Background: The regulatory light chains of smooth muscle myosin are phosphorylated at Ser19 and Thr18.
Results: Phosphorylation at Thr18 does not increase force elicited by Ser19 phosphorylation, but reduces the rate of relaxation.
Conclusion: Diphosphorylation slows relaxation compared with monophosphorylation at Ser19.
Significance: Knowledge of the functional effects of myosin diphosphorylation is important for understanding the underlying causes of hypercontractility.
Keywords: Calcium, Myosin, Protein Kinases, Protein Phosphorylation, Smooth Muscle
Abstract
The principal signal to activate smooth muscle contraction is phosphorylation of the regulatory light chains of myosin (LC20) at Ser19 by Ca2+/calmodulin-dependent myosin light chain kinase. Inhibition of myosin light chain phosphatase leads to Ca2+-independent phosphorylation at both Ser19 and Thr18 by integrin-linked kinase and/or zipper-interacting protein kinase. The functional effects of phosphorylation at Thr18 on steady-state isometric force and relaxation rate were investigated in Triton-skinned rat caudal arterial smooth muscle strips. Sequential phosphorylation at Ser19 and Thr18 was achieved by treatment with adenosine 5′-O-(3-thiotriphosphate) in the presence of Ca2+, which induced stoichiometric thiophosphorylation at Ser19, followed by microcystin (phosphatase inhibitor) in the absence of Ca2+, which induced phosphorylation at Thr18. Phosphorylation at Thr18 had no effect on steady-state force induced by Ser19 thiophosphorylation. However, phosphorylation of Ser19 or both Ser19 and Thr18 to comparable stoichiometries (0.5 mol of Pi/mol of LC20) and similar levels of isometric force revealed differences in the rates of dephosphorylation and relaxation following removal of the stimulus: t½ values for dephosphorylation were 83.3 and 560 s, and for relaxation were 560 and 1293 s, for monophosphorylated (Ser19) and diphosphorylated LC20, respectively. We conclude that phosphorylation at Thr18 decreases the rates of LC20 dephosphorylation and smooth muscle relaxation compared with LC20 phosphorylated exclusively at Ser19. These effects of LC20 diphosphorylation, combined with increased Ser19 phosphorylation (Ca2+-independent), may underlie the hypercontractility that is observed in response to certain physiological contractile stimuli, and under pathological conditions such as cerebral and coronary arterial vasospasm, intimal hyperplasia, and hypertension.
Introduction
Smooth muscle contraction is activated by an increase in cytosolic free Ca2+ concentration ([Ca2+]i), whereupon Ca2+ saturates the four Ca2+-binding sites of calmodulin (1). (Ca2+)4-calmodulin activates myosin light chain kinase (MLCK),2 which catalyzes phosphorylation of the motor protein myosin II at Ser19 of its two 20-kDa regulatory light chain subunits (LC20) (2). This simple phosphorylation reaction markedly increases the actin-activated MgATPase activity of myosin, which provides the energy for cross-bridge cycling and the development of force or shortening of the muscle (3). MLCK is also capable of phosphorylating LC20 at Thr18 in vitro, but this requires very high (unphysiological) concentrations of the kinase (4, 5). Relaxation follows the removal of Ca2+ from the cytosol, which inactivates MLCK, and myosin is dephosphorylated by myosin light chain phosphatase (MLCP), a type 1 Ser/Thr phosphatase (6).
We and others have demonstrated that smooth muscle contraction can be elicited in the absence of Ca2+ by treatment with inhibitors of type 1 protein phosphatases (7–19). For example, treatment of Triton-skinned rat caudal arterial smooth muscle strips with the membrane-impermeant phosphatase inhibitor microcystin in the absence of Ca2+ (presence of EGTA) elicited a slow, sustained contractile response that correlated with LC20 phosphorylation (16). Further investigation revealed that this Ca2+-independent phosphorylation occurred at both Ser19 and Thr18, referred to as diphosphorylation (16). The kinase responsible was shown not to be MLCK on the basis of the following observations: (i) purified MLCK is inactive in the absence of Ca2+ (20–22); (ii) LC20 diphosphorylation requires unphysiologically high MLCK concentrations (5); (iii) MLCK inhibitors have no effect on Ca2+-independent, microcystin-induced LC20 diphosphorylation and contraction of Triton-skinned tissue (16, 19); (iv) removal of endogenous calmodulin by treatment of Triton-skinned smooth muscle strips with the calmodulin antagonist trifluoperazine in the presence of Ca2+ does not affect Ca2+-independent, microcystin-induced LC20 diphosphorylation and contraction (23); (v) endogenous LC20 in smooth muscle myofilaments is phosphorylated in the absence of Ca2+ at Ser19 or Thr18 alone, as well as at both sites (16), whereas purified MLCK (at high concentration) only phosphorylates Thr18 after Ser19 has been phosphorylated (4); (vi) stimuli that induce maximal activation of MLCK in smooth muscle tissues (e.g. membrane depolarization of intact vascular smooth muscle strips with an optimal KCl concentration, or addition of a maximal concentration of Ca2+ to permeabilized strips) induce LC20 phosphorylation exclusively at Ser19 (23, 24); (vii) Ca2+-independent LC20 kinase activity can be separated from MLCK chromatographically (16); and (viii) the Ca2+-independent LC20 kinase, unlike MLCK, does not use ATPγS as a substrate (this study). We purified this Ca2+-independent LC20 kinase activity from chicken gizzard myofilaments and identified it as integrin-linked kinase (ILK) (17). Bacterially expressed ILK phosphorylated LC20 in intact myosin in a Ca2+-independent manner (17). Approximately 50% of cellular ILK was retained in Triton-skinned smooth muscle and may be associated with MLCP because purified phosphatase preparations contain co-purifying ILK (19). It should be noted that ILK has often been described as a pseudokinase (25), but the evidence for its bona fide kinase activity is substantial (26, 27). Zipper-interacting protein kinase (ZIPK) has also been implicated in the diphosphorylation of LC20 (18, 28), although inhibition of ZIPK activity in Triton-skinned rat caudal arterial smooth muscle did not affect microcystin-induced LC20 diphosphorylation or contraction (19), suggesting that ILK is likely the responsible kinase in these conditions.
The diphosphorylation site in LC20 is highly evolutionarily conserved: the sequence around Thr18–Ser19 (Arg-Ala-Thr-Ser-Asn-Val-Phe-Ala-Met-Phe; residues 16–25), is identical throughout the animal kingdom and is also found in a homolog of LC20 (29) in the genome of the unicellular choanoflagellate Monosiga brevicollis (30); choanoflagellates appear to be the closest living relatives of metazoans (30, 31). LC20 isoforms are also found in non-muscle myosin II, and contain phosphorylation sites corresponding to Thr18 and Ser19 of smooth muscle LC20 that play an important role in regulation of motility (32).
The functional effects of phosphorylation of LC20 at Ser19 and Thr18 have been investigated in vitro using purified LC20 or intact myosin as substrates at high concentrations of MLCK. Ikebe and Hartshorne (4) showed that the actin-activated MgATPase activity of diphosphorylated myosin was 2–3-fold greater than that of myosin phosphorylated exclusively at Ser19. This increase in actomyosin MgATPase activity can be attributed to a doubling of the Vmax when both sites are phosphorylated (33–35). In the in vitro motility assay, however, myosin phosphorylated at both Ser19 and Thr18 moved actin filaments at a rate similar to myosin phosphorylated at Ser19 alone (35, 36).
LC20 diphosphorylation has been observed in various smooth muscle tissues treated with a variety of contractile stimuli (37–41), and several instances of diphosphorylation of LC20 have been reported in pathological conditions associated with hypercontractility (42–46). This prompted us to further investigate the functional effects of LC20 diphosphorylation in vascular smooth muscle.
EXPERIMENTAL PROCEDURES
Materials
All chemicals were analytical grade unless otherwise indicated and purchased from EMD Chemicals (Gibbstown, NJ). Triton X-100 and ATPγS were purchased from Sigma, microcystin-LR from Alexis Biochemicals (San Diego, CA), calyculin-A and okadaic acid from Calbiochem, and dithiothreitol (DTT) from ICN Biochemicals (Aurora, OH). Calmodulin (47) and MLCK (48) were purified from chicken gizzard as previously described. Antibodies to LC20 (polyclonal anti-pan LC20) were from Santa Cruz Biotechnology (Santa Cruz, CA) and used at 1:500 dilution; phosphospecific antibodies to LC20 phosphorylated at Ser19 (monoclonal anti-pS19-LC20) were from Cell Signaling (Danvers, MA) and used at 1:1,000 dilution; phosphospecific antibodies to LC20 phosphorylated at Thr18 (polyclonal anti-pT18-LC20) were from 21st Century Biochemicals (Marlboro, MA) and used at 1:2,000 dilution; phosphospecific antibodies to LC20 phosphorylated at both Thr18 and Ser19 (polyclonal anti-pT18,pS19-LC20) were from Cell Signaling and used at 1:500 dilution. Polyclonal phosphospecific antibodies to MYPT1 phosphorylated at Thr697 or Thr855 were purchased from Upstate USA (Charlottesville, VA) and used at 1:1,000 dilution. Polyclonal anti-actin was from Cytoskeleton Inc. (Denver, CO) and used at 1:1,000 dilution. Secondary antibodies coupled to horseradish peroxidase were purchased from Chemicon (Temecula, CA).
