Background: Tyrosine-liganded hemoproteins typically are hydroperoxidases. They do not oxygenate their substrates.
Results: In the presence of an oxygen donor, tyrosine-liganded allene oxide synthase stereospecifically oxygenated polyunsaturated fatty acids.
Conclusion: A catalase-related enzyme can act as a monooxygenase.
Significance: A catalase hemoprotein, with tyrosine as the proximal heme ligand, can exhibit monooxygenase activity, a property usually associated with a cysteinate heme ligand, as in cytochromes P450.
Keywords: Arachidonic Acid, Cytochrome P450, Eicosanoid Structure, Enzyme Catalysis, Enzyme Mechanisms, Compound I, Catalase, Iodosylbenzene, Monooxygenase, Proximal Heme Ligand
Abstract
The ability of hemoproteins to catalyze epoxidation or hydroxylation reactions is usually associated with a cysteine as the proximal ligand to the heme, as in cytochrome P450 or nitric oxide synthase. Catalase-related allene oxide synthase (cAOS) from the coral Plexaura homomalla, like catalase itself, has tyrosine as the proximal heme ligand. Its natural reaction is to convert 8R-hydroperoxy-eicosatetraenoic acid (8R-HPETE) to an allene epoxide, a reaction activated by the ferric heme, forming product via the FeIV–OH intermediate, Compound II. Here we oxidized cAOS to Compound I (FeV=O) using the oxygen donor iodosylbenzene and investigated the catalytic competence of the enzyme. 8R-hydroxyeicosatetraenoic acid (8R-HETE), the hydroxy analog of the natural substrate, normally unreactive with cAOS, was thereby epoxidized stereospecifically on the 9,10 double bond to form 8R-hydroxy-9R,10R-trans-epoxy-eicosa-5Z,11Z,14Z-trienoic acid as the predominant product; the turnover was 1/s using 100 μm iodosylbenzene. The enantiomer, 8S-HETE, was epoxidized stereospecifically, although with less regiospecificity, and was hydroxylated on the 13- and 16-carbons. Arachidonic acid was converted to two major products, 8R-HETE and 8R,9S-eicosatrienoic acid (8R,9S-EET), plus other chiral monoepoxides and bis-allylic 10S-HETE. Linoleic acid was epoxidized, whereas stearic acid was not metabolized. We conclude that when cAOS is charged with an oxygen donor, it can act as a stereospecific monooxygenase. Our results indicate that in the tyrosine-liganded cAOS, a catalase-related hemoprotein in which a polyunsaturated fatty acid can enter the active site, the enzyme has the potential to mimic the activities of typical P450 epoxygenases and some capabilities of P450 hydroxylases.
Introduction
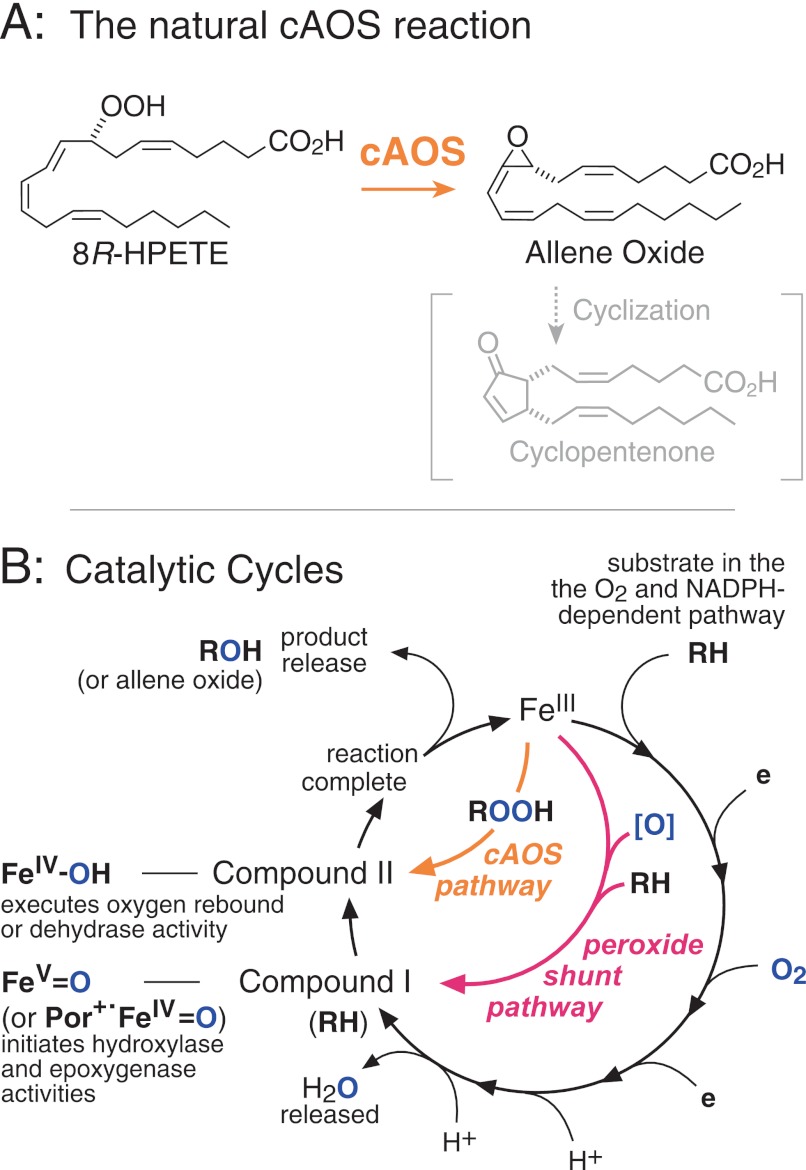
The distinctive hydroxylase and epoxygenase capabilities of the cytochrome P450s and other heme-thiolate monooxygenases are generally considered to be a special attribute conferred partly by unique properties of cysteine as the proximal ligand to the heme (e.g. Refs.1 and 2). Catalase hemoproteins, by contrast, have a tyrosine as the proximal heme ligand, and none are monooxygenases (3). Their prototypical activity is the breakdown of hydrogen peroxide, and they also exhibit a weak peroxidatic activity with very small molecule substrates, for example the oxidation of ethanol to acetaldehyde; reaction with larger substrates is precluded by the restricted access to the active site heme (4). Catalase-related allene oxide synthase (cAOS)2 from the coral Plexaura homomalla is atypical among the tyrosine-liganded hemoproteins in that its natural substrate is a long chain fatty acid hydroperoxide (5). It converts 8R-hydroperoxy-eicosatetraenoic acid (8R-HPETE) to an allene epoxide, an intermediate in cyclopentenone biosynthesis (Fig. 1A) (6). Its mechanism is directly analogous to the unconventional cytochrome P450 enzymes that metabolize fatty acid hydroperoxides, for example, CYP74A, an AOS in plants, and thromboxane synthase (CYP5) and prostacyclin synthase (CYP8A) in mammals (7). Instead of utilizing molecular oxygen, these enzymes react with the oxygens in their specific fatty acid peroxide substrates.
FIGURE 1.
Allene oxide synthase and its relationship to the catalytic cycle of cytochrome P450s. A, cAOS catalyzes transformation of its natural substrate 8R-HPETE to an allene oxide, which can subsequently rearrange to a cyclopentenone. B, the circular route represents the conventional monooxygenase pathway, involving molecular oxygen and NADPH and leading to Compound I, which initiates reaction with substrate. The peroxide shunt pathway uses an oxygen donor, [O], to form Compound I and bypass the need for O2 and NADPH. In fatty acid peroxide-metabolizing enzymes (CYP 5, 8A, 74, and cAOS), the ferric enzyme initiates reaction and forms Compound II directly. Por+· represents a radical cation on the heme porphyrin ring.
