Abstract
Despite recent population data, the influence of dietary folate supplementation on colon cancer risk remains controversial. This study examines the effects of folate deficiency, in combination with choline, methionine and vitamin B12 depletion, on intestinal tumorigenesis in ApcMin/+ mice. Methyl donor sufficient (MDS) and deficient (MDD) diets were started at 5 or 10 weeks of age and tumors evaluated at 16 weeks. MDD suppressed intestinal tumor formation in ApcMin/+ mice (~80%) when started at 5 weeks of age. The protective effect was lost when MDD was initiated at 10 weeks of age, indicating an important time-dependency on cancer suppression. Concomitant with cancer protection, MDD restricted body weight gain. Therefore, a second study was conducted in which MDS was given ad libitum or pair-fed with MDD. While small intestinal tumors were reduced 54% in pair-fed MDS mice, MDD caused a further reduction (96%). In colon, although MDD did not affect tumor numbers, tumor size was reduced. Gene expression profiling of normal-appearing colonic mucosa after 11 weeks on MDD identified a total of 493 significantly down-regulated genes relative to the MDS group. Pathway analysis placed many of these genes within general categories of inflammatory signaling and cell cycle regulation, consistent with recently published human data obtained during folate depletion (1). Further studies are warranted to investigate the complex interplay of methyl donor status and cancer protection in high-risk populations.
Keywords: Folic acid, Methyl donors, dietary depletion, colon, colorectal carcinogenesis, 1-carbon metabolism, inflammation, gene expression profiling
Introduction
Epidemiological studies have suggested that low folate levels are associated with an increased risk of colorectal cancer (2–6) and other health risks such as congenital neural tube defects. To reduce the incidence of neural tube defects the FDA instituted mandatory folate fortification program in 1998 leading to near doubling of median folate levels. Because folate is an essential co-factor in DNA methylation and nucleotide synthesis, there is ongoing concern that folate fortification may lead to increased risk for some forms of cancer, particularly among subjects harboring neoplastic foci. Indeed, early research in the 1940s demonstrated that administration of folic acid to patients with malignancy actually results in rapid progression of the disease (7). One recent clinical study showed acceleration in the development of premalignant adenomas (8) and two other clinical trials observed an increase in cancer incidence (9, 10) among subjects randomly assigned to receive folic acid supplements. Nevertheless, two contemporary post-fortification studies showed that compared to individuals on relatively low folate diet, individuals on adequate folate diets had reduced risk of colorectal cancer (11, 12), suggesting that adverse effects of folate might be limited to populations consuming large amounts of folic acid.
The folate controversy stems in part from the relative lack of mechanistic data regarding specific cellular pathways through which either low or high folate intake modulates the risk of colorectal cancer. We recently demonstrated in human subjects that changing folate availability by dietary depletion or supplementation alters the expression of a number of genes involved in the inflammatory and immune response in the colonic mucosa (1). Another human study showed that the administration of 1.2 mg folic acid for 12 weeks increases the levels of serum proteins involved in the regulation and activation of immune function and the complement cascade (13). Additionally, in the Aspirin/Folate Prevention of Large Bowel Polyps trial, folic acid administration was shown to antagonize the suppressive effects of aspirin on circulating inflammatory markers (14). These folate-associated molecular changes may provide clues as to how folate status might modulate the risk of colorectal carcinogenesis and neoplastic progression, but additional studies examining tumor outcomes are needed.
The aim of the present study was to determine the effects of folate/methyl donor depletion on intestinal tumorigenesis in ApcMin/+ mice. We hypothesized that folate/methyl donor depletion would attenuate the formation of intestinal tumors, and based on our recently published human data (1), reduce the expression of a panel of immune-related genes within the colonic mucosa. The use of antibiotics was avoided, thus preventing the severe methyl donor deficiency associated with suppression of the gut microflora (15, 16). Overall, our data show that folate/methyl donor deficiency markedly suppresses intestinal tumorigenesis in ApcMin/+ mice. The effect is not solely dependent on reduced caloric intake, however, as further demonstrated by a pair-feeding study with MDS diet. Importantly, the protection afforded to the intestine is highly dependent upon the timing and duration of the dietary intervention.
Materials and methods
Animals, diets and experimental design
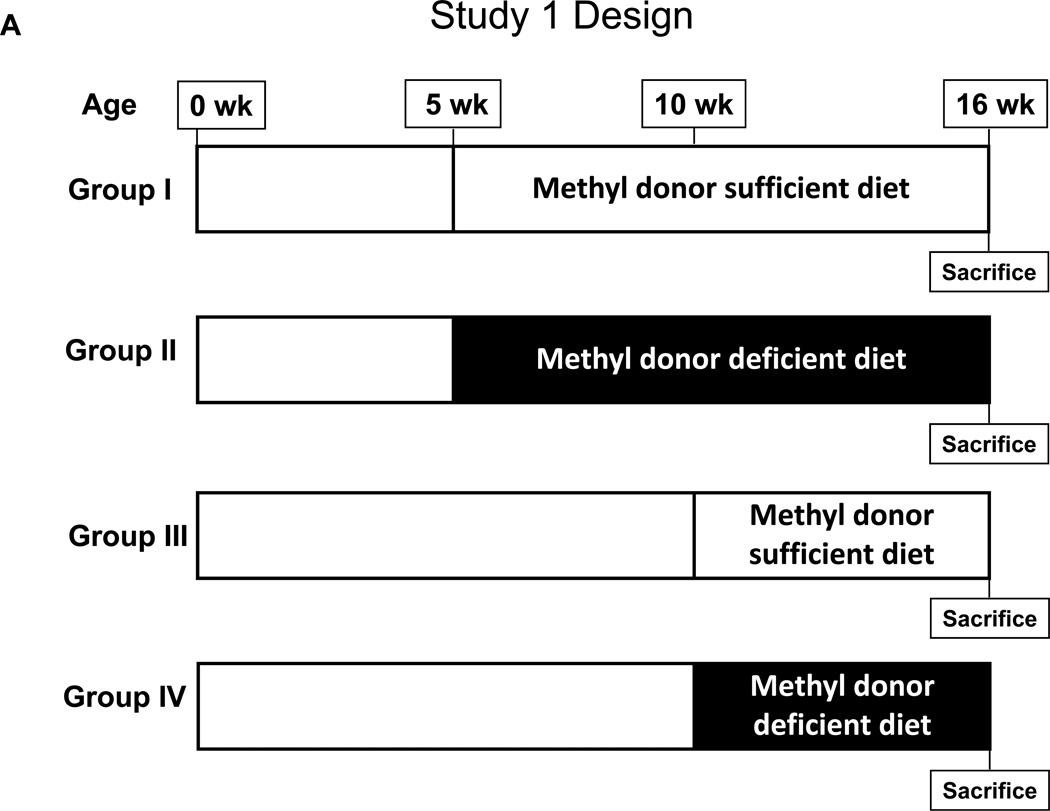
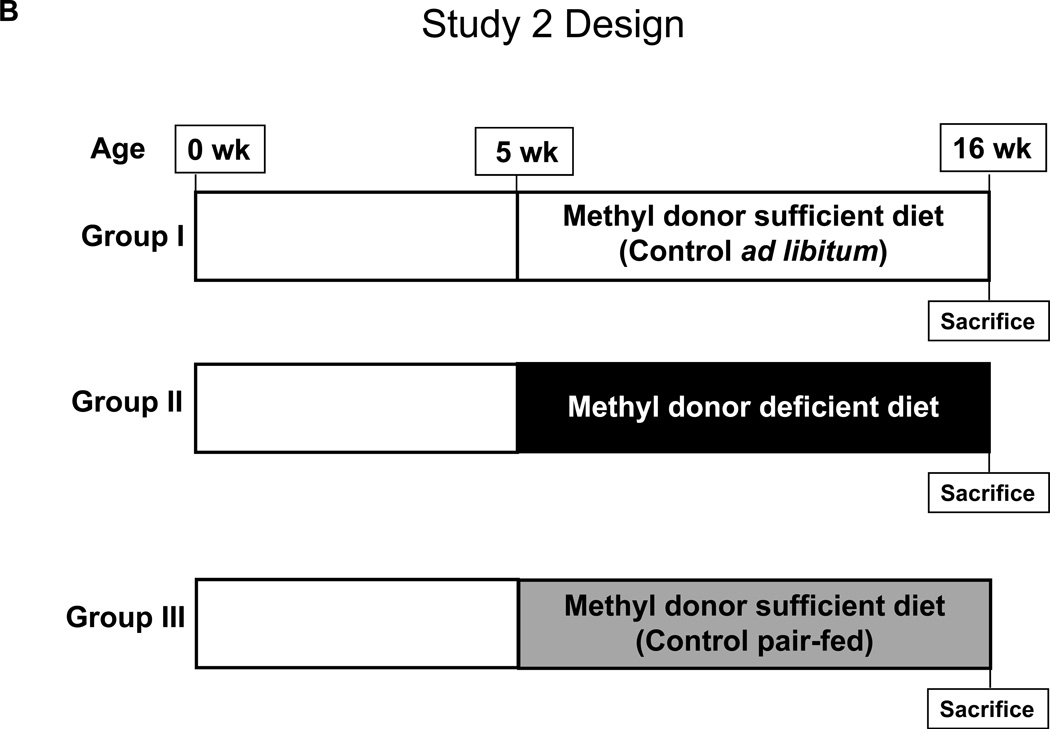
Animal experiments were conducted with approval from the Center for Laboratory Animal Care Committee (CLACC), The University of Connecticut Health Center. ApcMin/+ mice, originally purchased from The Jackson Laboratory, were maintained on a C57BL/6J background. Genotyping was performed by tail biopsy using PCR-based assays to determine the Apc gene status. As outlined in Figure 1A, in Study 1, a total of 39 ApcMin/+ mice were randomized at 5 weeks of age and placed into four experimental groups. In Groups 1 and 2, experimental diets were initiated at five weeks of age. In Group 1, 12 ApcMin/+ mice were fed a methyl donor sufficient diet (MDS; TD.99366, Harlan Laboratories, Madison WI) for a total of 11 weeks. In Group 2, 14 ApcMin/+ mice were fed a methyl donor deficient diet (MDD; TD.00605), devoid of folate, choline, methionine and vitamin B12 (complete details of the diets are available in Supplemental Data, Table S1), for a total of 11 weeks. In Group 3, a total of 5 ApcMin/+ mice received the MDS beginning at 10 weeks of age, while in Group 4, 8 ApcMin/+ mice received the MDD beginning at 10 weeks of age. In Study 2, as outlined in Figure 1B, a total of 37 ApcMin/+ mice were randomized at 5 weeks of age and placed into three experimental groups. In each group, experimental diets were initiated in mice at five weeks of age for a total of 11 weeks. In Group 1, ApcMin/+ mice (n=13) were fed the MDS ad libitum. In Group 2, ApcMin/+ mice (n=12) were fed a MDD diet ad libitum. Finally, in Group 3, to prevent weight differences between the experimental and control diets, ApcMin/+ mice (n=12) were pair-fed with MDS diet to the same amount consumed by the Group 2 animals. All mice in each group were sacrificed at 16 weeks of age. Body weights of mice were recorded weekly and reported as shown in Figure 2, Supplemental Figure S1, and Figure 3.