Buffer Compositions
HEPES-Tyrode (H-T) buffer contained 137 mm NaCl, 2.7 mm KCl, 1 mm MgCl2, 1.8 mm CaCl2, 5.6 mm glucose, 10 mm HEPES, pH 7.4. Ca2+-free H-T buffer contained 140.6 mm NaCl, 2.7 mm KCl, 1 mm MgCl2, 5.6 mm glucose, 10 mm HEPES, pH 7.4. Buffer A contained 30 mm TES, 0.5 mm DTT, 50 mm KCl, 5 mm K2EGTA, 150 mm sucrose, pH 7.4. pCa 9 solution contained 4 mm K2EGTA, 5.83 mm MgCl2, 0.5 mm dithioerythritol, 20 mm TES, pH 6.9, and an ATP regenerating system composed of 3.9 mm Na2ATP, 7.56 mm potassium propionate, 16.2 mm phosphocreatine, and 30 units/ml of creatine kinase. The free [Ca2+] of this pCa 9 solution was determined to be 6 nm using fura-2. pCa 4.5 solution contained 4 mm CaEGTA, 5.66 mm MgCl2, 0.5 mm dithioerythritol, 20 mm TES, pH 6.9, and the ATP regenerating system.
Tissue Preparation and Force Measurements
Caudal arteries were removed from male Sprague-Dawley rats (300–350 g) that had been anesthetized with halothane and euthanized according to protocols consistent with the standards of the Canadian Council on Animal Care and approved by the University of Calgary Animal Care and Use Committee. The arteries were cleaned of excess adventitia and adipose tissue in Ca2+-free H-T buffer. Segments were placed over a 0.31-mm needle and moved back and forth 40 times to remove the endothelium, cut into helical strips (1.5 × 6 mm), mounted on a Grass isometric force transducer (model FT03C) connected to a PowerLab (ADInstruments) 8-channel recording device with a resting tension of 0.45 g and incubated for 20 min in H-T buffer (bath volume = 0.8 ml). Tissues were stimulated at least twice with H-T buffer containing 87 mm KCl (the increase in [KCl] was balanced by a decrease in [NaCl]) with a 20-min interval of relaxation in Ca2+-free H-T buffer. Muscle strips were then incubated in Ca2+-free H-T buffer and either used for experiments with intact tissue or were skinned (demembranated) as follows. Tissues for skinning were incubated for 5 min in Buffer A and subsequently demembranated by incubation for 2 h in Buffer A containing 1% (v/v) Triton X-100. Skinned tissues were then washed 3 times (5 min each) in pCa 9 solution prior to treatments described in the figure legends.
Quantification of LC20 Phosphorylation Levels
At selected times during experimental protocols, tissues were immersed in cold 10% trichloroacetic acid, acetone, 10 mm DTT, washed three times (1 min each) with acetone/DTT, and lyophilized for 36 h. Dried tissues were immersed in 1 ml of SDS gel sample buffer (2% (w/v) SDS, 100 mm DTT, 10% (v/v) glycerol, 0.01% bromphenol blue, 60 mm Tris-HCl, pH 6.8), heated to 95 °C for 2 min, cooled to room temperature, and rotated overnight at 4 °C. Samples (40 μl) were subjected to phosphate affinity SDS-PAGE using an acrylamide-pendant phosphate-binding tag (Phos-tag SDS-PAGE with 12.5% acrylamide) at 30 mA/gel for 70 min in mini-gels in which 0.05 mm Phos-tag acrylamide (NARD Institute, Japan) and 0.1 mm MnCl2 were incorporated into the running gel (49). Separated proteins were transferred to PVDF membranes (Roche Applied Science) overnight at 27 volts and 4 °C in 25 mm Tris-HCl, pH 7.5, 192 mm glycine, 10% (v/v) methanol. Proteins were fixed on the membrane by treatment with 0.5% glutaraldehyde in phosphate-buffered saline (137 mm NaCl, 2.68 mm KCl, 10 mm Na2HPO4, 1.76 mm KH2PO4) for 45 min. Membranes were then incubated with 5% nonfat dried milk in Tris-buffered saline containing Tween (TBST: 20 mm Tris-HCl, pH 7.5, 137 mm NaCl, 3 mm KCl, 0.05% Tween 20) for 1–2 h, followed by primary antibody in TBST overnight at 4 °C. Following washout of the primary antibody, membranes were incubated with secondary antibody (anti-rabbit or anti-mouse IgG-horseradish peroxidase conjugate in TBST at 1:10,000 dilution) for 2 h at room temperature, washed with TBST (4 × 5 min), and then with TBS (1 × 5 min) before chemiluminescence signal detection using the Super-Signal West Femto reagent (Thermo Scientific, Rockford, IL). The emitted light was detected and quantified with a chemiluminescence imaging analyzer (LAS3000mini; Fujifilm) and images were analyzed with MultiGauge version 3.0 software.
Data Analysis
Values are presented as the mean ± S.E., with n indicating the number of animals used; several muscle strips were used from each animal. Statistical analyses were performed with SigmaPlot and data were analyzed by Student's t test, with p < 0.05 considered to indicate statistically significant differences.
RESULTS
Ca2+-independent, Microcystin-induced LC20 Diphosphorylation and Contraction
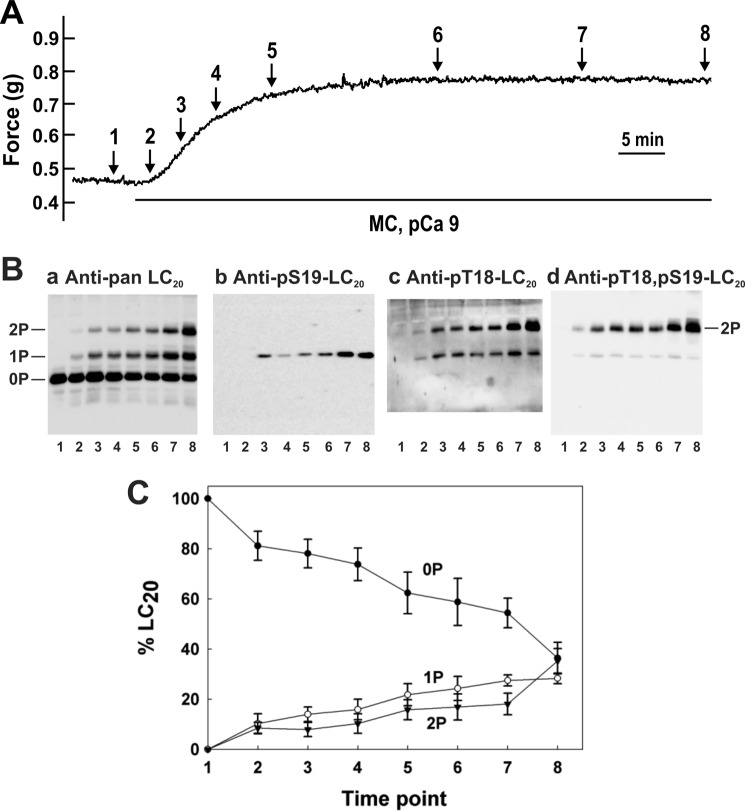
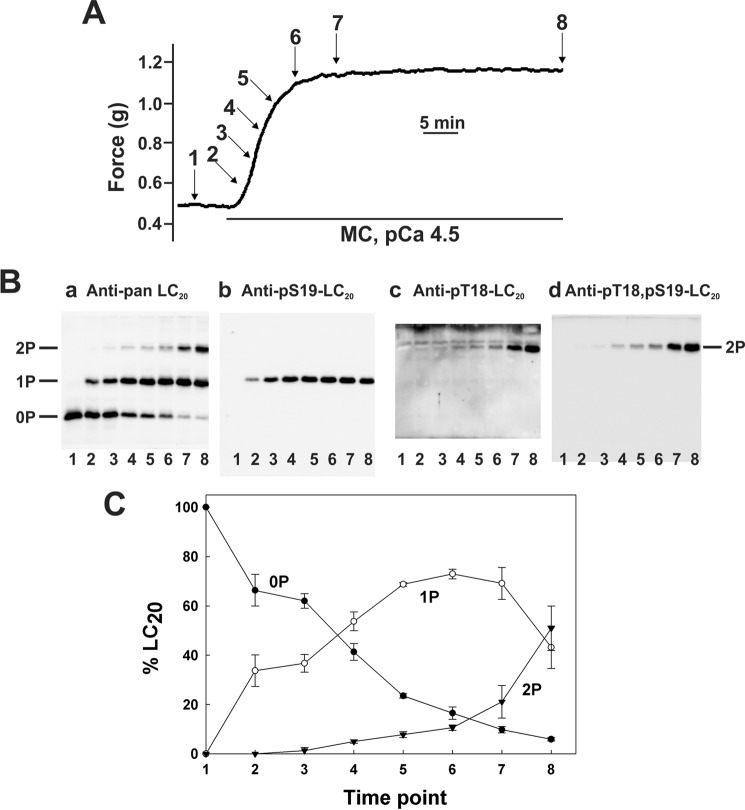
Fig. 1A show the time course of Ca2+-independent contraction of Triton-skinned rat caudal arterial smooth muscle strips in response to the phosphatase inhibitor microcystin (t½ = 451.1 ± 13.4 s (n = 8)). Tissues were immersed in TCA/acetone/DTT at the indicated times during the contractile response, washed with acetone, lyophilized, and tissue proteins were extracted in SDS gel sample buffer. Phosphorylated and unphosphorylated forms of LC20 were separated by Phos-tag SDS-PAGE (49) and detected by Western blotting with anti-pan LC20, which recognizes all forms of the protein (Fig. 1B, panel a). The three separated bands were identified by Western blotting with phosphospecific antibodies to LC20 (Fig. 1B, panels b-d). In resting tissue in the absence of Ca2+ (lane 1), only unphosphorylated LC20 was detected. Treatment with microcystin in the absence of Ca2+ induced a time-dependent increase in mono- and diphosphorylated LC20. The monophosphorylated band contained a mixture of LC20 phosphorylated exclusively at Ser19 (Fig. 1B, panel b) and LC20 phosphorylated exclusively at Thr18 (Fig. 1B, panel c). The antibody to Thr(p)18-LC20 also recognized diphosphorylated LC20 (Fig. 1B, panel c), identified as containing both Thr(P)18 and Ser(P)19 in Fig. 1B, panel d. The cumulative quantitative data in Fig. 1C show the time-dependent increase in mono- and diphosphorylation, and the corresponding decrease in unphosphorylated LC20 in response to microcystin in the absence of Ca2+.
