Abstract
By the peak of the CD8+ T cell response, the effector cell pool consists of a heterogeneous population of cells that includes both those with an increased propensity to become long-lived memory cells (memory precursor effector cells, MPEC) and those that are terminally differentiated cells (short-lived effector cells, SLEC). Numerous studies have established the critical role that functional avidity plays in determining the in vivo efficacy of CD8+ effector cells. Currently, how functional avidity differs in MPEC versus SLEC and the evolution of this property within these two populations during the expansion and contraction of the response are unknown. The data presented here show that at the peak of the effector response generated following poxvirus infection, SLEC were of higher functional avidity than their MPEC counterpart. Over time however, SLEC exhibited a decrease in peptide sensitivity. This is in contrast to MPEC which showed a modest increase in peptide sensitivity as the response reached equilibrium. The decrease in functional avidity in SLEC was independent of CD8 modulation or the amount of antigen receptor expressed by the T cell. Instead the loss in sensitivity was correlated with decreased expression and activation of ZAP-70 and Lck, critical components of T cell receptor membrane proximal signaling. These results highlight the potential contribution of avidity in the differentiation and evolution of the T cell effector response following viral infection.
INTRODUCTION
A critical aspect of the anti-viral immune response is the ability to generate long-lived memory CD8+ T cells that can rapidly respond following secondary antigen encounter. Recent studies have defined markers that identify effectors with increased potential to become memory cells (1–3). These cells express the IL-7 receptor alpha-chain (CD127), which is upregulated following transient loss as a result of activation. Effector cells with an intrinsically low survival and proliferative potential have been termed short-lived effector cells (SLEC) and are marked by the expression of killer cell lectin-like receptor G1 (KLRG1). Effector cells that express neither have been termed early effector cells (EEC)(4).
While these markers allow identification of cells that differ in their capacity to give rise to memory cells, the properties/signals that result in the differentiation and maintenance of cells along the MPEC versus SLEC pathway are only beginning to be unraveled. Recent studies identified high expression of CD25 as a marker of cells with a propensity to become SLEC (4–6). CD25 was found to be heterogeneously expressed in a transient manner within the responding effector population. The isolation and transfer of CD25high cells at early times postinfection, i.e. prior to selective upregulation of KLRG1 or CD127, revealed that this population preferentially differentiated into SLEC (5). While IL-2 signaling appears to promote differentiation into SLEC, the mechanistic basis for sustained high level CD25 expression is unclear. For example, whether the capacity for high and sustained expression of CD25 is associated with intrinsic properties of those cells or whether it is the result of stochastic encounter with antigen remains to be defined.
The CD8+ T cell response generated following virus infection is an amalgam of a number of individual clones that undergo rapid expansion. This yields a population of cells that are heterogeneous with regard to their functional properties. Heterogeneity within the polyclonal response can take the form of differences in the pattern of cytokines produced as well as the cytolysis exerted in response to TCR engagement. Increased breadth in the effector functions present in responding cells is associated with increased efficacy in vivo (7). An additional attribute that is predictive of efficacy is the sensitivity to peptide antigen, i.e. functional avidity (8–18). Among the polyclonal virus-specific effector population there exist cells that differ substantially in the amount of peptide required in order to induce lysis or secrete cytokines. In vitro cells can be identified that differ in peptide requirement by up to 5-logs (8). The difference in peptide sensitivity among these effectors is likely defined by both intrinsic properties, e.g. TCR affinity, as well as modulation that occurs as a result of peptide encounter, e.g. changes in CD8 level/isoform or differences in the regulation of signaling cascades.
At present, there is no information regarding how peptide sensitivity within the responding effector impacts the differentiation program with regard to SLEC versus MPEC generation. It is reasonable to speculate that interaction of higher avidity cells with APC results in a quantitatively or qualitatively different signal compared to that generated in low avidity cells. Increased signaling in responding cells could lead to increased CD25 expression, as has been reported under conditions of high level TCR engagement (19,20), thereby promoting SLEC commitment in these cells (5).
A number of studies support a role for tuning of TCR signaling in effector cell differentiation. For example, the tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1 (SHP-1), an important negative regulator of TCR signaling, has been shown to play a role in SLEC formation (21). In the absence of SHP-1, SLEC were increased suggesting that indeed augmented or prolonged signals from the TCR can drive an effector cell towards terminal differentiation (21). Thus an attractive model was that cells with intrinsically higher avidity would be more likely to receive strong activating signals and as such, would have an increased propensity to differentiate along the SLEC pathway.
Here we report the results of our studies evaluating the regulation of structural and functional avidity in the MPEC and SLEC populations present following viral infection. Our results suggest that the SLEC versus MPEC differ in peptide sensitivity; however, this is independent of TCR affinity or the level of expression of CD8 and TCR. Further, we find that cells exhibiting the MPEC phenotype during the acute response exhibit a modest increase in avidity during the establishment of the memory response. In contrast SLEC significantly decrease avidity over this same period of time. The decreased avidity observed in SLEC at later times is associated with decreased expression of both Lck and ZAP-70. Together these data suggest that avidity plays a role in the fate of effector cells in vivo following viral infection and that avidity within these the SLEC and MPEC populations evolves as the memory response is established.
MATERIALS AND METHODS
Mice and infections
Six-ten week old C57Bl/6 mice (Frederick Cancer Research Facility, National Cancer Institute, Fredrick, MD) were used throughout this study. Mice were maintained in the Wake Forest University School of Medicine animal facilities, under specific-pathogen-free conditions and in accordance with approved IACUC protocols. Mice received 1×106 plaque-forming units of (PFU) of VACV-GP33 (22) or 2×105 PFU of LCMV Armstrong by intraperitoneal injection.
Tetramer dissociation
A total of 1×106 spleen cells from vaccinia infected mice were stained for one hour at room temperature with B8R tetramer (NIH tetramer core facility, Emory University, Atlanta, GA), KLRG1(Abcam), CD127, and CD44 (Biolegend) antibodies. Cells were then washed and resuspended in media containing 50μg/ml anti-MHC-I antibody (Biolegend) to prevent tetramer rebinding. Cells were incubated at 37°C for the indicated times with additional CD127 antibody added to prevent the apparent loss of MPEC population, which occurred over time due to dissociation of the CD127 antibody. Cells were subsequently washed and fixed prior to staining with CD8α (BD Biosciences) for 30 minutes on ice.
Intracellular cytokine staining and flow cytometry
A total of 1×106 spleen cells from vaccinia virus or LCMV infected mice were cultured for 5 hours in media containing Golgi Plug (BD Biosciences) and graded concentrations of the immunodominant B8R peptide. Cells were then incubated for 30 minutes on ice with CD127, CD44 (Biolegend), KLRG1 (Abcam) and CD8α (BD Biosciences) and in some cases CD8β (Biolegend) antibodies. Following washing, cells were permeabilized using Cytofix/Cytoperm (BD Biosciences) and then stained for IFNγ (BD Biosciences). Samples were acquired on a BD FACSCanto II. 1×106 events were routinely acquired. Data were analyzed using FlowJo software (Tree Star, Inc).
