as the SLC6 and SLC7 families of transporters according to phylogenetic proximity to characterized amino acid transporters (HUGO nomenclature). Members of these families have been identified as important apical and basolateral parts of transepithelial essential amino acid absorption in the metazoan alimentary canal. Synergistically, they play critical physiological roles as essential substrate providers to diverse metabolic processes, including generic protein synthesis. This review briefly clarifies the requirements for amino acid transport and a variety of amino acid transport mechanisms, including the aforementioned families. Further it focuses on the large group of Nutrient Amino acid Transporters (NATs), which comprise a recently identified subfamily of the Neurotransmitter Sodium Symporter family (NSS or SLC6). The first insect NAT, cloned from the caterpillar gut, has a broad substrate spectrum similar to mammalian B0 transporters. Several new NAT-SLC6 members have been characterized in an effort to explore mechanisms for the essential amino acid absorption in model dipteran insects. The identification and functional characterization of new B0-like and narrow specificity transporters of essential amino acids in fruit fly and mosquitoes leads to a fundamentally important insight: that NATs evolved and act together as the integrated active core of a transport network that mediates active alimentary absorption and systemic distribution of essential amino acids. This role of NATs is projected from the most primitive prokaryotes to the most complex metazoan organisms, and represents an interesting platform for unraveling the molecular evolution of amino acid transport and modeling amino acid transport disorders. The comparative study of NATs elucidates important adaptive differences between essential amino acid transportomes of invertebrate and vertebrate organisms, outlining a new possibility for selective targeting of essential amino acid absorption mechanisms to control medically and economically important arthropods and other invertebrate organisms.
Keywords: Insect nutrition, Essential amino acids, Epithelial absorption, Transporters, Midgut, Alimentary canal
1. Introduction
The present review summarizes the progress made over the last decade in our understanding of the molecular physiology of amino acid transport in insects, with emphasis on essential amino acid transport. The earlier reviews by Castagna et al., published in 1997 (Castagna et al., 1997), and by Wolfersberger, published in 2000 (Wolfersberger, 2000), are suggested as a starting point and background reference, revisiting an important initial stage in the study of mammalian and insect amino acid transport systems and transporters. The present review omits redundant description of the earlier data and transporter systematics, taking off from the revolutionary expression cloning and heterologous expression analysis of the first NATs resembling K-activated amino acid transporter 1 (KAAT1) from the gut of the caterpillar, Manduca sexta, in 1998 (Castagna et al., 1998). Subsequent studies of KAAT1, and its paralog CAATCH1, revealed many important functional and structural aspects of the SLC6 protein family, which were recently reviewed (Castagna et al., 2009) and are not included here. In contrast, this review includes some details about earlier studies of essential amino acid transport in insects, an initial description of essential amino acid transport systems in taxonomically different model organisms, a putative electrochemical integration of essential amino acid transport systems in the model alimentary canal of mosquito larvae, and selected comparative data elucidating molecular and adaptive differences of metazoan amino acid transport systems.
2. Essential and conditionally essential amino acids in insects
The set of essential amino acids has been experimentally determined in several insect species. Ten proteinogenic l-amino acids, including aromatic (F, W, H), aliphatic (L, I, V, T), sulfur containing (M), and basic (R, K) are essential in mammals and also in mosquitoes (Akov, 1962; Golberg and Demeillon, 1948; Singh and Brown, 1957) and other insects (Friend and Dadd, 1982; Gordon, 1968; House, 1962; Phillips, 1929) (Table 1). They are the limiting factors in larval and egg development as well as key signaling and regulatory messengers of developmental processes (Akov, 1962; Hansen et al., 2004; Harrington et al., 2001; Lea et al., 1956; Singh and Brown, 1957). The amino acid demands increase and expand beyond the essential set in rapidly developing tissues and species. For example, Culex pipiens larvae fail to pass the 2nd instar larval stage in the absence of any of the essential amino acids, as well as dispensable glycine, serine, or asparagine (Clements, 1992; Dadd, 1978; Merritt et al., 1992), which they are unable to synthesize in sufficient amounts. The dietary restriction of bioenergetically important proline also delays development and leads to high mortality in late instar mosquito larvae (Lea and Delong, 1956; Singh and Brown, 1957). Interestingly, an artificial diet with high concentrations of amino acids (>0.9–1.4% w/v) is also deleterious for mosquitoes, hampering both larval viability and adult vigor (Dadd, 1978; Merritt et al., 1992). The mechanisms behind the acquisition, balance, and toxicity of the nutrient amino acids in insects most likely involve transmembrane transport processes.
Table 1.
The essential and dispensable proteinogenic amino acids.
| ST | EQ | MS | Formula | F% | ||
|---|---|---|---|---|---|---|
| W | Trp | 12 | 79 | 204 | C11H12N2O2 | 1.3 |
| F | Phe | 9 | 63 | 165 | C9H11NO2 | 4.0 |
| Y* | Tyr | 9 | 57 | 181 | C9H11NO3 | 3.3 |
| L | Leu | 7 | 33 | 131 | C6H13NO2 | 7.6 |
| H | His | 1 | 33 | 155 | C6H9N3O2 | 2.9 |
| V | Val | 4 | 25 | 177 | C5H11NO2 | 6.8 |
| C* | Cys | 9 | 25 | 121 | C3H7NO2S | 3.3 |
| I | Ile | 11 | 20 | 131 | C6H13NO2 | 3.8 |
| K | Lys | 10 | 19 | 146 | C6H14N2O2 | 7.2 |
| M | Met | 9 | 19 | 149 | C5H11NO2S | 1.8 |
| R | Arg | 10 | 18 | 174 | C6H14N4O2 | 4.2 |
| T | Thr | 6 | 6 | 119 | C4H9NO3 | 6.2 |
| S | Ser | 3 | 15 | 105 | C3H7NO3 | 8.1 |
| G | Gly | 4 | 14 | 75 | C2H5NO2 | 7.4 |
| A | Ala | 1 | 13 | 89 | C3H7NO2 | 7.4 |
| P | Pro | 4 | 12 | 115 | C5H9NO2 | 5.0 |
| Q | Glt | 2 | 9 | 146 | C5H10N2O3 | 3.7 |
| E | Glu | 1 | 8 | 147 | C5H9NO4 | 5.8 |
| N | Asn | 1 | 4 | 132 | C4H8N2O3 | 4.4 |
| D | Asp | 1 | 1 | 133 | C4H7NO4 | 5.9 |
Table notes: Essential and conditionally essential amino acids are highlighted by a gray background; Amino acids are shown as 1 and 3-letters letter codes. ST, the numbers of enzymatic steps required for the synthesis in autotrophs. Data were summarized from (Boudko, 2010; Craig and Weber, 1998; Neidhardt et al., 1990); precursors and synthesis cost from(Stryer, 1989); EQ, total energy of synthesis in ATPs equivalents, estimated as ATP, NAD, NADPH yield + consumption; MS, molecular mass; F%, overage frequency per protein sequence residue; Y* and C*, are conditionally essential for which F and M, are essential metabolic precursors; Ur, indicates that part of essential arginine can be recycled via urea cycle intermediates.
3. Transport versus anabolism of proteinogenic amino acids
A post-genomic survey reveals that half of the proteinogenic amino acid synthesis cascades were irreversibly lost in the majority of heterotrophic organisms, including animals (Payne and Loomis, 2006). The fact of a massive and uniform extinction suggests an earlier acquisition and stabilization of this trait in metazoan evolution and also raises two fundamental questions: Why was such an obvious disadvantage selected in the evolution of the most metabolically complex, environmentally labile, and successful organisms on the planet and how do multicellular metazoan organisms deal with the consequences of this metabolic problem, given that each metazoan cell now needs to acquire a balanced combination of essential proteinogenic amino acids in order to survive and reproduce? The answer is simple if we look at the bioenergetics of amino acid synthesis and the possible compensation for their combined loss via transport mechanisms. The set of essential amino acids in animals has explicit correlation with the complexity and energetic costs of their synthesis in bacteria and plants (Table 1). For example, bacterial tryptophan synthesis requires ~12 enzymatic steps and 79 ATPs in biological energy equivalent (Table 1, EQ) (Craig and Weber, 1998; Neidhardt et al., 1990). In contrast, a secondary (electrochemical energy coupled) membrane transporter can translocate environmental tryptophan against a 1000-fold concentration gradient using the electrochemical motive energy of the Na+ ion, which is subsequently recycled via the Na/K-ATPase (Cox and Helman, 1986a–c) or NHE/H+ V-ATPase tandem (Okech et al., 2008a) at the cost of ~1/3 ATP hydrolysis. The compensation of “expensive and slow” autotrophic synthesis by “cheap and fast” heterotrophic acquisition is a fundamental invention of life that cuts time and energy, as well as simplifies metabolic and genetic mechanisms, dedicated to the synthesis of proteinogenic amino acids, which together substantially accelerated metazoan evolution and environmental adaptations. Hence, the primary goal of animals’ heterotrophy is acquisition of energetically expensive essential amino acids rather than opportunistic nutrient resources of energy, carbon, and nitrogen. The energetic benefit from the salvage of environmentally available amino acids for protein synthesis is apparent, considering that autotrophs may invest over 80% of their total energy budget toward the synthesis of amino acids, and the lion’s share of that energy is needed to synthesize amino acids that are essential in animals. However, as a result of heterotrophic generalization animals require external supplies of essential amino acids to maintain protein synthesis, as well as many other metabolic processes that compete for essential amino acids. Moreover, metazoan organisms require a system that can transport essential amino acids across multiple membrane barriers. The range of putative amino acid transporters can now be identified by means of phylogenetic querying of completed insect genomes and functional inferences from characterized amino acid transporters.
4. General properties of essential amino acid transport
The intracellular acquisition of essential amino acids by metazoan cells generally requires three transport events (Fig. 1): absorption via apical membranes of epithelial cells in the alimentary canal [1] basolateral secretion from the cell into the internal cavity or circulation [2] and absorption via the plasma membranes of somatic cells [3]. Additional intracellular compartmentalization events [4] involving endomembrane transporters may be important to maintain intracellular absorption of essential amino acids and related metabolic processes (Fig. 1). All of these events rely on specific transport proteins, since the passive diffusion of essential amino acids across the lipid bilayers of cellular membranes is inadequate to support cellular and organism metabolism (~10−12 mol s−1 m−2) (Chakrabarti and Deamer, 1992, 1994). For example, it would take thousands of years for mosquito larvae to double their body weight without the transporter-assisted acquisition of essential amino acids. In contrast, tropical mosquito larvae are able to gain approximately 1000 times their body weight in around 10 days. Hence, alimentary and cellular absorption of essential amino acids in mosquito larvae involves high capacity transport mechanisms. Moreover, protein synthesis requires sub-centimolar intracellular concentrations of canonical amino acids, which may be 2–3 orders higher vs. typically available extracellular concentrations in the digestive and circulatory systems. Thus, amino acid absorption requires energy-coupled mechanisms working against notable concentration gradients of transported substrates. These properties present in secondary transporters that energize amino acid absorption via electrochemical motive forces of major alkali ions (Na+ and K+) or Proton Motive Force (PMF). In contrast, if concentration gradients of amino acids correspond with a desired transport direction then the electrochemical energy-coupled transporters can be substituted by permease (amino acid selective channel) or amino acid exchanging mechanisms.
Fig. 1.
Major components of metazoan amino acid transport and relative distribution of amino acid transporters from 10 SLC families. The larger characters indicate specific numbers of SLC families that mediate majority of essential amino acid transport across apical, basolateral, and plasma membranes. Tips of transparent triangles show directions of chemical gradients for essential amino acids.