FIGURE 1.
Ca2+-independent contraction and LC20 diphosphorylation in Triton-skinned rat caudal arterial smooth muscle in response to microcystin at pCa 9. A, Triton-skinned rat caudal arterial smooth muscle strips mounted on a force transducer in pCa 9 solution were treated with microcystin (1 μm). A typical contractile response is shown. Separate tissues were harvested at the indicated times during the contraction for analysis of LC20 phosphorylation by Phos-tag SDS-PAGE and Western blotting (B) with antibodies to LC20 (panel a), Ser(P)19-LC20 (panel b), Thr(P)18-LC20 (panel c), and Thr(P)18,Ser(P)19-LC20 (panel d). Numbers below the gel lanes correspond to the time points in A. C, cumulative quantitative data showing the proportions of unphosphorylated (0P, closed circles), mono- (1P, open circles), and diphosphorylated LC20 (2P, closed inverted triangles) as a function of time. Values represent the mean ± S.E. (n = 3).
Ca2+-independent, Calyculin-A-induced LC20 Diphosphorylation and Contraction
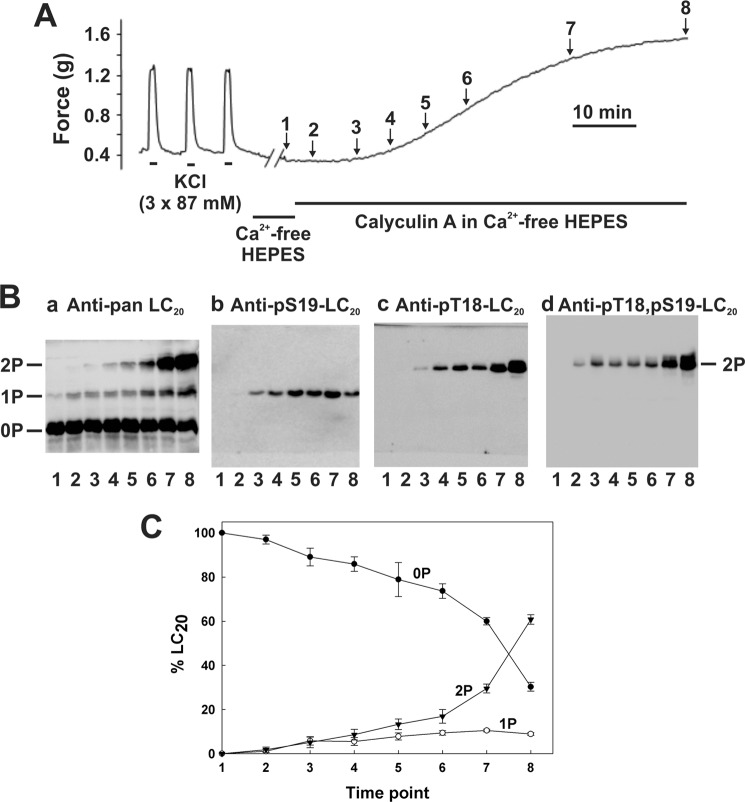
Treatment of intact rat caudal arterial smooth muscle with the membrane-permeant phosphatase inhibitor calyculin-A in Ca2+-free solution also induced LC20 mono- and diphosphorylation, which correlated with force development with a t½ of 1326 ± 96 s (n = 6) (Fig. 2). In this case, the amount of monophosphorylated LC20 detected was significantly less (Fig. 2C) than was observed in the Triton-skinned tissue in response to microcystin (Fig. 1C). It is also noteworthy that the steady-state force achieved in response to calyculin-A in the absence of Ca2+ appeared to be significantly higher than the force induced by a strong depolarizing stimulus (87 mm KCl) (Fig. 2A). This prompted us to address the question: does LC20 diphosphorylation elicit more steady-state isometric force than monophosphorylation?
FIGURE 2.
Ca2+-independent contraction and LC20 diphosphorylation in intact rat caudal arterial smooth muscle in response to calyculin-A in Ca2+-free solution. A, intact rat caudal arterial smooth muscle strips mounted on a force transducer in Ca2+-containing H-T buffer were induced to contract 3 times with 87 mm KCl. The tissue was then washed extensively with Ca2+-free H-T buffer (which emptied intracellular Ca2+ stores because the contractile response to caffeine was abolished; data not shown) prior to treatment with calyculin-A (0.5 μm) in Ca2+-free solution. Separate tissues were harvested at the indicated times during the contraction for analysis of LC20 phosphorylation by Phos-tag SDS-PAGE and Western blotting (B) with antibodies to LC20 (panel a), Ser(P)19-LC20 (panel b), Thr(P)18-LC20 (panel c), and Thr(P)18,Ser(P)19-LC20 (panel d). Numbers below the gel lanes correspond to the time points in A. C, cumulative quantitative data showing the proportions of unphosphorylated (0P, closed circles), mono- (1P, open circles), and diphosphorylated LC20 (2P, closed inverted triangles) as a function of time. Values represent the mean ± S.E. (n = 3).
KCl-induced LC20 Monophosphorylation and Contraction
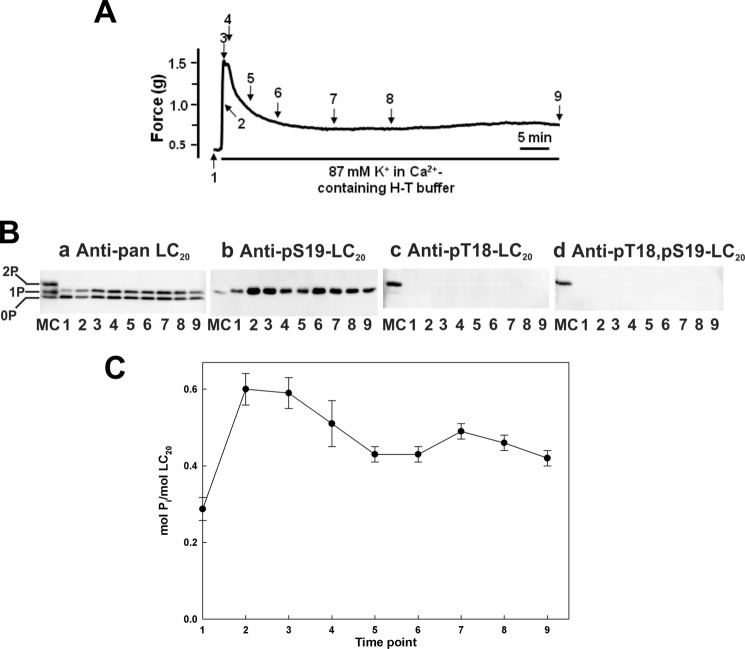
We first demonstrated that an increase in cytosolic free Ca2+ concentration induced exclusively monophosphorylation of LC20 at Ser19. Ca2+ entry via voltage-gated Ca2+ channels was activated by KCl-induced membrane depolarization of intact rat caudal arterial smooth muscle strips, which induced a rapid contractile response (t½ = 10.2 ± 0.2 s (n = 29)) (Fig. 3A). Analysis of the LC20 phosphorylation time course revealed phosphorylation at Ser19 (Fig. 3B, panel b) with no phosphorylation at Thr18 (Fig. 3B, panel c) or diphosphorylation at Thr18 and Ser19 (Fig. 3B, panels a and d). LC20 phosphorylation stoichiometry peaked at ∼0.6 mol of Pi/mol of LC20 (Fig. 3C).
FIGURE 3.
Contraction and LC20 phosphorylation in intact rat caudal arterial smooth muscle in response to KCl-induced depolarization in the presence of Ca2+. A, intact rat caudal arterial smooth muscle strips were treated with 87 mm KCl in Ca2+-containing H-T buffer and the contractile response was recorded. Separate tissues were harvested at the indicated times during the contraction for analysis of LC20 phosphorylation by Phos-tag SDS-PAGE and Western blotting with antibodies to LC20 (panel a), Ser(P)19-LC20 (panel b), Thr(P)18-LC20 (panel c), and Thr(P)18,Ser(P)19-LC20 (panel d). Numbers below the gel lanes correspond to the time points in A. MC denotes control tissue (Triton-skinned rat caudal arterial smooth muscle treated with microcystin at pCa 9 for 60 min) to identify unphosphorylated, mono-, and diphosphorylated LC20 bands. C, cumulative quantitative data showing the time course of LC20 phosphorylation stoichiometry; as shown in panel b, phosphorylation occurred exclusively at Ser19 under these conditions. Values represent the mean ± S.E. (n = 3).