TCR Vβ Analysis
A total of 1×106 spleen cells from vaccinia virus infected mice (d7 p.i.) were stained with antibodies against CD8α, CD44, CD127, KLRG1, and Vβ (2–14) together with B8R tetramer for 30 minutes on ice. Samples were then washed twice and acquired on a BD FACSCanto II. Data were analyzed using FlowJo software (Tree Star, Inc).
Analysis of Lck and ZAP-70 levels
Splenocytes were harvested at d7 or d30 postinfection. Cells were labeled with anti-CD8α, anti-CD44, anti-CD127, anti-KLRG1, and B8R tetramer APC. Following surface staining, cells were fixed with Lyse/Fix buffer (BD Biosciences) for 10 minutes at room temperature. Immediately after, cells were centrifuged and resuspended in 100μl of Cytofix/Cytoperm (BD Biosciences) for 20 minutes on ice. Cells were then stained with PE conjugated anti-Lck antibody (BD Biosciences) or purified anti-ZAP-70 antibody (Cell Signaling) for 20 minutes. In the case of ZAP-70, antibody was detected with an Alexa Fluor 488-conjugated secondary antibody (Molecular Probes). For analysis of phosphorylated protein, CD8+CD44hiKLRG1+ or CD127+ populations were sorted on a FACSAria instrument following enrichment of CD8+ cells using a CD8+ T cell isolation kit II (Miltenyi Biotech). Isolated cells were stained on ice with B8R tetramer and washed with cold FMF. At 1minute following removal from cold conditions, fix/lyse buffer (BD Biosciences) was added and cells incubated for 10 minutes at 37°C. Cells were permeabilized with 90% ice cold methanol for 30 minutes on ice prior to staining with antibodies specific to phosphorylated ZAP-70 (BD Biosciences) or phosphorylated Lck (anti-phospho-src (tyr 416), EMD Millipore). In the case of anti-phospho-src, antibody was detected by addition of an Alexa Fluor 488-conjugated secondary antibody (Molecular Probes). Cells were also analyzed at a later time, 10 minutes. Relative differences among the populations at this time followed the same pattern, although overall levels of phosphorylated molecules had decreased.
Statistical analysis
All significance analysis was performed using a two tailed Student’s t test.
RESULTS
Differentiation of MPEC and SLEC following vaccinia virus infection
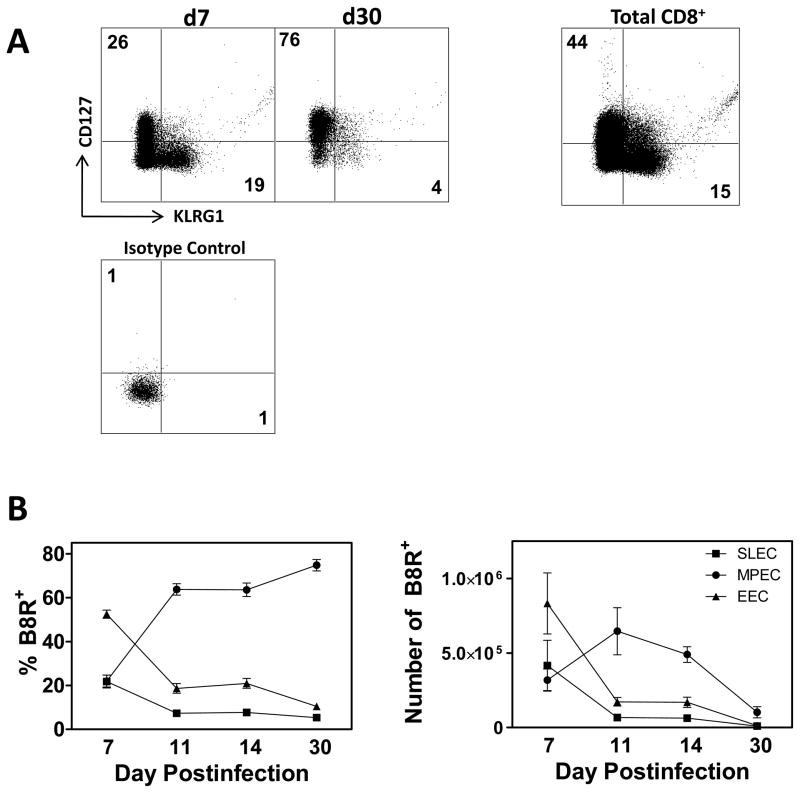
Current data suggest that CD8+ T cells possess the potential to differentiate along both the SLEC and MPEC pathways (23). That said, it is clear that within a polyclonal response a subset of cells will commit to the SLEC pathway whereas others will become memory precursors. Whether peptide sensitivity is associated with one pathway versus another remains to be determined. Prior to addressing this issue, we first established the kinetics of differentiation in our model system of intraperitoneal infection with vaccinia virus (VACV). C57BL/6 mice were infected with 106 PFU of VACV. Infections were staggered to allow for concurrent analysis of the populations present at each timepoint. On d7, 11, 14, and 30 postinfection, spleens were isolated and the frequency and number of EEC, MPEC and SLEC specific for the immunodominant B8R epitope determined by tetramer staining. On d7 following infection, most of the cells present, assessed by both percentage and number were found within the EEC population (Fig. 1). By d11, however, there was a sharp decrease in both the number and percentage of both EEC and SLEC, with a majority now displaying the MPEC phenotype (Fig. 1B). The distribution of effectors among the MPEC and SLEC populations remained relatively constant between days 11 and 30, although absolute numbers were diminishing in all populations. This suggests the three populations are undergoing cell loss throughout this time period. As expected, these data show an early predominance of SLEC compared to MPEC followed by contraction of this population coupled with generation of MPEC as the immune response progresses.
Figure 1. SLEC undergo rapid contraction after the peak of the CD8+ T cell response.
C57Bl/6 mice were infected with 1×106 PFU of VACV and analyzed on d7, 11, 14, and 30 p.i. Infections were staggered to allow for analysis on the same day. Cells are pre-gated on CD8+CD44+ cells. (A) Representative plots showing the frequency of B8R tetramer+ cells that express either CD127 (MPEC) or KLRG1 (SLEC). KLRG1+ and CD127+ staining on the total CD8+ population is shown for comparison. (B) Frequency (left panel) and number (right panel) of B8R tetramer+ CD8+ T cells that are MPEC (CD127+), SLEC (KLRG1+), or EEC (double negative). All data are a mean and SEM of at least 6 mice/group from at least 2 independent experiments.