The catalytic cycle of conventional cytochrome P450s is usually represented by the circular route in Fig. 1B (e.g. Refs. 1 and 2). After the ferric enzyme (FeIII) binds substrate (RH), the first two-thirds of the cycle involves oxygen activation, during which nothing happens to RH. After O2 is split and H2O is eliminated, the active form of the heme, Compound I, initiates reaction with RH by a hydrogen abstraction. This forms Compound II and a substrate radical, which instantly react, forming hydroxylated or epoxidized product by oxygen rebound and returning the enzyme to the ferric form. Pointedly, the same chemical transformation can be catalyzed without the need for O2 and the reducing equivalents from NADPH; this is achieved using the “peroxide shunt pathway” in which a peroxide co-substrate or other oxygen donor is used to form Compound I, after which the usual reactions take place (Fig. 1B). Distinct from these pathways, as the writings of Ullrich make clear (8), is the route taken by the fatty acid peroxide-metabolizing P450s, (CYP5, CYP8A, CYP74) and by cAOS. Here the ferric enzyme is the active species of the hemoprotein; it initiates the transformation and produces, directly, Compound II and a substrate intermediate (Fig. 1B) (7, 9). These two react instantly, completing the transformation. So, in this case, Compound I is never formed.
The objectives of the work reported here were to examine the consequences of forming Compound I in cAOS and to determine whether cAOS could thus become a hydroxylase or epoxygenase. We used iodosylbenzene (PhIO) as an oxygen donor to activate the peroxide shunt pathway to Compound I. PhIO has been used commonly for this purpose in P450 research (10, 11) and was the reagent used by Ullrich and co-workers (12) to demonstrate that the peroxide-metabolizing P450, thromboxane synthase (CYP5), is capable of hydroxylating a substrate analog. Herein we demonstrate that cAOS exhibits a robust regio- and stereospecific monooxygenase activity in the presence of iodosylbenzene, thus revealing a previously unrecognized catalytic potential of a tyrosine-liganded hemoprotein.
EXPERIMENTAL PROCEDURES
Materials
Arachidonic acid was purchased from Nu-Chek Prep Inc. (Elysian, MN). Iodosylbenzene (PhIO) was kindly provided by Dr. F. Peter Guengerich, who prepared it from iodosobenzene diacetate (13). cAOS was prepared by expression in Escherichia coli, as described (14). 8R-HETE was synthesized using the P. homomalla 8R-lipoxygenase domain from the cAOS-lipoxygenase fusion protein, expressed in E. coli (14). Also, both 8R-HETE and 8S-HETE were prepared by vitamin E-controlled autoxidation of methyl arachidonate (15), SP-HPLC separation of the HETE isomers, chiral column separation of the 8-HETE enantiomers (16), and alkaline hydrolysis of the methyl esters to yield the free acids, which were repurified by SP-HPLC prior to use.
Incubations with Iodosylbenzene
A fresh working solution of PhIO in MeOH (0.1 m) was prepared daily and kept on ice prior to dilution into the enzyme reaction. Incubations were conducted in pH 7.5 Tris or HEPES buffer containing 150 mm NaCl at room temperature. Typically, 8-HETE or arachidonic acid was added to 1–20 ml of buffer from ethanol solutions (5 or 10 mg/ml) to achieve a final concentration of 50 μm. cAOS was diluted 100-fold from stock solutions to achieve 1–2 μm final concentration. After 3 min at room temperature, samples were placed on ice, and any remaining PhIO was eliminated by the addition of ∼5 mg/ml sodium bisulfite (17). Samples were applied, without adjusting the pH, to a preconditioned C18 extraction cartridge (Waters, Oasis, 100 mg, or 0.5 g of Bond-Elut C18 for 10–20-ml volumes), and after washing with water, the fatty acid derivatives were eluted with MeOH and stored at −20 °C prior to HPLC analysis.
Chemical Epoxidation Using mCPBA
8R-HETE or arachidonic acid in dichloromethane (1 mg/ml) were reacted with a 2-fold molar excess of mCPBA for 30 min on ice. The dichloromethane was then washed twice with 0.1 m NaHCO3, twice with water and then taken to dryness and dissolved in methanol prior to HPLC analysis. Some of the epoxy products from 8R-HETE free acid proved difficult to retrieve from the RP-HPLC column solvent without undergoing partial hydrolysis, and therefore the initial reactions were conducted on 8R-HETE methyl ester. RP-HPLC was then conducted without acetic acid in the running solvent (MeOH/H2O, 80:20 v/v), which helped considerably in the recovery of intact epoxides.
HPLC Analyses
Typically, aliquots of the extracts were analyzed initially by RP-HPLC using a Waters Symmetry C18 column (25 × 0.46 cm) with a solvent of MeOH/H2O/HAc in the proportions 80/20/0.01, or 75/25/0.01 by volume for free acids and 80/20 (MeOH:H2O) for methyl esters. We used a flow rate of 1 ml/min and on-line UV detection (Agilent 1100 series diode array detector) with wavelengths set at 205, 220, 235, and 270 nm. Quantities of 0.5–1 mg of total fatty acids were injected for collection of products. Further analysis and purification was achieved by SP-HPLC using a Beckman Ultrasphere 5-μm silica column or a Thomson Instrument Co. Advantage 5-μm silica column (25 × 0.46 cm) with a solvent of hexane/isopropyl alcohol/glacial acetic acid (100/5/0.02 for free acids and 100/2 for methyl esters). Although the products were not enantiomeric, a Diacel Chiralpak AD chiral column (25 × 0.46 cm) gave excellent separation and purification of the diastereomers; a solvent system of hexane/ethanol/methanol (100:5:5, by volume) was used for epoxyalcohol methyl esters, giving retention volumes of 10–30 ml for different isomers. In attempts to better resolve the mixture of products obtained using chemical epoxidation with mCPBA, we investigated the use of acetonitrile/water solvent on RP-HPLC (CH3CN/H2O/HAc, 60:40:0.01, by volume). We note that the products 1 and 2 described under “Results” elute in the opposite order in the acetonitrile solvent, i.e. 2 before 1.
UV Spectroscopy
HETEs were quantified by UV spectroscopy from the conjugated diene (ϵ = 25,000 m−1 cm−1). Rates of reaction of 8-HETE with cAOS + PhIO were estimated by monitoring the disappearance of the conjugated diene chromophore at 235 or 250 nm versus time. The high absorbance of PhIO at 235 nm limited the useable range of PhIO concentrations at this wavelength, and therefore in most experiments, the wavelength was set at 250 nm, where 8-HETE gives about half of its maximal absorbance, and the total absorbance remained below 2 absorbance units. 8R-HETE (50 μm) and cAOS (1 μm) were added to 1 ml of buffer, pH 7.5, in a UV cuvette in a PerkinElmer Life Sciences Lambda-35 spectrophotometer at room temperature, and absorbance was recorded versus time. At 10 s, reaction was initiated by the addition of PhIO (10 μl of a 100-fold higher PhIO concentration in MeOH), and the initial rate of reaction was recorded.
Some caveats in the accuracy of this assay included that the addition of PhIO alone to buffer resulted in slow absorbance changes over time, although the rates were at least an order of magnitude slower than following 8R-HETE addition and were insignificant over the initial ∼10 s of reaction of 8R-HETE. This effect was most evident using Tris buffer, which appeared to react with PhIO, then HEPES, and least using phosphate buffer, which showed real, although minor (≤10%) absorbance changes over 30 min. Whether these rates increase upon reaction with substrate are unknown, although it seems possible. Reassuringly, use of arachidonic acid as substrate in place of 8R-HETE resulted in absorbance increases at 235 nm, in accord with some of its products having a conjugated diene chromophore, whereas 8S-HETE gave relatively weak absorbance decreases, compatible with many of its products retaining the conjugated diene.
GC-MS Analysis
GC-MS analysis of the methyl ester trimethylsilyl derivatives was carried out in the positive ion electron impact mode (70 eV) using a ThermoFinnigan DSQ mass spectrometer. The initial temperature was set for 150 °C, held for 1 min, and then increased to 300 °C at 20 °C/min increment and held at 300 °C for 3 min.