Figure 1. Study design.
A) Study 1: At five weeks of age, a total of 39 ApcMin/+ mice were randomized and placed into four experimental groups. Group I (n=12) received methyl donor sufficient diet (MDS) and Group II (n=14) received methyl donor deficient diet (MDD), for 11 weeks starting at 5 weeks of age. Group III (n=5) received MDS and Group IV (n=8) received MDD, for 6 weeks starting at 10 weeks of age. All mice were sacrificed at 16 weeks of age.
B) Study 2: At five weeks of age, a total of 37 ApcMin/+ mice were randomized and placed into three experimental groups. Group I (MDS ad libitum, n=13) received methyl donor sufficient diet ad libitum, Group II (MDD, n=12) received methyl donor deficient diet and Group III (MDS pair-fed, n=12) were pair-fed with MDS diet to the same amount consumed by the Group II animals, for 11 weeks starting at 5 weeks of age. All mice were sacrificed at 16 weeks of age.
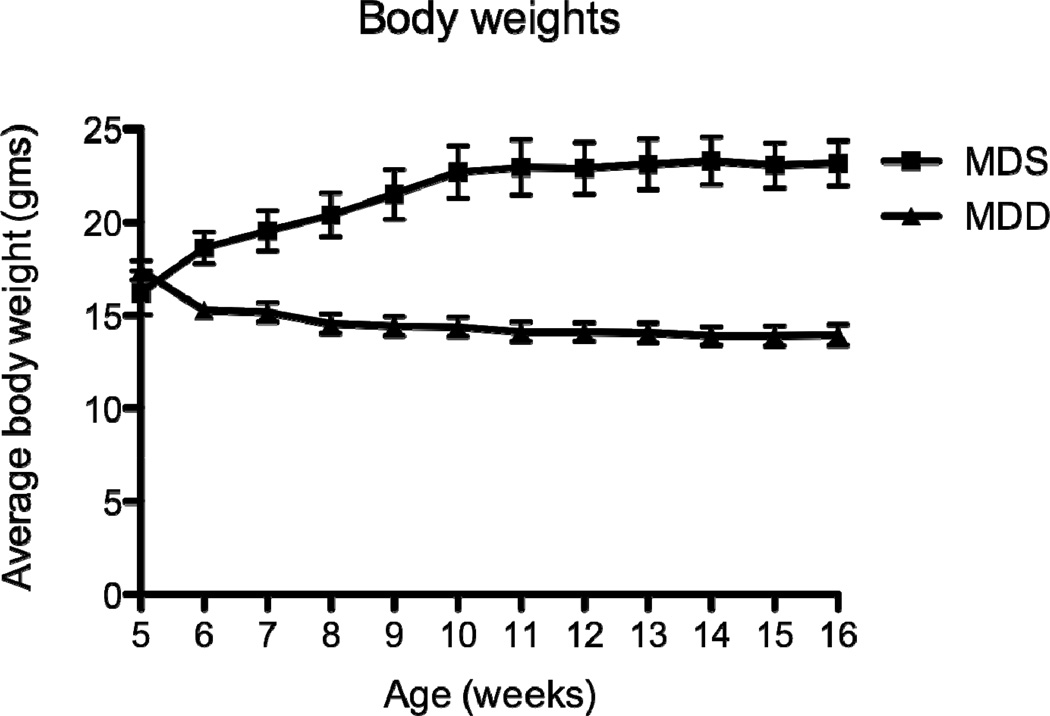
Figure 2. Body weight changes in ApcMin/+ mice maintained for 11 weeks on MDS or MDD diet (Study 1).
Mice were placed on MDS or MDD diet beginning at 5 weeks of age. Body weights were recorded weekly throughout the entire experimental period. Each data point represents the average body weight ± SEM for Group I (n=12) and Group II (n=14). Note that at the end of the study, there was an ~ 40% reduction in body weight in mice maintained on the MDD diet.
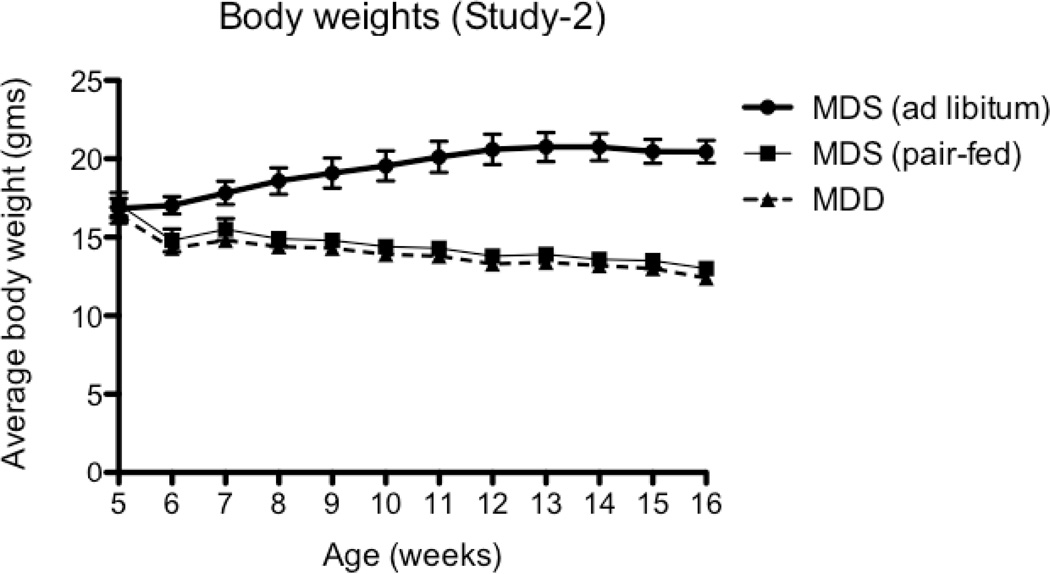
Figure 3. Body weight changes in ApcMin/+ mice maintained for 11 weeks on MDS or MDD diet (Study 2).
Mice were placed on MDS or MDD diet beginning at 5 weeks of age. Body weights were recorded weekly during the entire experimental period. Each data point represents the average body weight ± SEM for Group I (MDS ad libitum, n=13), Group II (MDD, n=12) and Group III (MDS pair-fed, n=12). Average body weights for Group II and Group III overlapped throughout the entire experimental period. Note that at the end of the study, there was an ~ 40% reduction in body weight in mice maintained on MDS pair-fed and MDD diets.
Serum folate levels
At the time of sacrifice, blood was collected from the posterior vena cava and centrifuged at 10,000 × g for 10 min at 4°C. Serum was collected and stored at −80°C until analysis. Serum folate concentrations were measured by a microtiter plate assay using Lactobacillus casei, as described previously (17).
Tumor incidence and multiplicity
Mice were sacrificed at 16 weeks of age. The small intestine and colon were harvested, flushed immediately with ice-cold PBS and slit open longitudinally. Specimens were fixed-flat in 10% buffered formalin and stored in 70% ethanol. For tumor quantitation, fixed tissues were stained with 0.2% methylene blue and examined under a dissecting microscope.