5. Molecular specificity and diversity of the metazoan amino acid transportomes
In prokaryotic cells, amino acid absorption involves two major categories of transport mechanisms: “primary” transporters, or metabolic-energy-coupled pumps, and electrochemical-energy-coupled “secondary” transporters. In eukaryotic cells, amino acid absorption is mediated exclusively by secondary transporters, which are divided on active or ion electrochemical-energy-coupled symporter mechanisms (also termed as electrogenic or, more appropriately, electrophoretic), exchangers that could have active and passive components but cannot perform significant accumulative transport, and passive uniporters that facilitate membrane transport of amino acids down a chemical gradient (Wolfersberger, 1994). Phylogenetic analysis identifies ~50 families of secondary transporters (Hediger et al., 2004), 10 of which contribute at least one member to the transport of amino acids or their metabolized derivatives (Boudko, 2010; Boudko et al., 2005b). These families have been summarized in the Transporter Classification Data Base (TCDB) (Busch and Saier, 2003; Saier, 1999, 2000) and the SoLute Carrier family systematics (Hediger et al., 2004) (SLC; originally proposed by the Human Genome Organization (HUGO) Nomenclature Committee). TCDB nomenclature is more comprehensive and broadly used in referencing prokaryotic transporters. While the SLC families are included within the TCDB classification system under the Major Facilitator Superfamily (MFS, 2.A) (Chang et al., 2004), the SLC family systematics nomenclature is more focused on mammalian transporters, and is more appropriate for phylogenomic comparison of eukaryotic transporters, including the amino acid transport systems summarized in this review. The diversity of the mammalian representatives of these families was summarized in a set of short reviews of individual SLC families, including SLC#: 1(Kanai and Hediger, 2004), 6 (Chen et al., 2003), 7 (Verrey et al., 2004), 15 (Daniel and Kottra, 2003), 16 (Halestrap and Meredith, 2003), 17 (Reimer and Edwards, 2003), 18 (Eiden et al., 2003), 32 (Gasnier, 2003), 36 (Boll et al., 2004), 38 (Mackenzie and Erickson, 2003), and 43 (Bodoy et al., 2005). In addition to the apparent phylogenetic delineation, the amino acid transporters from these families differ by substrate specificity, electrochemical coupling, partitioning in specific cellular membranes (e.g.: apical, basal, axonal, somatic) as well as spatial, temporal, tissue, and cell specific expression patterns. Hence, amino acid transportomes comprise a set of non-redundant transport mechanisms that mediate vectorial transport and satisfy distinct requirements for amino acids in organisms, tissues, and individual cells. The majority of the aforementioned parameters remain conserved within each family, providing an opportunity to use phylogenetic analysis-derived functional inferences of putative transporters across different organisms. Fig. 1 summaries the distribution of members of different amino acid transporter families across the membranes of metazoan organisms, and Table 2 summarizes the functional specificities, systematic, and relative expansion of these families in selected insect species referenced to the human genome (Table 2, Hs).
Table 2.
Putative amino acid transporter families and systems in insects.
| The HGNC solute carrier family series subfamily | SLC # | Specific subfamily |
AA Systems | Ae | Ag | Dm | Am | Tc | Hs |
|---|---|---|---|---|---|---|---|---|---|
| Acidic and small neutral (dispensable) AA transporter family | SLC1 | EA, ASC | X− | 4 | 3 | 3 | 7 | 3 | 7 |
| Chaperoning subunits for heterogenic amino acid transporters HAT-SLC7a | SLC3 | See SLC7 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Sodium dependent nutrient amino acids and neurotransmitter transporter family without pseudo gene | SLC6 | 20 | 17 | 20 | 17 | 16 | 19 | ||
| NTT, CreatinT, TaurineT | NTT | GABA | 3 | 3 | 3 | 3 | 5 | 9 | |
| AAT GlyT, PROT, B0+cluster | AAT | B0+, Gly | 2 | 2 | 2 | 2 | 2 | 4 | |
| Mammalian and insect NAT | NAT | B0, IMINO | 2 | 2 | 2 | 2 | 2 | 5 | |
| Insect specific INE | INE | - | 1 | 1 | 1 | 1 | 1 | - | |
| Insect specific NAT | NAT | B0, (W, F, Mb) | 9 | 7 | 6 | 5 | 2 | - | |
| Insect specific BLOT | BLOT | - | 2 | 2 | 2 | 2 | 2 | - | |
| Cationic amino acid transporters/glycoprotein-associated family without pseudo gene. | SLC7 | 12 | 13 | 12 | 12 | 11 | 13 | ||
| Cationic AA transporters | CAT | y+ | 5 | 5 | 5 | 4 | 5 | 5 | |
| Heterodimeric AA transporters | HAT | y+L, L, x−, b0+ | 7 | 8 | 7 | 4 | 6 | 8 | |
| Proton oligopeptide cotransporter family | SLC15 | - | PEPT | 2 | 2 | 3 | 1 | 4 | 4 |
| Organic and inorganic acid transporters. | SLC17 | - | 16 | 16 | >20 | 17 | >20 | 8 | |
| Vesicular glutamate (1–3) | x− (vGLUT) | 1 | 1 | 1 | 1 | 1 | 3 | ||
| Vesicular inhibitory AA transporter family | SLC32c | - | vGAT | 1 | 1 | 1 | 1 | 1 | 1 |
| Proton-coupled AA transporter family | SLC36c | PAT | 12 | 12 | 11 | 6 | 12 | 4 | |
| System A and N sodium-coupled neutral AA transporters | SLC38c | - | A, N | 3 | 4 | 2 | 2 | 3 | 11 |
| Na+-independent, system-l transporters | SLC43 | - | LAT | ns | ns | ns | ns | ns | 3 |
| Total number | 10 | 74 | 70 | >79 | 65 | >75 | 75 |
Abbreviations: Hs, Homo sapiens; Ae, Aedes aegypti; Ag, Anopheles gambiae; Dm, Drosophila melanogaster; Am, Apis mellifera; Tc; Tribolium castaneum; ns, no significant similarity found.
A few SLC3 members identified a transport function obligatory auxiliary subunit for particular HAT-SLC7 transporters but not perform transport function per se, the majority of the SLC3 related protein share significant homology with amylases.
W, F, M represent new tryptophan/indole -, phenylalanine/phenol, and methionine/sulfur-contained amino acid selective transport systems identified in mosquitoes.
SLC32 (vGAT), SLC36 (PAT) and SLC38 families are phylogenetically related (e < 10−3; Pairwise % identity in Dm 22.4% in Hs 20.5%) and represent subfamilies of a unified family transporters.
6. Amino acid transporters in insects
A phylogenomic analysis suggests that of 10 SLC families participating in mammalian amino acid transport, 9 are present in insects (Table 2). These families include ~70 genes/per genome, which is approximately 25% of the total pool of secondary transporters in these organisms. However, functional inference indicates that only ~60% of identified genes are potentially involved in amino acid transporters, and only ~15% may participate in the transport of essential amino acids. The rest of these genes encode alternative transporters for amino acid derived metabolites, transport other organic and inorganic solutes, mediate signaling functions without apparent transport, or denote remains of transporter evolution, pseudo genes. The SLC1, 32, 36, and 38 families, with limited inferred roles in the transport of essential amino acids, were previously summarized (Boudko, 2010; Boudko et al., 2005b). SLC15 members have been characterized as proton driven oligopeptide and histidine transporters in the mammalian alimentary canal and other tissues (Boll et al., 1996; Daniel and Kottra, 2003). Current characterization of insect SLC15 is limited to one OPT1 (oligopeptide transporter 1) that has been cloned from D. melanogaster and characterized as a broad substrate transporter with preference for l-alanine and alanine-derived small peptides (Roman et al., 1998). The SLC15 family has 1–4 members per insect genome that mediate proton-dependent alimentary absorption of di- and tri-peptides and may support initial absorption of essential amino acids present in form of peptides derived from alimentary protein digestion. However, it should be considered that SLC15 transporters have low specificity for peptide sequences and are unlikely to significantly contribute to systemic distribution and cell-specific delivery of essential amino acids. In addition, the high alkaline environment in the alimentary canals of dipteran and many other insects is less suitable for the proton motive force (PMF)-driven absorption mechanism of SLC15 transporters. Therefore, the SLC6 and SLC7 families are of immediate interest for understanding essential amino acid transport mechanisms because their members serve as the majority of characterized apical, basolateral and plasma membrane components of the essential amino acid transport system (Fig. 1).
The SLC7 family members play roles as basolateral or cell-specific permeases and exchangers of metabolic and nutrient amino acids (Closs et al., 2006; Fernandez et al., 2003; Hansen et al., 2011; Palacin and Kanai, 2003; Verrey et al., 2004). They are also expressed in apical membranes of mammalian epithelial tissues and plasma membranes of somatic cells. However, data about polar distribution and contribution of SLC7 members in the insect alimentary system are currently limited. SLC7 combines two major subfamilies: Cationic Amino acid Transporters (CATs) (Closs et al., 2006), which have a 14 transmembrane domain (TMD) structure and differentially mediate transport of cationic amino acids Arg, Lys, and His; and Heterodimeric Amino acid Transporters (HATs), which have 12 TMDs and represent a part of the L-amino acid transport system (transporting branched-chain small and large AAs), y+ (arginine, ornithine, lysine, histidine, and dibasic AA transport system), asc (small neutral L- and D-AA transport system), and b0+ systems transporting the majority of essential amino acids. Function of mammalian SLC7-HATs (Verrey et al., 2004) may require association with an auxiliary subunit from the SLC3 family (Palacin and Kanai, 2003). Similarly, it has been shown that paired expression of heavy-chain SLC3 subunit CG2791 is necessary to induce l-system amino acid transport by the Dm SLC7-HAT transporter JhI-21 in Xenopus oocytes (Reynolds et al., 2009). SLC7 members undergo complex spatial and developmental expression and are regulated by nutrient and hormonal signaling. For example, two DmHAT transcripts, JhI-21 and minidiscs (mnd), representing a light subunit of the HAT subfamily, show developmental profiles that are consistent with juvenile hormone activity and are overexpressed in cultured Drosophila cells and ovarian nurse cells exposed to juvenile hormone or its synthetic analog, methoprene (Dubrovsky et al., 2002). In addition to amino acid transport, dipteran SLC7 members have been implicated in nutrient amino acid sensing pathways as transceptors (Hundal and Taylor, 2009; Kriel et al., 2011; Pinilla et al., 2011). For example, HAT-SLC7 minidiscs (Martin et al., 2000) and CAT-SLC7 slimfast (slif; CG11128) (Colombani et al., 2003) are expressed in the fat body and regulated by nutrition and growth factors of the fruit fly. Two CAT-SLC7s transporters, slimfast and iCAT2, are essential to the onset of blood meal-mediated TOR signaling in the females of yellow fever mosquitoes, Aedes aegypti (Ae), which subsequently triggers vitellogenic gene expression and oogenesis (Attardo et al., 2006). One of these genes has been recently heterologously expressed and characterized as a permease with particular selectivity to essential l-histidine (Hansen et al., 2011).
The SLC6 family includes a number of mechanisms that transport essential or conditionally essential amino acids as well as products of essential amino acids metabolism that mediate neurohumoral and neurochemical signal transduction or are coupled to osmotic and bioenergetic balance. SLC6 is prominent in bacteria and archaea, whose genomes may include 1–4 SLC6 paralogs serving in environmental absorption of the limited proteinogenic amino acids, often with focused selectivity for the most difficult to synthesized and most energetically expensive W (Androutsellis-Theotokis et al., 2003), F (Chye et al., 1986), Y (Quick et al., 2006), M (Trotschel et al., 2008) amino acids and with some phenotypes exhibiting transport ability for a more broad range of neutral amino acids (for a review see (Burkovski and Kramer, 2002)). Depriving the organism of such amino acids, or knocking out bacterial SLC6 transporters, limits the exponential growth of bacterial populations. In contrast to SLC7 members, metazoan amino acid transporters of the SLC6 family act in the apical membrane of the alimentary canal or the plasma membrane of neuronal and other somatic cells, using the Na+ motive force to drive substrates against large chemical gradients (Fig. 1). The SLC6 family is expanded in metazoan organisms and includes ~20 genes per genome, some of which have a number of splicing variants. The metazoan SLC6 family can be divided to several phylogenetic clusters with two clearly identifiable subfamilies present in all metazoan organisms (Fig. 2). The subfamily representing more evolutionary recent offspring exists exclusively in Metazoa. It combines several orthologous clusters of catecholamine (dopamine, norepinephrine, and octopamine), indolamine (serotonin), and GABA NeuroTransmitter Transporters (Fig 2, NTT). The basal subfamily, comprising Nutrient Amino acid Transporters (NAT) exists in Bacteria, Archaea, Protozoa, Fungi and Metazoa (Fig 2). Several mammalian representatives of these clusters have been cloned and characterized recently (Broer, 2006; Verrey et al., 2005) (Bohmer et al., 2005; Broer et al., 2004, 2006b; Kowalczuk et al., 2005; Ristic et al., 2006). These studies revealed the genetic linkage of the mammalian NATs to heritable metabolic and mental disorders (Broer et al., 2006a; Kleta et al., 2004; Romeo et al., 2005; Seow et al., 2004) and their definitive roles in the sodium-dependent broad substrate spectra absorption of large neutral essential amino acids and proline, known as the B0 and IMINO system substrates, respectively. This cluster includes pairs of orthologous transporters found in insect genomes and defined here as mammalian and insect NATs (Fig. 2, miNAT). B0-like functionality was also reported earlier in two NATs cloned from Manduca sexta (Ms): KAAT1 (Castagna et al., 1998) and CAATCH1 (Quick and Stevens, 2001). The caterpillar transporters form an insect-specific NAT cluster with 2–9 genes found in genomes of other insect species (Fig. 2, iNAT). Several other SLC6 members that are phylogenetically close to the iNAT cluster have been analyzed in model insects using genetic approaches. Intriguingly, some of these genes are also specific to invertebrate genomes and have no orthologs or apparent functional homologs in vertebrates. They play important roles in insect physiology and development; however, transport roles for many of these genes remain unknown (Fig. 2, Orphan genes or transporters). The following paragraph briefly summarizes existing analyses of the orphan SLC6 members in insects.