Effects on Force and LC20 Phosphorylation of Sequential Treatment with Ca2+ and Microcystin
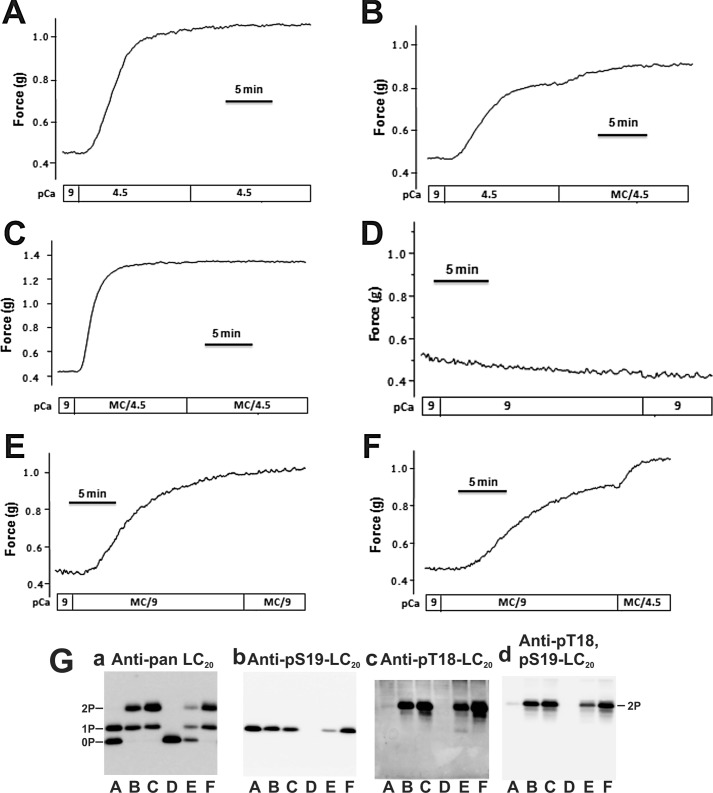
Similarly, addition of Ca2+ to Triton-skinned rat caudal arterial smooth muscle induced phosphorylation of LC20 exclusively at Ser19 (Fig. 4G, lanes A in panels a-d) with a t½ of 151.7 ± 4.8 s (n = 23) and an LC20 phosphorylation level of ∼0.5 mol of Pi/mol of LC20 (Table 1). Addition of microcystin at the plateau of a Ca2+-induced contraction resulted in a further increase in force of ∼25% (Fig. 4B and Table 2), which correlated with LC20 diphosphorylation (Fig. 4G, lanes B in panels a–d, and Table 1). If microcystin and Ca2+ were added together, a rapid contraction occurred (t½ of 65.3 ± 2.3 s (n = 15) compared with 151.7 ± 4.8 s (n = 23) for Ca2+ alone and 451.1 ± 13.4 s (n = 8) for microcystin at pCa 9), which was again accompanied by LC20 diphosphorylation (Fig. 4G, lanes C in panels a–d, and Table 1). No force development or LC20 phosphorylation was observed in the absence of Ca2+ and phosphatase inhibitor (Fig. 4, D and G, lanes D in panels a–d, and Table 1). If contraction was evoked by addition of microcystin in the absence of Ca2+, subsequent addition of Ca2+ elicited further force development (∼20%; Fig. 4F and Table 2) and LC20 diphosphorylation (Fig. 4G, lanes F in panels a–d, and Table 1) compared with control (Fig. 4, E and G, lanes E, and Tables 1 and 2). A more detailed analysis of the (Ca2+ + microcystin)-induced contraction revealed rapid phosphorylation of LC20 at Ser19 that can be attributed to MLCK activation by Ca2+, and a slower rate of phosphorylation at Thr18, due to ILK activity that is unmasked by the phosphatase inhibitor (Fig. 5).
FIGURE 4.
Effect of microcystin on Ca2+-induced contraction and Ca2+ on microcystin-induced contraction of Triton-skinned rat caudal arterial smooth muscle. A–F, Triton-skinned rat caudal arterial smooth muscle strips mounted on a force transducer in pCa 9 solution were treated as indicated. G, LC20 phosphorylation at the end of the protocols shown in A–F was analyzed by Phos-tag SDS-PAGE and Western blotting with antibodies to LC20 (panel a), Ser(P)19-LC20 (panel b), Thr(P)18-LC20 (panel c), and Thr(P)18,Ser(P)19-LC20 (panel d). Letters below the gel lanes correspond to panels A–F. Results are representative of at least 3 independent experiments.
TABLE 1.
Quantification of LC20 mono- and diphosphorylation in Triton-skinned rat caudal arterial smooth muscle
LC20 phosphorylation levels were quantified by Phos-tag SDS-PAGE (see Fig. 4G, panel a) in tissues treated as described in the legend to Fig. 4, A–F. Values indicate the levels of unphosphorylated (0P), monophosphorylated (1P), and diphosphorylated LC20 (2P) under the conditions indicated.
| Conditions | % Total LC20 |
n | ||
|---|---|---|---|---|
| 0P | 1P | 2P | ||
| pCa4.5/pCa 4.5 | 52.1 ± 3.2 | 47.9 ± 3.2 | 0 | 4 |
| pCa 4.5/MC, pCa 4.5 | 0 | 40.3 ± 2.6 | 59.6 ± 2.7 | 5 |
| MC, pCa 4.5/MC, pCa 4.5 | 0 | 27.8 ± 3.8 | 71.0 ± 4.3 | 4 |
| pCa 9/pCa 9 | 100 | 0 | 0 | 3 |
| MC, pCa 9/MC, pCa 9 | 41.7 ± 3.1 | 30.6 ± 2.5 | 27.7 ± 2.5 | 5 |
| MC, pCa 9/MC, pCa 4.5 | 0 | 34.3 ± 4.3 | 64.4 ± 4.6 | 5 |
TABLE 2.
The effects on steady-state isometric force of sequential treatment of Triton-skinned rat caudal arterial smooth muscle with Ca2+ and microcystin
Steady-state force measurements were made under the conditions indicated in the legend to Fig. 4, A–F. Values of Force (%) indicate the levels of steady-state force at the end of the protocol compared to that before transfer to the final bathing solution. For example, in the case of Fig. 4B, where the tissue was contracted with pCa 4.5 and then transferred to pCa 4.5 solution containing microcystin, steady-state force in the presence of Ca2+ and microcystin was 124.5 ± 2.2% of that in the presence of Ca2+ alone.
| Conditions | Force (%) | n |
|---|---|---|
| pCa 4.5/pCa 4.5 | 105.1 ± 1.4 | 4 |
| pCa 4.5/MC, pCa 4.5 | 124.5 ± 2.2 | 5 |
| MC, pCa 4.5/MC, pCa 4.5 | 100.8 ± 0.3 | 4 |
| MC, pCa 9/MC, pCa9 | 106.1 ± 1.8 | 4 |
| MC, pCa 9/MC, pCa 4.5 | 121.4 ± 3.0 | 5 |
FIGURE 5.
Contraction and LC20 diphosphorylation in Triton-skinned rat caudal arterial smooth muscle in response to microcystin at pCa 4.5. A, Triton-skinned rat caudal arterial smooth muscle strips mounted on a force transducer in pCa 9 solution were treated with microcystin (1 μm) at pCa 4.5. Separate tissues were harvested at the indicated times during the contraction for analysis of LC20 phosphorylation by Phos-tag SDS-PAGE and Western blotting (B) with antibodies to LC20 (panel a), Ser(P)19-LC20 (panel b), Thr(P)18-LC20 (panel c), and Thr(P)18,Ser(P)19-LC20 (panel d). Numbers below the gel lanes correspond to the time points in A. C, cumulative quantitative data showing the proportions of unphosphorylated (0P, closed circles), mono- (1P, open circles), and diphosphorylated LC20 (2P, closed inverted triangles) as a function of time. Values represent the mean ± S.E. (n = 3).
Effects on Force and LC20 Phosphorylation of Combined Treatment with KCl and Calyculin-A
Calyculin-A treatment of intact rat caudal arterial smooth muscle in the presence of extracellular Ca2+ elicited a slow, sustained contraction (Fig. 6, green trace) with a t½ of 1206 ± 102 s (n = 6), which was indistinguishable from the calyculin-A-induced contraction in Ca2+-free solution (t½ = 1326 ± 96 s (n = 6)) (Fig. 2A). Membrane depolarization in the presence of extracellular Ca2+ elicited a rapid increase in force (t½ = 10.2 ± 0.2 s (n = 29)), which subsequently declined to a steady-state level (Figs. 3A and 6, red trace). The simultaneous application of KCl and calyculin-A in the presence of extracellular Ca2+ elicited a contractile response (Fig. 6, black trace) that matched the superimposed contractions due to membrane depolarization (Fig. 6, red trace) and phosphatase inhibition (Fig. 6, green trace): the initial rapid contractile response in the presence of KCl and calyculin-A occurred with a t½ of 11.2 ± 0.6 s (n = 6), i.e. similar to the contraction induced by KCl treatment alone (t½ = 10.2 ± 0.2 s (n = 29)), whereas the slow, sustained contractile response occurred with a t½ of 1110 ± 84 s (n = 3), i.e. similar to the contraction induced by calyculin-A in Ca2+-free solution (t½ = 1326 ± 96 s (n = 6)). We hypothesize that the biphasic contractile response to KCl and calyculin-A involves two distinct mechanisms: the rapid response is attributable to membrane depolarization-mediated Ca2+ entry and MLCK activation, and the slow response to calyculin-A-mediated inhibition of MLCP with unmasking of Ca2+-independent LC20 kinase activity. These mechanisms are supported by measurements of site-specific LC20 phosphorylation during the time course of contraction in the presence of extracellular Ca2+ and following addition of both KCl and calyculin-A (Fig. 7). Thus, there was a rapid initial increase in LC20 monophosphorylation (Fig. 7B, panel a), which occurred exclusively at Ser19 (Fig. 7B, panels b and c), followed by a slight dephosphorylation (Fig. 7C) leading to partial relaxation (Fig. 7A). It was only at prolonged incubation times that diphosphorylation of LC20 was observed (Fig. 7B, panels a and d), which correlated with the slow, sustained phase of contraction (Fig. 7A).
FIGURE 6.
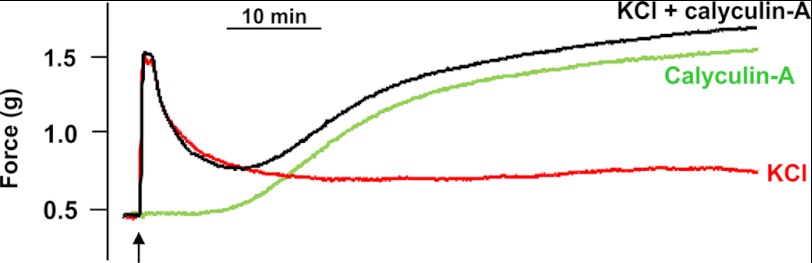
Comparison of the time courses of contraction of intact rat caudal arterial smooth muscle in response to: (i) KCl in the presence of extracellular Ca2+, (ii) calyculin-A in the presence of extracellular Ca2+, and (iii) a combination of KCl and calyculin-A in the presence of extracellular Ca2+. Membrane-intact rat caudal arterial smooth muscle strips, mounted on a force transducer in Ca2+-containing H-T buffer, were treated with KCl (87 mm) (red trace), calyculin-A (0.5 μm) (green trace), or both KCl and calyculin-A (black trace). The arrow indicates the time of application of the contractile stimulus.