RNA extraction and gene expression analyses
Sections of excised colons with no macroscopic evidence of tumors from the Study 1 MDS Group 1 (n=3) and MDD Group 2 (n=5) were maintained in liquid nitrogen until analysis. Total RNA was extracted using the Trizol Reagent (Invitrogen, Carlsbad, CA). All RNA samples were assessed for quality on an Agilent 2100 Bioanalyzer (Agilent Technologies). The Microarray Core at Children’s Hospital (Boston, MA) performed array processing and cRNA hybridization. Briefly, total RNA was converted into cRNA labeled with biotin and hybridized onto the Mouse-Ref8 v1.1 Sentrix BeadChip Array (Illumina Corp.) for approximately 18 hours. The BeadChip Array was then washed and stained with Streptavidin-Cy3, and scanned by the Illumina BeadArray Reader (Illumina Corp.). Each sample was hybridized to two separate BeadChips. Images were quantified using the Illumina BeadStudio software and values were reported as average signal for each gene.
Expression data were analyzed by Genespring software (Agilent Technologies, Inc, Santa Clara, CA) after normalization. Prior to analysis, the arrays were normalized to the 50th percentile per chip (array) and median per gene. Quality control was performed by analyzing gene expression correlation coefficients, and samples with coefficient <0.97 were excluded (one control and one intervention animal). Biological duplicate samples signals were averaged. The differences in gene expression levels were determined using t-tests, adjusted by the Benjamini-Hochberg method at a false discovery rate (FDR) of less than 0.1. The significant gene list was subjected to hierarchical clustering using squared Euclidian distance and a centroid linkage rule (18). This microarray data set has been deposited in the NCBI Gene Expression Omnibus with the accession number GSE37010. Subsequently, Gene Set Enrichment Analysis (GSEA) was used. GSEA is a computational method that determines whether an a priori defined set of genes show statistically significant differences between two phenotypes. We ranked gene expression differences between MDS and MDD groups to identify gene sets that were significantly enriched after methyl donor depletion (19, 20). FDR-q was used to rank the enrichment results.
Quantitative real-time analysis
For reverse transcriptase PCR (RT-PCR), duplicate 1-µg samples of total RNA were used as a template for cDNA synthesis using Superscript III First Strand kit (Invitrogen). cDNA was diluted in RT buffer and amount corresponding to 100 ng of original RNA was used for gene expression by quantitative RT-PCR. All qRT-PCR reactions used TaqMan® Gene Expression Assay probes and primers (Applied Biosystems) and the ABI Prism 7500 RT-PCR system at the Liver Center (Yale University). Three genes that are related to inflammation were chosen from among the top ten most down-regulated genes (TNF-alpha, CXCL1 and CD28) and were measured by qRT-PCR to validate the expression array data. Quantification of expression changes used the delta method, and 18S mRNA was used as endogenous controls. Two independent RT-PCR reactions were used for each sample to calculate results.
Statistics
Statistical analysis of the gene expression arrays is described above. For the comparison of serum folate levels, the number of intestinal polyps and qRT-PCR data, statistical analyses were performed using an unpaired t-test and P-values are indicated in the Figures. In Study 2, one- way ANOVA with Bonferroni’s post-test was used to compare the number of intestinal polyps between groups. The difference in colon tumor size in response to methyl donor depletion was tested using Chi-square test (size cutoff of 2mm).
Results
Effects of dietary methyl donor depletion on intestinal tumor formation
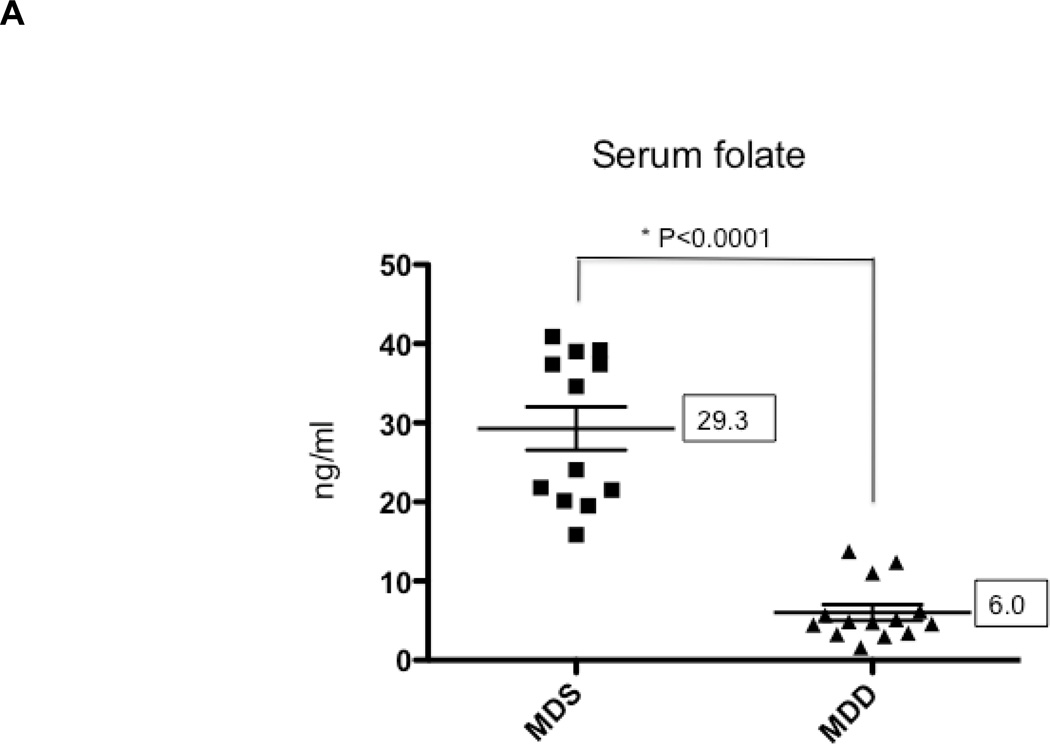
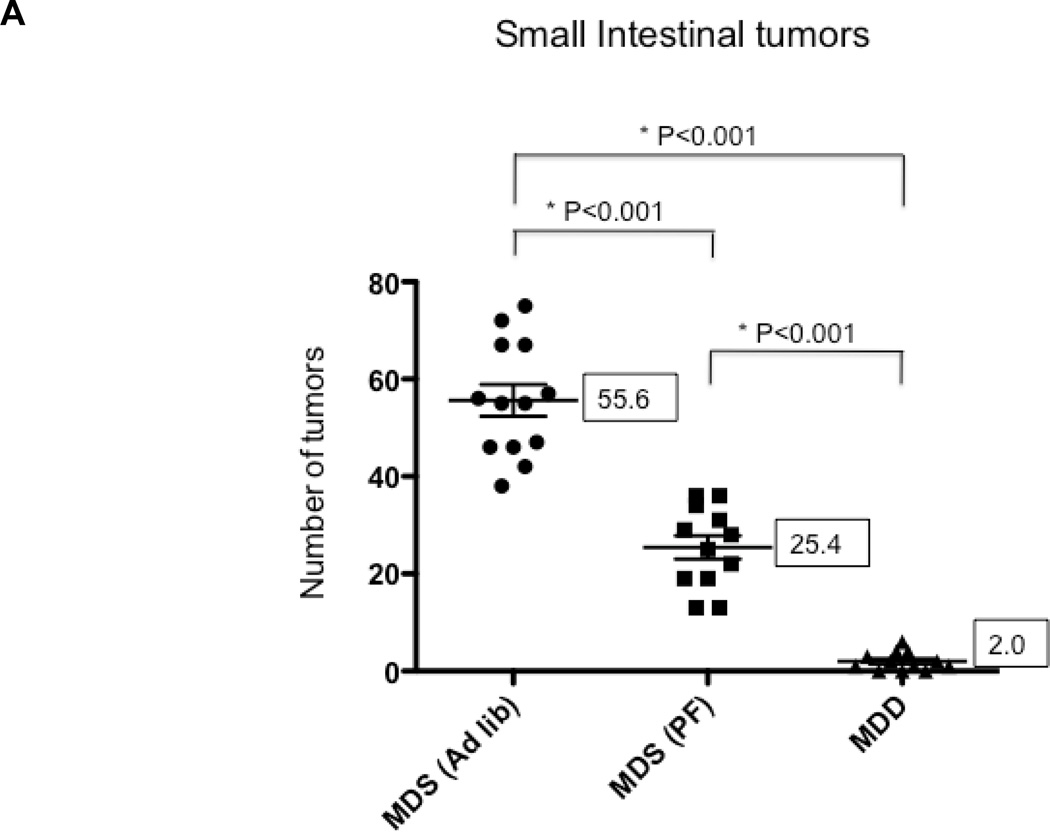
In Study 1, ApcMin/+ mice were maintained on either the MDS or MDD diet, beginning at either 5 or 10 weeks of age (Figure 1A). Regardless of timing, mice lost weight on the MDD diet, although the weight loss leveled off after 8 weeks on the experimental diet (Figure 2). At the end of the study period, there was almost a 40% body weight difference between the MDS and MDD groups (Figure 2), consistent with previous folate depletion studies in rodents (15, 21–23). Although the MDD diet caused a significant reduction in body weight gain, there were no premature deaths recorded. In order to confirm the effects of dietary folate depletion, the serum concentrations were also measured. As shown in Figure 4A, the mean serum folate concentration in the MDD group was reduced by approximately 80% compared to the MDS dietary group (P<0.0001) when dietary intervention with the MDD diet was initiated at 5 weeks of age.