Fig. 2.
Phylogenomic tree of selected insect and mammalian SLC6 families. The tree includes 14 Caenorhabditis elegans (CE), 17 Anopheles gambiae (Ag), 20 Aedes aegypti (Ae), 21 Drosophila melanogaster (CG prefix), 20 Homo sapiens (Hs), and 5 characterized Manduca sexta members. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). The bootstrap consensus tree inferred from 2000 replicates is taken to represent the evolutionary history of the taxa (Felsenstein, 1985). The proportions of replicate trees in which the associated taxa clustered together in the bootstrap test (2000 replicates) are shown next to the branches. The tree is drawn in the same order as those of the evolutionary distances. The evolutionary distances were computed using the Poisson correction method (Zuckerkandl and Pauling, 1965) and are in the units of the number of amino acid substitutions per site. The analysis involved 93 amino acid sequences. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 489 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 (Tamura et al., 2011). The red, blue and green branches, respectively, render neurotransmitter transporters (NTT), mammalian insect NATs clustered together with characterized mammalian B0 and IMINO transporters, and insect-specific NATs with characterized B0-like and narrow specificity transporters. Background color highlight: broad (gray), narrow Tryptophan (magenta); Phenylalanine (green), and Methionine (yellow). Pink stars indicate cloned and functionally characterized transporters. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The bloated tubules gene (blot, CG3897, DmBLOT) is expressed in ectodermally derived epithelia, including Malpighian tubules, and in the nervous system. It exhibits complex and dynamic expression patterns during embryogenesis and supports organization of the apical cytocortex processes, which are critical for cell division, differentiation and epithelial morphogenesis (Johnson et al., 1999). blot mutants are larval lethal, showing swollen Malpighian tubule cells with an enlarged and disorganized apical surface. Embryos lacking maternal blot expression die during early embryogenesis, showing an inability to form actin filaments in the apical cortex, which results in impaired syncytial nuclear divisions and severe cytoskeleton defects. DmBLOT has an apparent paralog in the Drosophila genome and pairs of related genes in other insect genomes. This paralog is named bedraggled (bdg, CG8291) and appears to be obligatory for both embryo viability and the initial stages of imaginal disc development, and influences the tissue polarity complex during the R3/R4 fate decision in the Drosophila eye (Rawls et al., 2007). Drosophila inebriated (ine, DmINE, CG15444), which causes the increased motoneuron excitability phenotype (Huang et al., 2002; Huang and Stern, 2002; Soehnge et al., 1996; Stern and Ganetzky, 1992), has a single ortholog in each currently available insect genome. One such ortholog, caterpillar MsINE, has been expressed in a heterologous system and characterized as a hyperosmotic stress sensitive transporter-like protein (Chiu et al., 2000). The DmINE also has also been deduced as a carcinine transporter based on genetic and pharmacological assays (Gavin et al., 2007), again without explicit demonstration of its transport phenomenon. Recently, two additional DmSLC6 members have been described with references to their unique roles in fly biology. Male germ-line-specific SLC6 transporter CG7075, expressed in developing sperm, has been inferred, based on phylogenetic analysis, as a glycine transporter necessary to supply polyglycylation of the massive amounts of tubulin in the giant sperm cells (Chatterjee et al., 2011). CG15088 transporter-like protein has been identified by a lithium induced shift of its expression level (Kasuya et al., 2009a). It is expressed in the adult brain and required for lithium resistance (Kasuya et al., 2009b). However, putative transporter roles for both of these proteins have not been directly demonstrated. The distribution of Drosophila SLC6 members has been examined by in situ hybridization and relative RT-PCR of isolated adult tissues (Thimgan et al., 2006). In particular, this study revealed that fly NATs have unique expression profiles in the alimentary canal, male and female reproductive tissues, and Central Nervous System (CNS) (Thimgan et al., 2006). The Drosophila SLC6 and SLC7 members have also been analyzed with emphasis on their expression and roles in the fly visual system using a combination of an array of specific probes on Northern blots and behavioral assays of deletion mutants of selected genes(Romero-Calderon et al., 2007). This study has concluded that at least one NAT participates in visual signal transduction.
Current study of insect SLC6 transporters beyond dipteran model organisms is very limited. In addition to the aforementioned B0-like MsKAAT1 and CAATCH1, a proline selective transporter (MasPROT) that belongs to an intermediate phylogenetic cluster between NATs and NTTs subfamilies, sometime referred as the Glycine-proline subfamily or Amino Acid Transporter subfamily (Fig. 2, AAT), has been cloned and functionally characterized (Sandhu et al., 2002a). It is expressed predominantly in the caterpillar CNS and flight muscles, which may excessively use proline as a metabolic energy fuel (Sandhu et al., 2002a). Positional cloning in an effort to identify molecular components responsible for resistance of Bombyx mori (Bm) caterpillar to Bm adenovirus type 2 lead to isolation of a BmNAT (Ito et al., 2008). The authors showed that deletion of this gene induces absolute virus resistance and suggests that this protein is a receptor for BmDNTV-2. However, the physiological role of this protein remains unknown. Summarizing current evidence, the important contributions of the NAT-SLC6 subfamily and their phylogenetic offspring to a range of biological processes have been assessed. However, the fragmented nature and scattering of available data made it impossible to define the principal role of NATs in the absorption of essential amino acids. That role becomes much more evident after identification and comparative functional analysis of several new NATs from dipteran insects.
7. Insect NAT-SLC6 members with broad substrate spectra (B0 sytem-like transporters)
MsKAAT1 (NSBI, AAC24190) is the first characterized NAT of the SLC6 family (Castagna et al., 1998). It was identified by expression cloning of RNA from caterpillar midgut of Manduca sexta, heterologously expressed in Xenopus oocytes and revealed a substrate specificity profile similar to the earlier reported profile of the amino acid uptake in Brush Border Membrane Vesicles (BBMV)(Wolfersberger, 1993) isolated from midgut epithelium of M. sexta (Hennigan et al., 1993a,b) and the mammalian B0 amino acid transport system. The capital case character “B” and “0” define the Na+-coupled broad substrate spectra neutral amino acid specificity of this transport system, respectively. However, despite its broad substrate spectrum, MsKAAT1 showed a notably different substrate selectivity and affinity profile vs. mammalian B0. More importantly, it can efficiently use the inward K+ gradient in addition to the canonical Na+ coupling of the mammalian B0 system (Castagna et al., 1998). The heterologously expressed MsKAAT1 mechanism has l-amino acid selectivity with approximately even transport capacity for absorption of l-enantiomers of F, L (KmLeu = 123 µM), M and I (Castagna et al., 1998). The extended substrate profile determined by competitive inhibition of l-Leucine uptake also included W, V, A, and with lower inhibition efficacy C, H, N, S, T, and G (Vincenti et al., 2000). High expression of MsKAAT1 in principal columnar cells of the caterpillar gut and its unusual opportunistic Na+ and/or K+ coupling showed both the principal role and adaptation of this transporter for acquisition of nutrient amino acids in the caterpillar alimentary canal which deals with low Na+ and high K+ plant diets. MsKAAT1 is Cl− sensitive, revealing overall transport stoichiometry of 2 Na+ (or 2 K+):1 Cl− :1 l-amino acid (Castagna et al., 1998). MsKAAT1 mediated transport was maximal at high pH values, which indicates adaptation to an alkaline midgut environments that are common in caterpillars and many other insects (Castagna et al., 1997; Peres and Bossi, 2000).
MsCAATCH1 (NCBI, AF013963) was also cloned from the caterpillar gut (Feldman et al., 2000). It shares 90.3% identical amino acids with the MsKAAT1 protein, but exhibits different substrate selectivity that is determined by symported cations. For example it prefers Thr with K+ ion but Pro with Na+ ion (Km[Na+]Pro = 330, Km[Na+]Thr = 35, Km[K+]Pro = 1900, and Km[K+]Thr = 235 in µM) (Feldman et al., 2000). In addition, MsCAATCH1 combines amino acid transport with a trait of thermodynamically uncoupled ligand-gated leak channels, exhibiting a carrier-independent leakage current that is completely blocked by some substrates (Quick and Stevens, 2001). MsCAATCH1 is moderately chloride dependent (Bette et al., 2008), but its preferable ion/amino acid coupling stoichiometry is unclear due to the increased difficulty in determining these characteristics imposed by the uncoupled current component. The caterpillar transporters became a prominent model in the mutagenesis-mediated studies of molecular physiology and structure function relationships, leading to multiple insights about the functional significance of structural components of the SLC6 family (recently reviewed by Castagna et al. (2009)). Presently we do not know how many NATs genes exist in the caterpillar genome. However, pBLAST of MsNATs identifies two complete sequences of SLC6 transporters in a draft annotation of the Bombyx mori genome (SilkDB), indicating the presence of two independent BmNATs closely related to the described M. sexta transporters. One of these genes, NCBI protein BAG29453 (identified previously as viral receptor of BmDNV-1) (Ito et al., 2008), appears to be paralogous to both MsNATs. Deletion of this gene exhibits a viable phenotype, but induces absolute resistance to BmDNV-1 viral infection. This fact suggests reciprocal compensation of NAT functions in caterpillars. A pair of putative NAT from the B. mori genome also resides within the miNAT cluster (data not shown). Studies of chimerically bound MsKAAT1 and MsCAATCH1 showed functional expression of such constructs with possible formation of oligomeric structures (Bossi et al., 2007). Binary homo-dimerization is a documented phenomenon for several NTT-SLC6 members (Horschitz et al., 2003; Schmid et al., 2001) and is evident from the crystallization of the first bacterial SLC6 representative (Yamashita et al., 2005). The hetero-oligomerization of MsNATs is interesting, but lack of data about relative expression and localization of these transporters obscures the potential biological significance of the MsNAT oligomerization.
AeAAT1 (NCBI AAR08269) was the first characterized mosquito B0-like NAT. It was cloned from cDNA prepared from midgut tissue of Ae. aegypti larvae, and characterized by comparative phylogenomic analysis, functional expression in Xenopus laevis oocytes, and in situ hybridization in whole mount preparations of mosquito larvae (Boudko et al., 2005a). The phylogenomic analysis showed that insect NATs form a paralogous group, e.g. identified insect transporters from mosquito, fruit fly, and caterpillar were phylogenetically separated from mammalian homologs and putative nematode NATs (Boudko et al., 2005a). Even NATs from different insect genera form phylogenetically separate paralogous clusters. This pattern counters the strong orthology and functional conservation observed among NTT-SLC6 members and suggests that NATs evolved with a higher degree of evolutionary freedom, allowing functional adaptive plasticity of their transport phenotypes and providing an integrated transport network for absorption of nutrient amino acids. Incidentally, AeNAT1 and DmNAT1, two dipteran transporters described next, represent the two nearest phylogenetic relatives of MsKAAT1 and MsCAATCH1 in the root of the NAT-SLC6 cluster. Hence, the broad substrate specificity of these transporters may allow us to elucidate an ancestral phenotype.
