Background: The terminal complement pathway contributes to cerebral malaria (CM) pathogenesis.
Results: The terminal pathway is activated in CM through a unique mechanism independent of the C5 convertases.
Conclusion: We propose that enzymes of the coagulation system activate C5 in CM.
Significance: Inhibition of complement at the level of C5 represents a unique therapeutic target in CM.
Keywords: Complement, Infectious Diseases, Innate Immunity, Malaria, Neuroimmunology, Neurological Diseases, Anaphylatoxins, Convertases, Membrane Attack Complex
Abstract
Cerebral malaria (CM) is the most severe manifestation of clinical malaria syndromes and has a high fatality rate especially in the developing world. Recent studies demonstrated that C5−/− mice are resistant to experimental CM (ECM) and that protection was due to the inability to form the membrane attack complex. Unexpectedly, we observed that C4−/− and factor B−/− mice were fully susceptible to disease, indicating that activation of the classical or alternative pathways is not required for ECM. C3−/− mice were also susceptible to ECM, indicating that the canonical C5 convertases are not required for ECM development and progression. Abrogation of ECM by treatment with anti-C9 antibody and detection of C5a in serum of C3−/− mice confirmed that C5 activation occurs in ECM independent of C5 convertases. Our data indicate that activation of C5 in ECM likely occurs via coagulation enzymes of the extrinsic protease pathway.
Introduction
Plasmodium falciparum malaria is one of the most important public health burdens facing the global community. More than 2.5 billion people live in areas where they are at risk from P. falciparum infection (1), and there are between 100 and 300 million clinical cases and more than one million malaria-related deaths each year (44), with the majority of deaths occurring in children in sub-Saharan Africa. The overwhelming majority of malaria mortality is caused by P. falciparum, and cerebral malaria (CM)2 is one of the most severe clinical complications of P. falciparum malaria. Cerebral malaria has a case fatality rate of 15–30%, and more than 10% of children who recover from CM have ongoing neurological complications such as cognitive impairment (reviewed in Refs. 2 and 3). Despite the importance of CM, our understanding of the molecular causes of its pathology is limited.
A potential role for complement in the development and progression of malaria and CM has been studied for some time; however, the approach has largely been correlative (examining for changes in the levels of complement proteins and activation fragments) rather than mechanistic (reviewed in Ref. 4). These studies demonstrate that both the classical and the alternative complement pathways are activated in malaria, whereas the mannose-binding protein pathway is not significantly involved (4). Recent studies have shown that mice naturally deficient in C5 are resistant to experimental cerebral malaria (ECM) (5, 6) and that the terminal complement pathway contribution to ECM immunopathology is mediated by the membrane attack complex (MAC) and not C5a (6). However, these observations have not clarified the role of the activation pathways and C3, the central component of the complement system, in ECM.
The present study demonstrates that neither the classical nor the alternative complement pathways are required for disease development. Surprisingly, C3−/− mice developed severe ECM, reinforcing the observation that the early activation pathways are not required for disease. Importantly, the inability of C3−/− mice to generate C3b indicates that severe ECM in these mice occurs through activation of C5 independent of complement C5 convertases. These results suggest that C5 is activated in ECM through the extrinsic protease pathway (7–10).
EXPERIMENTAL PROCEDURES
Mice, Malaria Parasites, and ECM
C4−/−, factor B−/−, C3−/−, and sCrry/GFAP mice have been described previously (11–14). All mice were backcrossed to C57BL/6 mice for eight or more generations. Male and female mice between the ages of 8 and 12 weeks were used for all experiments. All studies were performed with approval from the University of Alabama (UAB) Institutional Animal Care and Use Committee.
Plasmodium berghei ANKA was maintained by passage in BALB/c mice as described previously (15). ECM was induced by injecting mice intraperitoneally with 5 × 105 PbA-infected RBCs. Parasitemia was monitored on day 6 after infection by Giemsa-stained, thin blood smears. Mice were monitored twice daily for clinical signs of neurologic disease, in a blinded fashion, using the following scoring scale: 0, asymptomatic; 1, symptomatic (ruffled fur); 2, mild disease (slow righting); 3, moderate disease (difficulty righting); 4, severe disease (ataxia, seizures, coma); 5, dead. Mice observed having seizures were given a score of 4 regardless of other clinical signs of disease, and moribund animals were scored 4.5 and humanely sacrificed. Mice were classified as having ECM if they displayed these symptoms between days 5 and 9 after infection and had a corresponding drop in external body temperature or succumbed to infection.
Cytokine and C5a Serum Protein Levels and Analysis of Leukocytes from Brains
Whole blood was collected via retro-orbital bleed on day 6 after ECM induction. Samples were assayed for IFN-g, IL-1b, and IL-6 using Bio-Plex mouse cytokine assays (Bio-Rad) performed according to the manufacturer's instructions. C5a was quantitated using a solid-phase ELISA as described previously (16). Mice were transcardially perfused with PBS for 2 min, and brains were processed for flow cytometry as described previously (17).