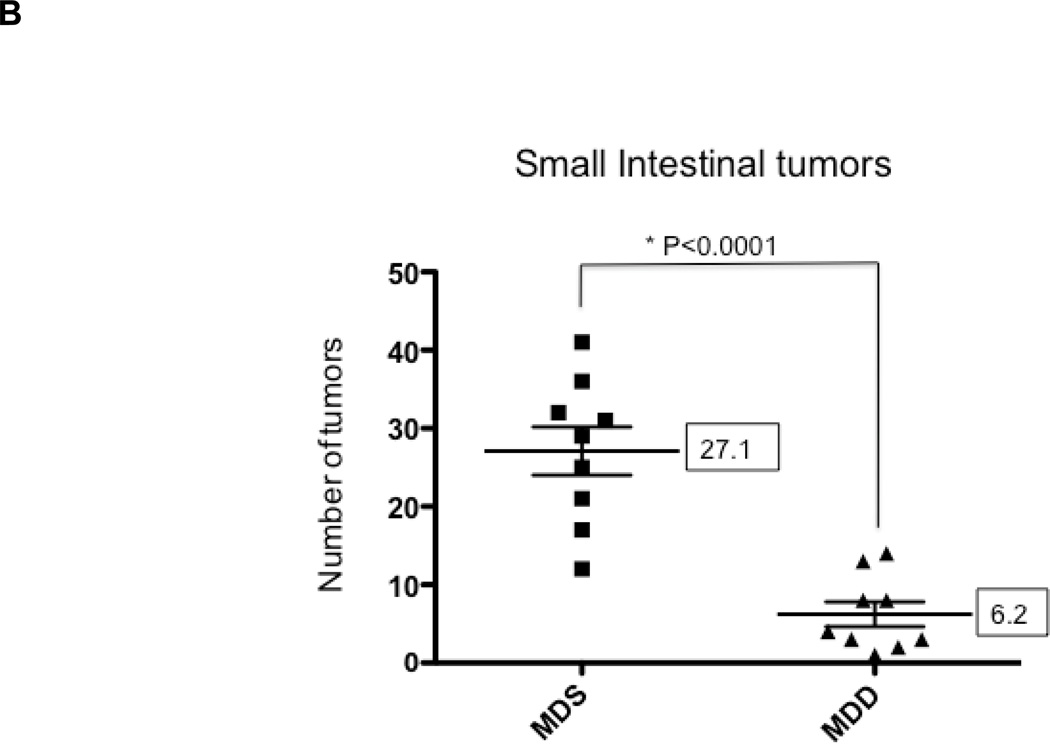
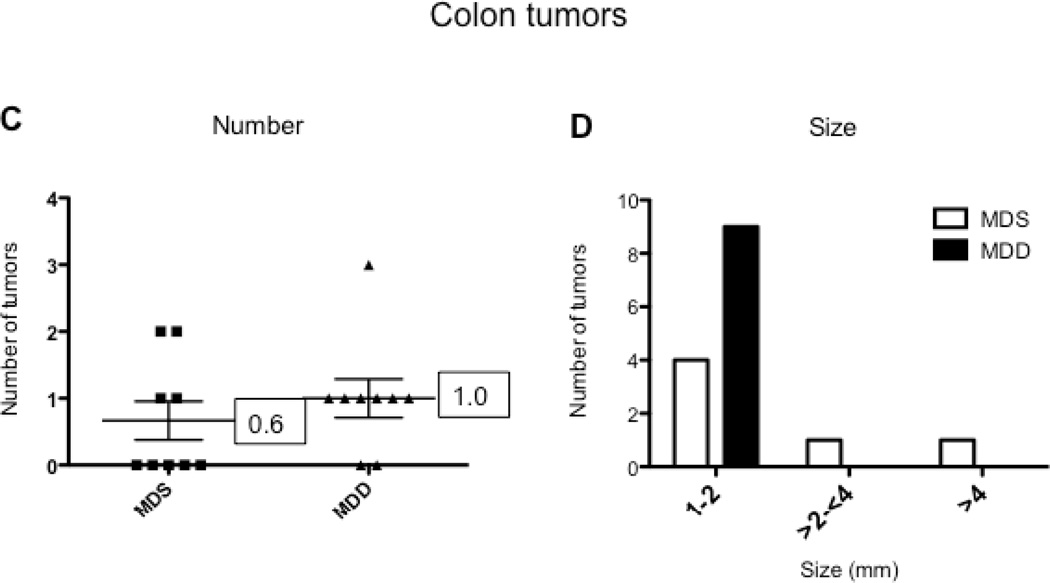
Figure 4. The effects of methyl donor depletion for 11 weeks on serum folate concentrations and intestinal tumor formation.
A) Serum folate concentrations were determined by a microtiter plate assay using Lactobacillus casei, as described under Materials and Methods; B) and C) Total number of tumors per mouse in the small intestine (B) and colon (C) from MDS and MDD (n=9 per each group) mice, respectively. D) Colon tumors were classified by size as indicated. Note that there were no medium (between 2 and 4mm) or large-sized (≥4mm) colon tumors in the MDD group. Each data point represents an individual mouse and the numbers indicate the mean value for each group. P-values are indicated in the Figure and determined by unpaired t-tests.
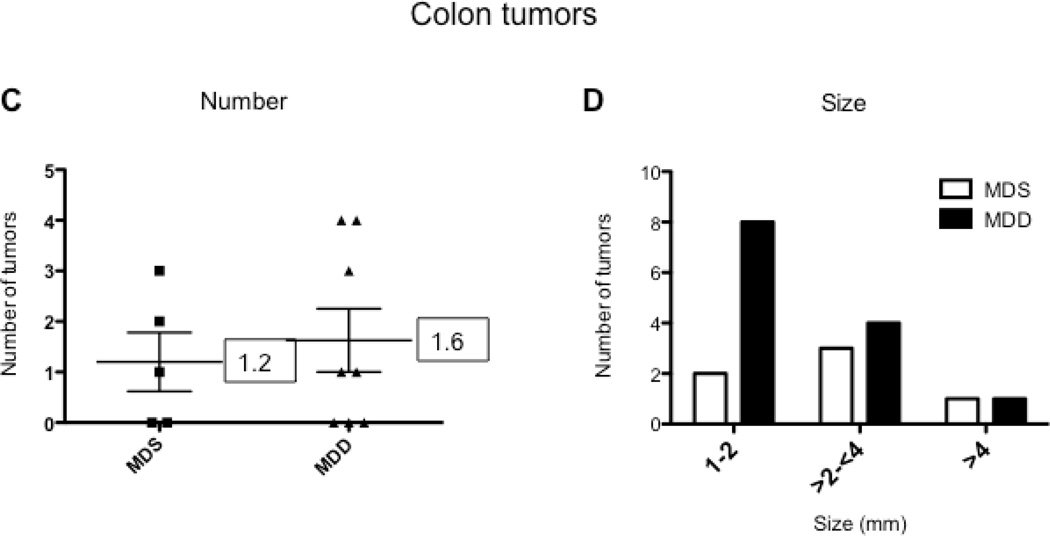
To determine the effects of methyl donor depletion on ApcMin/+-induced tumorigenesis, the number and size of small intestinal and colonic tumors were examined in all four dietary treatment groups. As shown in Figure 4B, analysis of methylene blue-stained whole mounts of the small intestine revealed a significant reduction (80%, P<0.0001) in tumor numbers in mice maintained for 11 weeks on the MDD diet compared to the MDS diet. Despite this marked suppression in tumor numbers, methyl donor depletion did not affect the size of tumors in the small intestine (data not shown). Consistent with previous studies in ApcMin/+ mice (24–26), tumors were mainly confined to the small intestine and their distribution was not affected by dietary methyl donor status. In the colon, although the total number of tumors was not affected by methyl donor depletion (Fig. 4C), there was a trend towards a reduction in the size of colon tumors in the MDD diet group (P=0.063). As shown in Figure 4D, there were no medium-(between 2 and 4mm) or large-sized (≥4mm) colon tumors in the MDD group, suggesting that methyl donor deficiency may prevent the progression of colon tumors in ApcMin/+ mice.