FIGURE 7.
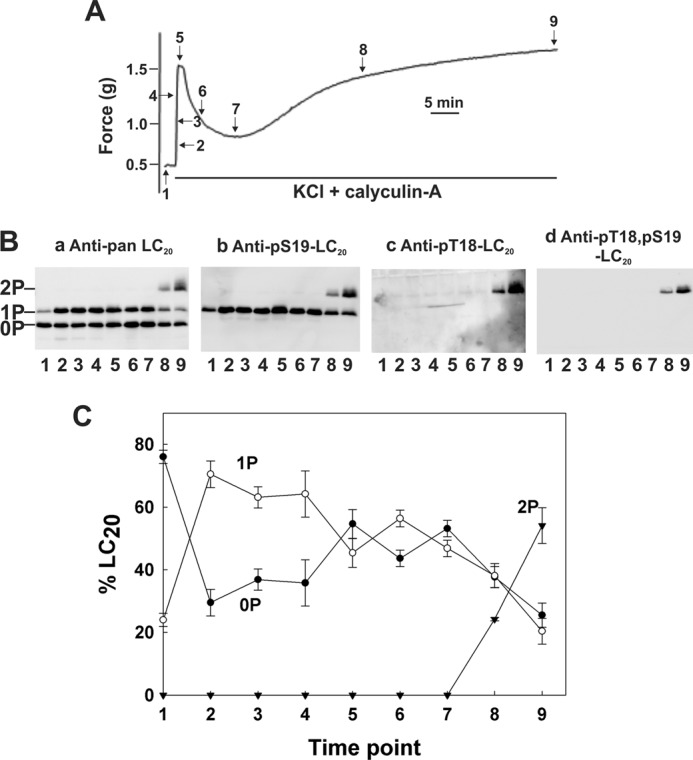
Contraction and LC20 diphosphorylation in intact rat caudal arterial smooth muscle in response to KCl and calyculin-A in the presence of extracellular Ca2+. A, membrane-intact rat caudal arterial smooth muscle strips, mounted on a force transducer in Ca2+-containing H-T buffer, were treated with KCl (87 mm) and calyculin-A (0.5 μm). Separate tissues were harvested at the indicated times during the contraction for analysis of LC20 phosphorylation by Phos-tag SDS-PAGE and Western blotting (B) with antibodies to LC20 (panel a), Ser(P)19-LC20 (panel b), Thr(P)18-LC20 (panel c), and Thr(P)18,Ser(P)19-LC20 (panel d). Numbers below the gel lanes correspond to the time points in A. C, cumulative quantitative data showing the proportions of unphosphorylated (0P, closed circles), mono- (1P, open circles), and diphosphorylated LC20 (2P, closed inverted triangles) as a function of time. Values represent the mean ± S.E. (n = 3).
Stoichiometric Phosphorylation of LC20 at Ser19 in Triton-skinned Tissue
The results described above suggest that phosphorylation of LC20 at Thr18 may increase the level of force that is achieved in intact or Triton-skinned rat caudal arterial smooth muscle as a result of Ser19 phosphorylation. Alternatively, the observed increases in force could be due to an increase in the total level of Ser19 phosphorylation, rather than phosphorylation at Thr18. To distinguish between these possibilities, it would be necessary to achieve stoichiometric phosphorylation exclusively at Ser19 and then observe whether or not phosphorylation at Thr18 has an additional effect on steady-state force. The next step, therefore, was to achieve stoichiometric phosphorylation exclusively at Ser19. Unfortunately, treatment of intact tissue with an optimal KCl concentration to elicit a maximal increase in [Ca2+]i, leading to maximal activation of MLCK, does not lead to stoichiometric phosphorylation of LC20 at Ser19 (Fig. 3). This is due to competing dephosphorylation of LC20 by MLCP, which is constitutively active. Likewise, in Triton-skinned tissue, addition of a maximal [Ca2+] fails to elicit stoichiometric LC20 phosphorylation at Ser19 for the same reason (Fig. 4G, lane A in panel a, and Table 1). We tested the possibility that the stoichiometry of LC20 phosphorylation could be increased by addition of exogenous calmodulin and MLCK to Triton-skinned tissue in the presence of Ca2+, recognizing the caveat that, if the MLCK concentration was too high, it would phosphorylate Thr18 as well. Whereas the addition of calmodulin in the absence or presence of MLCK did increase LC20 phosphorylation slightly, there remained a significant amount of unphosphorylated LC20, and a low level of LC20 diphosphorylation was observed (supplemental Fig. S1 and Table S1). This approach was, therefore, unsuitable for achieving stoichiometric phosphorylation at Ser19 in the absence of Thr18 phosphorylation.
An alternative approach to achieve stoichiometric LC20 phosphorylation was to use ATPγS to thiophosphorylate LC20: MLCK uses ATPγS as a substrate (50), but the thiophosphorylated protein is not a substrate for MLCP (51). This approach was used successfully with Triton-skinned rat caudal arterial smooth muscle (Fig. 8). Triton-skinned tissues were shown to be viable by contraction at pCa 4.5 in the presence of ATP and an ATP regenerating system, and relaxation following removal of Ca2+ (Fig. 8A). Following removal of ATP, incubation with ATPγS in the presence of Ca2+, but absence of ATP or an ATP regenerating system, resulted in stoichiometric thiophosphorylation of LC20 at Ser19 (Fig. 8B, lanes 2 and 3). It is noteworthy that thiophosphorylated LC20 migrates more rapidly upon Phos-tag SDS-PAGE than does phosphorylated LC20, which enables clear discrimination between phosphorylated and thiophosphorylated forms of the protein.
FIGURE 8.
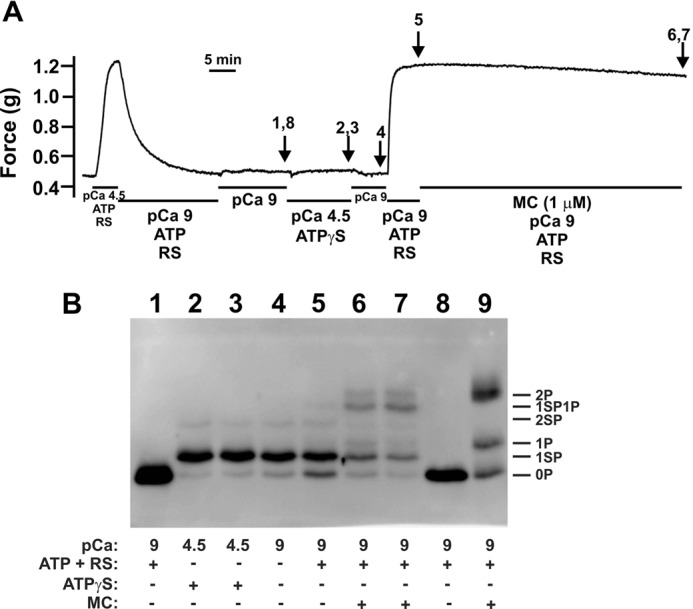
Stoichiometric thiophosphorylation of LC20 at Ser19 in Triton-skinned rat caudal arterial smooth muscle. A, the viability of Triton-skinned rat caudal arterial smooth muscle strips was initially verified by transfer from relaxing solution (pCa 9) to pCa 4.5 solution containing ATP and an ATP regenerating system (RS), which induced a contractile response. Tissues were then relaxed by 3 washes in pCa 9 solution containing ATP and RS. ATP was then removed by 6 washes in pCa 9 solution without ATP or RS. Tissues were then incubated in pCa 4.5 solution containing ATPγS (4 mm) in the absence of ATP and RS. Excess ATPγS was then removed by washing twice with pCa 9 solution without ATP or RS. Contraction was evoked by transfer to pCa 9 solution containing ATP and RS. Once steady-state force was established, microcystin (1 μm) was added in pCa 9 solution containing ATP and RS. Tissues were harvested at the indicated times during this protocol for Phos-tag SDS-PAGE and Western blotting with anti-pan LC20 (B), as shown by the arrows in A (the numbers correspond to the lanes in B): (i) lanes 1 and 8, tissue incubated at pCa 9 showing exclusively unphosphorylated LC20; (ii) lanes 2 and 3, pCa 4.5 + ATPγS in the absence of ATP and RS; (iii) lane 4, pCa 9 in the absence of ATP and RS following thiophosphorylation; (iv) lane 5, at the plateau of force development following transfer to pCa 9 solution containing ATP and RS; (v) lanes 6 and 7, following treatment with microcystin at pCa 9 in the presence of ATP and RS. An additional control is included in lane 9: Triton-skinned tissue treated with microcystin at pCa 9 for 60 min to identify unphosphorylated (0P), monophosphorylated (1P), and diphosphorylated (2P) LC20 bands. Thiophosphorylated forms of LC20 are indicated as follows: 1SP, monothiophosphorylated LC20; 2SP, dithiophosphorylated LC20; 1SP1P, LC20 thiophosphorylated at one site and monophosphorylated at the other. Data are representative of 8 independent experiments.
ATPγS is not hydrolyzed by activated myosin and therefore does not support cross-bridge cycling and contraction (20, 52, 53). Stoichiometric thiophosphorylation at Ser19 (Fig. 8B, lanes 2 and 3) was, therefore, not accompanied by contraction (Fig. 8A). Transfer to pCa 9 solution containing ATP and an ATP regenerating system following washout of ATPγS resulted in a rapid contractile response (t½ = 21.2 ± 0.2 s (n = 8)) and steady-state force corresponding to 85.4 ± 1.9% (n = 8) of the pCa 4.5-induced contraction (Fig. 8A). Once the steady-state force was achieved, microcystin was added at pCa 9 in the presence of ATP and an ATP regenerating system. No additional force development was observed (77.3 ± 4.2% (n = 5) of pCa 4.5-induced contraction), although significant di(thio)phosphorylation of LC20 did occur (Fig. 8B, lanes 6 and 7, and Table 3).