The heterologous expression and characterization of AeAAT1 reveals B0-like properties. However, in contrast to relatively flat B0 substrate selectivity, AeAAT1 has notably elevated apparent affinity and transport velocity for F and is virtually insensitive to N, P and L (AeAAT1 selectivity profile: F ≫ C > H > A≈S > M > I > Y > T ≈ G ≫ N ≈ P > L; KmPhe = 0.45 mM; KmNa+ = 38 mM; ηNa+/ηPhe ≈ 2/1). The ability to discriminate between L vs. I and M is unusual for B0 type transporters. Moreover, electrochemical profiles of AeAAT1 differ from the MsNAT transporters. For example, the Cl− dependency and ability to utilize K+ instead Na+ electrochemical gradient are absent in AeAAT1 (Boudko et al.,2005a). Whole mount in situ hybridization confirms specific spatial transcription of AeAAT1 in the posterior midgut, which correlates with apical absorption of digested amino acids from the larval gut (Clements, 1992). In addition, it has been identified in secretory parts of the larval alimentary canal, including salivary glands and cardia, and was found in the larval brain (Boudko et al., 2005a). The characterization of AeAAT1 provides additional insight into the adaptive diversity of metazoan essential amino acid transport systems. It indicates that the phylogenetic distance of iNAT cluster (Fig. 2) may correlate with adaptive differences in substrate specificities. It also shows that in addition to alimentary absorption, NATs might support other functions, including epithelial secretion and neuronal nutrition (Boudko et al., 2005a).
DmNAT1 (NCBI AAY56384, FlyBase CG3252) was the first NAT characterized in the fruit fly, D. melanogaster. It is a member of an insect specific NAT cluster that includes six Drosophila genes (Miller et al., 2008). Heterologous functional expression and characterization of DmNAT1 reveals another interesting adaptive aspect of broad substrate spectra NATs. Specifically, DmNAT1 is adapted to transport both l- and d-isomers of essential amino acids with virtually equal apparent affinities and transport velocities. This is quite interesting because many insects, including Drosophila, can substitute essential l-amino acids with d-enantiomers (Geer, 1966). Hence, DmNAT1 may substantially benefit nutrition via absorption of substrates from fermented and symbiotically modified diets, and may contribute to the unique capacity of fruit fly larvae to survive on artificial diets with complete substitutions of essential l-amino acids by their d-enantiomers. In contrast, the closest relative of DmNAT1 in mosquitoes, AeAAT1 has limited capacity for transporting d-enantiomers of essential amino acids. DmNAT1 is expressed in the posterior midgut and salivary gland, quite similar to expression profiles identified for mosquito NATs. Strong ubiquitous transcription in the brain suggests roles for DmNAT1 in neuronal nutrition as well as sub-synaptic clearance of dl-neutral amino acids, specifically endogenous d-serine that modulates NMDA receptor-coupled signal transduction. The characterization of DmNAT1 extends our knowledge of the biological roles of the insect NAT-SLC6 members, revealing adaptations for the absorption of d-isomers of the essential amino acids.
Summarizing the above, the broad substrate spectra NATs are present in all analyzed genomes of insects. They are adapted to transport essential and conditionally essential amino acids with relatively uniform affinity and velocity. In some herbivorous insects, e.g. moth and beetles, NATs are limited to a single duplication, perhaps requiring broad substrate spectrum phenotypes in order to sufficiently supply the nutrient demands of these organisms. In addition to alimentary absorption, insect B0-like NATs are expressed in other tissues where they can serve as essential substrate providers, supporting cellular processes with elevated consumption of essential amino acids.
8. Insect NAT-SLC6 members with narrow substrate specificity
Comparative phylogenomic analysis of emerging insect genomes revealed significant paralogous expansion of NATs that form insect specific clusters (Boudko et al., 2005a). In particular, such expansions are prominent in dipteran genomes, including D. melanogaster (6), An. gambiae (7), Ae. aegypti (9), and Culex quinquefasciatus (8 NATs). In addition, each of these arthropods has orthologous pairs of orphan NATs that phylogenetically reside within the miNAT cluster (Fig. 2). Molecular cloning and characterization of NATs from the iNAT cluster reveled several unique transport phenotypes with narrow specialization for absorption of essential amino acids.
AgNAT8 (NCBI AAN40409) is the first characterized metazoan transporter with narrow substrate specificity. It was cloned from the larval midgut cDNA of An. gambiae, an important tropical disease vector mosquito and genomic model (Meleshkevitch et al., 2006). The An. gambiae genome (Holt et al., 2002) includes 7 putative NATs that form a condensed tandem gene cluster in chromosome 3 without interruption by any other known gene (Meleshkevitch et al., 2006). NAT specific expansion and dense gene clustering is also present in genomes of other insect species, suggesting that it is a common trait and supporting a theory of cluster gene duplication and possible metabolon-like regulation of NAT activities. In contrast to B0-like NATs, AgNAT8 expressed in Xenopus oocytes has an unusually narrow substrate preference, with particularly high transport velocity and apparent affinity to L-phenylalanine (K0.5Phe ~ 0.2 mM, K0.5Na+ ~ 26 mM, stoichiometry 2:1) and the metabolically derived phenyl-branched l-tyrosine and L-DOPA (Meleshkevitch et al., 2006). Such a substrate profile had never been reported in earlier studies of transport systems with Brush-Border Membrane Vesicles (BBMV), implying that such a transporter exists only in mosquitoes or that the BBMV assay cannot resolve individual NAT activity from synergistic NAT action. For example, AgNAT8 might act with other NATs to form a broad spectra mechanism similar to the mammalian B0 and insect B0-like transport systems. The idea of synergistic cumulative activities of multiple narrow substrate spectra NATs has emerged. Such a system of morphologically and energetically independent NATs could alter substrate absorption via modulation of the relative expression (densities) of individual transporters, which would improve the organism’s nutrition in changing nutrient environments (Meleshkevitch et al., 2006). The in situ hybridization assay confirmed elevated expression of AgNAT8 transcript in absorptive and secretory regions of the larval alimentary canal and also showed cell specific NAT expression in the neuronal system, including central ganglia and neuronal components of visual and mechanosensory signal transduction pathways. AgNAT8 characterization indicated that a plausible reason for the escalation of NAT numbers in insect genomes is excessive duplication followed by the selective retention of NATs with narrow substrate spectra. It also showed that NATs might play specific roles in central and peripheral nervous systems. At the same time, it raised the possibility that all NATs are redundant transporters with slightly increased degrees of specialization for the absorption of phenol-branch substrates.
AgNAT6 (NCBI CAF25029) is the first identified metazoan tryptophan and indole-branch selective transporter. Characterization of this transporter showed that NATs with narrow substrate specificities could potentially have significant shifts in substrate spectra toward other essential aromatic substrates (Meleshkevitch et al., 2009b). AgNAT6 is the closest “short-term” paralog of AgNAT8, the two sharing 52.9% pairwise protein sequence identities. However, in contrast to the phenyl branch-preferring AgNAT8, it absorbs indole-branch substrates, with an especially high affinity for l-tryptophan (K0.5Trp = 1.3 vs. K0.5 Phe = 430 µM) and its metabolic derivative 5-hydroxytryptophan (5-HTP). Remarkably, the difference in the substrate coordinating framework involves only 3 out of total 12 substrate-coordinating residues (Meleshkevitch et al., 2009b). Thus, AgNAT6 has specifically adapted to absorb l-Trp with minor interference from the more highly concentrated l-Phe and l-Tyr. Moreover, the molecular evolution of such a trait required only minor alteration of the transporter sequence. The in situ hybridization assay of AgNAT6 transcript showed elevated expression in the alimentary canal and CNS, but more importantly revealed significant differences when compared to AgNAT8 in spatial and cellular expression patterns. The characterization of AeAAT1, AgNAT6, and AgNAT8 demonstrated incremental substrate specialization of NATs, but also raised the possibility that mosquito NATs are all aromatic transporters with variations in their substrate selectivity.
AeNAT5 (NCBI ABZ81825) is the first metazoan methionine selective transporter (Meleshkevitch et al., 2009a). The characterization of AeAAT1, AgNAT6, and AgNAT8 demonstrated incremental substrate specialization of NATs, but also raised the possibility that mosquito NATs are all aromatic transporters with some adaptive variations in their selectivity profiles. Hence, the identification and characterization of AeNAT5 and its Anopheles ortholog AgNAT5 (ABZ81817), both with narrow selectivity to sulfur-containing amino acids, was a theoretically important breakthrough showing that NAT specialization actually expands beyond aromatic substrates. The Anopheles and Aedes NAT5 orthologs have 69.8% pairwise protein identity and absolutely identical amino acid sequences in their substrate binding pockets. AeNAT5 has very high transport velocity and apparent affinity to l-Met (K0.5 Met ~21 µM). In addition, it accepts homocysteine and l-cysteine (K0.5DL-hCys ~ 0.9 mM, K0.5L-Cys ~ 2.2 mM), but is virtually insensitive to aromatic and other B0-system substrates (Fig. 3). The AeNAT5 mechanism has a of ~46 mM, an estimated stoichiometry of 1:1 and is expressed in the alimentary and neuronal systems.
Fig. 3.
An example of NAT characterization based on data from the heterologous expression of AeNAT5, a l-Met selective transporter from Aedes aegypti. A. AeNAT5-coupled substrate-induced currents. Acronyms: single letter code for amino acids; hC, homocysteine; DOPA, (3,4-dihydroxy-L-phenylalanine); 5HT, serotonin; 5HTP, 5-hydroxytryptophan, DA, dopamine; dM, dF, enantiomers of L-Met and L-Phe respectively; GABA, -Aminobutyric acid; Tau, taurine; OA, octopamine; TA, tyramine. B. A staircase test of increasing l-Met concentrations. C. Typical stimulatory voltage (top) and response current (bottom) signals along with a resulting I/V plot (right). D. Saturable kinetic characteristics of AeNAT5 that were acquired using protocol similar to that shown on the B panel. Data points (mean + Std. Errors) were fitted with a Hill function: F = aX^/(K0.5^+X^); from which the apparent affinity (K0.5) and Hill constant (, transport order) were calculated. E. Saturable sodium kinetics of AeNAT5 at 1 mmol l−1 concentration of l-Met.
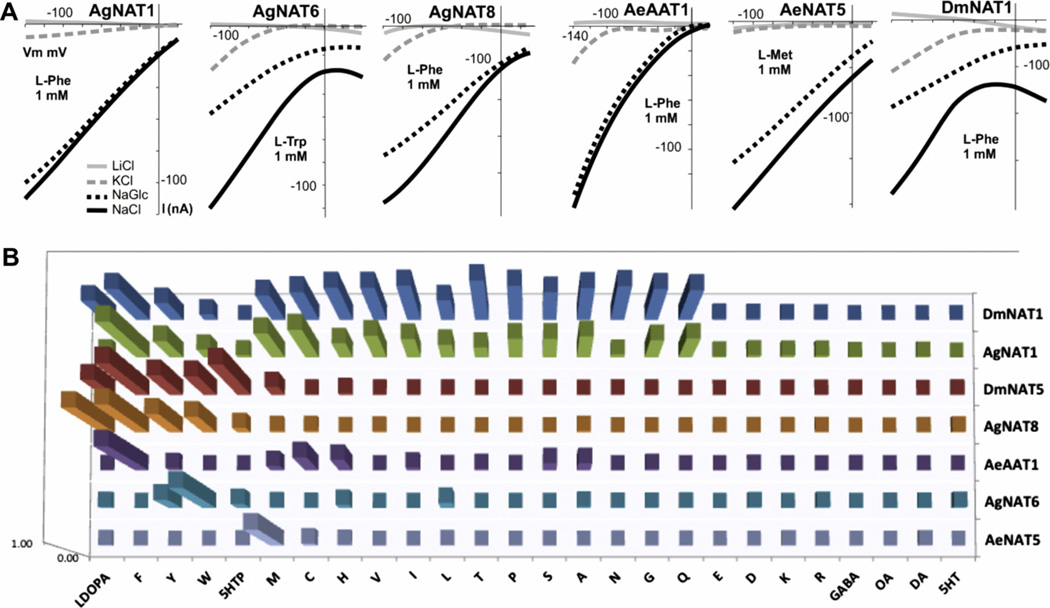
Insect NAT-SLC6 members specialize in the active absorption of essential amino acids. The functional characterization of new dipteran NATs showed that in addition to ubiquitous B0-like transporters, the NAT-SLC6 subfamily includes a number of mechanisms with narrow specialization for the active apical absorption and subsequent systemic distribution of the most underrepresented essential aromatic (W, F), sulfur-containing (M), and conditionally essential (Y, C) amino acids (summarized in Fig. 4). Although presently we still have limited comparative data about specific and integrated roles of NATs in most metazoan organisms, and initial analyses of NATs in model dipteran insects and mammals is quite fragmented, the consensus of the available information clearly elucidates that the NAT-SLC6 subfamily is a critical component of an essential amino acid transport network in metazoan organisms, and that NATs are likely unique in this quality across the superfamilies of secondary transporters. This network likely integrates many transport mechanisms, including members of SLC7 and other amino acid transporter families. However, the NAT-SLC6 subfamily is particularly important because it integrates as a universal active core of this network and plugs it into organismal bioenergetics and metabolism. Evidently, NATs undergo rapid gene multiplication substitution that enables a high degree of evolutionary plasticity of nutrient amino acid uptake mechanisms and facilitates nutrient adaptations of organisms in a dynamic environment. In addition, analysis of dipteran NATs shows a range of adaptive variations in the electrochemical coupling, alkali ion preference, chloride dependency, and preferred stoichiometry. The segregation of substrate absorption between several transporters and electrochemical adaptations can also potentiate nutrient absorption and facilitate accumulation of essential amino acids, which are major limiting factors in insect development and are particularly critical for aquatic mosquito larvae.