Initial differentiation of effector cells along the MPEC versus SLEC pathways is impacted by Vβ usage
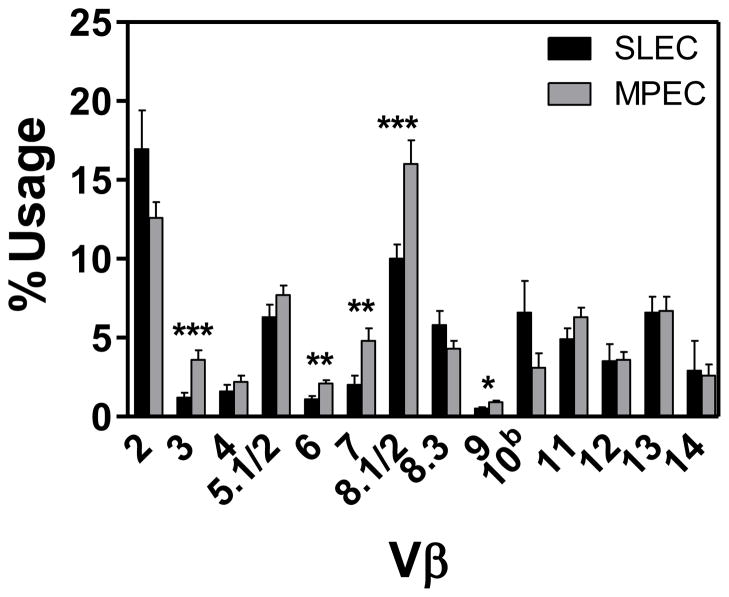
The effector pool generated as a result of infection is the result of the recruitment and expansion of distinct naïve precursors. Although a single cell can give rise to both MPEC and SLEC (23), it is not clear whether there are differences with regard to the propensity to do so in the context of a polyclonal response. We used TCR Vβ analysis to follow the differentiation of cells within the polyclonal response. We reasoned that if individual clones differed in their propensity to give rise to SLEC vs. MPEC that this may be reflected in the Vβ distribution among the various effector cell populations. We first tested whether Vβ usage correlated with the differentiation into SLEC vs. MPEC. We assessed the responses on d7 postinfection, when the effector population was at its peak and both SLEC and MPEC populations were readily detected. As above, B8R-specific SLEC and MPEC were identified by staining with KLRG1 and CD127 antibodies, respectively. While Vβ2 and Vβ8.1/2 were the dominant regions utilized by both MPEC and SLEC populations, comparison of the usage across the repertoire for the MPEC and SLEC populations revealed a bias in the usage of Vβ3, 6, 7, 8.1/2, and 9, with these regions being more highly represented within the MPEC populations (Fig. 2). These data are consistent with nonrandom selection within the effector population with regard to differentiation into SLEC vs. MPEC.
Figure 2. TCR Vβ usage following VACV infection.
Spleens were harvested on d7 following infection with 1×106 PFU of VACV. TCR Vβ analysis of B8R-specific SLEC or MPEC was determined using a panel of Vβ-specific antibodies. Data shown are the mean and SEM from four independent experiments each with 3 mice. Mean and SEM are shown. *, p≤0.05; **, p≤0.01; ***, p≤0.002.
SLEC present at d7 are of higher avidity than MPEC
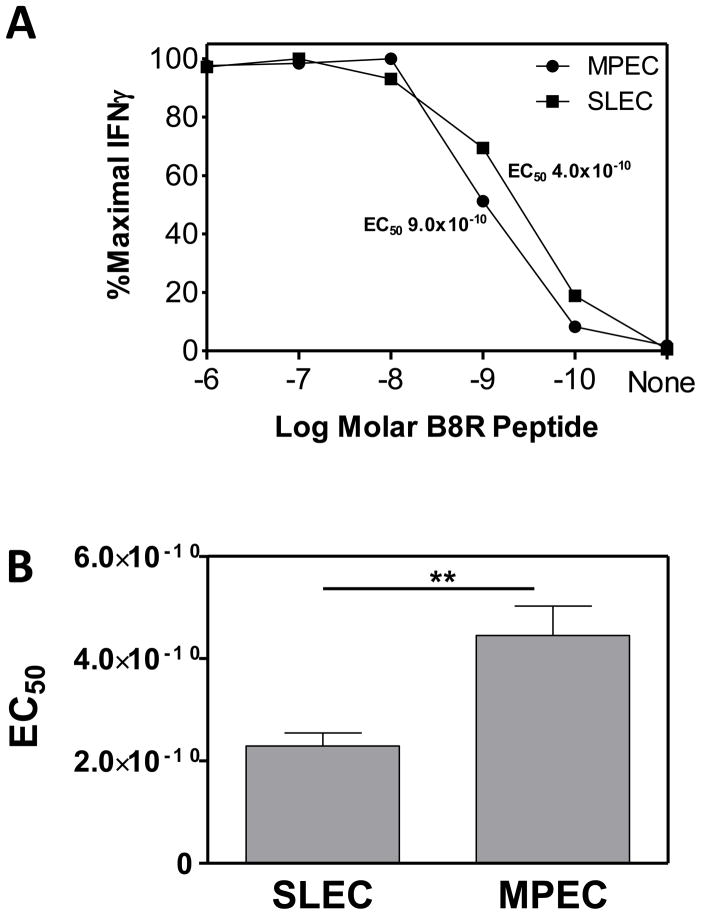
Given that avidity may influence the number of antigen contacts that a cell receives, coupled with our understanding that repeated antigen contact can drive a cell towards a more terminally differentiated phenotype, we tested the hypothesis that functional avidity could contribute to the differentiation fate of an effector cell. To determine if functional avidity differed within MPEC and SLEC populations, splenocytes were isolated at the peak of the effector response, i.e. d7 postinfection, and stimulated with titrated concentrations of B8R peptide. The peptide dose response curves for MPEC and SLEC from a representative animal (Fig. 3A) as well as averaged data (Fig. 3B) are shown. We found a similar increase in the sensitivity of SLEC vs. MPEC in the mediastinal lymph node at d7 p.i. (supplemental Figure 1). Interestingly cells in the periphery, i.e. the lung, showed no skewing in SLEC towards higher avidity (supplemental Fig. 1). This may reflect selection for SLEC with inherently lower avidity for either entry into or survival within this tissue. Alternatively it may reflect regulation of these cells in the lung. This possibility is intriguing given the negative regulation of effector cells that has been reported in this tissue (24–27). Together these analyses revealed a reproducible and significant difference in functional avidity between SLEC and MPEC populations in lymphoid organs at early times postinfection, with SLEC exhibiting increased sensitivity to peptide antigen compared to their MPEC counterpart.
Figure 3. MPEC and SLEC differ in functional avidity on d7 p.i.
C57Bl/6 mice were infected with 1×106 PFU of VACV and on d7 p.i. avidity was assessed by stimulation with titrated concentrations of B8R peptide. IFNγ production was determined by ICCS. Data from a representative mouse are shown in (A). (B) Average EC50 for MPEC vs. SLEC (n=31). The EC50 represents the amount of peptide needed to achieve the 50% maximal percentage of IFNγ producing cells. **, p≤0.01.