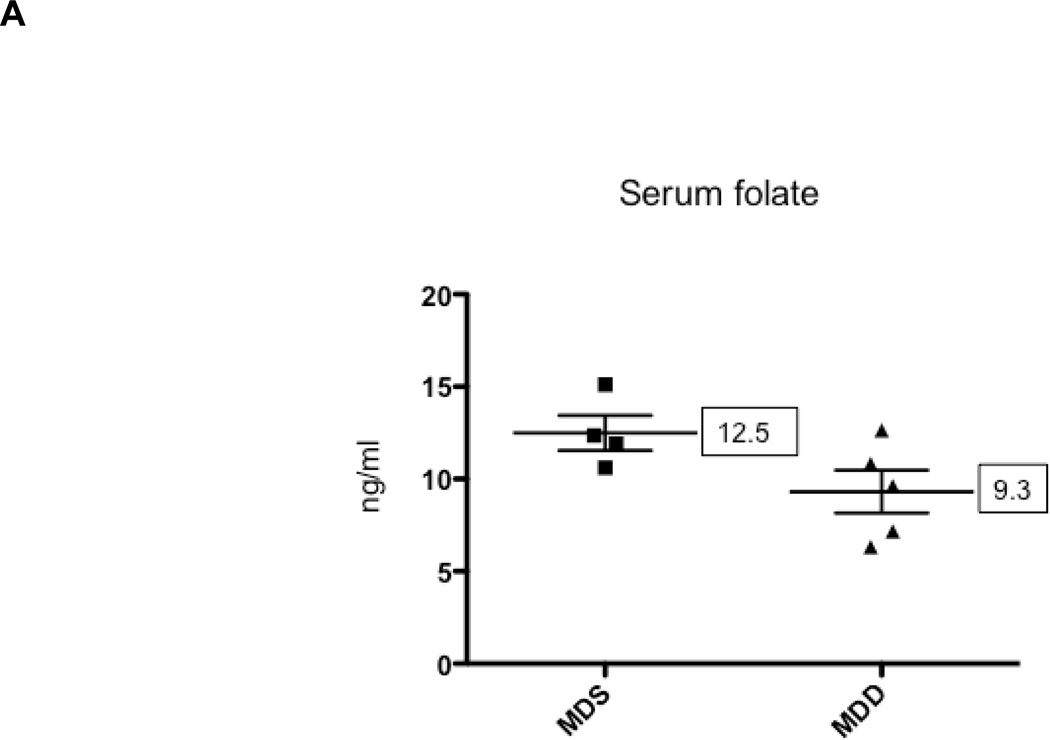
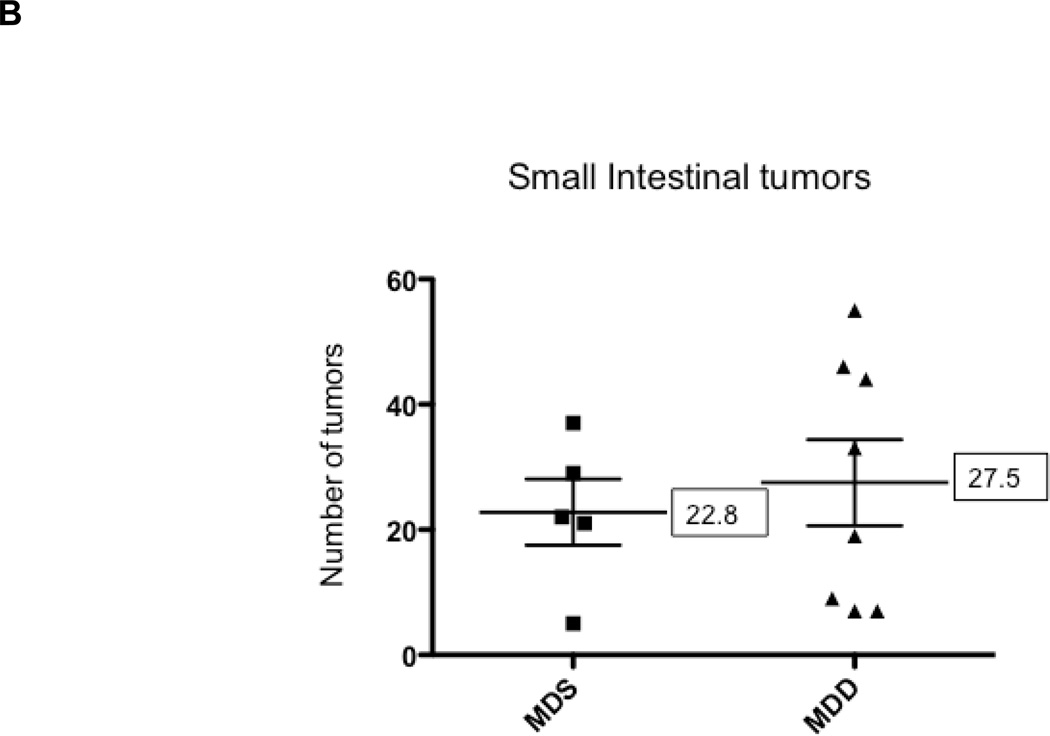
When the start of the MDD diet was delayed until 10 weeks of age, serum folate was reduced by less than 25% in the MDD group (P<0.08) as shown in Figure 5A. However, cancer suppression was largely lost in the small intestine and colon (Figures 5B and 5C). The inhibitory effect on colon tumor growth was also lost (Fig. 5D). The lack of protection, however, may be related to the mild reduction in serum folate levels. These results indicate that the timing and duration of methyl donor depletion is a critical factor in suppression of intestinal tumor formation in the ApcMin/+ mouse model.
Figure 5. The effects of methyl donor depletion for 6 weeks on serum folate concentrations and intestinal tumor formation.
A) Serum folate concentrations were determined by a microtiter plate assay using Lactobacillus casei, as described under Materials and Methods; B) and C) Total number of tumors per mouse in the small intestine (B) and colon (C) from MDS (n=5) and MDD (n=8) mice, respectively. D) Colon tumors were classified by size as indicated. Each data point represents an individual mouse and the numbers indicate the mean value for each group. No statistically significant differences were observed between the groups.
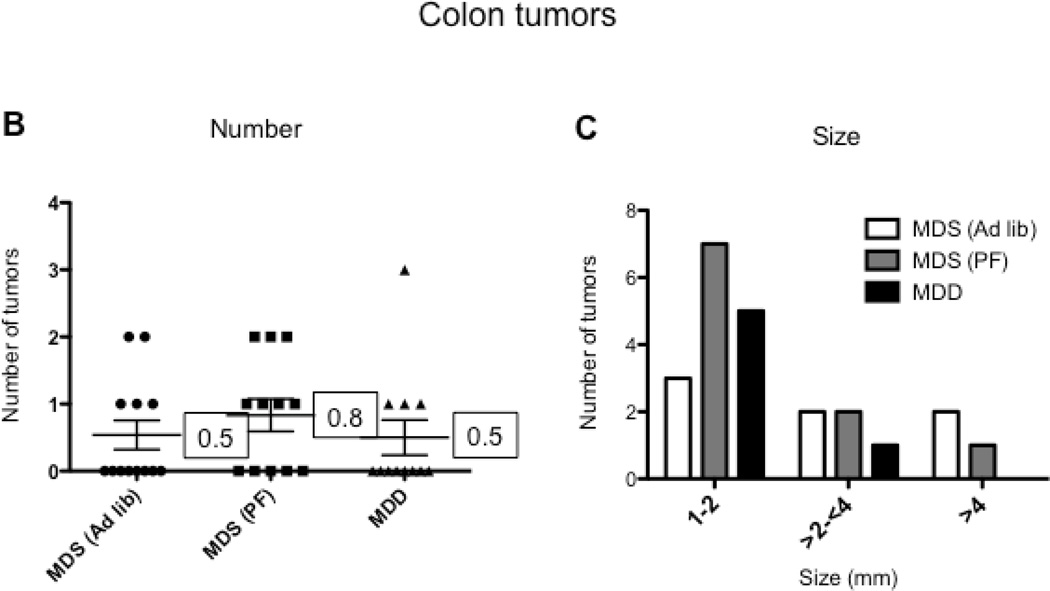
Because we observed extensive weight loss with the MDD diet, we conducted a second study (Study 2) using a pair-feeding design (Figure 1B) to adjust for the potential effect of weight loss on intestinal tumorigenesis. As shown in Figure 3, pair-fed animals showed the same reduction in body weight gain when compared to the MDD group. However, even after adjusting for reduced body weight gain, the MDD diet induced a striking and significant further tumor reduction (~96%, P<0.0001), as shown in Figure 6A. Also, as anticipated, the calorie-restricted animals (i.e., MDS pair-fed group) showed a significant reduction (~54%, P<0.0001) in tumor burden (Figure 6A). In the colon, although the total number of tumors was not affected by methyl donor depletion (Fig. 6B), there was a non-significant (P=0.285) reduction in medium-sized (between 2 and 4 mm) tumors and an absence of large-sized (≥4 mm) tumors in the MDD group (Figure 6C). These data suggest that methyl donor deficiency may prevent the progression of colon tumors in ApcMin/+ mice, a conclusion that is consistent with our findings in Study 1.
Figure 6. The effect of reduced caloric intake and methyl donor depletion on intestinal tumor formation.
A) and B) Total number of tumors per mouse in the small intestine (A) and colon (B) from MDS ad libitum (n=13), MDS pair-fed (n=12) and MDD (n=12) mice, respectively. C) Colon tumors were classified by size as indicated. Note that there were no large-sized (≥4mm) colon tumors in the MDD group. Each data point represents an individual mouse and the numbers indicate the mean value for each group. P-values are indicated in the Figure as determined using one-way analysis of variance followed by Bonferroni’s multiple comparison tests.