TABLE 3.
Thiophosphorylation of LC20 in Triton-skinned rat caudal arterial smooth muscle
Values represent percentage of total LC20 ± S.E. (n = 3). *, #, and ^ indicate values are not statistically significantly different from each other; 0P, unphosphorylated LC20; 1SP, monothiophosphorylated LC20; 1P, monophosphorylated LC20; 1SP1P, LC20 thiophosphorylated at one site and phosphorylated at the other; 2P, diphosphorylated LC20; RS, ATP regenerating system; MC, microcystin.
| Conditions | 0P | 1SP | 1P | 1SP1P | 2P |
|---|---|---|---|---|---|
| ATPγS, pCa 4.5, no RS | 6.0 ± 2.5* | 88.4 ± 4.8# | 0 | 0 | 5.6 ± 4.3^ |
| Then pCa 9, no RS | 11.1 ± 3.4* | 81.6 ± 3.9# | 0 | 0 | 7.2 ± 2.7^ |
| Then pCa 9, RS | 16.6 ± 4.7* | 80.0 ± 4.4# | 0 | 0 | 3.4 ± 1.9^ |
| Then MC, pCa 9, RS | 13.9 ± 3.2 | 54.7 ± 4.0 | 3.4 ± 2.1 | 14.0 ± 9.2 | 13.9 ± 8.6 |
The identities of the thiophosphorylated LC20 species as depicted in Fig. 8B were verified by the use of phosphospecific antibodies (supplemental Fig. S2). Incubation of Triton-skinned rat caudal arterial smooth muscle strips with ATPγS and microcystin at pCa 9, in the absence of ATP and an ATP regenerating system, failed to elicit thiophosphorylation of LC20 (supplemental Fig. S3, lanes 3 and 4). This is in contrast to incubation with ATPγS at pCa 4.5, in the absence of ATP and an ATP regenerating system, which led to LC20 monothiophosphorylation (supplemental Fig. S3, lane 2) at Ser19 (supplemental Fig. S2, lanes 3–5).
Effects of Diphosphorylation of LC20 on the Rates of Dephosphorylation and Relaxation
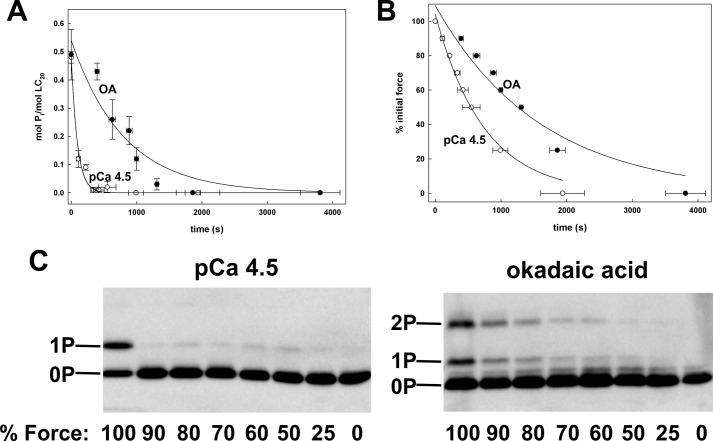
Finally, we investigated the possibility that LC20 diphosphorylation may affect relaxation, rather than contraction, by comparing the rates of dephosphorylation and relaxation of Triton-skinned rat caudal arterial smooth muscle following monophosphorylation of LC20 at pCa 4.5 or diphosphorylation of LC20 at pCa 9 in the presence of okadaic acid. Okadaic acid was chosen as the phosphatase inhibitor for these experiments, rather than microcystin, because its effects are readily reversible (54), whereas microcystin can covalently modify the catalytic subunit of type 1 protein phosphatase, resulting in irreversible inhibition of the phosphatase (55). Indeed, we have observed that microcystin-induced contractions cannot be reversed by washout of the inhibitor (data not shown).
Comparable levels of phosphorylation of LC20 were achieved with pCa 4.5 (0.48 ± 0.02 mol of Pi/mol of LC20 (n = 4)) or okadaic acid treatment at pCa 9 (0.49 ± 0.09 mol of Pi/mol of LC20 (n = 5)), with monophosphorylation occurring exclusively in response to Ca2+ and both mono- and diphosphorylation being detected in the presence of okadaic acid, as expected (Fig. 9C). The steady-state force generated by okadaic acid at pCa 9 was 83.3 ± 1.4% (n = 9) of that at pCa 4.5 (supplemental Fig. S4). Relaxation was initiated by transfer to pCa 9 solution and the time courses of LC20 dephosphorylation and relaxation were quantified (Fig. 9, A and B, respectively). The rate of dephosphorylation of LC20 was markedly reduced in the tissues in which LC20 had been diphosphorylated compared with tissues containing exclusively monophosphorylated LC20 (Fig. 9A): t½ values were 83.3 s for Ca2+-treated tissue and 560 s for okadaic acid-treated tissue. This correlated with a reduction in the rate of relaxation (Fig. 9B): t½ values were 560 s for Ca2+-treated tissue and 1293 s for okadaic acid-treated tissue. The slower rate of dephosphorylation following okadaic acid treatment cannot be explained by slow washout of the inhibitor because MYPT1-Thr697 and -Thr855 (the inhibitory phosphorylation sites in the myosin targeting subunit of MLCP) (56) were maximally dephosphorylated at the first time point analyzed during the relaxation, i.e. when force was at 90% (supplemental Fig. S5).
FIGURE 9.
Comparison of the time courses of relaxation and LC20 dephosphorylation in Triton-skinned rat caudal arterial smooth muscle following contraction with Ca2+ or okadaic acid in the absence of Ca2+. Triton-skinned tissues that had been contracted with Ca2+ (open circles) or okadaic acid (20 μm) at pCa 9 (closed circles) were transferred to pCa 9 solution and the time courses of dephosphorylation (A) and relaxation (B) were followed. Tissues were harvested at 10, 20, 30, 40, 50, 75, and 100% relaxation and LC20 phosphorylation levels were quantified by Phos-tag SDS-PAGE and Western blotting with anti-pan LC20. Values represent the mean ± S.E. (n = 5). Representative Western blots are shown in C.
DISCUSSION
LC20 diphosphorylation has been observed in several smooth muscle tissues treated with various contractile stimuli, including carbachol- (37) and neurally stimulated bovine tracheal smooth muscle (38), prostaglandin-F2α-stimulated rabbit thoracic aorta (39, 40), and angiotensin II-stimulated rat renal efferent arterioles (41). LC20 diphosphorylation has also been observed in pathological cases of smooth muscle hypercontractility, for example, coronary artery spasm (44, 45), cerebral vasospasm after subarachnoid hemorrhage (43, 46), and intimal hyperplasia (42). More recently, Cho et al. (57) provided evidence for enhanced Ca2+-independent LC20 diphosphorylation and force generation in β-escin-permeabilized mesenteric arterial smooth muscle rings of spontaneously hypertensive rats compared with normotensive Wistar Kyoto controls. Furthermore, phenylephrine induced significant LC20 diphosphorylation in the spontaneously hypertensive rat arteries. Evidence was also presented that ZIPK contributes to the Ca2+-independent LC20 diphosphorylation through phosphorylation of MYPT1 at Thr697 and possibly direct phosphorylation of LC20, and the expression level of ZIPK, but not ILK, was greater in spontaneously hypertensive rats than Wistar Kyoto tissues (57). Collectively, these data suggest that LC20 diphosphorylation may account for the hypercontractility observed in smooth muscle tissues in response to certain contractile stimuli and in pathological situations. It was, therefore, important to determine the functional effect of LC20 phosphorylation on smooth muscle contractility. The results of these studies led to the following conclusions.
(i) Treatment of Triton-skinned rat caudal arterial smooth muscle with the phosphatase inhibitor microcystin in the absence of Ca2+ induced a slow, sustained contraction, as previously observed (16), which correlated with LC20 phosphorylation at Ser19 and Thr18 (Fig. 1).
(ii) Similar results were obtained when intact tissues were treated with the membrane-permeant phosphatase inhibitor calyculin-A in the absence of extracellular and stored Ca2+ (Fig. 2). However, an interesting difference between the Triton-skinned and intact tissues was observed: microcystin treatment of skinned tissue induced monophosphorylation at Ser19 and Thr18 at similar rates (Fig. 1B, panels b and c), in addition to diphosphorylation (Fig. 1B, panel d), whereas no monophosphorylation was observed at Thr18 following calyculin-A treatment of intact tissue in the absence of extracellular Ca2+ (Fig. 2B, panel c), but instead Ser19 monophosphorylation was followed by Thr18 phosphorylation to form the diphosphorylated species (Fig. 2B). This suggests that LC20 phosphorylation at the two sites was random in the Triton-skinned tissue experiments but sequential in the intact tissue experiments. A possible explanation would be that distinct kinases are involved in the two situations, the most likely candidates being ILK and ZIPK, and we have provided evidence that ILK is responsible for microcystin-induced Ca2+-independent contraction of Triton-skinned rat caudal arterial smooth muscle (19).
(iii) The level of steady-state force induced by calyculin-A in the absence of Ca2+ is significantly greater than that induced by a maximally effective concentration of KCl, i.e. an optimal Ca2+ signal (Fig. 2A). This would be consistent with diphosphorylation of LC20 increasing steady-state force compared with Ser19 monophosphorylation. Indeed, addition of microcystin to Triton-skinned tissue pre-contracted at pCa 4.5 (Fig. 4B), or of Ca2+ to tissue pre-contracted with microcystin in the absence of Ca2+ (Fig. 4F), evoked a significant increase in steady-state force (Table 2), which correlated with increases in LC20 diphosphorylation (Fig. 4G and Table 1). However, Ser19 phosphorylation stoichiometry also increased under these conditions (from ∼0.5 mol of Pi/mol of LC20 to ∼1 mol of Pi/mol of LC20) (Table 1), suggesting that the enhanced force responses could be due to increased phosphorylation at Ser19 (whether in the form of monophosphorylated or diphosphorylated LC20).