SLEC and MPEC diverge in functional avidity over the course of the response
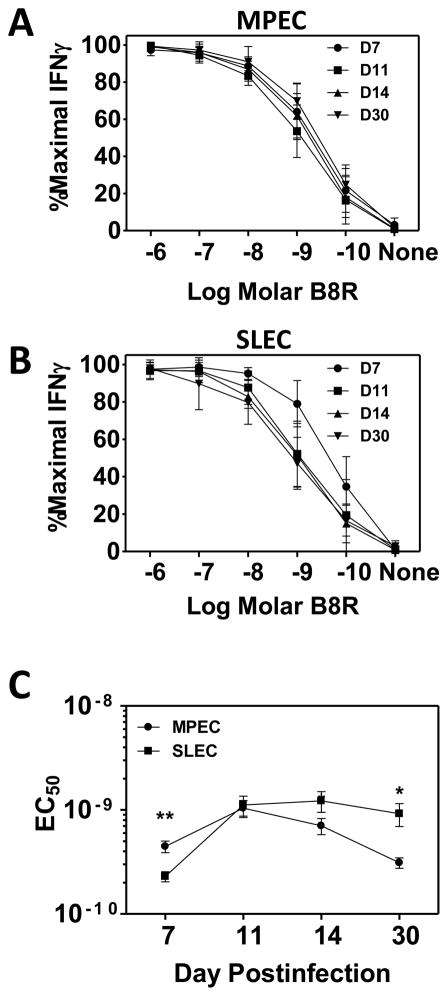
We next tested the possibility that avidity was altered over time as MPEC and SLEC populations evolved and contracted (d14 p.i. and beyond). To assess this possibility, we analyzed the functional avidity within the MPEC and SLEC populations between d7 and d30 p.i (Fig. 4, representative primary data are shown in supplemental Fig. 2). MPEC demonstrated a significant, although modest increase in avidity between the peak of the MPEC response (d11) versus post-contraction (d30), requiring 3.3-fold less peptide for production of IFNγ (Fig. 4A and C). These findings show a movement towards higher avidity within the MPEC population. In contrast, SLEC exhibited a significant decrease in avidity between their peak response at d7 and d30 (Fig. 4B). SLEC present at d30 required approximately 4-fold more peptide required to elicit effector function compared SLEC present at d7 (Fig. 4C). The evolution of avidity over time within the two populations resulted in a 3.1-fold difference in avidity between the MPEC and SLEC present at d30, with MPEC now being the higher avidity population.
Figure 4. Late stage SLEC exhibit decreased functional avidity.
C57Bl/6 mice were infected with 1×106 PFU of VACV. Infections were staggered to allow for concurrent analysis. On d7, 11, 14 and 30 following infection, spleens were harvested and cells stimulated with titrated concentrations of B8R peptide. Results are expressed as a percentage of the maximum observed following stimulation with 10−6M peptide. Dose response curves for MPEC (A) and SLEC (B) at d7, 11, 14, or 30 following infection. (C) Average EC50 for MPEC and SLEC populations over time. All data are the mean of at least 9 mice analyzed in 3 independent experiments. Mean and SEM are shown. *, p≤0.05; ***, p≤0.001.
Changes in functional avidity within MPEC or SLEC populations over time are not associated with altered TCR levels
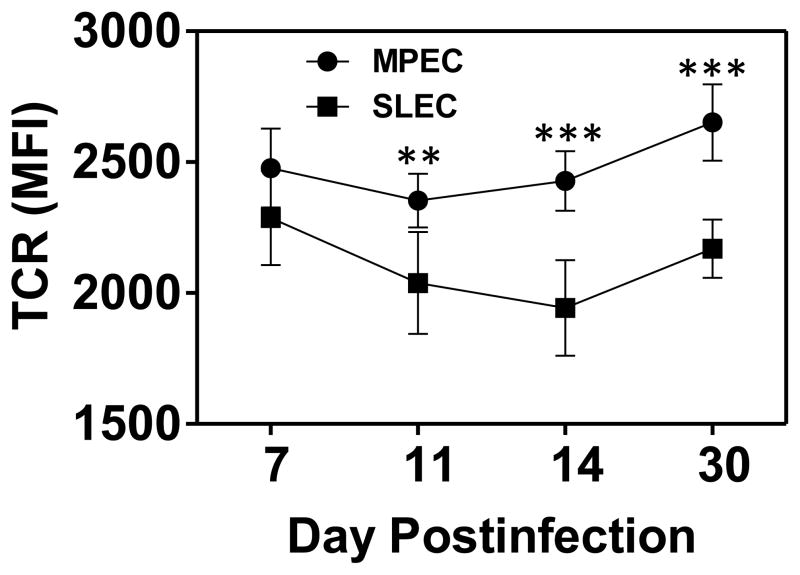
We were interested in the mechanism responsible for the differences in avidity present at the various times postinfection. As a first step, we tested the possibility that changes in the level of TCR expression correlated with the observed differences in peptide sensitivity. Splenocytes were isolated at d7-30 p.i. and stained with antibodies specific to CD8, CD44, CD127, KLRG1, TCRβ, along with tetramer. As shown in figure 5, TCR levels on B8R-specific cells were similar within the SLEC populations across all timepoints. This was also the case for MPEC. Thus changes in the level of TCR over time could not account for the changes in peptide sensitivity that occurred in the MPEC or SLEC population over time. There were differences in the level of TCR when comparing MPEC to SLEC populations at days 11–14, with MPEC expressing higher levels of TCR compared to SLEC. However, when comparing the avidity of the two populations over time, there was not a consistent correlation with avidity.
Figure 5. Time-dependent changes in functional avidity are independent of TCR levels.
TCR levels were measured on B8R tetramer+ MPEC and SLEC at d7, 11, 14, and 30 following infection by staining with a pan TCRβ antibody. Significant differences were observed between MPEC and SLEC on days 11–30. **, p≤0.01; ***, p≤0.001.
Changes in functional avidity are not associated with altered structural avidity
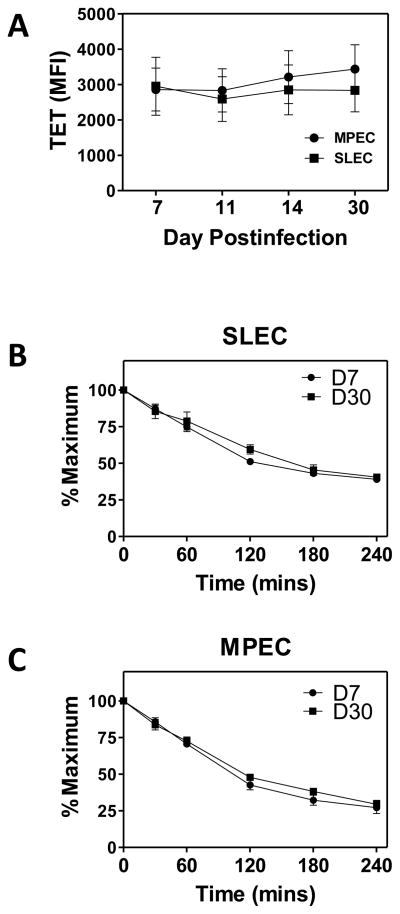
We then tested the possibility that, as the response evolved, there was a selection for T cells bearing receptors with different affinity, i.e. lower in the case of SLEC or higher in the case of MPEC or that changes occurred in the organization of the TCR within the membrane that altered the ability to interact efficiently with the APC. Either of these would change the ability of cells to bind and retain tetramer. We first determined the level of tetramer binding within the SLEC and MPEC populations at d7, 11, 14, and 30 p.i. (Fig. 6A). No significant differences were found in the intensity of tetramer staining either over the course of the response for MPEC or SLEC or between the two cell types.