Effects of methyl donor status on global gene expression patterns
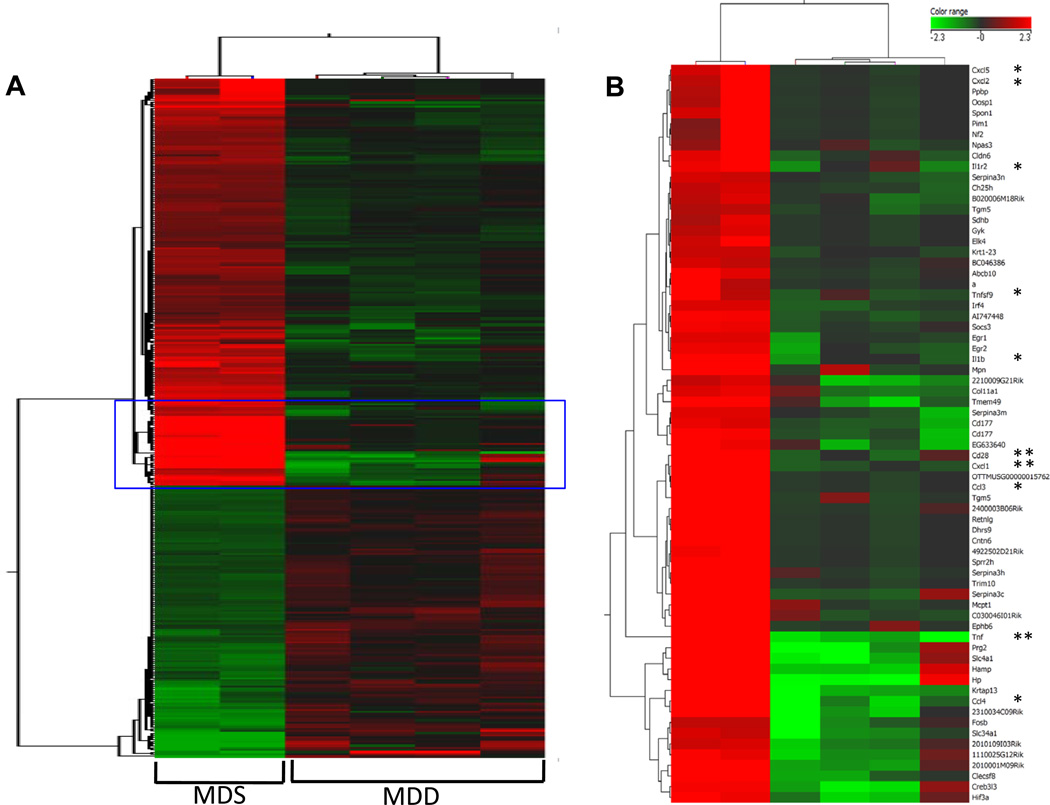
RNA was isolated from normal-appearing colonic mucosa from Study 1 mice maintained on the MDS and MDD diets for 11 weeks. A total of 1,009 genes were differentially expressed between the control and MDD groups at FDR < 0.1 (refer to Supplemental data for complete results); 516 genes were up-regulated and 493 genes were down-regulated. Hierarchical clustering of the most significantly altered genes represented as individual animals is shown in Figure 7A. Among the significantly differentially regulated genes, many were down-regulated and showed distinct clustering patterns (Fig. 7B). Within this set were a number of down-regulated genes that are involved in various aspects of immune cell function and inflammation, as well as cell cycle control.
Figure 7. The effect of MDD diet on global gene expression patterns in the colons of ApcMin/+ mice.
A) Total RNA was isolated from normal-appearing colonic mucosa from mice maintained on MDS or MDD diets for 11 weeks. Significantly expressed genes between the MDS and MDD groups, identified by adjusted t-tests, were clustered using a squared Euclidean algorithm. B) A set of genes that are related to inflammation and chemokine signaling are shown enlarged in the right panel. “*” Indicates genes related to chemokine pathways and “* *” indicates the genes that were further validated by qRT-PCR. The colored scale shown in the Figure represents the change in signal intensities.
Validation of differentially expressed genes by qRT-PCR
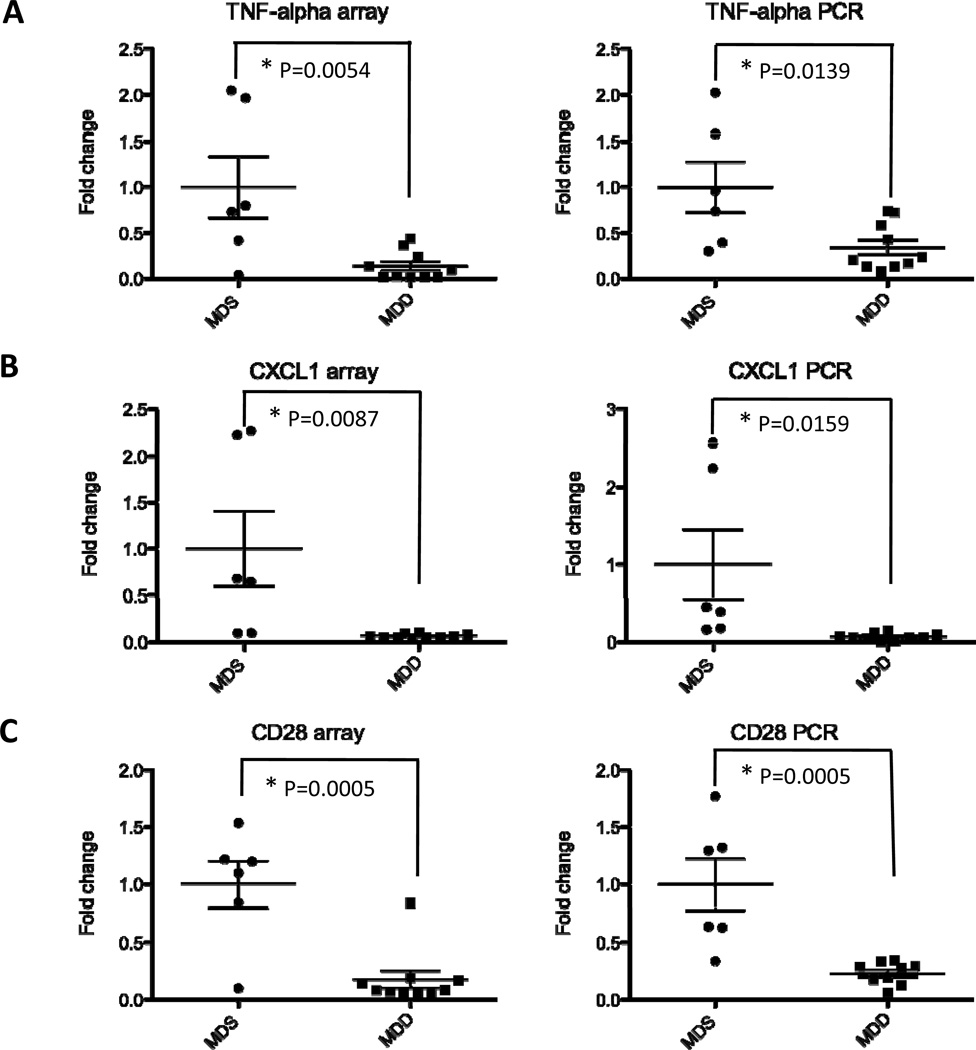
To provide further validation of the microarray data, we selected three differentially regulated genes from the most significantly altered genes identified earlier by t-tests. Included in this analysis are TNF-α, CXCL1 and CD28. As shown in Figure 8, there was a marked down-regulation in the expression of TNF-α, CXCL1 and CD28 (P=0.0139, P=0.0159 and P=0.0005 respectively). These data confirm the gene expression changes identified in Figure 7B.
Figure 8. Validation of gene expression changes induced by the MDD diet in ApcMin/+ colons.
Total RNA was isolated from the colons of ApcMin/+ mice as described under Materials and Methods. To validate the array data obtained from Groups I and II, real-time PCR analysis was performed on three of the most significantly down-regulated genes within the pro-inflammatory network, including TNF-alpha (A), CXCL1 (B) and CD28 (C). Each data point represents the expression fold-change value for an individual mouse, with P values as indicated in the Figure. The left panels show the microarray data and the right panels show the qRT-PCR data.
Gene Set Enrichment Analysis
To gain additional insight into the molecular alterations that may be associated with methyl donor depletion and tumor suppression, we performed a GSEA for Gene Ontology categories within the two dietary groups. Again, Gene Ontology showed that among the most down-regulated categories were those related to immune cell function and inflammatory response. Other down-regulated functional categories include cell turnover, comprised of chemokine and cytokine activity, cell cycle progression and mitosis. The most up-regulated categories were related to extracellular structure and ribosome organization. GSEA for canonical pathway analysis showed that the most down-regulated pathways included Toll-like receptor and T-cell receptor signaling, cytokine-cytokine receptor, cell adhesion molecules, and p53 signaling.