Many of the hydroxy-epoxy products characterized under “Results” gave multiple peaks on GC analysis, and hydrogenation was often associated with marked hydrogenolysis, giving a diol and other breakdown products (cf. supplement to Ref. 18). For these products, their identification relied on LC-MS to establish the molecular weight and NMR for structural analysis.
LC-MS Analysis
The molecular weights of the reaction products of 8-HETE and arachidonic acid were established from the M-H anions measured by negative ion electrospray LC-MS using a ThermoFinnigan TSQ Quantum instrument.
NMR Analysis
1H NMR and 1H,1H COSY NMR spectra were recorded on a Bruker 600-MHz spectrometer at 298 K. The parts/million values are reported relative to residual nondeuterated solvent (δ = 7.16 ppm for C6D6, 7.26 ppm for CDCl3). Spectral simulations were run using ACD/Labs software.
RESULTS
Reactions of cAOS + Iodosylbenzene with 8R-HETE
Native cAOS is remarkably specific in its allene oxide synthase activity with fatty acid hydroperoxides, showing a strong preference for its natural substrate, 8R-HPETE (14). The native enzyme is completely unreactive with polyunsaturated fatty acids or their hydroxylated analogs. Obviously, 8R-HPETE must fit well into the active site and, accordingly, we selected its hydroxy analog, 8R-HETE, as an initial substrate to test the catalytic activity of cAOS oxidized with PhIO as the oxygen donor.
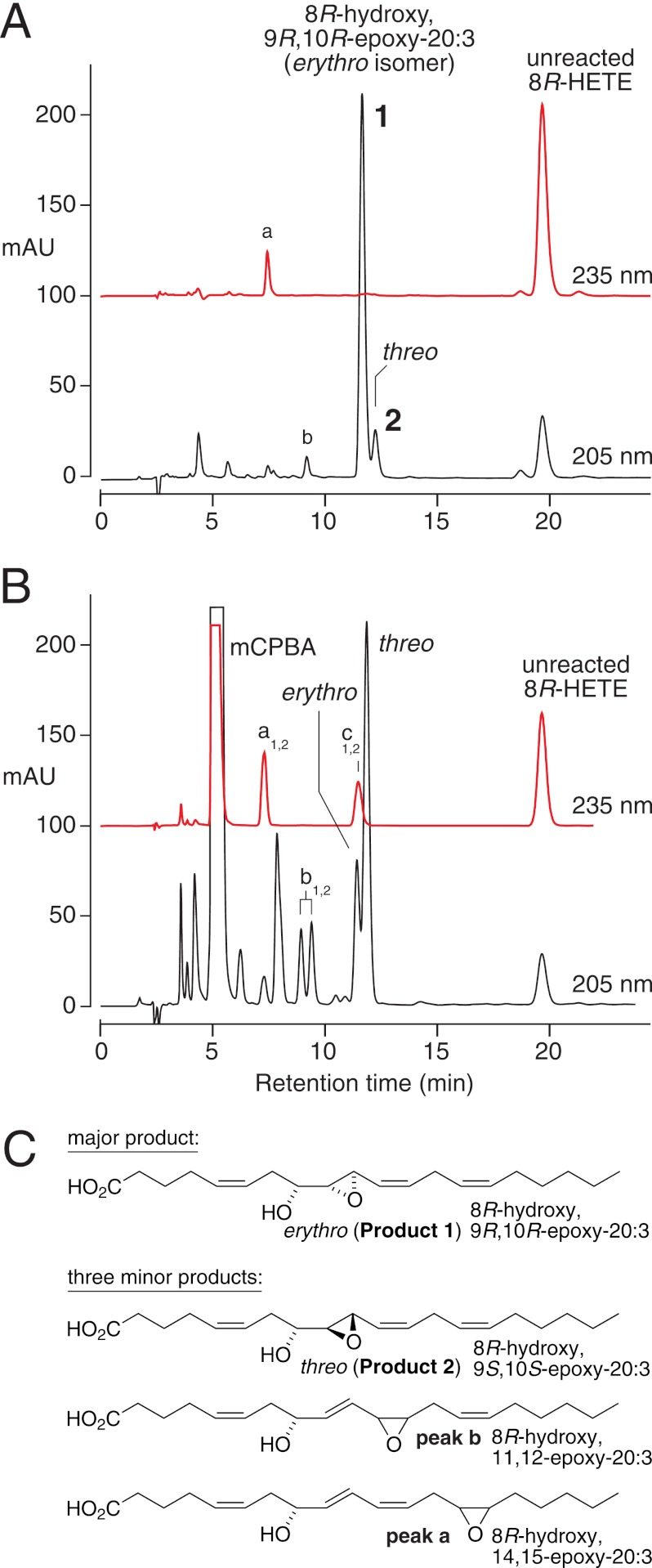
Incubation of cAOS (1 μm), 8R-HETE (100 μm) and equimolar PhIO (100 μm) in pH 7.5 buffer for 3 min at room temperature led to the disappearance of most of the 8R-HETE substrate and formation of a remarkably selective product profile. Upon RP-HPLC analysis, the profile was dominated by two closely eluting peaks at about 12 min, present in a ratio of 10:1, and designated as 1 and 2 (Fig. 2A). The 12-min retention time in this isocratic RP-HPLC system is less polar than a dihydroxy derivative, more compatible with an epoxidation of 8R-HETE. Furthermore, the 235-nm chromophore of the HETE substrate was lost in 1 and 2, consistent with an epoxidation in the 9E,11Z-conjugated diene. LC-MS analysis indicated that the two products weighed 16 atomic mass units more than 8-HETE, compatible with the addition of an atom of oxygen. The structures and stereochemistry of 1 and 2 were established by comparison with the products of chemically epoxidized 8R-HETE as described in the next subsection. In control experiments, no products were formed upon incubation of 8R-HETE with PhIO in the absence of cAOS enzyme.
FIGURE 2.
RP-HPLC analysis of products formed from 8R-HETE by cAOS/PhIO (A) and mCPBA (B). A, cAOS (1 μm), 8R-HETE (100 μm), and PhIO (100 μm) were reacted for 5 min in pH 7.5 Tris buffer and extracted using a C18 cartridge, and an aliquot was analyzed using a Waters Symmetry column (25 × 0.46 cm), a solvent of MeOH/H2O/HAc (80/20/0.01 by volume), and a flow rate of 1 ml/min, with on-line UV detection (Agilent 1100 series diode array detector) at 205 and 235 nm. The UV traces are at the same sensitivity and offset for clarity. mAU, milliabsorbance units. B, 8R-HETE (1 mg/ml) was reacted with an equivalent of mCPBA in dichloromethane for 30 min at 0 °C and extracted, and an aliquot was analyzed by RP-HPLC with UV detection as above. Larger quantities were isolated, further purified by SP-HPLC or using a Chiralpak AD chiral column, and characterized by NMR (with NMR data in the supplemental material, including products a1,2, b1,2, and c1,2). C, products formed from 8R-HETE by cAOS/PhIO.