Figure 6. Changes in functional avidity are independent of structural avidity.
Structural avidity in B8R-specific MPEC and SLEC was assessed by tetramer binding and dissociation. (A) Tetramer binding of SLEC and MPEC at saturating conditions. For tetramer dissociation analyses, cells were stained with tetramer, washed, and resuspended in media containing anti-MHC I antibody. Samples were incubated at 37°C for 0.5, 1, 2, 3 or 4 hours with additional anti-CD127, followed by staining with anti-CD8 antibody. The level of tetramer binding as a percent of the 0h value is shown for SLEC (B) and MPEC (C). Data are the average of 6 mice analyzed in two independent experiments.
Although the level of tetramer binding under saturating conditions was similar, a more sensitive approach for measuring TCR affinity/structural avidity is found in the analysis of tetramer dissociation. Splenocytes were isolated at d7 or d30 postinfection and stained for 1 hour at room temperature with B8R tetramer together with CD127, KLRG1 and CD44 antibodies. Cells were then washed and resuspended in media containing anti-MHC class I antibodies to prevent rebinding of tetramer during the dissociation period. To assess loss of tetramer binding, cells were incubated at 37°C, conditions under which high affinity TCR clones selectively maintain tetramer binding. CD127 antibody was included during the dissociation period to optimize detection of MPEC, as we had noted in initial studies that there was a time dependent loss in MPEC during the assay period due to CD127 antibody dissociation. At the indicated times over a 4 hour period, samples were fixed and subsequently stained with antibody to CD8α. No significant difference in tetramer dissociation in SLEC (Fig. 6B) or MPEC (Fig. 6C) present at d7 versus d30 was observed. Of note, there was also no difference when comparing dissociation in MPEC vs. SLEC. Thus changes in structural avidity could not account for the altered functional avidity.
Contribution of CD8 to time-dependent changes in functional avidity in SLEC
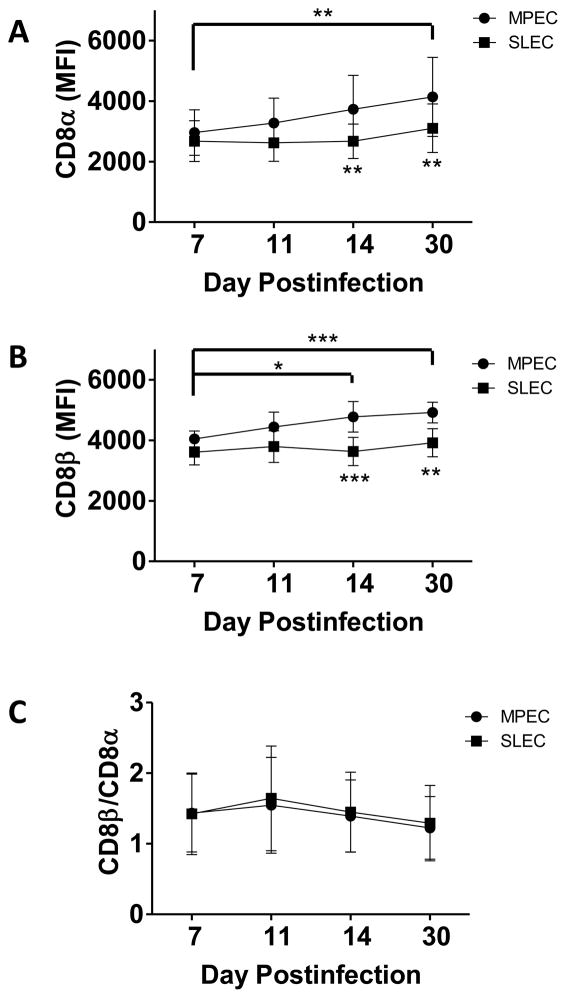
CD8 is expressed on the cell surface as either a heterodimer consisting of αβ chains or as an αα homodimer. A number of studies have indicated that expression of the heterodimeric form of CD8 is associated with increased sensitivity to peptide antigen (28–30). In addition, we have previously shown that an increased ratio of CD8β:α staining is associated with higher avidity (29). Based on the potential for changes in either the absolute level or isoform of CD8 to alter avidity, we investigated CD8 regulation as a mechanism to account for the changes in avidity observed in MPEC and SLEC over time. In SLEC, neither CD8α (Fig. 7A), nor CD8β (Fig. 7B) levels were significantly modulated over the time course assessed. In contrast, the level of both CD8α and β increased significantly over time in the MPEC population. Evaluation of the ratio of CD8β:CD8α did not reveal significant changes, suggesting that the representation of CD8αβ vs. αα was constant (Fig. 7C). This increase in CD8 may contribute to the increased peptide sensitivity observed in MPEC present at later times postinfection.
Figure 7. Altered avidity in MPEC, but not SLEC, present at d30 correlates with CD8 levels.
C57Bl/6 mice were infected with 1×106 PFU of VACV and on d7, 11, 30, or 30 p.i. B8R-specific MPEC and SLEC were analyzed. Levels of CD8α (A) and CD8β (B) as well as the ratio of CD8β:α (C) are shown. Data are the mean and SEM from 9 animals analyzed in 3 independent experiments. Asterisks below symbols refer to comparisons between MPEC and SLEC populations at a given timepoint. Connected bars with asterisks denote significant changes in CD8 expression on MPEC at that time compared to d7. There were no significant changes in CD8 expression in SLEC. *, p≤0.05; **, p≤0.01; ***, p≤0.001.
D30 SLEC exhibit reduced levels of both total and activated Lck and ZAP-70 protein
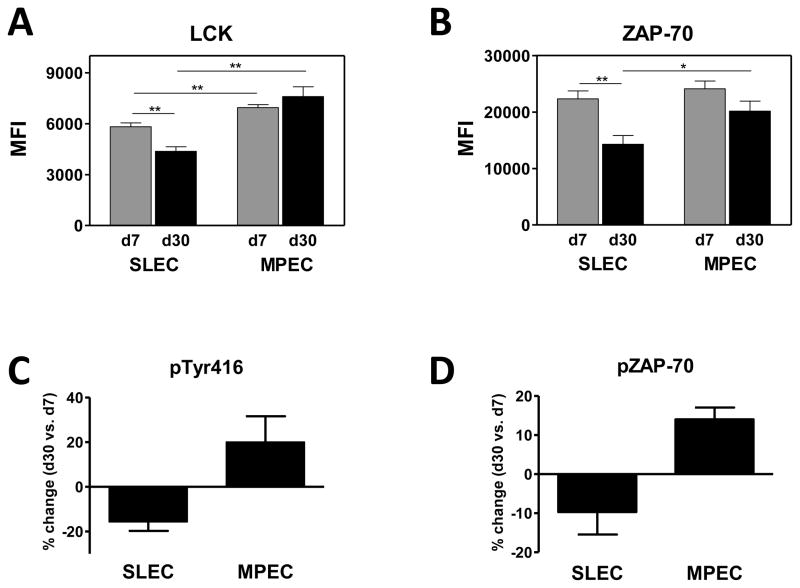
We next tested the hypothesis that differences in the expression of signal transduction molecules could account for the reduced responsiveness of late stage SLEC. Splenocytes were isolated on d7 or d30 postinfection and the presence of MPEC and SLEC within the B8R specific population identified by antibody staining. The level of Lck and ZAP-70, two membrane proximal molecules involved in TCR signaling, was analyzed. Firstly, we noted that the level of Lck was modestly higher (1.2 fold) in MPEC versus SLEC at d7 postinfection. In contrast to Lck, levels of ZAP-70 did not differ between these early populations.