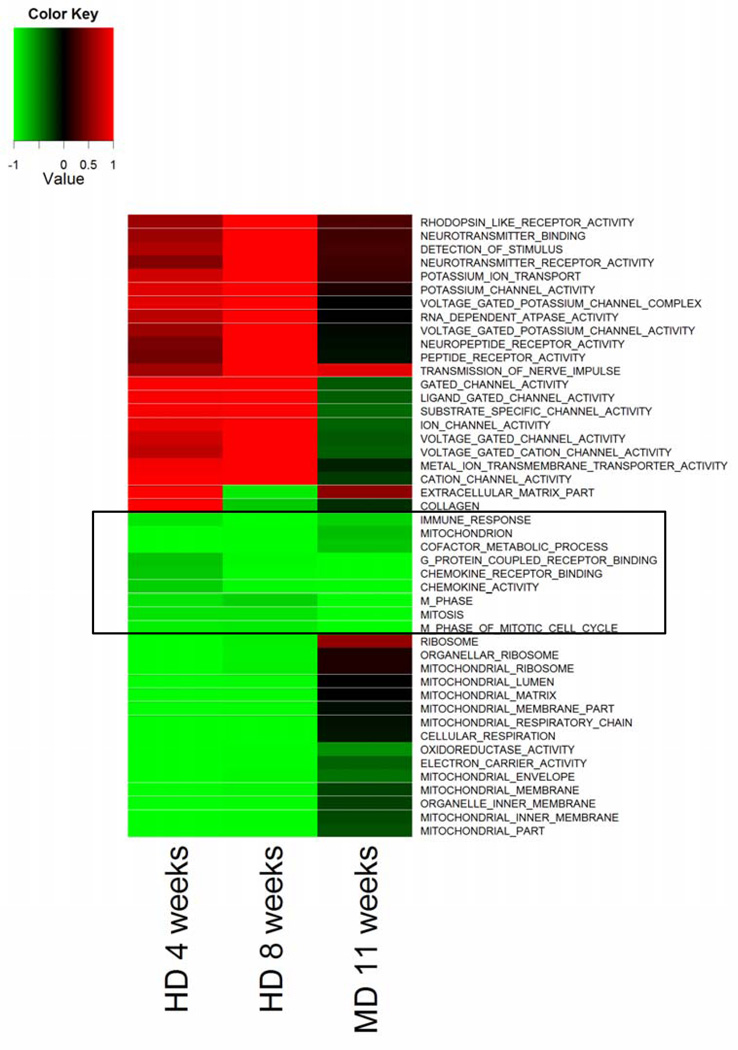
During the course of these studies, we began to observe a remarkable overlap in gene expression profiles within the colons of methyl donor-depleted ApcMin/+ mice and that of a folate depletion study that was recently performed in human subjects (1). Based on these similarities, we compared mouse GSEA data to our recently published human folate depletion study (1). Although we observed significant differences in gene expression patterns in the colons of mice maintained on the MDS and MDD diets, we cannot exclude the possibility that these changes may be due in part to the marked suppression in body weight gain observed in the MDD group. Nevertheless, a direct comparison of GSEA for both Gene Ontology categories and canonical pathways across the two species showed that folate and/or methyl donor depletion was associated with a significant down-regulation of gene ontology categories and pathways involved in the inflammatory response and mucosal immunity, including components of the innate immune response (Fig. 9).
Figure 9. Heatmap of Gene Set Enrichment Analysis for Gene Ontology comparing folate/methyl donor deficient mice to a human folate depletion study.
The Figure compares significantly enriched GO categories in the human 4-week (HD 4 weeks) and 8-week (HD 8 weeks) dietary folate depletion studies to the present 11-week folate/methyl donor depletion study. Only categories with an FDR q-value of less than 0.001 in at least one condition are shown. Colors indicate down-regulation (green) or up-regulation (red), and values are represented as 1 - P (with down-regulation assigned negative values). Note that gene categories related to immune function and inflammation are down-regulated by folate/methyl depletion in the mouse colon and closely resemble the human data.
Discussion
The present study demonstrates that dietary restriction of methyl donors, including folate, suppresses intestinal tumorigenesis in ApcMin/+ mice. Although the tumor suppression is most dramatic in the small intestine, the primary site of tumors in this model, some trend towards a reduction in the size of tumors was also observed in the colon. Importantly, the protective effect of methyl donor depletion is strongly dependent upon the timing and duration of the dietary treatment. Gene expression profiling within the normal colonic mucosa identified a striking down-regulation by dietary methyl donor depletion of a large number of genes involved in the immune and inflammatory response. These observed gene expression changes in mice overlapped extensively with expression changes observed within the human colorectum obtained from subjects maintained on low dietary folate (1), validating the use of this mouse model.
We observed that animals in the methyl-depletion group exhibited a reduced body weight gain during the experimental period. Previous nutritional studies in rodents have also shown that folate deficiency results in a reduction in body weight gain (15, 21–23). There is an extensive body of literature describing the effects of caloric restriction on protection from experimentally-induced carcinogenesis, beginning with the seminal studies in the 1940s by Tannenbaum and co-workers (27) (reviewed by Kritchevsky in (28)). Thus, we speculated that the tumor suppressive effects observed in the ApcMin/+ mice maintained on the MDD diet might be an indirect result of caloric restriction, especially since body weight gain was reduced by almost 40% during the course of treatment in the present study. For example, in a recent study in primates, caloric restriction was found to significantly reduce the incidence of age-related diseases, including a suppression of gastrointestinal adenocarcinomas, the most common cancer found in rhesus monkeys, by ~50% compared to a control diet (29). In fact, caloric restriction was shown to reduce the frequency of intestinal polyps in ApcMin/+ mice by almost 60%, an effect that was mediated in part through reduced levels of IGF-1 and leptin (30). Caloric restriction may also afford protection against colon carcinogenesis in human populations. In a study of colonic proliferation in obese subjects, caloric restriction significantly reduced markers of colonic proliferation (31). Importantly, these cancer preventive effects are also highly dependent on the extent and duration of caloric restriction, as well as the age at which the restriction was initiated in animal models (32–34). However, in a follow-up pair-feeding study in which ApcMin/+ mice on MDS diet demonstrated comparable weight loss to the MDD dietary group, we clearly demonstrated that the cancer suppressive effects of the MDD diet extend beyond the moderate protection observed in the calorie-restricted group.
Therefore, the tumor suppressive effects observed in the present study are unlikely to be due entirely to reduced food intake and the associated reduction in body weight gain. Furthermore, when methyl donor deficiency was delayed until 10 weeks of age, mice continued to lose weight (up to 40% at the end of the study) as shown in Supplementary Fig. S1, yet the cancer protection was essentially lost. In addition, low serum folate levels were comparable in both study groups. Moreover, the colons of methyl deficient animals exhibited gene expression profiles resembling published human data, yet human subjects were under strict dietary control and did not lose weight during the three-month study (1). Thus these data indicate that the tumor suppressive effect is most likely related to the timing and extent of methyl donor deficiency rather than simply caloric restriction.
In previous studies, the timing of folate intervention was also found to influence the number of intestinal tumors in ApcMin/+ mice. When moderate folate supplementation was started prior to the formation of neoplastic foci in three week-old mice, there was a significant decrease in the number of intestinal and colonic tumors (35). However, when folate levels were moderately reduced in six week-old mice, after neoplastic foci have developed, only small intestinal adenomas were reduced (35). The same group also reported that dietary folate supplementation at weaning suppresses both ileal and colonic tumor formation (24). The present study shows that methyl donor depletion suppresses tumor formation when the dietary intervention was initiated at 5 weeks of age, at just about the time when neoplastic foci are established in this model (35). In contrast to a previous study (35), however, the suppressive effect was lost when the MDD intervention was initiated at 10 weeks of age after establishment of neoplastic foci. Additionally, Lindzon et al. (36) tested the effect of folate supplementation in an azoxymethane-treated rat model. Supplementation with folic acid beginning six weeks after carcinogen treatment was found to promote the growth of pre-neoplastic aberrant crypt foci and tumors (36).
In the present study, we describe significant alterations occurring within a number of signaling networks associated with the colonic mucosa in ApcMin/+ mice that result from methyl donor deficiency. Using Gene Set Enrichment Analysis, we demonstrate that several of the most significantly enriched gene categories are related to immune and inflammatory response. Within these categories of inflammatory genes showing significant down-regulation are a large number of cytokines and chemokines (Fig. 7B). Canonical pathway analyses showed that chemokine receptor signaling and innate and mucosal immune responses were also down-regulated, in remarkable agreement with our recent human study in which dietary folate levels were reduced for up to 8 weeks (Fig. 9) (1). In the human study, folate depletion also resulted in reduced expression of a number of immune response genes, including most notably cytokines and chemokines (1). Interestingly, folate supplementation in the same human study increased the expression of inflammatory and immune response genes (1). In a related human study, the administration of 1.2 mg folic acid for twelve weeks increased the levels of serum proteins involved in the regulation and activation of immune function such as acute phase reactants and complement fixation (13). The analysis of circulating inflammatory markers obtained from the human Aspirin/Folate Polyp Prevention trial suggested that folic acid administration antagonizes the colon cancer suppressive effects of aspirin (14). Taken together, these studies suggest that folate/methyl donor status may modulate the intestinal inflammatory response.
Although we found that methyl donor deficiency reduced the expression of a panel of genes associated with the immune response, the impact of these expression changes on the inflammatory response within the colonic mucosa is not known at the present time. There is abundant evidence supporting the association of chronic inflammation and colon cancer risk (37), and numerous inflammatory signals are important modulators of colon cancer risk. In fact, the suppression of intestinal inflammation in ApcMin/+ mice has already been shown to reduce tumor burden (26, 38, 39). In addition, the use of anti-inflammatory agents such as NSAIDs can also reduce this risk, but the significant potential for serious adverse effects limit the long-term use of these agents for chemoprevention (40, 41). Our mouse data clearly show that folate/methyl donor depletion does in fact markedly reduce tumor incidence in a high-risk animal model, but further studies are necessary to more definitely establish the implications of this dietary intervention on the human colonic mucosa.