Fig. 4.
Electrochemical properties and substrate specificities of selected mosquito and fruit fly NATs. A. A set of selected I/V plots showing variations in ion and voltage dependencies for characterized insect NATs. Each plot combines four I/V graphs from ion substitution experiments using 100 mM media with different major salts (left plot insert). I/V graphs in which “background” currents (in the absence of organic substrates) were subtracted from currents in the presence of 1 mM substrate are shown. B. Normalized (to maximum response 1) relative substrate transport efficiencies of selected insect NATs as a function of substrate induced currents.
9. Localization of insect NATs in epithelial membranes of the alimentary canal
In situ hybridization of whole mount insect preparations with NAT specific mRNA probes showed elevated expression of, and predicted a role for, NATs in the epithelial and neuronal tissues (Boudko et al., 2005a; Meleshkevitch et al., 2006, 2009a,b; Miller et al., 2008). In particular, these localization studies revealed high, quasi-uniform, expression of all analyzed NATs in the posterior and anterior regions of the larval alimentary canal, which correlates with nutrient amino acid absorption (posterior midgut) and secretory functions (salivary gland and cardia) (Clements, 1992). qPCR of AeAAT1 (Boudko et al., 2005a), AgNAT6 (Meleshkevitch et al., 2009b) and AgNAT8 (Meleshkevitch et al., 2006) confirmed the results of in situ hybridization and also showed that NATs are expressed in adult tissues. However, transcript localization and quantification did not resolve the membrane distribution of NATs that is important for rendering their contribution in epithelial functions. For example, alternative apical or basolateral docking of a NAT would support transepithelial transport in opposite directions. Immunolocalization of AgNAT6 and AgNAT8 has been examined by immunolabeling of frozen sections and whole mount mosquito preparations with transporter epitope specific antibodies that provide a more detailed image of spatial and polar distribution of these transporters (Okech et al., 2008b). This study confirmed the anticipated apical localization of NATs in absorptive regions of the larval posterior midgut and Malpighian tubules. In contrast, basal localization of both transporters has been identified in the cardia, which secretes peritrophic membrane, and in salivary glands, which perform excessive synthesis of salivary proteins. These data support roles for both transporters as substrate providers in secretory functions in which basal NATs absorb amino acids from hemolymph and feed them to the synthesis of peptides that are subsequently secreted via an apical exocytosis mechanism. Moreover, immunolabeling revealed notable differences in relative densities and spatial distributions of the aromatic amino acid transporters, which suggests unique morphological profiles of essential amino acid absorption by different NATs (Fig. 5). The apparent segregation of spatial expression in absorptive epithelia has been suggested as an additional adaptive mechanism that may reduce bioenergetic competition of NATs and, therefore, improve absorption of the most underrepresented essential amino acids, tryptophan and phenylalanine(Okech et al., 2008b). Similar spatial segregation of different B0 and IMINO system transporters has also been demonstrated in the proximal tubule epithelia of the mammalian kidney (Vanslambrouck et al., 2010).
Fig. 5.
The summary of spatial (left panel) and polar (right panel) distributions of AgNAT6 and AgNAT8 in the alimentary canal of Anopheles larvae, unraveled with a set of transporter-specific affinity-purified antibodies. The left panel represents green immuno-fluorescence in the whole mount larval gut after labeling with pre-immune serum (A), AgNAT6-specific antibodies (B), or AgNAT8-specific antibodies (C). (D) Relative fluorescence ratios from the above preparations showed as black, green, or red lines, respectively. The right panel represents immunolabeling of frozen sections prepared from specified parts of the larval gut (related loci are indicated by fine black arrows). The top and bottom parts correspond to AgNAT6 and AgNAT8 labeling, respectively, which are also reflected in the reconstruction diagram (middle insert). On the diagram, green lines denote the exact location of transport mechanisms and green/red arrows represent transport directions upon apical/basal docking of the transport mechanisms. On the section pictures green, red and blue signals correspond with AgNAT-, nuclei- and actin-specific labeling, respectively. Yellow signals are overlaps of actin and NAT signals in the Malpighian tubules. White arrows indicate apical membranes. Abbreviations are: AMG, anterior midgut; PMG, posterior midgut; GC, gastric caeca; RC, rectum; SG, salivary gland; MT, Malpighian tubules; pm, peritrophic membrane; cm- caecal membrane; A/B plus arrow marks indicate apical and basal membranes. Summarized with modification from JEB (Okech et al., 2008b). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
10. Electrochemical integration of NAT mechanisms in alimentary functions
From the results of heterologous functional characterization we have learned that insect NATs represent normally rectifying (unidirectional) mechanisms that are predominantly driven by Na+ motive force (Fig. 4 A). Many insects NATs are also capable of using an inward K+ motive force that may, theoretically, exist in specific conditions when high extracellular activity of K+ ions and large negative transmembrane voltages reverse K+ flux. For example, the alimentary canal of an herbivorous caterpillar experiences high dietary loads of K+ but only trace Na+. A Na+ dependent mechanism in such a condition is ineffective for bulk absorption of nutrient amino acids. The H+ V-ATPase, which generates a large transmembrane voltage at the apical membrane of goblet cells in the caterpillar gut, eventually inverts the K+ gradient, creating a motive force that can be used for the bulk absorption of nutrient amino acids (Castagna et al., 1998). In contrast, fresh water mosquito larvae are dealing with low concentrations of both alkali metal cations in their habitats, and plausibly use effective recycling of these physiologically important cations. Hence, the mosquito NAT population represents a transitional phenotype in that all are able to use the Na+ gradient, but some can also use the K+-gradient, similar to caterpillar NATs. The capacity of mosquito NATs to use both Na+ and K+ can be explained as an adaptation to varied concentrations of these cations. Many mosquitoes tolerate or have even adapted to high salinity and, therefore, might predominantly use Na+ dependent transporters. NATs, such as in the mammalian B0ATs (Broer et al., 2004, 2006b) and caterpillar KAAT1 (Castagna et al., 1998), also reflect adaptations of these organisms to a high (in mammals) vs. trace (in caterpillars) availability of Na+ in the animals’ diets. The availability of Na+ and K+ also varies over the life history of insects. For example, the role of Na+ is expected to be very low in adults feeding on plants; but it might increase after a blood meal.
High extracellular Cl− concentrations facilitate the functions of some insect NATs (Fig. 4, AgNAT6, AgNAT8, AeNAT5, and DmNAT1), while several broad substrate spectra NATs, such as AeAAT1 and AgNAT1, are less sensitive to this ion (Fig. 4A, AgNAT1 and AeAA1). The studies on Cl− dependency of insect NATs revealed a possibility for bidirectional (electroneutral) Cl− transport, in which thermodynamically favorable outward Cl− movement would restore the initial conformation of the transporter, facilitating the overall transport kinetics (Meleshkevitch et al., 2009b). A similar putative mechanism was described in SLC1 transporters that likely use K+ to reset their initial conformations [reviewed by Kanai and Hediger (Kanai and Hediger, 2004)]. The structural basis of Cl− interaction with SLC6 members was the subject of recent analysis, which suggested a possible replacement of Cl− with another negatively charged ion in chloride-independent members of SLC6 (Forrest et al., 2007; Zomot et al., 2007). However, the exact roles of this anion in SLC6 functions, and in particular in NAT activity, remain to be clarified.
The high expression and activity of NATs imply that they are major users of Na+ and/or K+ ions present in the lumen of the insect alimentary canal (for example, Fig. 6). NAT activity is linked to a previously proposed anterior midgut alkalinization pathway (Harvey et al., 2009; Okech et al., 2008a) and is plausibly involved in cation recycling (Fig. 6, red loop arrows). In fact, the major role of anterior midgut (AMG) alkalinization (Fig. 6, pH > 10 area) may be to support NAT activity and nutrient amino acid absorption in the posterior midgut (PMG). Reciprocally, an additional important role for NATs could be to recycle sodium ions and maintain Na+ ion homeostasis in insect bodies. A simplified putative model that integrates alkalinization and nutrient amino acid absorption in the mosquito alimentary canal is presented in Fig. 6. According to this model, high alkalinity in the AMG is generated via means of nutrient acquisition and region specific transepithelial uptake of Na+ via the combined actions of the basal Na/H exchanger (NHE) or Na channel (NC) and apical Na/K ATPase, which are energized by the H + V-ATPase, coupled to the uptake of Cl− from the lumen in this area. The sodium ions electrophoretically and mechanically moved to the PMG are used by apically expressed NATs and subsequently uploaded to hemolymph via basal Na/K ATPase or an NHE, which is energized by the apical V-ATPase. This model is well supported by morphological studies of involved acid/base balance transporters and pumps (see figure legend for references).
Fig. 6.
A simplified tentative electrochemical integration of NATs with mineral ion homeostasis in the alimentary canal of An. gambiae larvae. Blue and red lines show putative anionic (Cl−) and cationic (Na+) pathways that induce alkalinization in the anterior midgut (AMG, pH > 10) by secretion of Na+ ion and resorption of Cl− ion. Line thickness represents a predictable generalization of ion fluxes that are associated with apical NAT function in the posterior midgut (PMG) and mineral ion recycling in AMG. Positions of AE (Cl−/HCO3− anion exchanger) (Linser et al., 2009), ClC (Cl− plasma membrane or paracellular conductance channel) (Beyenbach et al., 2010; O’Connor and Beyenbach, 2001), sodium proton exchanger NHE (Harvey et al., 2009; Okech et al., 2008a; Pullikuth et al., 2003; Rheault et al., 2007), Na/K ATPase (Patrick et al., 2006), V-ATPase (Boudko et al., 2001a,b; Patrick et al., 2006; Wieczorek et al., 1999; Zhuang et al., 1999) are rendered based on the available literature (see more detailed description in the text).
11. Structural aspects of essential amino acid transporters of the NAT-SLC6 subfamily
The crystal structure of the first NAT from the thermophilic bacterium Aquifex aeolicus, AeLeuT, has been solved (Yamashita et al., 2005), revealing substrate and ion-binding sites in substrate occlusion and outside open conformations (Singh et al., 2007) (NCBI accession LeuT, PDBs 2A65 and 3GWW). Now we can use simple alignment to reveal the molecular specificities of the principal functional domains of insect NATs (Fig. 7 and Fig. 8). The canonical structure of SLC6 members (Fig. 7 and Fig. 9A) includes 12 transmembrane domains (TMDs) with intracellular N and C termini. TMD1 and 6 have irregular helices with uncoiled central regions that form a cavity in the middle of the NAT proteins. In outside open conformation this cavity is connected to the outward vestibule, allowing extracellular substrates to enter and interact with specific Na+ and amino acid coordinating residues of the NAT protein. There are 12 residues that maintain the amino acid binding network and form amino acid binding pocket (Fig. 8, green shape); 4 residues that first bind sodium, which may be facultative for insect and mammalian NAT action (Fig. 8, yellow shape); and 5 residues that bind a second sodium (Fig. 8, red shape), which likely is obligatory for NAT action due to its involvement in interaction with the amino acid substrate(Yamashita et al., 2005). Approximately 40% of those residues are conserved across NATs. Naturally occurring mutations in the remaining residues determine the substrate binding affinity and cavity volume, which together affect the substrate specificity of NATs (Fig. 8). Thus, modification of only a few or even one of these residues may substantially modify the shape, volume, and electrostatic properties of substrate binding pocket, as well as modality of a transporter’s action. The NAT transport process can be described in terms of a putative conformational cycle (Yamashita et al., 2005). Apparently, outside open conformation is stable, and Na+ binding has little effect on this conformation despite the immense inward electrostatic motive forces acting on the Na+ ions. Amino acid binding destabilizes the outside open conformation, which under action of Na+ motive forces collapses to a short term occlusion conformation and subsequently propagates to an inside open conformation, ejecting substrates into the cytosol. Finally, the transporter recycles to an outside open conformation set for the next cycle.