Chemical Epoxidation of 8R-HETE, Identification of 1 and 2
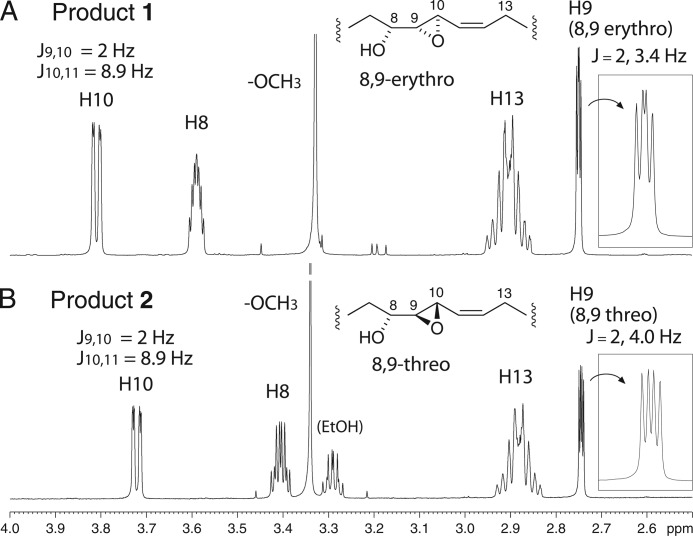
For comparison with the enzymatic reaction and to produce larger quantities of products for analysis, 8R-HETE was treated with the chemical epoxidizing agent mCPBA in dichloromethane. In addition to a series of slightly more polar derivatives, this gave the same two products 1 and 2, except in 1:3 abundance (Fig. 2B). On SP-HPLC, the peaks were fully resolved, eluting in the same order, 1 followed by 2 (supplemental Fig. S1). The 1H NMR and COSY spectra allowed assignment of the structures and stereochemistry, showing that products 1 and 2 are, respectively, the erythro and threo diastereomers of 8R-hydroxy-9,10-trans-epoxy-eicosa-5Z,11Z,14Z-trienoate (Figs. 2C and 3, with NMR data listed in the supplemental material). The chemical shifts of C9 and C10 and their coupling constant (J9,10 = 2 Hz) indicated that both diastereomers are 9,10-trans-epoxides (19). The erythro/threo stereochemistry at C8/C9 could be assigned based on precedents for epoxyalcohols that are trans-epoxides (20): (i) the erythro diastereomer is less polar than threo on SP-HPLC (product 1 is less polar than 2), (ii) the geminal hydroxy proton in the erythro diastereomer is the farther downfield (H8 is at 3.59 in 1, at 3.405 in 2), and (iii) the erythro diastereomer has a smaller coupling constant to the vicinal epoxide proton (J8,9 = 3.2 Hz in 1, 4.0 Hz in 2). In fact the appearance of the H9 signal in the threo diastereomer (product 2, Fig. 3) matches that of the analogous signal in 13,14-trans-epoxy-threo-15S-hydroxy-eicosatrienoate (illustrated in Ref. 21); both appear as a doublet of doublets with four equally spaced signals (in contrast to the erythro diastereomer, product 1, Fig. 3). Furthermore, the more prominent threo product using mCPBA conforms with chemical precedent (22). Thus, with the reasonable assumption that the configuration of the 8R-hydroxy is retained in the products (and the coupling constants support the double bonds as cis), 1 has the structure 8R-hydroxy-9R,10R-epoxy-eicosa-5Z,11Z,14Z-trienoic acid, and product 2 is the 8R,9S,10S diastereomer. The identity of the cAOS products 1 and 2 with their chemically epoxidized counterparts was established by their indistinguishable UV spectra and mass spectra, their co-chromatography on RP-HPLC (Fig. 2) and SP-HPLC (supplemental Fig. S1), and in the case of the major cAOS product 1, by its identical NMR spectrum to the mCPBA product 1 (supplemental Fig. S2).
FIGURE 3.
Partial 1H NMR spectra of products 1 and 2, 8R-hydroxy-9,10-epoxy-20:3 diastereomers. The inset (right side) on each spectrum shows an expanded view of H9. The 2-Hz coupling for J9,10 establishes the trans-epoxide configuration in both 1 and 2. The more downfield chemical shift of H8 in 1 and the 3.4- and 4.0-Hz couplings for J8,9 support the erythro and threo assignments for 1 and 2, respectively (see main text) (20).
mCPBA also epoxidized the other double bonds in 8R-HETE. The 14,15-epoxy, 11,12-epoxy, and 5,6-epoxy derivatives were characterized (Fig. 2B, products a1,2, b1,2, and c1,2, with NMR data listed in the supplemental material). Very minor products of the enzymatic reaction with 8R-HETE, in addition to the 8R,9S,10S threo diastereomer, were thus identified as single diastereomers of 8R-hydroxy-14,15-epoxide and 8R-hydroxy-11,12-epoxide (Fig. 2, A and C, peaks a and b, respectively).
Rates of Reaction with 8R-HETE
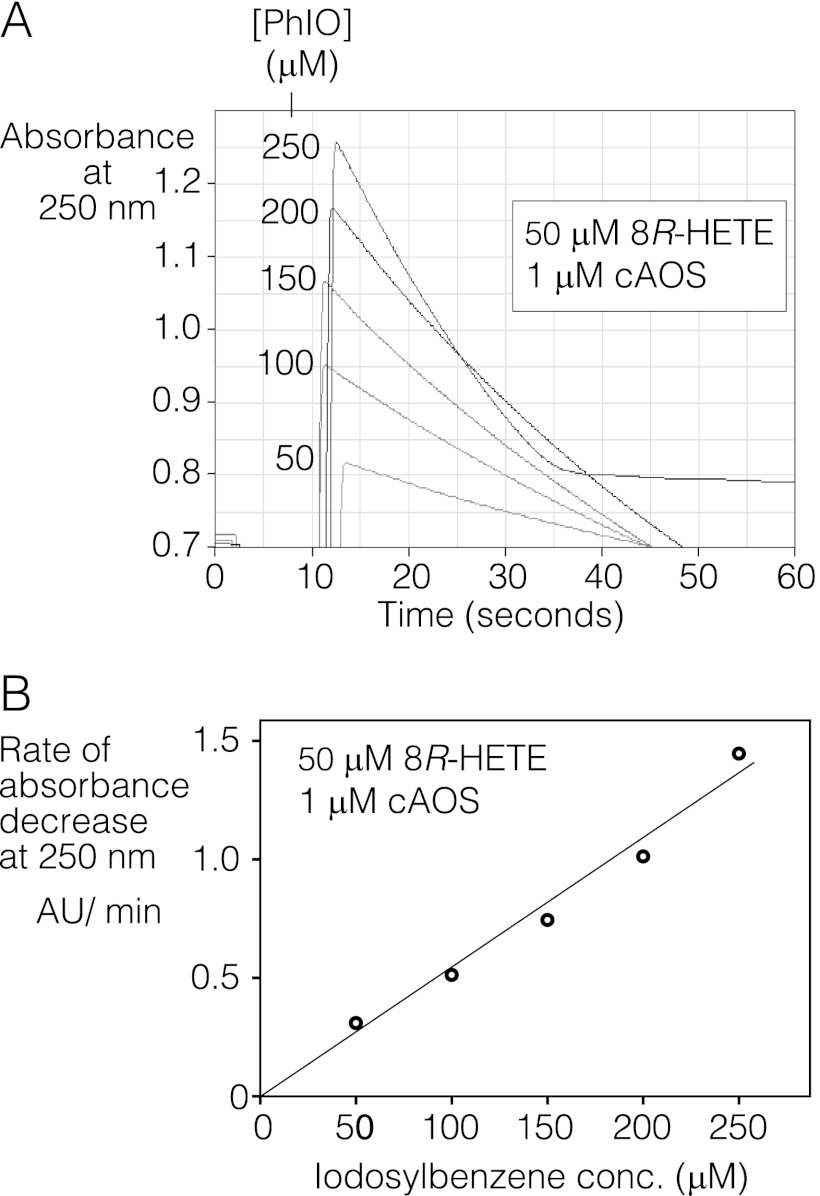
Because the main products 1 and 2 have lost the conjugated diene chromophore, the rate of reaction of 8R-HETE with cAOS and PhIO could be estimated from the loss of absorbance at 235 nm versus time. With 50 μm 8R-HETE and 1 μm cAOS in the cuvette, the reaction rate was linearly dependent on the concentration of PhIO in the range 0–250 μm, with initial turnover rates of 0.98/s (± 0.11 S.E., n = 5) per 100 μm PhIO (Fig. 4).
FIGURE 4.
Rate of reaction of 8R-HETE with cAOS and effect of iodosylbenzene concentration. Reaction was monitored by UV spectroscopy as decrease in 8-HETE absorbance versus time. A, representative traces showing, at 10 s, the addition of PhIO at the indicated concentrations (μm) to 50 μm 8R-HETE and 1 μm cAOS in 50 mm phosphate buffer, pH 7.5. B, rates of absorbance decrease at 250 nm versus PhIO concentration (conc.) in a representative experiment in which 2–6 recordings were averaged at each PhIO concentration. Use of the 250-nm wavelength (to reduce absorbance due to PhIO) is explained under “Experimental Procedures.” The rate of 8R-HETE transformation in this experiment was 0.7 s−1 per 100 μm PhIO and averaged 1 s−1 in five experiments. mAU, milliabsorbance units.