In summary, the present study addresses the influence of folate/methyl donor status on intestinal tumorigenesis. We demonstrate that this dietary restriction suppresses intestinal tumorigenesis in ApcMin/+ mice. Although the tumor suppression is most dramatic in the small intestine, the primary site of tumor formation in this model, a trend towards tumor protection was also observed in the colon. Importantly, the protective effect of methyl donor depletion is strongly dependent upon the timing and duration of the dietary intervention. Gene expression profiling within the normal colonic mucosa identified a striking down-regulation by dietary methyl donor depletion of a large number of genes involved in the immune and inflammatory response. These gene expression changes in mice generally resemble the results of our human folate depletion study (1), validating the use of this mouse model to study the interplay between dietary interventions and colon cancer risk.
Supplementary Material
Beginning at 10 weeks of age, mice were placed on MDS or MDD diets. Body weights were recorded weekly during the entire experimental period. Each data point represents the average body weight ± SEM for Group III (n=5) and Group IV (n=8). Note that at the end of the study, there was an ~ 40% reduction in body weight in mice maintained on the MDD diet.
Acknowledgments
Supported in part by NIH Grant 1R01CA125691, a generous gift from the Garre Family to the University of Connecticut Foundation and the Neag Comprehensive Cancer Center.
Footnotes
Competing Interest: None to declare for all authors
References
- 1.Protiva P, Mason JB, Liu Z, Hopkins ME, Nelson C, Marshall JR, et al. Altered folate availability modifies the molecular environment of the human colorectum: implications for colorectal carcinogenesis. Cancer Prev Res (Phila) 2011;4:530–543. doi: 10.1158/1940-6207.CAPR-10-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason JB. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr Rev. 2009;67:206–212. doi: 10.1111/j.1753-4887.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulrich CM, Potter JD. Folate and cancer--timing is everything. Jama. 2007;297:2408–2409. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132:2350S–2355S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 5.Harnack L, Jacobs DR, Jr, Nicodemus K, Lazovich D, Anderson K, Folsom AR. Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer. 2002;43:152–158. doi: 10.1207/S15327914NC432_5. [DOI] [PubMed] [Google Scholar]
- 6.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005;113:825–828. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- 7.Farber S, Cutler EC, Hawkins JW, Harrison JH, Peirce EC, 2nd, Lenz GG. The Action of Pteroylglutamic Conjugates on Man. Science. 1947;106:619–621. doi: 10.1126/science.106.2764.619. [DOI] [PubMed] [Google Scholar]
- 8.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 9.Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. Jama. 2009;302:2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101:432–435. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson TM, Weinstein SJ, Pfeiffer RM, Hollenbeck AR, Subar AF, Schatzkin A, et al. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutr. 2011;94:1053–1062. doi: 10.3945/ajcn.110.002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens VL, McCullough ML, Sun J, Jacobs EJ, Campbell PT, Gapstur SM. High levels of folate from supplements and fortification are not associated with increased risk of colorectal cancer. Gastroenterology. 2011;141:98–105. e1. doi: 10.1053/j.gastro.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Duthie SJ, Horgan G, de Roos B, Rucklidge G, Reid M, Duncan G, et al. Blood folate status and expression of proteins involved in immune function, inflammation, and coagulation: biochemical and proteomic changes in the plasma of humans in response to long-term synthetic folic acid supplementation. J Proteome Res. 2010;9:1941–1950. doi: 10.1021/pr901103n. [DOI] [PubMed] [Google Scholar]
- 14.Ho GY, Xue X, Cushman M, McKeown-Eyssen G, Sandler RS, Ahnen DJ, et al. Antagonistic effects of aspirin and folic acid on inflammation markers and subsequent risk of recurrent colorectal adenomas. J Natl Cancer Inst. 2009;101:1650–1654. doi: 10.1093/jnci/djp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walzem RL, Clifford AJ. Folate deficiency in rats fed diets containing free amino acids or intact proteins. J Nutr. 1988;118:1089–1096. doi: 10.1093/jn/118.9.1089. [DOI] [PubMed] [Google Scholar]
- 16.Rong N, Selhub J, Goldin BR, Rosenberg IH. Bacterially synthesized folate in rat large intestine is incorporated into host tissue folyl polyglutamates. J Nutr. 1991;121:1955–1959. doi: 10.1093/jn/121.12.1955. [DOI] [PubMed] [Google Scholar]
- 17.Tamura T. Microbiological assay of folate. In: Picciano MF, Stokstad ELR, Gregory JF, editors. Folic acid metabolism in health and disease. New York: Wiley-Liss; 1990. pp. 121–137. [Google Scholar]
- 18.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 21.Clifford AJ, Wilson DS, Bills ND. Repletion of folate-depleted rats with an amino acid-based diet supplemented with folic acid. J Nutr. 1989;119:1956–1961. doi: 10.1093/jn/119.12.1956. [DOI] [PubMed] [Google Scholar]
- 22.Kim YI, Hayek M, Mason JB, Meydani SN. Severe folate deficiency impairs natural killer cell-mediated cytotoxicity in rats. J Nutr. 2002;132:1361–1367. doi: 10.1093/jn/132.6.1361. [DOI] [PubMed] [Google Scholar]
- 23.Kim YI, Shirwadkar S, Choi SW, Puchyr M, Wang Y, Mason JB. Effects of dietary folate on DNA strand breaks within mutation-prone exons of the p53 gene in rat colon. Gastroenterology. 2000;119:151–161. doi: 10.1053/gast.2000.8518. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60:5434–5440. [PubMed] [Google Scholar]
- 25.Hao X, Sun Y, Yang CS, Bose M, Lambert JD, Ju J, et al. Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer. 2007;59:62–69. doi: 10.1080/01635580701365050. [DOI] [PubMed] [Google Scholar]
- 26.Chae WJ, Bothwell AL. IL-17F deficiency inhibits small intestinal tumorigenesis in Apc(Min/+) mice. Biochem Biophys Res Commun. 2011;414:31–36. doi: 10.1016/j.bbrc.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Tannenbaum A. Effects of varying caloric intake upon tumor incidence and tumor growth. Annals of the New York Academy of Sciences. 1947;49:5–18. [Google Scholar]
- 28.Kritchevsky D. Caloric restriction and experimental carcinogenesis. Toxicol Sci. 1999;52:13–16. doi: 10.1093/toxsci/52.2.13. [DOI] [PubMed] [Google Scholar]
- 29.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mai V, Colbert LH, Berrigan D, Perkins SN, Pfeiffer R, Lavigne JA, et al. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res. 2003;63:1752–1755. [PubMed] [Google Scholar]
- 31.Steinbach G, Heymsfield S, Olansen NE, Tighe A, Holt PR. Effect of caloric restriction on colonic proliferation in obese persons: implications for colon cancer prevention. Cancer Res. 1994;54:1194–1197. [PubMed] [Google Scholar]
- 32.Tannenbaum A, Silverstone H. The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer Res. 1949;9:724–727. [PubMed] [Google Scholar]
- 33.Cheney KE, Liu RK, Smith GS, Meredith PJ, Mickey MR, Walford RL. The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J Gerontol. 1983;38:420–430. doi: 10.1093/geronj/38.4.420. [DOI] [PubMed] [Google Scholar]
- 34.Klurfeld DM, Welch CB, Davis MJ, Kritchevsky D. Determination of degree of energy restriction necessary to reduce DMBA-induced mammary tumorigenesis in rats during the promotion phase. J Nutr. 1989;119:286–291. doi: 10.1093/jn/119.2.286. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Sohn KJ, Medline A, Ash C, Gallinger S, Kim YI. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/−Msh2−/− mice. Cancer Res. 2000;60:3191–3199. [PubMed] [Google Scholar]
- 36.Lindzon GM, Medline A, Sohn KJ, Depeint F, Croxford R, Kim YI. Effect of folic acid supplementation on the progression of colorectal aberrant crypt foci. Carcinogenesis. 2009;30:1536–1543. doi: 10.1093/carcin/bgp152. [DOI] [PubMed] [Google Scholar]
- 37.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 38.Barbour KW, Davis T, White A, Baumann H, Berger FG. Haptoglobin, inflammation, and tumorigenesis in the MIN mouse. Redox Rep. 2001;6:366–368. doi: 10.1179/135100001101536553. [DOI] [PubMed] [Google Scholar]
- 39.Murphy EA, Davis JM, McClellan JL, Gordon BT, Carmichael MD. Curcumin's effect on intestinal inflammation and tumorigenesis in the ApcMin/+ mouse. J Interferon Cytokine Res. 2011;31:219–226. doi: 10.1089/jir.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1728–1735. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992;327:749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Beginning at 10 weeks of age, mice were placed on MDS or MDD diets. Body weights were recorded weekly during the entire experimental period. Each data point represents the average body weight ± SEM for Group III (n=5) and Group IV (n=8). Note that at the end of the study, there was an ~ 40% reduction in body weight in mice maintained on the MDD diet.