Fig. 7.
Alignment of selected insect NAT protein sequences relative to the structure of a crystalized bacterial transporter, AeLeuT (2a65). Transmembrane domains (TMD1–12) and conserved structural features including putative amino acid and cotransporter ion binding sites were identified based on alignment with AeLeuT sequence related structural data (2a65) (Yamashita et al., 2005). The alignment was generated by Clustal X with manual improvement and visualized using Geneious Pro 5 software (Biomatters, Auckland, New Zealand). Increasing background intensity indicates an increase in sequence similarity. Filled shapes along green, yellow, red, and blue lines indicate: amino acid, first Na+, second Na+, and Cl− ion binding sites. Black and empty shapes indicate conserved charged pairs that form extracellular and cytoplasmic entrances of the transporters. Red arrows indicate relative positions of transmembrane helices. AeLeut sequence was removed from the alignment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 8.
Substrate binding sites of selected insect NAT-SLC6 transporters. Motifs interacting with amino acid, Na+, and Cl− substrates were isolated from sequence alignment which was prepared iterating phylogenetic and 3D-structural alignments of selected transporters (Fig. 1). The shapes inserted on top of the alignment indicate transmembrane domains (TMD) distribution and inferred roles of the aligned sites. The inferences of amino acid and Na+ binding sites are based on published occlusion state of AaLeuT/3F3D (Yamashita et al., 2005), as well as on experimentally determined Cl− binding sites (Forrest et al., 2007). Relative numbers of amino acids positions in the AaLeuT and AeNAT5 sequences are shown in upper and lower lines, respectively. Only amino acids that exhibit disagreement with the consensus sequence, and could potentially contribute to the unique transporter phenotypes, are shown in color; all others sites are shown in gray. A gray background indicates characterized transporters that represent specific transport systems, including B0-lke system and newly identified narrow substrate insect systems with W (indole), F (phenol), and M (sulfur-containing) amino acid selectivities. Empty shapes outline the putative sites that may contribute to the unique methionine selectivity of W, F, and M system transporters. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 9.
2D and 3D structural aspects of aromatic AgNATs and substrate docking results. A. 2D architecture of NATs. TMDs are shown as cylinders with different colors. Spheres represent Na+ ions and the green shape represents amino acids in the substrate-binding site between TMDs 1 and 6. B. An in silico generated 3D model of an AgNAT8 homodimer and its position in the plasma membrane (inner and outer surfaces of lipid layers are indicated by blue and red dots). Each subunit has 12 TMDs, shown in alternate colors; a molecular surface only is shown for the second subunit. The characteristic for SLC6 homo-dimerization AgNAT8 transporters was confirmed by Western blot analyses of native and heterologously expressed transporters (Okech et al., 2008b). AgNAT8 and AgNAT6 were modeled using iterative modeling and “loop” protein optimization with Modeller 8.6 (Fiser and Sali, 2003; Sali and Blundell, 1993) and Yasara Structure (Krieger et al., 2002). Final ERATA scores are 82% and 91% for AgNAT8 and AgNAT6, respectively. Two subunits of AgNAT8 were spatially superimposed according to available crystallographic data from the 2A65 structure and optimized by energy minimization. C. AgNAT6 and AgNAT8 substrate binding pockets (SBP) outlined by a transparent molecular surface. The location of SBPs in AgNAT8 and AgNAT6 structures was identified by superimposition with a SBP of LeuT. Selected substrates were docked in the NAT SBPs using ArgusLab a molecular modeling, graphics, and drug design program (Planaria Software LLC, Seattle, WA) and free energies of ligand–protein interactions were calculated. Optimal docking of selected substrates and free energy values are shown on the figure. D. Experimentally determined normalized substrate transport efficiency profiles for AgNAT6 and AgNAT8 (blue and red patterns, respectively). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The bacterial NATs share ~20% sequence identities, with a notable increase of conservation in the principal substrate-binding TMDs 1, 6, and 7 (Fig. 7). These conditions have been found sufficient for the homology modeling of NTT structures, with a focus on the substrate-binding core, and for performing in silico experiments for simulation of transporter behavior and pharmacophore interaction. Using these approaches, 3D models of NATs can be built and used to determine their substrate and pharmacophore specificities (Fig. 9).
12. Conclusions and perspectives
Identification of caterpillar NATs and subsequent characterization of several NATs from different insect species, along with demonstration of their specific expression profiles in the larval alimentary canal, secretory cells, and other tissues defined the role of NATs as the active core of the essential amino acid transport system (Boudko, 2010). They showed that insect NATs include a category of previously unknown transporters with narrow substrate specificities. The observed differences in substrate profiles and spatial densities provided new insight into the integration and regulation of the NAT-SLC6 subfamily. This regulation occurs via unique patterns of spatial expression and polar docking of individual NATs. Alternative spatial distribution minimizes the overlap of transporters, further reducing their competition for the electrochemical energy of transmembrane ion gradients. At the same time, regulated expression of several transporters in the same cell may assist in the localized altering of the essential amino acid absorption spectra(Okech et al., 2008b). Obviously, the recent progress exposes just the tip of the iceberg, and substantial studies should be accomplished to improve our understanding of the essential amino acid transport network’s evolution, adaptation, integration, regulation, and specificity in different organisms.
A number of scientific and practical fields may substantially benefit from additional studies of NATs in model insects. The explicit role of NATs, in combination with an easily accessible insect model organism, provides an outstanding platform for unraveling the integrative biology of essential amino acid transport and analyzing the role of essential amino acid transport in specific biological processes, including alimentary, neuronal, developmental, immune etc., some of which can be extrapolated to analogous processes in mammalian physiology and human health. Moreover, the accessibility to the electrochemical activity of insect NATs in a heterologous expression system provides an outstanding opportunity to analyze the structural aspects that are responsible for NAT action. Additionally, insect NATs are readily available for exploring structural aspects of the molecular evolution and adaptation of essential amino acid transporters in metazoan organisms. The structural analysis that is now possible also provides a methodology for unraveling structural aspects of NAT substrate/pharmacophore specificity and molecular engendering of NATs with specific functions.
Due to their crucial role in insect nutrition and phylogenetic specificity, NATs are considered to be excellent targets for the development of lineage-specific and environmentally safe insecticides. For example, narrow substrate spectra NATs compose apparently orthologous groups in two vector mosquitoes, An. gambiae and Ae. aegypti, but not in other dipteran, hymenopteran, coleopteran, or lepidopteran insects, including analyzed fruit fly, honey bee, red flour beetle, and silk moth genomes (unpublished personal observations). Thus, the inhibition of such a transporter may be quite deleterious, and specific, to rapidly developing mosquito larvae. Alternatively, allosteric regulation and optimization of NAT activity could be applied to improve nutrition and productivity in agriculturally employed arthropods, such as honey bees or silkworms.
Acknowledgements
I am delighted to provide this personal tribute to my mentor and friend William R. Harvey who is not merely a brilliant researcher and teacher but also inventor and leader in molecular and integrative studies of membrane transport. Supported by Research Grants AI-52436 and AI-30464 from the NIH.
References
- Akov S. A qualitative and quantitative study of the nutritional requirements of Aedes aegypti L. larvae. Journal of Insect Physiology. 1962;8:319–335. [Google Scholar]
- Androutsellis-Theotokis A, Goldberg NR, Ueda K, Beppu T, Beckman ML, Das S, Javitch JA, Rudnick G. Characterization of a functional bacterial homologue of sodium-dependent neurotransmitter transporters. Journal of Biological Chemistry. 2003;278:12703–12709. doi: 10.1074/jbc.M206563200. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Shiao SH, Raikhel AS. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. Journal of Experimental Physiology. 2006;209:3071–3078. doi: 10.1242/jeb.02349. [DOI] [PubMed] [Google Scholar]
- Bette S, Castagna M, Bossi E, Peres A, Sacchi VF. The SLC6/NSS family members KAAT1 and CAATCH1 have weak chloride dependence. Channels. 2008;2:358–362. doi: 10.4161/chan.2.5.6901. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annual Review of Entomology. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. Journal of Biological Chemistry. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Bohmer C, Broer A, Munzinger M, Kowalczuk S, Rasko JEJ, Lang F, Broer S. Characterization of mouse amino acid transporter B(0)AT1 (slc6a19) Biochemical Journal. 2005;389:745–751. doi: 10.1042/BJ20050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll M, Daniel H, Gasnier B. The SLC36 family: proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Archiv – European Journal of Physiology. 2004;447:776–779. doi: 10.1007/s00424-003-1073-4. [DOI] [PubMed] [Google Scholar]
- Boll M, Herget M, Wagener M, Weber WM, Markovich D, Biber J, Clauss W, Murer H, Daniel H. Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proceedings National Academy of Sciences USA. 1996;93:284–289. doi: 10.1073/pnas.93.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi E, Soragna A, Miszner A, Giovannardi S, Frangione V, Peres A. Oligomeric structure of the neutral amino acid transporters KAAT1 and CAATCH1. American Journal of Physiology – Cell Physiology. 2007;292:C1379–C1387. doi: 10.1152/ajpcell.00473.2006. [DOI] [PubMed] [Google Scholar]
- Boudko DY. Molecular ontology of amino acid transport. In: Gerencser G, editor. Epithelial Transport Physiology. Springer, NY: Humana-Springer Verlag; 2010. pp. 379–472. [Google Scholar]
- Boudko DY, Kohn AB, Meleshkevitch EA, Dasher MK, Seron TJ, Stevens BR, Harvey WR. Ancestry and progeny of nutrient amino acid transporters. Proceedings National Academy of Sciences USA. 2005a;102:1360–1365. doi: 10.1073/pnas.0405183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudko DY, Moroz LL, Harvey WR, Linser PJ. Alkalinization by chloride/bicarbonate pathway in larval mosquito midgut. Proceedings National Academy of Sciences USA. 2001a;98:15354–15359. doi: 10.1073/pnas.261253998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudko DY, Moroz LL, Linser PJ, Trimarchi JR, Smith PJ, Harvey WR. In situ analysis of pH gradients in mosquito larvae using non-invasive, self-referencing, pH-sensitive microelectrodes. Journal of Experimental Physiology. 2001b;204:691–699. doi: 10.1242/jeb.204.4.691. [DOI] [PubMed] [Google Scholar]
- Boudko DY, Stevens BR, Donly BC, Harvey WR. Nutrient amino acid and neurotransmitter transporters. In: Lawrence I, Gilbert KIaSSG, editors. Comprehensive Molecular Insect Science. first ed. Amsterdam: Elsevier; 2005b. pp. 255–309. [Google Scholar]
- Broer A, Cavanaugh JA, Rasko JEJ, Broer S. The molecular basis of neutral aminoacidurias. Pflügers Archiv – European Journal of Physiology. 2006a;451:511–517. doi: 10.1007/s00424-005-1481-8. [DOI] [PubMed] [Google Scholar]
- Broer A, Klingel K, Kowalczuk S, Rasko JEJ, Cavanaugh J, Broer S. Molecular cloning of mouse amino acid transport system B-0, a neutral amino acid transporter related to Hartnup disorder. Journal of Biological Chemistry. 2004;279:24467–24476. doi: 10.1074/jbc.M400904200. [DOI] [PubMed] [Google Scholar]