Reactions of cAOS + Iodosylbenzene with 8S-HETE
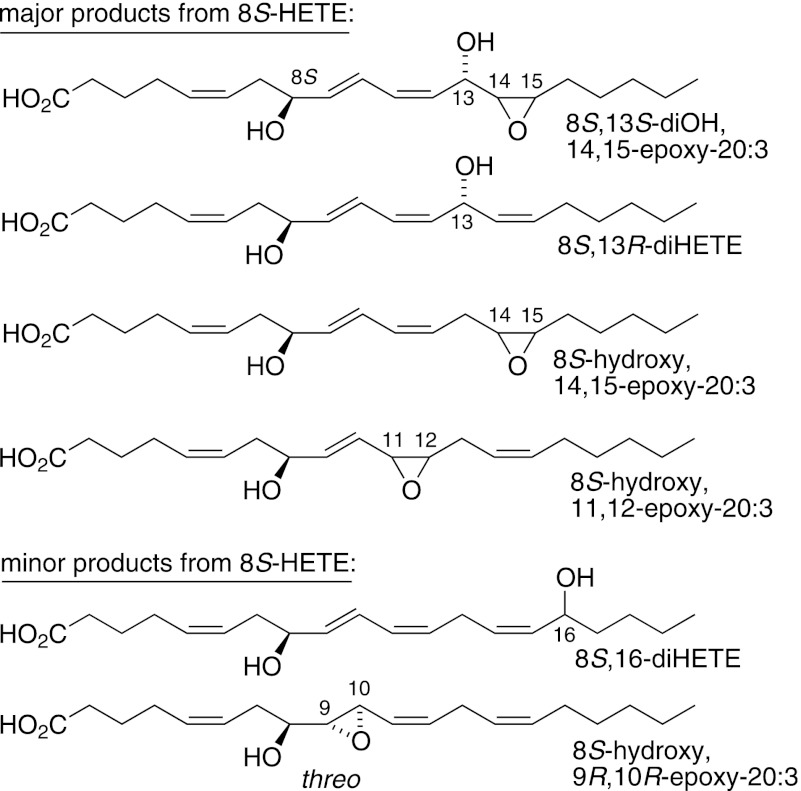
For comparison with the specificity of reaction with 8R-HETE, we also tested the enantiomer, 8S-HETE, as substrate. Although there was less regiospecificity as compared with 8R-HETE, the oxygenations were stereospecific, forming single diastereomeric products (Fig. 5, with RP-HPLC chromatogram (supplemental Fig. S3) and NMR and GC-MS data in the supplemental material). Also, of particular interest mechanistically, hydroxylation was a prominent transformation, mainly on the bis-allylic 13-carbon, with a minor allylic 16-hydroxylation (Fig. 5, supplemental Figs. S3–S6).
FIGURE 5.
Products formed from 8S-HETE by cAOS/PhIO. The supplemental material provides a typical RP-HPLC chromatogram (supplemental Fig. S3) together with NMR, GC-MS, and chiral analyses of the products (supplemental Figs. S4–S6).
Reactions of cAOS + Iodosylbenzene with Arachidonic Acid
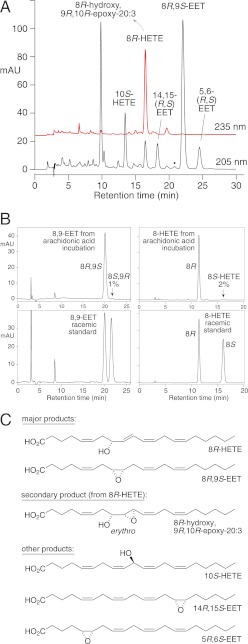
Having identified specific oxygenation products with 8-hydroxy analogs, we then tested the parent, achiral substrate, arachidonic acid. The cAOS charged with PhIO catalyzed regiospecific and stereospecific oxygenation. Two major chiral products were formed: 8R,9S-epoxyeicosatrienoic acid (8R,9S-EET) and 8R-HETE (Fig. 6, A and B), in 98 and 96% enantiomeric excess, respectively. Much of the 8R-HETE was further epoxidized to the erythro epoxyalcohol diastereomer, 8R-hydroxy-9R,10R-trans-epoxy-eicosa-5Z,11Z,14Z-trienoate; this is identical to product 1 of the 8R-HETE incubations, and as arachidonate has no 9,10 double bond, it can only be formed as a secondary oxygenation product from 8R-HETE.
FIGURE 6.
Products formed from arachidonic acid by cAOS/PhIO. A, RP-HPLC analysis of an aliquot using a Thomson Instrument Co. TLC-Advantage ODS column (25 × 0.46 cm), a solvent of MeOH/H2O/HAc (80/20/0.01 by volume), and a flow rate of 1 ml/min, with on-line UV detection (Agilent 1100 series diode array detector) at 205 and 235 nm. The black dot at ∼21 min marks the expected retention time of 11,12-EET (not present). mAU, milliabsorbance units. B, chiral analysis of 8,9-EET (left panels) and 8-HETE (right panels) formed by cAOS/PhIO from arachidonic acid. 8,9-EET was analyzed as the free acid using a Chiralpak AD column (25 × 0.46 cm) and a solvent of hexane/methanol/glacial acetic acid (100:2:0.05) at a flow rate of 1 ml/min with UV detection at 205 nm. 8-HETE was analyzed as the methyl ester using the same conditions except with a solvent of hexane/methanol (100:2, v/v) with UV detection at 235 nm. C, structures of the products. The supplemental material illustrates chiral analysis of 5,6-EET (supplemental Fig. S7), 14,15-EET (supplemental Fig. S8), and 10-HETE (supplemental Fig. S9).
Other products from arachidonic acid included the single EET enantiomer, 5R,6S-EET and 14,15-EET (76% 14R,15S) (Fig. 6C, supplemental Figs. S7 and S8). 11,12-EET was absent (its retention time is marked in Fig. 6A with a black dot). Besides 8R-HETE, there was prominent formation of specifically 10-HETE (83% 10S) (Fig. 6C, supplemental Fig. S9). Although reaction rate could not be determined using the UV assay, the extent of metabolism in the 5-min incubation was similar to that of 8R-HETE and 8S-HETE.
Reactions of cAOS + Iodosylbenzene with Stearic and Linoleic Acids
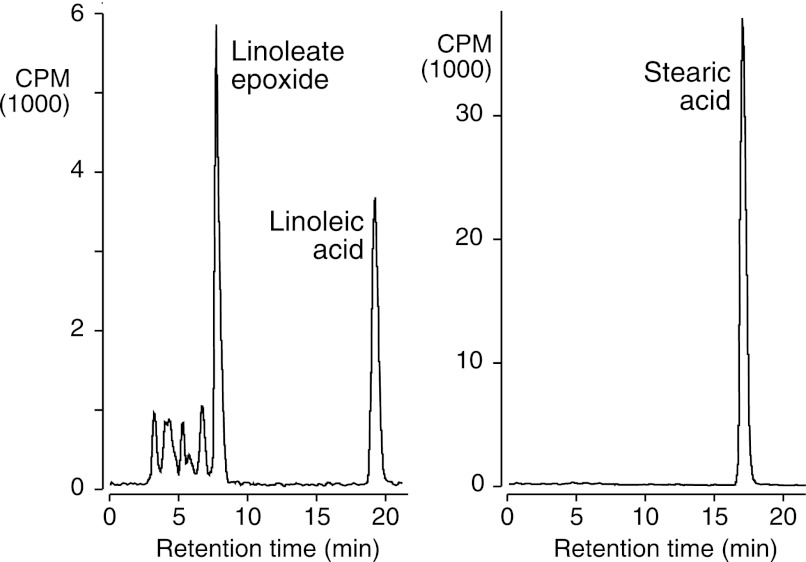
So far, the results reveal epoxidation and hydroxylation reactions by cAOS within the double bond system of eicosatetraenoates. To examine the possibility of hydroxylating a plain alkane, cAOS/PhIO was tested with [14C]stearic acid, using [14C]linoleic acid as a control. In side-by-side incubations, linoleate (18:2) was extensively oxygenated, whereas the stearate (18:0) was recovered unchanged, Fig. 7.