(iv) In intact (Fig. 3) and Triton-skinned tissue (Fig. 4A and G), Ca2+ elicited exclusively monophosphorylation of LC20 at Ser19, as expected.
(v) The fact that the rate of contraction of Triton-skinned rat caudal arterial smooth muscle in response to Ca2+ was significantly faster (t½ ∼ 150 s) than that in response to microcystin at pCa 9 (t½ ∼ 450 s) suggested that it may be possible to induce maximal phosphorylation at Ser19 before achieving diphosphorylation, and thereby determine more convincingly if diphosphorylation causes additional force development. Furthermore, treatment with microcystin at pCa 4.5 caused a significant increase in the rate of contraction (t½ ∼ 65 s) compared with Ca2+ alone (t½ ∼ 150 s) or microcystin alone (t½ ∼ 450 s). Detailed analysis of the (Ca2+ + microcystin)-induced contraction of Triton-skinned rat caudal arterial smooth muscle revealed rapid phosphorylation of LC20 at Ser19 (which can be attributed to MLCK activation by Ca2+) and a slower rate of phosphorylation at Thr18 (due to ILK activity that is unmasked by the phosphatase inhibitor) (Fig. 5). The observation that no additional force was evoked as diphosphorylated LC20 appeared argues that Thr18 phosphorylation likely does not increase steady-state force beyond that achieved by phosphorylation at Ser19.
(vi) The combination of KCl and calyculin-A in the presence of Ca2+ induced a biphasic contractile response of intact tissue (Fig. 6), which corresponds to the combined contractile responses to KCl in the presence of Ca2+ and calyculin-A in the absence or presence of Ca2+. In this case, the initial rapid phasic contraction correlated with Ser19 phosphorylation, and the slow sustained contractile response with the diphosphorylation of LC20 (Fig. 7). The contractile effects of KCl and calyculin-A, however, could be explained entirely by Ser19 phosphorylation.
It was necessary, therefore, to devise a way to achieve stoichiometric phosphorylation at Ser19 without Thr18 phosphorylation, and then observe whether subsequent phosphorylation at Thr18 has an effect on steady-state force development. This was achieved by using ATPγS to evoke close-to-stoichiometric thiophosphorylation at Ser19 with very little dithiophosphorylation (Fig. 8B and Table 3). Subsequent phosphorylation of LC20 at Thr18 (Fig. 8B) failed to elicit an increase in force (Fig. 8A). We conclude, therefore, that phosphorylation at Ser19 of LC20 accounts for maximal force development, and no further force results from additional phosphorylation at Thr18.
We then turned our attention to the possibility that diphosphorylation may affect relaxation rather than contraction by comparing the time courses of dephosphorylation of LC20 and relaxation of Triton-skinned muscle strips that had been pre-contracted under conditions that evoked phosphorylation exclusively at Ser19 or at both Ser19 and Thr18 to the same overall phosphorylation stoichiometry. The rates of dephosphorylation and relaxation were significantly slower in the case of diphosphorylated LC20 (Fig. 9). We conclude, therefore, that diphosphorylation of LC20 at Thr18 and Ser19 has a marked effect on relaxation compared with monophosphorylation at Ser19.
The mechanism underlying the reduction in the rate of dephosphorylation of diphosphorylated LC20 compared with Ser19-monophosphorylated LC20 remains to be determined. A possibility is that the Km of MLCP for diphosphorylated LC20 may be significantly higher than that for LC20 phosphorylated exclusively at Ser19. Although such kinetic comparisons have not been performed to date, in vitro assays indicated that dephosphorylation of diphosphorylated LC20 (whether free or in intact myosin) occurred by a random mechanism, with dephosphorylation at Ser19 and Thr18 occurring at similar rates (5).
The principal conclusions from this study are: (i) the level of steady-state force is dictated by the level of Ser19 phosphorylation and is unaffected by Thr18 phosphorylation; and (ii) Thr18 phosphorylation reduces the rate of LC20 dephosphorylation and relaxation, supporting a sustained contractile response. There is abundant literature indicating that most contractile stimuli elicit phosphorylation exclusively at Ser19 and this can be explained by Ca2+-induced activation of MLCK, with or without a modest degree of Ca2+ sensitization due to MLCP inhibition (58). Specific stimuli and pathophysiological situations associated with hypercontractility induce LC20 diphosphorylation at Thr18 and Ser19. This can be explained by increased MLCP inhibition, unmasking constitutive Ca2+-independent LC20 kinase activity (ILK and/or ZIPK), and potentially an increase in activity of Ca2+-independent LC20 kinases, leading to an increase in Ser19 phosphorylation (force) and Thr18 phosphorylation (sustained contraction). ILK and ZIPK are therefore potential therapeutic targets for the treatment of cerebral and coronary vasospasm, intimal hyperplasia, hypertension, and other conditions associated with hypercontractility.
Supplementary Material
Acknowledgment
We are grateful to Dr. Ryan Mills for helpful comments on the manuscript.
This work was supported in part by Canadian Institutes of Health Research Grant MOP-111262 (to M. P.W.).

This article contains supplemental Figs. S1–S5 and Table S1.
- MLCK
- myosin light chain kinase
- ATPγS
- adenosine 5′-O-(3-thiotriphosphate)
- CaM
- calmodulin
- ILK
- integrin-linked kinase
- LC20
- the 20-kDa regulatory light chains of smooth muscle myosin II
- MLCP
- myosin light chain phosphatase
- MYPT1
- myosin targeting subunit of MLCP
- ZIPK
- zipper-interacting protein kinase
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid.
REFERENCES
- 1. Berridge M. J. (2008) Smooth muscle cell calcium activation mechanisms. J. Physiol. 586, 5047–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen B. G., Walsh M. P. (1994) The biochemical basis of the regulation of smooth muscle contraction. Trends Biochem. Sci. 19, 362–368 [DOI] [PubMed] [Google Scholar]
- 3. Walsh M. P. (1991) The Ayerst Award Lecture 1990. Calcium-dependent mechanisms of regulation of smooth muscle contraction. Biochem. Cell Biol. 69, 771–800 [DOI] [PubMed] [Google Scholar]
- 4. Ikebe M., Hartshorne D. J. (1985) Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 260, 10027–10031 [PubMed] [Google Scholar]
- 5. Ikebe M., Hartshorne D. J., Elzinga M. (1986) Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J. Biol. Chem. 261, 36–39 [PubMed] [Google Scholar]
- 6. Hartshorne D. J., Ito M., Erdödi F. (2004) Role of protein phosphatase type 1 in contractile functions. Myosin phosphatase. J. Biol. Chem. 279, 37211–37214 [DOI] [PubMed] [Google Scholar]
- 7. Shibata S., Ishida Y., Kitano H., Ohizumi Y., Habon J., Tsukitani Y., Kikuchi H. (1982) Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscles under the condition of Ca deficiency. J. Pharmacol. Exp. Ther. 223, 135–143 [PubMed] [Google Scholar]
- 8. Ozaki H., Ishihara H., Kohama K., Nonomura Y., Shibata S., Karaki H. (1987) Calcium-independent phosphorylation of smooth muscle myosin light chain by okadaic acid isolated from black sponge (Halichondria okadai). J. Pharmacol. Exp. Ther. 243, 1167–1173 [PubMed] [Google Scholar]
- 9. Hirano K., Kanaide H., Nakamura M. (1989) Effects of okadaic acid on cytosolic calcium concentrations and on contractions of the porcine coronary artery. Br. J. Pharmacol. 98, 1261–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishihara H., Ozaki H., Sato K., Hori M., Karaki H., Watabe S., Kato Y., Fusetani N., Hashimoto K., Uemura D. (1989) Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J. Pharmacol. Exp. Ther. 250, 388–396 [PubMed] [Google Scholar]
- 11. Obara K., Takai A., Ruegg J. C., de Lanerolle P. (1989) Okadaic acid, a phosphatase inhibitor, produces a Ca2+ and calmodulin-independent contraction of smooth muscle. Pflügers Arch. 414, 134–138 [DOI] [PubMed] [Google Scholar]
- 12. Hori M., Magae J., Han Y. G., Hartshorne D. J., Karaki H. (1991) A novel protein phosphatase inhibitor, tautomycin. Effect on smooth muscle. FEBS Lett. 285, 145–148 [DOI] [PubMed] [Google Scholar]
- 13. Gong M. C., Cohen P., Kitazawa T., Ikebe M., Masuo M., Somlyo A. P., Somlyo A. V. (1992) Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J. Biol. Chem. 267, 14662–14668 [PubMed] [Google Scholar]
- 14. Suzuki A., Itoh T. (1993) Effects of calyculin A on tension and myosin phosphorylation in skinned smooth muscle of the rabbit mesenteric artery. Br. J. Pharmacol. 109, 703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shirazi A., Iizuka K., Fadden P., Mosse C., Somlyo A. P., Somlyo A. V., Haystead T. A. (1994) Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J. Biol. Chem. 269, 31598–31606 [PubMed] [Google Scholar]
- 16. Weber L. P., Van Lierop J. E., Walsh M. P. (1999) Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. J. Physiol. 516, 805–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng J. T., Van Lierop J. E., Sutherland C., Walsh M. P. (2001) Ca2+-independent smooth muscle contraction. A novel function for integrin-linked kinase. J. Biol. Chem. 276, 16365–16373 [DOI] [PubMed] [Google Scholar]