We next assessed whether there were changes in the expression of these two molecules over time in MPEC vs. SLEC. We found that MPEC present at d30 did not exhibit significant differences in either Lck or ZAP-70 compared to their d7 counterpart (Fig. 8A and B). In contrast, SLEC showed a substantial and significant decrease in both Lck and ZAP-70 levels at d30 compared to d7. On average, Lck levels were decreased 25% while ZAP-70 levels were decreased 36%. This is not reflective of a generalized decrease in protein levels in d30 SLEC, as no change in CD8 or TCR expression was detected in this population between d7 and d30 (Fig. 5 and 7).
Figure 8. Decreased avidity in d30 SLEC correlated with reduced levels of total and activated Lck and ZAP-70.
B8R-specific MPEC and SLEC present at d7 and d30 were analyzed for the expression of Lck (A) and ZAP-70 (B). Data represent the average MFI from 8–9 individual animals. For the analysis of phosphorylated molecules, CD8+CD44hiKLRG1+ or CD127+ cells were isolated by sorting. Cells were then incubated with tetramer to both identify the cells as well as initiate TCR signaling. Tetramer+ cells were analyzed following staining with phosphosrc-Tyr416 (C) or phospho-ZAP70 antibody (C). Data represent a total of 9 animals analyzed in 3 independent experiments. *, p≤0.05; **, p≤0.001.
The decreased levels of protein suggested that the level of activated Lck and ZAP-70 would be reduced in these cells following TCR engagement. To directly test this possibility KLRG1+ and CD127+ populations present on d7 and d30 were sorted and incubated with tetramer. Sorting was performed as our preliminary studies showed that KLRG1 and CD127 staining were lost during the permeabilization procedure, prohibiting identification of SLEC within the bulk population following permeabilization. The use of tetramer allowed both detection of antigen-specific cells as well as stimulation through the TCR. We analyzed these responses by comparing the level of phosphorylated molecules in d30 B8R-specific SLEC or MPEC populations to those at d7. Changes in the positive direction represent increased levels of phosphorylated molecules in the population present at d30, while a negative value represents a decreased level of phosphorylated protein. The data in figures 8C and D show that B8Rspecific SLEC present at d30 have reduced levels of phosphorylated Lck and ZAP-70 compared to the cells at d7, in agreement with changes in the total protein level. In contrast MPEC exhibited an increase in the levels of phospho-Lck and phospho-ZAP-70. This was intriguing given that we saw no significant increase in total protein and suggests augmented signaling efficiency in these cells. Together these data are consistent with a model wherein the decreased sensitivity in SLEC present at later times is a result of decreased signaling in the cells.
Avidity within the MPEC and SLEC is regulated in an epitope dependent fashion
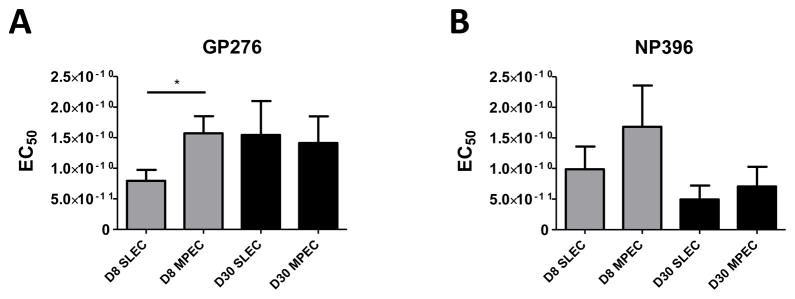
The preceding data establish the differential regulation of avidity within the VACV B8R-specific effector population during the acute response as well at later times as memory is established. We sought to determine whether the patterns observed for the B8R-specific response reflected general patterns with regard to effector cells or whether there were epitope-specific or virus-specific factors that regulated this process. To address this question, we evaluated the response to two epitopes (NP396–404 and GP276–286) at d8 and d30 postinfection intraperitoneal infection with the Armstrong strain of LCMV. Day 8 was chosen as this is the timepoint of the peak response. Figure 9 shows SLEC specific for the GP276 epitope present at d8 p.i. were of significantly higher avidity compared to MPEC, similar to what was observed for the B8R response. There was a similar trend with the NP396-specific response, although this did not reach statistical significance. Interestingly, the changes observed during the period of contraction and memory generation were variable when compared within the LCMV response as well as across infections, i.e. the vaccinia virus-specific response. While on average GP276-specific SLEC present at d30 required more peptide compared to their d7 counterpart, d30 NP396-specific SLEC trended towards increased peptide sensitivity. We also did not detect significant decreases in the peptide requirement of MPEC present at d30 vs. d7 for either epitope (Fig. 9). Thus while SLEC appear to generally be skewed toward high avidity during the acute response, the regulation of avidity within the effector population over time is dependent on the epitope assessed.
Figure 9. SLEC present at d7 following infection with LCMV are of higher avidity than MPEC.
Mice were infected i.p. with 2×105 PFU of LCMV Armstrong. On d8 or d30 p.i. avidity within the GP276–286 (A) and NP396–404 (B) specific MPEC and SLEC populations was assessed by measuring the production of IFNγ following stimulation with titrated concentrations of peptide. Data are the average of at least 8 mice analyzed in 3 three independent experiments. *, p≤0.05
DISCUSSION
Following activation, T cells undergo a process of differentiation during which a percentage of effectors acquire the potential to become long-lived memory cells. Recently, our understanding of the markers associated with this fate decision has increased substantially. For example, the earliest marker of cells that will become memory appears to be the reduced expression of CD25 (5). Subsequently these cells will re-express CD127, which promotes long term survival and proliferation via IL-7 signaling. These capabilities are dictated, at least in part, by the expression of the transcription factor Eomes (31–33). In contrast, cells with reduced memory potential express T-bet and Blimp-1 (1,33–35). Although these studies highlight the processes by which cells undergo differentiation along the SLEC or MPEC pathways, the mechanism through which cells initially choose one fate vs. the other and the factors that promote retention in the repertoire as well as function following this process remain relatively unknown.