FIGURE 7.
RP-HPLC analysis of the reactions of linoleic acid (left) and stearic acid (right) with cAOS/PhIO. The 14C-labeled fatty acids (50 μm) were reacted with cAOS (2 μm) and PhIO (50 μm) for 15 min at room temperature and extracted, and aliquots were analyzed by RP-HPLC (Waters Symmetry column, 25 × 0.46 cm) with on-line UV and radioactive detection. A solvent system of MeOH/H2O/HAc was used in the proportions 90:10:0.01 for linoleic acid and 95:5:0.01 for stearic acid. The main peak from linoleic acid was detected at 205 nm in the UV (no 235 nm absorbance) and was identified as a 3:1 mixture of 12,13- to 9,10-linoleate monoepoxides by separation on SP-HPLC.
DISCUSSION
Monooxygenase Activity
The catalytic capabilities of cAOS, with a tyrosine as the proximal heme ligand (5), are quite remarkable in a number of respects. In its natural activity, cAOS catalyzes the same chemistry as the allene oxide synthases of the cytochrome P450 family CYP74 (6, 7) and analogous reactions to thromboxane and prostacyclin synthases (CYP5, CYP8A) (23). Here we show that when charged with the single oxygen donor iodosylbenzene, cAOS mimics the monooxygenase activities of many of the cytochrome P450 epoxygenases and hydroxylases (24, 25). The cAOS/PhIO enzyme catalyzed 8R-HETE synthesis, as well as bis-allylic and allylic hydroxylation and epoxidation of double bonds. Usually, the catalytic activities of the cytochrome P450s are considered a unique attribute of the cysteinyl proximal heme ligand (1). Obviously, we have uncovered a mimic of some of these activities in the tyrosine-liganded cAOS.
Stereochemistry of the Oxygenations
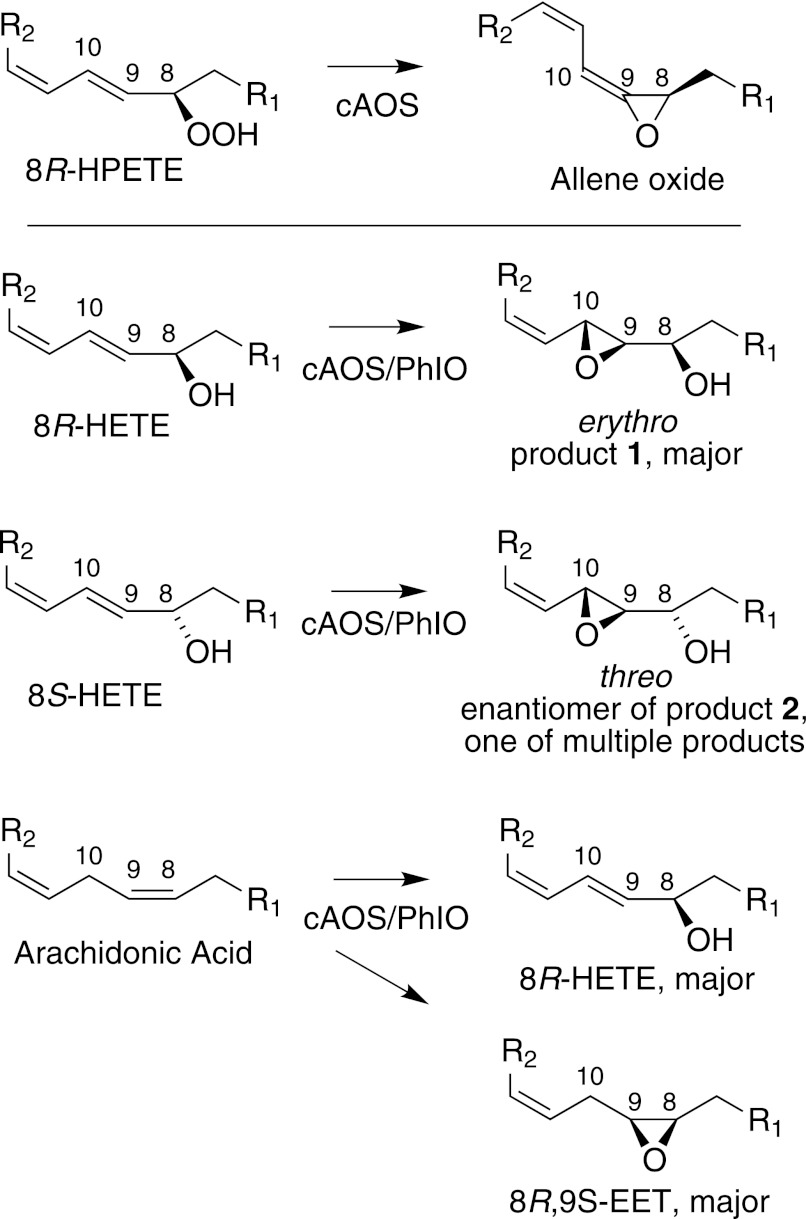
Equally remarkable to the monooxygenase capability of cAOS is the regiospecificity and stereoselectivity of the iodosylbenzene-supported reactions. With 8R-HETE and arachidonic acid as substrates, the oxygenation specificity was clearly related to the natural reaction with arachidonate 8R-HPETE (Fig. 8). 8R-HPETE must bind so that the heme iron can interact with and cleave the 8R-hydroperoxyl. In the monooxygenase activity, typically one expects a suprafacial relationship between the initiating hydrogen abstraction and the oxygen rebound giving the epoxide or hydroxy product (11, 26). One can deduce, therefore, that the binding of 8R-HETE and arachidonic acid must be in the same orientation as 8R-HPETE. This is in accord with the specific erythro 9R,10R epoxidation of the 9,10-trans double bond of 8R-HETE, the corresponding threo epoxidation as one of the products of 8S-HETE, and the formation of 8R,8S-EET and 8R-HETE from arachidonic acid (Fig. 8).
FIGURE 8.
Stereochemistry of the cAOS-PhIO products in relation to biosynthesis of allene oxide. Top, partial structures illustrating conversion of 8R-HPETE to allene oxide. Bottom, transformations of 8R-HETE, 8S-HETE, and arachidonic acid to chiral epoxy and hydroxy products. R1 = HO2C(CH2)4CH = CH, and R2 = CH2CH = CH(CH2)4CH3.
The less abundant EET products from arachidonic acid were also stereospecifically epoxidized on the same face of the molecule, forming 5R,6S-EET and 14R,15S-EET (Fig. 6). The odd one out in this respect is the 10-HETE, which was ∼80% 10S, representing mainly hydroxylation on the opposite face of the substrate. One might surmise that when arachidonic acid is bound in the active site such that the ferryl heme abstracts the 10R hydrogen, the resulting products, both formed via suprafacial oxygen rebound, are 8R,8S-EET and 8R-HETE. Only a small fraction of the overall complement of products is hydroxylated at C-10 on the same face of the molecule (20% of the total 10-HETE, 10R-HETE). Perhaps the 80% of the 10-HETE that is 10S is formed as a primary product from the small fraction of the reacting substrate undergoing 10S hydrogen abstraction.
Using 8S-HETE as substrate, there was a mixture of products, although each appeared to be the result of a stereospecific oxygenation. As mentioned above, epoxidation of the 9,10 double bond gave the threo epoxyalcohol (8S-hydroxy-9R,10R-epoxy), which represents oxygenation on the same face of the molecule as occurs with 8R-HETE (Figs. 5 and 8). The oxygenation at C-13 is also on the same face, a 13R hydroxylation (Fig. 5, supplemental Fig. S5). We did not determine the absolute configuration of all of the other products, but each was a single diastereomer as apparent from further HPLC purification and from NMR. The bis-allylic hydroxylation at the 13 or 16 positions (and at the 10-carbon using arachidonic acid as substrate) is typical of the NADPH-dependent oxygenation of arachidonic acid by liver microsomes and recombinant cytochrome P450s (25, 27).