- Broer A, Tietze N, Kowalczuk S, Chubb S, Munzinger M, Bak LK, Broer S. The orphan transporter v7-3 (slc6a15) is a Na+-dependent neutral amino acid transporter (B0AT2) Biochemical Journal. 2006b;393:421–430. doi: 10.1042/BJ20051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S. The SLC6 orphans are forming a family of amino acid transporters. Neurochemistry International. 2006;48:559–567. doi: 10.1016/j.neuint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Burkovski A, Kramer R. Bacterial amino acid transport proteins: occurrence, functions, and significance for biotechnological applications. Applied Microbiology Biotechnology. 2002;58:265–274. doi: 10.1007/s00253-001-0869-4. [DOI] [PubMed] [Google Scholar]
- Busch W, Saier MH., Jr . The IUBMB-endorsed transporter classification system. In: Yan Q, editor. Membrane Transporters: Methods and Protocols. Methods in Molecular Biology. Totowa, NJ: Humana Press; 2003. p. xii.p. 369. [DOI] [PubMed] [Google Scholar]
- Castagna M, Bossi E, Sacchi VF. Molecular physiology of the insect K-activated amino acid transporter 1 (KAAT1) and cation-anion activated amino acid transporter/channel 1 (CAATCH1) in the light of the structure of the homologous protein LeuT. Insect Molecular Biology. 2009;18:265–279. doi: 10.1111/j.1365-2583.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- Castagna M, Shayakul C, Trotti D, Sacchi VF, Harvey WR, Hediger MA. Molecular characteristics of mammalian and insect amino acid transporters: implications for amino acid homeostasis. Journal of Experimental Physiology. 1997;200(2):269–286. doi: 10.1242/jeb.200.2.269. [DOI] [PubMed] [Google Scholar]
- Castagna M, Shayakul C, Trotti D, Sacchi VF, Harvey WR, Hediger MA. Cloning and characterization of a potassium-coupled amino acid transporter. Proceedings National Academy of Sciences USA. 1998;95:5395–5400. doi: 10.1073/pnas.95.9.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti AC, Deamer DW. Permeability of lipid bilayers to amino acids and phosphate. Biochemical et Biophysical Acta. 1992;1111:171–177. doi: 10.1016/0005-2736(92)90308-9. [DOI] [PubMed] [Google Scholar]
- Chakrabarti AC, Deamer DW. Permeation of membranes by the neutral form of amino acids and peptides: relevance to the origin of peptide translocation. Journal of Molecular Evolution. 1994;39:1–5. doi: 10.1007/BF00178243. [DOI] [PubMed] [Google Scholar]
- Chang AB, Lin R, Keith Studley W, Tran CV, Saier MH., Jr Phylogeny as a guide to structure and function of membrane transport proteins. Molecular Membrane Biology. 2004;21:171–181. doi: 10.1080/09687680410001720830. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Rollins J, Mahowald AP, Bazinet C. Neurotransmitter transporter-like: a male germline-specific SLC6 transporter required for Drosophila spermiogenesis. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0016275. e16275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflügers Archiv – European Journal of Physiology. 2003;29:29. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Chiu C-S, Ross LS, Cohen BN, Lester HA, Gill SS. The transporter-like protein inebriated mediates hyperosmotic stimuli through intracellular signalling. Journal of Experimental Physiology. 2000;203:3531–3546. doi: 10.1242/jeb.203.23.3531. [DOI] [PubMed] [Google Scholar]
- Chye ML, Guest JR, Pittard J. Cloning of the aroP gene and identification of its product in Escherichia coli K-12. Journal of Bacteriology. 1986;167:749–753. doi: 10.1128/jb.167.2.749-753.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. London: Chapman and Hall Press; 1992. 5.3. Nutrition requirements; p. 509. [Google Scholar]
- Closs EI, Boissel JP, Habermeier A, Rotmann A. Structure and function of cationic amino acid transporters (CATs) Journal of Membrane Biology. 2006;213:67–77. doi: 10.1007/s00232-006-0875-7. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Cox TC, Helman SI. Na+ and K+ transport at basolateral membranes of epithelial cells. I. Stoichiometry of the Na, K-ATPase. The Journal of General Physiology. 1986a;87:467–483. doi: 10.1085/jgp.87.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TC, Helman SI. Na+ and K+ transport at basolateral membranes of epithelial cells. II. K+ efflux and stoichiometry of the Na, K-ATPase. The Journal of General Physiology. 1986b;87:485–502. doi: 10.1085/jgp.87.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TC, Helman SI. Na+ and K+ transport at basolateral membranes of epithelial cells. III. Voltage independence of basolateral membrane Na+ efflux. The Journal of General Physiology. 1986c;87:503–509. doi: 10.1085/jgp.87.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Weber RS. Selection costs of amino acid substitutions in ColE1 and ColIa gene clusters harbored by Escherichia coli. Molecular Biology and Evolution. 1998;15:774–776. doi: 10.1093/oxfordjournals.molbev.a025981. [DOI] [PubMed] [Google Scholar]
- Dadd RH. Amino acid requirements of the mosquito Culex pipiens: asparagine essential. Journal of Insect Physiology. 1978;24:25–30. doi: 10.1016/0022-1910(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflügers Archiv – European Journal of Physiology. 2003;7:7. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- Dubrovsky EB, Dubrovskaya VA, Berger EM. Juvenile hormone signaling during oogenesis in Drosophila melanogaster. Insect Biochemical Molecular Biology. 2002;32:1555–1565. doi: 10.1016/s0965-1748(02)00076-0. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflügers Archiv – European Journal of Physiology. 2003;24:24. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- Feldman DH, Harvey WR, Stevens BR. A novel electrogenic amino acid transporter is activated by K+ or Na+, is alkaline pH-dependent, and is Cl−-independent. Journal of Biological Chemistry. 2000;275:24518–24526. doi: 10.1074/jbc.M907582199. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Torrents D, Chillaron J, Martin Del Rio R, Zorzano A, Palacin M. Basolateral LAT-2 has a major role in the transepithelial flux of l-cystine in the renal proximal tubule cell line OK. Journal of the American Society of Nephrology. 2003;14:837–847. doi: 10.1097/01.asn.0000057852.35075.ac. [DOI] [PubMed] [Google Scholar]
- Fiser A, Sali A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymology. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Tavoulari S, Zhang YW, Rudnick G, Honig B. Identification of a chloride ion binding site in Na+/Cl -dependent transporters. Proceedings National Academy of Sciences USA. 2007;104:12761–12766. doi: 10.1073/pnas.0705600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend WG, Dadd RH. Insect nutrition: a comparative perspective. Advances in Nutritional Research. 1982;4:205–247. doi: 10.1007/978-1-4613-9934-6_8. [DOI] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflügers Archiv – European Journal of Physiology. 2003;16:16. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Gavin BA, Arruda SE, Dolph PJ. The role of carcinine in signaling at the Drosophila photoreceptor synapse. PLoS Genetics. 2007;3:e206. doi: 10.1371/journal.pgen.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer BW. Utilization of D-amino acids for growth by Drosophila melanogaster larvae. Journal of Nutrition. 1966;90:31–39. doi: 10.1093/jn/90.1.31. [DOI] [PubMed] [Google Scholar]
- Golberg L, Demeillon B. The nutrition of the larva of aedes aegypti linnaeus. 4. protein and amino-acid requirements. Biochemical Journal. 1948;43:379–387. [PMC free article] [PubMed] [Google Scholar]
- Gordon HT. Quantitative aspects of insect nutrition. American Zoologist. 1968;8:131–138. doi: 10.1093/icb/8.1.131. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Archiv – European Journal of Physiology. 2003;9:9. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Park JH, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proceedings National Academy of Sciences USA. 2004;101:10626–10631. doi: 10.1073/pnas.0403460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Boudko DY, Shiao SH, Voronov DA, Meleshkevitch EA, Drake LL, Aguirre SE, Fox J, Attardo GM, Raikhel AS. AaCAT1 of the yellow fever mosquito, Aedes aegypti: a novel histidine-specific amino acid transporter from the SLC7 family. Journal of Biological Chemistry. 2011;10803:10813. doi: 10.1074/jbc.M110.179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? Journal of Medical Entomology. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Harvey WR, Boudko DY, Rheault MR, Okech BA. NHE(VNAT): an H+ V-ATPase electrically coupled to a Na+:nutrient amino acid transporter (NAT) forms an Na+/H+ exchanger (NHE) Journal of Experimental Physiology. 2009;212:347–357. doi: 10.1242/jeb.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins introduction. Pflügers Archiv – European Journal of Physiology. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Hennigan BB, Wolfersberger MG, Harvey WR. Neutral amino acid symport in larval Manduca sexta midgut brush-border membrane vesicles deduced from cation-dependent uptake of leucine, alanine, and phenylalanine. Biochemical et Biophysical Acta. 1993a;1148:216–222. doi: 10.1016/0005-2736(93)90132-j. [DOI] [PubMed] [Google Scholar]
- Hennigan BB, Wolfersberger MG, Parthasarathy R, Harvey WR. Cation-dependent leucine, alanine, and phenylalanine uptake at pH 10 in brush-border membrane vesicles from larval Manduca sexta midgut. Biochemical et Biophysical Acta. 1993b;1148:209–215. doi: 10.1016/0005-2736(93)90131-i. [DOI] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O’Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Schloss P. Functional coupling of serotonin and noradrenaline transporters. Journal of Neurochemistry. 2003;86:958–965. doi: 10.1046/j.1471-4159.2003.01899.x. [DOI] [PubMed] [Google Scholar]
- House HL. Insect nutrition. Annual Review of Biochemistry. 1962;31:653–672. doi: 10.1146/annurev.bi.31.070162.003253. [DOI] [PubMed] [Google Scholar]
- Huang X, Huang Y, Chinnappan R, Bocchini C, Gustin MC, Stern M. The Drosophila inebriated-encoded neurotransmitter/osmolyte transporter: dual roles in the control of neuronal excitability and the osmotic stress response. Genetics. 2002;160:561–569. doi: 10.1093/genetics/160.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Stern M. In vivo properties of the Drosophila inebriated-encoded neurotransmitter transporter. Journal of Neuroscience. 2002;22:1698–1708. doi: 10.1523/JNEUROSCI.22-05-01698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. American Journal of Physiology-Endocrinology and Metabolism. 2009;296:E603–E613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Kidokoro K, Sezutsu H, Nohata J, Yamamoto K, Kobayashi I, Uchino K, Kalyebi A, Eguchi R, Hara W, Tamura T, Katsuma S, Shimada T, Mita K, Kadono-Okuda K. Deletion of a gene encoding an amino acid transporter in the midgut membrane causes resistance to a Bombyx parvo-like virus. Proceedings National Academy of Sciences USA. 2008;105:7523–7527. doi: 10.1073/pnas.0711841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Knust E, Skaer H. Bloated tubules (blot) encodes a Drosophila member of the neurotransmitter transporter family required for organisation of the apical cytocortex. Developmental Biology. 1999;212:440–454. doi: 10.1006/dbio.1999.9351. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflügers Archiv – European Journal of Physiology. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- Kasuya J, Kaas G, Kitamoto T. Effects of lithium chloride on the gene expression profiles in Drosophila heads. Neuroscience Research. 2009a;64:413–420. doi: 10.1016/j.neures.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya J, Kaas GA, Kitamoto T. A putative amino acid transporter of the solute carrier 6 family is upregulated by lithium and is required for resistance to lithium toxicity in Drosophila. Neuroscience. 2009b;163:825–837. doi: 10.1016/j.neuroscience.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleta R, Romeo E, Ristic Z, Ohura T, Stuart C, Arcos-Burgos M, Dave MH, Wagner CA, Camargo SR, Inoue S, Matsuura N, Helip-Wooley A, Bockenhauer D, Warth R, Bernardini I, Visser G, Eggermann T, Lee P, Chairoungdua A, Jutabha P, Babu E, Nilwarangkoon S, Anzai N, Kanai Y, Verrey F, Gahl WA, Koizumi A. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nature Genetics. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- Kowalczuk S, Broer A, Munzinger M, Tietze N, Klingel K, Broer S. Molecular cloning of the mouse IMINO system: an Na+- and Cl−-dependent proline transporter. Biochemical Journal. 2005;386:417–422. doi: 10.1042/BJ20050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger E, Koraimann G, Vriend G. Increasing the precision of comparative models with YASARA NOVA–a self-parameterizing force field. Proteins. 2002;47:393–402. doi: 10.1002/prot.10104. [DOI] [PubMed] [Google Scholar]