In the current studies we evaluated the functional and structural avidity of effectors present following poxvirus infection. We observed a statistically significant increase in the amount of peptide required by MPEC versus SLEC present at d7 postinfection, demonstrating that cells which had differentiated into SLEC possessed higher functional avidity. Two models could account for the increased avidity observed in SLEC. Firstly, an increase in the quality or quantity of signal that occurs, for example as a result of a higher avidity interaction with a target cell, may induce differentiation into SLEC. There is evidence to suggest that the nature of the signal that results from TCR engagement can impact cell fate decisions, with prolonged or sustained signaling driving SLEC generation (21,36). For example, antigen specific cells lacking SHP-1, a key negative regulator of TCR signaling have more SLEC present at the peak of the T cell response when compared to their wild type counterparts (21). Further, increasing the level of presented peptide, which likely increases signaling in a quantitative manner can promote SLEC generation in some (36), but not all cases (1). Finally, stronger and/or increased antigen contact drives CD25 expression (19,20), a known contributor to SLEC differentiation. Differences in TCR usage within the two populations would be consistent with the selection of a subpopulation of cells that possess intrinsic differences in antigen recognition, thereby selecting them for differentiation along the SLEC pathway. That said, the selection of these cells appears independent of structural avidity and CD8 levels, consistent with a model wherein differences in avidity are dictated by variations in signaling capabilities among the individual clones.
An alternative model to explain the difference in avidity is that the program associated with SLEC differentiation results in increased avidity. While signal transduction in SLEC vs. MPEC has not been directly compared, expression analysis did reveal the presence of a limited number of signaling molecules that exhibited increased expression in SLEC compared to MPEC (37). Among these were potential regulators of NFκB (MALT1) and ZAP-70 (Tyrobp) activation. In addition, it is clear that the organization of molecules within the membrane can have profound effects on T cell signaling and activation (e.g. (38–41)). Thus, an attractive possibility for increased sensitivity is the reorganization of molecules (both receptor and membrane associated cytoplasmic proteins) within lipid rafts resulting in optimal signal transduction.
In addition to these early differences in avidity that were observed as cells differentiated into SLEC vs. MPEC following VACV infection, we also detected changes in peptide sensitivity within each population over the course of the response. While MPEC increased their functional avidity over time, SLEC exhibited decreased avidity. This is intriguing given that SLEC were initially the higher avidity cell type. At the population level, effector T cells have been shown to undergo marked changes in functional avidity over the course of the response, albeit the large majority of the previously reported changes occurred prior to the peak of the response (42–45). Altered avidity at the population level can result from either selective survival of a subset of effector cells or from a global change in all effectors. At present, our data do not allow us to discriminate between these two possibilities. Since the number of cells present at the later times is decreased in both SLEC and MPEC, it is possible that the effector population present at the late times p.i. reflects a subset of cells with increased survival potential. While TCR analysis at d30 could shed light on this process, the decreased number of antigen specific SLEC made assessment of Vβ usage at this timepoint infeasible. For MPEC, the increased phosphorylation of ZAP-70 and Lck observed in d30 vs. d7 MPEC suggests that if there is selective survival of a subset of cells, those with increased signaling capabilities are at an advantage.
In contrast to selective survival of cells with intrinsic differences, an alternative model to account for changes in avidity over time is active regulation of avidity within individual cells. Such a scenario is consistent with our previous in vitro data demonstrating the ability of individual cells to actively modulate avidity following multiple antigen encounters (46). In further support of active modulation of as a means to control avidity, a study by Whitton and colleagues reported changes in functional avidity independent of changes in structural avidity or TCR affinity (42). One mechanism that appears to account for modulation is a change in the isoform or level of CD8 (29,30,46,47). We did observe a significant increase in the expression of both CD8α and β in MPEC over time that was associated with increased avidity. Overall higher expression of CD8 (reflected by increased expression both CD8α and β) may be an attribute of MPEC that have a survival advantage. Alternatively, cells may actively upregulate CD8 as they differentiate towards bona fide memory cells. In our studies, increased CD8 in MPEC was associated with augmented levels of both phosphorylated Lck and ZAP-70. Thus, increased CD8 levels appear to promote improved efficiency with regard to the initiation of TCR signaling.
While CD8 modulation may contribute to the regulation of avidity in MPEC, it cannot account for the changes observed in SLEC over time. This lower avidity population did not exhibit decreases in CD8, nor were there changes in TCR levels or tetramer dissociation compared to their d7 counterpart. Instead, the change in functional avidity in these cells was associated with decreased levels of two critical components of the membrane proximal signaling machinery, ZAP-70 and Lck. The decreased protein levels were coupled with decreased amounts of phosphorylated protein. Increases in Lck have been previously linked to increases in avidity (42). However, this is the first report to our knowledge that suggests active modulation in ZAP-70 by effectors as a mechanism to control the sensitivity to peptide antigen in vivo. While the induction of decreased peptide sensitivity or nonresponsiveness in CD8+ T cells in vivo has been reported, this does not appear to be regulated by modulation of Lck and ZAP-70 levels (48,49). Thus, this mechanism may be selectively utilized by SLEC to downregulate antigen sensitivity.
In summary, our results are consistent with a model wherein TCR usage and/or avidity are potential contributors to the initial fate decision in CD8+ T cell effectors. In support of this we found biased TCR usage as well as higher avidity in SLEC present at early times following virus infection compared to their MPEC counterpart. The peptide sensitivity of an effector plays a critical role in determining the efficiency of viral clearance. The lower avidity observed in MPEC at early times may be a mechanism to promote survival of these cells into the memory pool. Differences in avidity may be only one weapon in the arsenal of these effectors that reduces the likelihood that MPEC continue to engage antigen. This may contribute to the increased survival of these cells long-term, allowing them to populate a memory pool that can respond efficiently upon antigen re-encounter. Avidity was not static in MPEC and SLEC populations over time. B8R-specific SLEC generated following poxvirus infection showed progressively decreased avidity while MPEC increased avidity. These changes may reflect continued antigen encounter by SLEC which drives them toward decreased peptide sensitivity or death, effectively eliminating them from the secondary response. Deleting these highly sensitive SLEC may serve as a way for the immune system to protect against damage as the host recovers from infection. In contrast, increased sensitivity of MPEC may result in a memory population that has increased efficacy upon secondary virus encounter. Extension of our findings to another viral model, LCMV, suggested regulation of avidity over time within SLEC and MPEC populations occurs in an epitope dependent fashion. This result points out the importance of understanding how this process is regulated. The flexibility with regard to the regulation of avidity within the effector population opens the door to interventions that would allow modulation of avidity within the population as is desired in a given context, e.g. the decrease in avidity in the context of autoreactive cells.
Supplementary Material
Acknowledgments
We thank Dr. Karen Haas for helpful comments regarding this manuscript. We thank the NIH Tetramer Core Facility for provision of tetramer.