Rates of Reaction
The initial rate of reaction we observed for cAOS metabolism of 8R-HETE was linear with the oxygen donor concentration, and the ∼1/s value using 100 μm PhIO is about 2 orders of magnitude higher than reported for conventional P450 arachidonate monooxygenases using NADPH (e.g. Refs. 24, 28, and 29). On the other hand, the rates we observed are about 2 orders of magnitude below the rates achieved by bacterial P450BM3 (CYP102A1) in metabolism of fatty acid substrates (30). Arguably, BM3 attains its exceptional rates due to the efficient coupling with its fusion partner, the reductase that supplies the electrons from NADPH, whereas for mammalian P450s, the coupling with reductase may be rate-limiting in NADPH-supported oxygenations. When the NADPH requirement for conventional P450s is bypassed by substitution with PhIO as an oxygen donor, the rates usually increase considerably (cf. Refs, 31 and 32).
The Potential Scope of Hydroxylation Activity
The cAOS/PhIO enzyme did not react with the alkane chain of stearic acid. This apparent limitation should be further explored in direct comparison with P450 epoxygenases and hydroxylases that normally react within the arachidonate double bonds or with CYP5, CYP8A, or CYP74 enzymes charged with iodosylbenzene. Alkane hydroxylation is considered the tour-de-force reaction of the thiolate-liganded heme monooxygenases (33). Nonetheless, one might question whether all cytochrome P450s have the innate ability to hydroxylate plain alkanes. Also, tied to this, do all P450s have an extra catalytic capability as compared with the tyrosine-liganded cAOS?
In earlier work, the fact that the product profile of P450s can be selectively altered by active site mutations led to the suggestion that different catalytic species (e.g. Fe–OOH versus Compound I, Fe=O) were responsible for distinct P450 reactions (34). More recently, the balance of evidence supports the concept that Compound I is the only effective oxidant in typical P450 reactions (35, 36), and rather that the chemistry of the enzyme is modulated by the active site structure on the distal side of the heme (an alternative interpretation of the earlier studies (34, 37)). Added to this is the evidence that the ability to catalyze P450-type chemistry does not require a thiolate as the proximal heme ligand. For example, histidine-liganded myoglobin treated with cumene hydroperoxide under anaerobic conditions can catalyze the bis-allylic hydroxylation and epoxidation of linoleic acid; the resulting products are racemic, although the individual reactions involve suprafacial hydrogen abstraction and hydroxylation typical of a P450-type mechanism (38). Also, a myoglobin mutant has been designed that catalyzes a single turnover hydroxylation of an active site tryptophan (39), which the authors interpret as a P450-like oxygenation by the histidine-liganded myoglobin. Mutation of the proximal heme histidine in myoglobin to cysteine (P450-like) or tyrosine (catalase-like) had fairly minor effects on catalysis and certainly did not confer the mutant enzymes with the properties typical of a P450 or catalase (40). Also, a switch of the cysteine ligand of P450CAM or CYP119 with selenocysteine allowed most of the native P450 enzyme chemistry to be retained (41, 42).
Our results tend to support the developing concept that a thiolate proximal heme ligand is not required for cytochrome P450-like catalytic activity. Multiple cAOS-related enzymes have been identified, the normal reactions of which include the transformation of fatty acid hydroperoxides to allylic epoxides (43), including one with a bicyclobutane carbon chain (44). There may, therefore, be new catalytic possibilities in the reactions of these enzymes when treated with an oxidant that forms Compound I.
Supplementary Material
Acknowledgments
We thank Dr. Don F. Stec for assistance with the NMR and Dr. Claus Schneider for helpful comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM 074888 (to A. R. B.).

This article contains supplemental Figs. S1–S9, NMR data, and references.
- cAOS
- catalase-related allene oxide synthase
- EET
- epoxyeicosatrienoic acid
- HPETE
- hydroperoxyeicosatetraenoic acid
- HETE
- hydroxyeicosatetraenoic acid
- PhIO
- iodosylbenzene
- mCPBA
- meta-chloroperoxybenzoic acid
- RP-HPLC
- reversed phase high pressure liquid chromatography
- SP-HPLC
- straight phase high pressure liquid chromatography
- CYP
- cytochrome P450.
REFERENCES
- 1. Denisov I. G., Makris T. M., Sligar S. G., Schlichting I. (2005) Structure and chemistry of cytochrome P450. Chem. Rev. 105, 2253–2277 [DOI] [PubMed] [Google Scholar]
- 2. Guengerich F. P. (2007) Mechanisms of cytochrome P450 substrate oxidation: MiniReview. J. Biochem. Mol. Toxicol. 21, 163–168 [DOI] [PubMed] [Google Scholar]
- 3. Nicholls P., Fita I., Loewen P. C. (2001) Enzymology and structure of catalases. Adv. Inorg. Chem. 51, 51–106 [Google Scholar]
- 4. Putnam C. D., Arvai A. S., Bourne Y., Tainer J. A. (2000) Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J. Mol. Biol. 296, 295–309 [DOI] [PubMed] [Google Scholar]
- 5. Oldham M. L., Brash A. R., Newcomer M. E. (2005) The structure of coral allene oxide synthase reveals a catalase adapted for metabolism of a fatty acid hydroperoxide. Proc. Natl. Acad. Sci. U.S.A. 102, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tijet N., Brash A. R. (2002) Allene oxide synthases and allene oxides. Prostaglandins Other Lipid Mediat. 68–69, 423–431 [DOI] [PubMed] [Google Scholar]
- 7. Brash A. R. (2009) Mechanistic aspects of CYP74 allene oxide synthases and related cytochrome P450 enzymes. Phytochemistry 70, 1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ullrich V. (2003) Thoughts on thiolate tethering: tribute and thanks to a teacher. Arch. Biochem. Biophys. 409, 45–51 [DOI] [PubMed] [Google Scholar]
- 9. Cho K. B., Lai W., Hamberg M., Raman C. S., Shaik S. (2011) The reaction mechanism of allene oxide synthase: interplay of theoretical QM/MM calculations and experimental investigations. Arch. Biochem. Biophys. 507, 14–25 [DOI] [PubMed] [Google Scholar]
- 10. Lichtenberger F., Nastainczyk W., Ullrich V. (1976) Cytochrome P450 as an oxene transferase. Biochem. Biophys. Res. Commun. 70, 939–946 [DOI] [PubMed] [Google Scholar]
- 11. Ortiz de Montellano P. R. (2010) Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 110, 932–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hecker M., Baader W. J., Weber P., Ullrich V. (1987) Thromboxane synthase catalyses hydroxylations of prostaglandin H2 analogs in the presence of iodosylbenzene. Eur. J. Biochem. 169, 563–569 [DOI] [PubMed] [Google Scholar]
- 13. Saltzman H., Sharefkin J. G. (1973) Iodosobenzene. Org. Synth. Coll. 5, 658–659 [Google Scholar]
- 14. Boutaud O., Brash A. R. (1999) Purification and catalytic activities of the two domains of the allene oxide synthase-lipoxygenase fusion protein of the coral Plexaura homomalla. J. Biol. Chem. 274, 33764–33770 [DOI] [PubMed] [Google Scholar]
- 15. Peers K. E., Coxon D. T. (1983) Controlled synthesis of monohydroperoxides by α-tocopherol inhibited autoxidation of polyunsaturated lipids. Chem. Phys. Lipids 32, 49–56 [Google Scholar]