- Kriel J, Haesendonckx S, Rubio-Texeira M, Van Zeebroeck G, Thevelein JM. From transporter to transceptor: signaling from transporters provokes reevaluation of complex trafficking and regulatory controls: endocytic internalization and intracellular trafficking of nutrient transceptors may, at least in part, be governed by their signaling function. BioEssays. 2011;870:879. doi: 10.1002/bies.201100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea AO, Delong DM. Studies on the nutrition of Aedes aegypti larvae; Proceedings of the Tenth International Congress Entomology; 1956. pp. 299–302. [Google Scholar]
- Lea AO, Dimond JB, Delong DM. Role of diet in egg development by mosquitoes (Aedes aegypti) Science. 1956;123:890–891. doi: 10.1126/science.123.3203.890. [DOI] [PubMed] [Google Scholar]
- Linser PJ, Smith KE, Seron TJ, Neira Oviedo M. Carbonic anhydrases and anion transport in mosquito midgut pH regulation. Journal of Experimental Physiology. 2009;212:1662–1671. doi: 10.1242/jeb.028084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflügers Archiv – European Journal of Physiology. 2003;4:4. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Martin JF, Hersperger E, Simcox A, Shearn A. Minidiscs encodes a putative amino acid transporter subunit required non-autonomously for imaginal cell proliferation. Mechanisms of Development. 2000;92:155–167. doi: 10.1016/s0925-4773(99)00338-x. [DOI] [PubMed] [Google Scholar]
- Meleshkevitch E, Voronov D, Miller M, Fox J, Popova L, Boudko D. Novel methionine-selective transporters from the neurotransmitter sodium symporter family. Amino Acids. 2009a;37:84. [Google Scholar]
- Meleshkevitch EA, Assis-Nascimento P, Popova LB, Miller MM, Kohn AB, Phung EN, Mandal A, Harvey WR, Boudko DY. Molecular characterization of the first aromatic nutrient transporter from the sodium neurotransmitter symporter family. Journal of Experimental Physiology. 2006;209:3183–3198. doi: 10.1242/jeb.02374. [DOI] [PubMed] [Google Scholar]
- Meleshkevitch EA, Robinson M, Popova LB, Miller MM, Harvey WR, Boudko DY. Cloning and functional expression of the first eukaryotic Na+-tryptophan symporter, AgNAT6. Journal of Experimental Physiology. 2009b;212:1559–1567. doi: 10.1242/jeb.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annual Review of Entomology. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Miller MM, Popova LB, Meleshkevitch EA, Tran PV, Boudko DY. The invertebrate B(0) system transporter D. melanogaster NAT1, has unique d-amino acid affinity and mediates gut and brain functions. Insect Biochemical Molecular Biology. 2008;38:923–931. doi: 10.1016/j.ibmb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt FC, Ingraham JL, Schaechter M. Physiology of the bacterial cell : a molecular approach. Sunderland, Mass, U.S.A.: Sinauer Associates; 1990. [Google Scholar]
- O’Connor KR, Beyenbach KW. Chloride channels in apical membrane patches of stellate cells of Malpighian tubules of Aedes aegypti. Journal of Experimental Physiology. 2001;204:367–378. doi: 10.1242/jeb.204.2.367. [DOI] [PubMed] [Google Scholar]
- Okech BA, Boudko DY, Linser PJ, Harvey WR. Cationic pathway of pH regulation in larvae of Anopheles gambiae. Journal of Experimental Physiology. 2008a;211:957–968. doi: 10.1242/jeb.012021. [DOI] [PubMed] [Google Scholar]
- Okech BA, Meleshkevitch EA, Miller MM, Popova LB, Harvey WR, Boudko DY. Synergy and specificity of two Na+-aromatic amino acid symporters in the model alimentary canal of mosquito larvae. Journal of Experimental Physiology. 2008b;211:1594–1602. doi: 10.1242/jeb.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacin M, Kanai Y. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflügers Archiv – European Journal of Physiology. 2003;6:6. doi: 10.1007/s00424-003-1062-7. [DOI] [PubMed] [Google Scholar]
- Patrick ML, Aimanova K, Sanders HR, Gill SS. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. Journal of Experimental Physiology. 2006;209:4638–4651. doi: 10.1242/jeb.02551. [DOI] [PubMed] [Google Scholar]
- Payne SH, Loomis WF. Retention and loss of amino acid biosynthetic pathways based on analysis of whole-genome sequences. Eukaryotic Cell. 2006;5:272–276. doi: 10.1128/EC.5.2.272-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres A, Bossi E. Effects of pH on the uncoupled, coupled and pre-steady-state currents at the amino acid transporter KAAT1 expressed in Xenopus oocytes. Journal of Physiology-London. 2000;525:83–89. doi: 10.1111/j.1469-7793.2000.t01-1-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EF. Insect nutrition. Science. 1929;70:168. doi: 10.1126/science.70.1807.168-a. [DOI] [PubMed] [Google Scholar]
- Pinilla J, Aledo JC, Cwiklinski E, Hyde R, Taylor PM, Hundal HS. SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Frontier in Bioscience (Elite Ed) 2011;3:1289–1299. doi: 10.2741/e332. [DOI] [PubMed] [Google Scholar]
- Pullikuth AK, Filippov V, Gill SS. Phylogeny and cloning of ion transporters in mosquitoes. Journal of Experimental Physiology. 2003;206:3857–3868. doi: 10.1242/jeb.00641. [DOI] [PubMed] [Google Scholar]
- Quick M, Stevens BR. Amino acid transporter CAATCH1 is also an amino acid-gated cation channel. Journal of Biological Chemistry. 2001;276:33413–33418. doi: 10.1074/jbc.M104438200. [DOI] [PubMed] [Google Scholar]
- Quick M, Yano H, Goldberg NR, Duan L, Beuming T, Shi L, Weinstein H, Javitch JA. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter:sodium symporter from Fusobacterium nucleatum. Journal of Biological Chemistry. 2006;281:26444–26454. doi: 10.1074/jbc.M602438200. [DOI] [PubMed] [Google Scholar]
- Rawls AS, Schultz SA, Mitra RD, Wolff T. Bedraggled, a putative transporter, influences the tissue polarity complex during the R3/R4 fate decision in the Drosophila eye. Genetics. 2007;177:313–328. doi: 10.1534/genetics.107.075945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflügers Archiv – European Journal of Physiology. 2003;17:17. doi: 10.1007/s00424-003-1087-y. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Roversi P, Laynes R, Kazi S, Boyd CA, Goberdhan DC. Drosophila expresses a CD98 transporter with an evolutionarily conserved structure and amino acid-transport properties. Biochemical Journal. 2009;420:363–372. doi: 10.1042/BJ20082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheault MR, Okech BA, Keen SB, Miller MM, Meleshkevitch EA, Linser PJ, Boudko DY, Harvey WR. Molecular cloning, phylogeny and localization of AgNHA1: the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. Journal of Experimental Physiology. 2007;210:3848–3861. doi: 10.1242/jeb.007872. [DOI] [PubMed] [Google Scholar]
- Ristic Z, Camargo SM, Romeo E, Bodoy S, Bertran J, Palacin M, Makrides V, Furrer EM, Verrey F. Neutral amino acid transport mediated by ortholog of imino acid transporter SIT1/SLC6A20 in opossum kidney cells. American Journal of Physiology – Renal Physiology. 2006;290:F880–F887. doi: 10.1152/ajprenal.00319.2005. [DOI] [PubMed] [Google Scholar]
- Roman G, Meller V, Wu KH, Davis RL. The opt1 gene of Drosophila melanogaster encodes a proton-dependent dipeptide transporter. American Journal of Physiology-Cell Physiology. 1998;44:C857–C869. doi: 10.1152/ajpcell.1998.275.3.C857. [DOI] [PubMed] [Google Scholar]
- Romeo E, Dave MH, Kleta R, Ristic Z, Camargo SMR, Makrides V, Wagner CA, Verrey F. B(0)AT1 (SLC6A19) and other SLC6 orphan transporters: Hartnup disorder and beyond. FASEB Journal. 2005;19:A747. [Google Scholar]
- Romero-Calderon R, Shome RM, Simon AF, Daniels RW, DiAntonio A, Krantz DE. A screen for neurotransmitter transporters expressed in the visual system of Drosophila melanogaster identifies three novel genes. Developmental Neurobiology. 2007;67:550–569. doi: 10.1002/dneu.20342. [DOI] [PubMed] [Google Scholar]
- Saier MH. Genome archeology leading to the characterization and classification of transport proteins. Current Opinion in Microbiology. 1999;2:555–561. doi: 10.1016/s1369-5274(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Saier MH., Jr A functional-phylogenetic classification system for transmembrane solute transporters. Microbiology and Molecular Biology Reviews. 2000;64:354–411. doi: 10.1128/mmbr.64.2.354-411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restraints. Journal of Molecular Biology. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Ross LS, Gill SS. Molecular cloning and functional expression of a proline transporter from Manduca sexta. Insect Biochemistry and Molecular Biology. 2002;32:1391–1400. doi: 10.1016/s0965-1748(02)00059-0. [DOI] [PubMed] [Google Scholar]
- Schmid JA, Scholze P, Kudlacek O, Freissmuth M, Singer EA, Sitte HH. Oligomerization of the human serotonin transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. Journal of Biological Chemistry. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- Seow HF, Broer S, Broer A, Bailey CG, Potter SJ, Cavanaugh JA, Rasko JEJ. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nature Genetics. 2004;36:1003–1007. doi: 10.1038/ng1406. [DOI] [PubMed] [Google Scholar]
- Singh K, Brown A. Nutritional requirements of Aedes aegypti L. Journal of Insect Physiology. 1957;1:199–220. [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Soehnge H, Huang X, Becker M, Whitley P, Conover D, Stern M. A neurotransmitter transporter encoded by the Drosophila inebriated gene. Proceedings National Academy of Sciences USA. 1996;93:13262–13267. doi: 10.1073/pnas.93.23.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Ganetzky B. Identification and characterization of inebriated, gene affecting neuronal excitability in Drosophila. Journal of Neurogenetics. 1992;8:157–172. doi: 10.3109/01677069209083445. [DOI] [PubMed] [Google Scholar]
- Stryer L. Molecular Design of Life. New York: W.H, Freeman and Co.; 1989. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimgan MS, Berg JS, Stuart AE. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. Journal of Experimental Physiology. 2006;209:3383–3404. doi: 10.1242/jeb.02328. [DOI] [PubMed] [Google Scholar]
- Trotschel C, Follmann M, Nettekoven JA, Mohrbach T, Forrest LR, Burkovski A, Marin K, Kramer R. Methionine Uptake in Corynebacterium glutamicum by MetQNI and by MetPS, a novel methionine and alanine importer of the NSS neurotransmitter transporter family. Biochemistry. 2008;47:12698–12709. doi: 10.1021/bi801206t. [DOI] [PubMed] [Google Scholar]
- Vanslambrouck JM, Broer A, Thavyogarajah T, Holst J, Bailey CG, Broer S, Rasko JE. Renal imino acid and glycine transport system ontogeny and involvement in developmental iminoglycinuria. Biochemical Journal. 2010;428:397–407. doi: 10.1042/BJ20091667. [DOI] [PubMed] [Google Scholar]
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Archiv – European Journal of Physiology. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- Verrey F, Ristic Z, Romeo E, Ramadan T, Makrides V, Dave MH, Wagner CA, Camargo SM. Novel renal amino acid transporters. Annual Review of Physiology. 2005;67:557–572. doi: 10.1146/annurev.physiol.67.031103.153949. [DOI] [PubMed] [Google Scholar]
- Vincenti S, Castagna M, Peres A, Sacchi VF. Substrate selectivity and pH dependence of KAAT1 expressed in Xenopus laevis oocytes. Journal of Membrane Biology. 2000;174:213–224. doi: 10.1007/s002320001046. [DOI] [PubMed] [Google Scholar]
- Wieczorek H, Grüber G, Harvey WR, Huss M, Merzendorfer H. The plasma membrane H+-V-ATPase from tobacco hornworm midgut. Journal of Bioenergetics and Biomembranes. 1999;31:67–74. doi: 10.1023/a:1005448614450. [DOI] [PubMed] [Google Scholar]
- Wolfersberger M. Preparation and Partial Characterization of Amino Acid Transporting Brush Border Membrane Vesicles from the Larval Midgut of the Gypsy Moth (Lymantria Dispar) Archive of Insect Biochemistry and Physiology. 1993;24(3):139–147. doi: 10.1002/arch.940240304. [DOI] [PubMed] [Google Scholar]
- Wolfersberger MG. Uniporters, symporters and antiporters. Journal of Experimental Physiology. 1994;196:5–6. doi: 10.1242/jeb.196.1.5. [DOI] [PubMed] [Google Scholar]
- Wolfersberger MG. Amino acid transport in insects. Annual Review of Entomology. 2000;45:111–120. doi: 10.1146/annurev.ento.45.1.111. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Linser PJ, Harvey WR. Antibody to H(+) V-ATPase subunit E colocalizes with portasomes in alkaline larval midgut of a freshwater mosquito (Aedes aegypti) Journal of Experimental Physiology. 1999;202:2449–2460. doi: 10.1242/jeb.202.18.2449. [DOI] [PubMed] [Google Scholar]
- Zomot E, Bendahan A, Quick M, Zhao Y, Javitch JA, Kanner BI. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449:726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]
- Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins. In: Vogel VBaHJ., editor. Evolving Genes and Proteins. New York: Academic Press; 1965. pp. 97–166. [Google Scholar]