Reference List
- 1.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 3.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 8.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 10.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 11.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedlik C, Dadaglio G, Saron MF, Deriaud E, Rojas M, Casal SI, Leclerc C. In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity. J Virol. 2000;74:5769–5775. doi: 10.1128/jvi.74.13.5769-5775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neveu B, Debeaupuis E, Echasserieau K, Moullac-Vaidye B, Gassin M, Jegou L, Decalf J, Albert M, Ferry N, Gournay J, Houssaint E, Bonneville M, Saulquin X. Selection of high-avidity CD8 T cells correlates with control of hepatitis C virus infection. Hepatology. 2008;48:713–722. doi: 10.1002/hep.22379. [DOI] [PubMed] [Google Scholar]
- 14.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, Costagliola D, Rouzioux C, Agut H, Marcelin AG, Douek D, Autran B, Appay V. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A, Larsen M, Pancino G, Douek DC, Autran B, Saez-Cirion A, Appay V. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113:6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kattan T, MacNamara A, Rowan AG, Nose H, Mosley AJ, Tanaka Y, Taylor GP, Asquith B, Bangham CR. The avidity and lytic efficiency of the CTL response to HTLV-1. J Immunol. 2009;182:5723–5729. doi: 10.4049/jimmunol.0900069. [DOI] [PubMed] [Google Scholar]
- 17.Bullock TNJ, Mullins DW, Colella TA, Engelhard VH. Manipulation of avidity to improve effectiveness of adoptively transferred CD8+ T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J Immunol. 2001;167:5824–5831. doi: 10.4049/jimmunol.167.10.5824. [DOI] [PubMed] [Google Scholar]
- 18.Speiser DE, Kyburz D, Stubi U, Hengartner H, Zinkernagel RM. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J Immunol. 1992;149:972–980. [PubMed] [Google Scholar]
- 19.Cai Z, Brunmark A, Jackson MR, Loh D, Peterson PA, Sprent J. Transfected Drosophila cells as a probe for defining the minimal requirements for stimulating unprimed CD8+ T cells. Proc Natl Acad Sci USA. 1996;93:14736–14741. doi: 10.1073/pnas.93.25.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlin M, Sandalova E, Masucci MG, Levitsky V. Help signals provided by lymphokines modulate the activation and apoptotic programs induced by partially agonistic peptides in specific cytotoxic T lymphocytes. Eur J Immunol. 2005;35:2929–2939. doi: 10.1002/eji.200526330. [DOI] [PubMed] [Google Scholar]
- 21.Fowler CC, Pao LI, Blattman JN, Greenberg PD. SHP-1 in T cells limits the production of CD8 effector cells without impacting the formation of long-lived central memory cells. J Immunol. 2010;185:3256–3267. doi: 10.4049/jimmunol.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitton JL, Sheng N, Oldstone MB, McKee TA. A “string-of-beads” vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Arimilli S, Sharma SK, Yammani R, Reid SD, Parks GD, Alexander-Miller MA. Pivotal Advance: Nonfunctional lung effectors exhibit decreased calcium mobilization associated with reduced expression of ORAI1. J Leukoc Biol. 2010 doi: 10.1189/jlb.0809575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray PM, Arimilli S, Palmer EM, Parks GD, Alexander-Miller MA. Altered function in CD8+ T cells following paramyxovirus infection of the respiratory tract. J Virol. 2005;79:3339–3349. doi: 10.1128/JVI.79.6.3339-3349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 27.Fulton RB, Olson MR, Varga SM. Regulation of cytokine production by virus-specific CD8 T cells in the lungs. J Virol. 2008;82:7799–7811. doi: 10.1128/JVI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaki S, Tanabe M, Nakauchi H, Takiguchi M. Beta-chain broadens range of CD8 recognition for MHC class I molecule. J Immunol. 1992;149:1613–1618. [PubMed] [Google Scholar]
- 29.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: Correlation with CD8αβ versus CD8αα expression. J Immunol. 2001;167:2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 30.Kroger CJ, Alexander-Miller MA. Dose-dependent modulation of CD8 and functional avidity as a result of peptide encounter. Immunology. 2007;122:167–178. doi: 10.1111/j.1365-2567.2007.02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 32.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 33.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Obar JJ, Lefrancois L. Early events governing memory CD8+ T-cell differentiation. Int Immunol. 2010;22:619–625. doi: 10.1093/intimm/dxq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croom HA, Denton AE, Valkenburg SA, Swan NG, Olson MR, Turner SJ, Doherty PC, Kedzierska K. Memory precursor phenotype of CD8+ T cells reflects early antigenic experience rather than memory numbers in a model of localized acute influenza infection. Eur J Immunol. 2011;41:682–693. doi: 10.1002/eji.201040625. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cawthon AG, Kroger CJ, Alexander-Miller MA. High avidity CD8+ T cells generated from CD28-deficient or wildtype mice exhibit a differential dependence on lipid raft integrity for activation. Cell Immunol. 2004;227:148–155. doi: 10.1016/j.cellimm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Drake DR, III, Braciale TJ. Cutting edge: lipid raft integrity affects the efficiency of MHC class I tetramer binding and cell surface TCR arrangement on CD8+ T cells. J Immunol. 2001;166:7009–7013. doi: 10.4049/jimmunol.166.12.7009. [DOI] [PubMed] [Google Scholar]
- 40.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 41.Horejsi V. The roles of membrane microdomains (rafts) in T cell activation. Immunol Rev. 2003;191:148. doi: 10.1034/j.1600-065x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 42.Slifka MK, Whitton JL. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 43.Raue HP, Slifka MK. CD8+ T cell immunodominance shifts during the early stages of acute LCMV infection independently from functional avidity maturation. Virology. 2009;390:197–204. doi: 10.1016/j.virol.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray PM, Parks GD, Alexander-Miller MA. High avidity CD8+ T cells are the initial population elicited following viral infection of the respiratory tract. J Immunol. 2003;170:174–181. doi: 10.4049/jimmunol.170.1.174. [DOI] [PubMed] [Google Scholar]
- 45.Freeman ML, Lanzer KG, Cookenham T, Peters B, Sidney J, Wu TT, Sun R, Woodland DL, Sette A, Blackman MA. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J Virol. 2010;84:2881–2892. doi: 10.1128/JVI.02229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroger CJ, Alexander-Miller MA. Cutting edge: CD8+ T cell clones possess the potential to differentiate into both high- and low-avidity effector cells. J Immunol. 2007;179:748–751. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- 47.Cawthon AG, Alexander-Miller MA. Optimal colocalization of TCR and CD8 as a novel mechanism for the control of functional avidity. J Immunol. 2002;169:3492–3498. doi: 10.4049/jimmunol.169.7.3492. [DOI] [PubMed] [Google Scholar]
- 48.Guillaume S, Tuosto L, Tanchot C, Di B, Acuto VO, Rocha B. Proximal changes in signal transduction that modify CD8+ T cell responsiveness in vivo. Eur J Immunol. 2003;33:2551–2556. doi: 10.1002/eji.200324196. [DOI] [PubMed] [Google Scholar]
- 49.Welke J, Zavazava N. P59(fyn) is upregulated in anergic CD8+ T cells. Hum Immunol. 2002;63:834–843. doi: 10.1016/s0198-8859(02)00455-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.