- 16. Schneider C., Boeglin W. E., Brash A. R. (2000) Enantiomeric separation of hydroxy eicosanoids by chiral column chromatography: effect of the alcohol modifier. Anal. Biochem. 287, 186–189 [DOI] [PubMed] [Google Scholar]
- 17. Gelb M. H., Toscano W. A., Jr., Sligar S. G. (1982) Chemical mechanisms for cytochrome P-450 oxidation: spectral and catalytic properties of a manganese-substituted protein. Proc. Natl. Acad. Sci. U.S.A. 79, 5758–5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu Z., Schneider C., Boeglin W. E., Marnett L. J., Brash A. R. (2003) The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc. Natl. Acad. Sci. U.S.A. 100, 9162–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mercier J., Agoh B. (1974) Behavior of long chain allylic hydroperoxides in the presence of certain transition metal complexes. II. Structure of epoxy alcohols formed from the hydroperoxides of cis and trans octadec-9-enoate methyl esters in the presence of vanadyl acetylacetonate (translated from the French). Chem. Phys. Lipids 12, 239–248 [Google Scholar]
- 20. Bernart M. W., Gerwick W. H. (1994) Eicosanoids from the tropical red alga Murrayella periclados. Phytochemistry 36, 1233–1240 [Google Scholar]
- 21. Hamberg M., Herman R. P., Jacobsson U. (1986) Stereochemistry of two epoxy alcohols from Saprolegnia parasitica. Biochim. Biophys. Acta 879, 410–418 [Google Scholar]
- 22. Rossiter B. E., Verhoeven T. R., Sharpless K. B. (1979) Stereoselective epoxidation of acyclic allylic alcohols. A correction of our previous work. Tetrahedron Lett. 20, 4733–4736 [Google Scholar]
- 23. Ullrich V., Brugger R. (1994) Prostacyclin and thromboxane synthase: new aspects of hemethiolate catalysis. Angew. Chem. Int. Ed. Engl. 33, 1911–1919 [Google Scholar]
- 24. Zeldin D. C., DuBois R. N., Falck J. R., Capdevila J. H. (1995) Molecular cloning, expression, and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch. Biochem. Biophys. 322, 76–86 [DOI] [PubMed] [Google Scholar]
- 25. Bylund J., Kunz T., Valmsen K., Oliw E. H. (1998) Cytochromes P450 with bis-allylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. J. Pharmacol. Exp. Ther. 284, 51–60 [PubMed] [Google Scholar]
- 26. Oliw E. H., Brodowsky I. D., Hörnsten L., Hamberg M. (1993) Bis-allylic hydroxylation of polyunsaturated fatty acids by hepatic monooxygenases and its relation to the enzymatic and nonenzymatic formation of conjugated hydroxy fatty acids. Arch. Biochem. Biophys. 300, 434–439 [DOI] [PubMed] [Google Scholar]
- 27. Brash A. R., Boeglin W. E., Capdevila J. H., Yeola S., Blair I. A. (1995) 7-HETE, 10-HETE, and 13-HETE are major products of NADPH-dependent arachidonic acid metabolism in rat liver microsomes: analysis of their stereochemistry, and the stereochemistry of their acid-catalyzed rearrangement. Arch. Biochem. Biophys. 321, 485–492 [DOI] [PubMed] [Google Scholar]
- 28. Rifkind A. B., Lee C., Chang T. K., Waxman D. J. (1995) Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch. Biochem. Biophys. 320, 380–389 [DOI] [PubMed] [Google Scholar]
- 29. Luo G., Zeldin D. C., Blaisdell J. A., Hodgson E., Goldstein J. A. (1998) Cloning and expression of murine CYP2Cs and their ability to metabolize arachidonic acid. Arch. Biochem. Biophys. 357, 45–57 [DOI] [PubMed] [Google Scholar]
- 30. Ost T. W., Miles C. S., Murdoch J., Cheung Y., Reid G. A., Chapman S. K., Munro A. W. (2000) Rational redesign of the substrate binding site of flavocytochrome P450 BM3. FEBS Lett. 486, 173–177 [DOI] [PubMed] [Google Scholar]
- 31. Gustafsson J. A., Rondahl L., Bergman J. (1979) Iodosylbenzene derivatives as oxygen donors in cytochrome P-450 catalyzed steroid hydroxylations. Biochemistry 18, 865–870 [DOI] [PubMed] [Google Scholar]
- 32. Yamazaki H., Ueng Y. F., Shimada T., Guengerich F. P. (1995) Roles of divalent metal ions in oxidations catalyzed by recombinant cytochrome P450 3A4 and replacement of NADPH–cytochrome P450 reductase with other flavoproteins, ferredoxin, and oxygen surrogates. Biochemistry 34, 8380–8389 [DOI] [PubMed] [Google Scholar]
- 33. Groves J. T. (2003) The bioinorganic chemistry of iron in oxygenases and supramolecular assemblies. Proc. Natl. Acad. Sci. U.S.A. 100, 3569–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaz A. D., McGinnity D. F., Coon M. J. (1998) Epoxidation of olefins by cytochrome P450: evidence from site-specific mutagenesis for hydroperoxo-iron as an electrophilic oxidant. Proc. Natl. Acad. Sci. U.S.A. 95, 3555–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Franke A., Fertinger C., van Eldik R. (2008) Which oxidant is really responsible for P450 model oxygenation reactions? A kinetic approach. Angew. Chem. Int. Ed. Engl. 47, 5238–5242 [DOI] [PubMed] [Google Scholar]
- 36. Shaik S., Cohen S., Wang Y., Chen H., Kumar D., Thiel W. (2010) P450 enzymes: their structure, reactivity, and selectivity – modeled by QM/MM calculations. Chem. Rev. 110, 949–1017 [DOI] [PubMed] [Google Scholar]
- 37. Jin S., Bryson T. A., Dawson J. H. (2004) Hydroperoxoferric heme intermediate as a second electrophilic oxidant in cytochrome P450-catalyzed reactions. J. Biol. Inorg. Chem. 9, 644–653 [DOI] [PubMed] [Google Scholar]
- 38. Hamberg M. (1997) Myoglobin-catalyzed bis-allylic hydroxylation and epoxidation of linoleic acid. Arch. Biochem. Biophys. 344, 194–199 [DOI] [PubMed] [Google Scholar]
- 39. Watanabe Y., Nakajima H., Ueno T. (2007) Reactivities of oxo and peroxo intermediates studied by hemoprotein mutants. Acc. Chem. Res. 40, 554–562 [DOI] [PubMed] [Google Scholar]
- 40. Adachi S., Nagano S., Ishimori K., Watanabe Y., Morishima I., Egawa T., Kitagawa T., Makino R. (1993) Roles of proximal ligand in heme proteins: replacement of proximal histidine of human myoglobin with cysteine and tyrosine by site-directed mutagenesis as models for P-450, chloroperoxidase, and catalase. Biochemistry 32, 241–252 [DOI] [PubMed] [Google Scholar]
- 41. Jiang Y., Sivaramakrishnan S., Hayashi T., Cohen S., Moënne-Loccoz P., Shaik S., Ortiz de Montellano P. R. (2009) Calculated and experimental spin state of seleno cytochrome P450. Angew. Chem. Int. Ed. Engl. 48, 7193–7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aldag C., Gromov I. A., García-Rubio I., von Koenig K., Schlichting I., Jaun B., Hilvert D. (2009) Probing the role of the proximal heme ligand in cytochrome P450cam by recombinant incorporation of selenocysteine. Proc. Natl. Acad. Sci. U.S.A. 106, 5481–5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao B., Boeglin W. E., Zheng Y., Schneider C., Brash A. R. (2009) Evidence for an ionic intermediate in the transformation of fatty acid hydroperoxide by a catalase-related allene oxide synthase from the Cyanobacterium Acaryochloris marina. J. Biol. Chem. 284, 22087–22098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneider C., Niisuke K., Boeglin W. E., Voehler M., Stec D. F., Porter N. A., Brash A. R. (2007) Enzymatic synthesis of a bicyclobutane fatty acid by a hemoprotein lipoxygenase fusion protein from the cyanobacterium Anabaena PCC 7120. Proc. Natl. Acad. Sci. U.S.A. 104, 18941–18945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.