- 18. Niiro N., Ikebe M. (2001) Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J. Biol. Chem. 276, 29567–29574 [DOI] [PubMed] [Google Scholar]
- 19. Wilson D. P., Sutherland C., Borman M. A., Deng J. T., Macdonald J. A., Walsh M. P. (2005) Integrin-linked kinase is responsible for Ca2+-independent myosin diphosphorylation and contraction of vascular smooth muscle. Biochem. J. 392, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh M. P., Bridenbaugh R., Hartshorne D. J., Kerrick W. G. (1982) Phosphorylation-dependent activated tension in skinned gizzard muscle fibers in the absence of Ca2+. J. Biol. Chem. 257, 5987–5990 [PubMed] [Google Scholar]
- 21. Ngai P. K., Walsh M. P. (1984) Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J. Biol. Chem. 259, 13656–13659 [PubMed] [Google Scholar]
- 22. Walsh M. P. (1985) Limited proteolysis of smooth muscle myosin light chain kinase. Biochemistry 24, 3724–3730 [DOI] [PubMed] [Google Scholar]
- 23. Wilson D. P., Sutherland C., Walsh M. P. (2002) Ca2+ activation of smooth muscle contraction. Evidence for the involvement of calmodulin that is bound to the Triton-insoluble fraction even in the absence of Ca2+. J. Biol. Chem. 277, 2186–2192 [DOI] [PubMed] [Google Scholar]
- 24. Mita M., Yanagihara H., Hishinuma S., Saito M., Walsh M. P. (2002) Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem. J. 364, 431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wickström S. A., Lange A., Montanez E., Fässler R. (2010) The ILK/PINCH/parvin complex. The kinase is dead, long live the pseudokinase! EMBO J. 29, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maydan M., McDonald P. C., Sanghera J., Yan J., Rallis C., Pinchin S., Hannigan G. E., Foster L. J., Ish-Horowicz D., Walsh M. P., Dedhar S. (2010) Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3β) phosphorylation. PLoS One 5, e12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hannigan G. E., McDonald P. C., Walsh M. P., Dedhar S. (2011) Integrin-linked kinase. Not so “pseudo” after all. Oncogene 30, 4375–4385 [DOI] [PubMed] [Google Scholar]
- 28. Moffat L. D., Brown S. B., Grassie M. E., Ulke-Lemée A., Williamson L. M., Walsh M. P., MacDonald J. A. (2011) Chemical genetics of zipper-interacting protein kinase reveal myosin light chain as a bona fide substrate in permeabilized arterial smooth muscle. J. Biol. Chem. 286, 36978–36991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walsh M. P. (2011) Vascular smooth muscle myosin light chain diphosphorylation. Mechanism, function, and pathological implications. IUBMB Life 63, 987–1000 [DOI] [PubMed] [Google Scholar]
- 30. King N., Westbrook M. J., Young S. L., Kuo A., Abedin M., Chapman J., Fairclough S., Hellsten U., Isogai Y., Letunic I., Marr M., Pincus D., Putnam N., Rokas A., Wright K. J., Zuzow R., Dirks W., Good M., Goodstein D., Lemons D., Li W., Lyons J. B., Morris A., Nichols S., Richter D. J., Salamov A., Sequencing J. G., Bork P., Lim W. A., Manning G., Miller W. T., McGinnis W., Shapiro H., Tjian R., Grigoriev I. V., Rokhsar D. (2008) The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451, 783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang B. F., O'Kelly C., Nerad T., Gray M. W., Burger G. (2002) The closest unicellular relatives of animals. Curr. Biol. 12, 1773–1778 [DOI] [PubMed] [Google Scholar]
- 32. Vicente-Manzanares M., Ma X., Adelstein R. S., Horwitz A. R. (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikebe M., Koretz J., Hartshorne D. J. (1988) Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J. Biol. Chem. 263, 6432–6437 [PubMed] [Google Scholar]
- 34. Kamisoyama H., Araki Y., Ikebe M. (1994) Mutagenesis of the phosphorylation site (serine 19) of smooth muscle myosin regulatory light chain and its effects on the properties of myosin. Biochemistry 33, 840–847 [DOI] [PubMed] [Google Scholar]
- 35. Bresnick A. R., Wolff-Long V. L., Baumann O., Pollard T. D. (1995) Phosphorylation on threonine 18 of the regulatory light chain dissociates the ATPase and motor properties of smooth muscle myosin II. Biochemistry 34, 12576–12583 [DOI] [PubMed] [Google Scholar]
- 36. Umemoto S., Bengur A. R., Sellers J. R. (1989) Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J. Biol. Chem. 264, 1431–1436 [PubMed] [Google Scholar]
- 37. Colburn J. C., Michnoff C. H., Hsu L. C., Slaughter C. A., Kamm K. E., Stull J. T. (1988) Sites phosphorylated in myosin light chain in contracting smooth muscle. J. Biol. Chem. 263, 19166–19173 [PubMed] [Google Scholar]
- 38. Miller-Hance W. C., Miller J. R., Wells J. N., Stull J. T., Kamm K. E. (1988) Biochemical events associated with activation of smooth muscle contraction. J. Biol. Chem. 263, 13979–13982 [PubMed] [Google Scholar]
- 39. Seto M., Sasaki Y., Sasaki Y. (1990) Stimulus-specific patterns of myosin light chain phosphorylation in smooth muscle of rabbit thoracic artery. Pflügers Arch. 415, 484–489 [DOI] [PubMed] [Google Scholar]
- 40. Seto M., Sasaki Y., Hidaka H., Sasaki Y. (1991) Effects of HA1077. A protein kinase inhibitor, on myosin phosphorylation and tension in smooth muscle. Eur. J. Pharmacol. 195, 267–272 [DOI] [PubMed] [Google Scholar]
- 41. Takeya K., Loutzenhiser K., Wang X., Kathol I., Walsh M. P., Loutzenhiser R. (2011) Differing effects of angiotensin II on myosin light chain phosphorylation in renal afferent and efferent arterioles. FASEB J. 25, 817a [Google Scholar]
- 42. Seto M., Yano K., Sasaki Y., Azuma H. (1993) Intimal hyperplasia enhances myosin phosphorylation in rabbit carotid artery. Exp. Mol. Pathol. 58, 1–13 [DOI] [PubMed] [Google Scholar]
- 43. Harada T., Seto M., Sasaki Y., London S., Luo Z., Mayberg M. (1995) The time course of myosin light-chain phosphorylation in blood-induced vasospasm. Neurosurgery 36, 1178–1182 [DOI] [PubMed] [Google Scholar]
- 44. Katsumata N., Shimokawa H., Seto M., Kozai T., Yamawaki T., Kuwata K., Egashira K., Ikegaki I., Asano T., Sasaki Y., Takeshita A. (1997) Enhanced myosin light chain phosphorylations as a central mechanism for coronary artery spasm in a swine model with interleukin-1β. Circulation 96, 4357–4363 [DOI] [PubMed] [Google Scholar]
- 45. Shimokawa H., Seto M., Katsumata N., Amano M., Kozai T., Yamawaki T., Kuwata K., Kandabashi T., Egashira K., Ikegaki I., Asano T., Kaibuchi K., Takeshita A. (1999) Rho kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc. Res. 43, 1029–1039 [DOI] [PubMed] [Google Scholar]
- 46. Obara K., Nishizawa S., Koide M., Nozawa K., Mitate A., Ishikawa T., Nakayama K. (2005) Interactive role of protein kinase C-δ with Rho kinase in the development of cerebral vasospasm in a canine two-hemorrhage model. J. Vasc. Res. 42, 67–76 [DOI] [PubMed] [Google Scholar]
- 47. Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. (1984) Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem. J. 224, 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ngai P. K., Carruthers C. A., Walsh M. P. (1984) Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem. J. 218, 863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takeya K., Loutzenhiser K., Shiraishi M., Loutzenhiser R., Walsh M. P. (2008) A highly sensitive technique to measure myosin regulatory light chain phosphorylation. The first quantification in renal arterioles. Am. J. Physiol. Renal Physiol. 294, F1487–1492 [DOI] [PubMed] [Google Scholar]
- 50. Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. (1978) Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry 17, 4411–4418 [DOI] [PubMed] [Google Scholar]
- 51. Gratecos D., Fischer E. H. (1974) Adenosine 5′-O-(3-thiotriphosphate) in the control of phosphorylase activity. Biochem. Biophys. Res. Commun. 58, 960–967 [DOI] [PubMed] [Google Scholar]
- 52. Cassidy P., Hoar P. E., Kerrick W. G. (1979) Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATPγS. J. Biol. Chem. 254, 11148–11153 [PubMed] [Google Scholar]
- 53. Walsh M. P., Bridenbaugh R., Kerrick W. G., Hartshorne D. J. (1983) Gizzard Ca2+-independent myosin light chain kinase. Evidence in favor of the phosphorylation theory. Fed. Proc. 42, 45–50 [PubMed] [Google Scholar]
- 54. Bialojan C., Rüegg J. C., Takai A. (1988) Effects of okadaic acid on isometric tension and myosin phosphorylation of chemically skinned guinea pig taenia coli. J. Physiol. 398, 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Craig M., Luu H. A., McCready T. L., Williams D., Andersen R. J., Holmes C. F. (1996) Molecular mechanisms underlying the interaction of motuporin and microcystins with type-1 and type-2A protein phosphatases. Biochem. Cell Biol. 74, 569–578 [DOI] [PubMed] [Google Scholar]
- 56. Grassie M. E., Moffat L. D., Walsh M. P., MacDonald J. A. (2011) The myosin phosphatase targeting protein (MYPT) family. A regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch. Biochem. Biophys. 510, 147–159 [DOI] [PubMed] [Google Scholar]
- 57. Cho Y. E., Ahn D. S., Morgan K. G., Lee Y. H. (2011) Enhanced contractility and myosin phosphorylation induced by Ca2+-independent MLCK activity in hypertensive rats. Cardiovasc. Res. 91, 162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Somlyo A. P., Somlyo A. V. (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II. Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.