Anti-C9 Antibody and CR2-Crry Treatment
C3−/− mice were infected as described above. Starting on day 4 after infection, mice were treated with either 400 μg of rabbit anti-mouse C9 antibody or 400 μg of rabbit IgG isotype control antibody. Mice were injected daily until the onset of symptoms (day 6 after infection) as described previously (6). Wild type mice were treated with CR2-Crry (100 mg) (18) or PBS on days 5 and 6 after infection.
Statistical Analysis
Statistical significance of ECM survival was calculated using the log rank test using Prism 4 (GraphPad Software, Inc.). Student's t test (one-way) and Wilcoxon rank sum tests were used to determine significance for parametric and nonparametric data. Data are shown as mean ± S.E. A value of p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Deletion of C4, Factor B, or C3 Does Not Alter Susceptibility to ECM
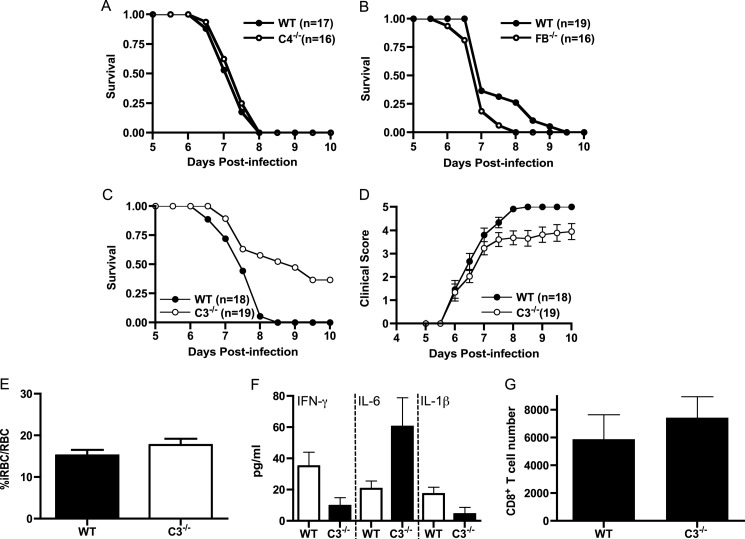
Previous studies have demonstrated that C5−/− mice are highly resistant to ECM (5, 6, 19–21) and that terminal pathway-mediated mechanisms contribute to inflammation and immunopathology in this disease model (6). To determine which complement activation pathway (classical or alternative) leads to C5 activation in ECM, we followed the course of disease in C4−/− (11) and factor B−/− mice (12). We found that both complement mutant mice were fully susceptible to ECM; however, factor B−/− mice progressed to fatal ECM a day earlier than wild type mice (Fig. 1, A and B). The trend toward more severe disease in factor B−/− mice supports a protective role for complement as expected in most infectious disease settings. These results demonstrate that C5 activation in ECM occurs independent of the classical and alternative pathways and raised questions regarding the role of C5 convertases in ECM development. To assess this latter possibility, we performed ECM using C3−/− mice (13). Survival of C3−/− mice was greater than wild type mice (7/19 versus 0/18); however, the difference did not reach statistical significance (p = 0.21, log rank test, Fig. 1C). Clinical scores were very similar between the two groups of mice throughout the course of disease but were significantly lower in C3−/− mice from day 7.5 onward (p < 0.05, Wilcoxon rank sum test, Fig. 1D). Wild type and C3−/− mice had similar levels of parasitemia, pro-inflammatory cytokine production, and CNS infiltration of CD8+ T cells at the peak of disease (Fig. 1, E–G). Thus, despite a modest increase in survival, ECM is markedly more severe in C3−/− mice as compared with C5−/− mice (6) and very similar to the disease course seen in wild type mice. These data suggest that C5 cleavage is occurring in C3−/− mice in the absence of C5 convertases leading to MAC-mediated immunopathology.
FIGURE 1.
C4−/− and factor B−/− mice are fully susceptible to the development of ECM, whereas C3−/− mice are partially resistant. Wild type, C4−/−, factor B−/− (FB−/−), and C3−/− mice were injected intraperitoneally with 5 × 105 PbA-iRBC, and clinical scores and survival were monitored twice daily for 10 days as described under “Experimental Procedures.” A, C4−/− mice (n = 16) were fully susceptible to disease-induced mortality as compared with wild type mice (n = 17). B, factor B−/− mice (n = 16) were fully susceptible to disease-induced mortality as compared with wild type mice (n = 19). C, C3−/− mice (n = 19) are not significantly protected from disease-induced mortality (p = 0.21, log rank test) as compared with wild type mice (n = 18). D, clinical scores for wild type and C3−/− mice are significantly different from day 7.5 through day 10 (p < 0.05, Wilcoxon rank sum test). E, parasitemia on day 6 after infection was not significantly different between wild type and C3−/− mice (p > 0.05, Student's t test). F, INF-γ, IL-6, and IL-1 levels were determined by ELISA (n = 3–5 mice/group). G, brain tissue was isolated from PBS-perfused wild type and C3−/− mice at day 6 (n = 4/group) and subjected to flow cytometric analysis as described previously (43). The number of CD8+ T cells in the brains of wild type and C3−/− mice was not significantly different (p > 0.05, Student's t test).
The Terminal Pathway Is Activated and Contributes to ECM Development in C3−/− Mice
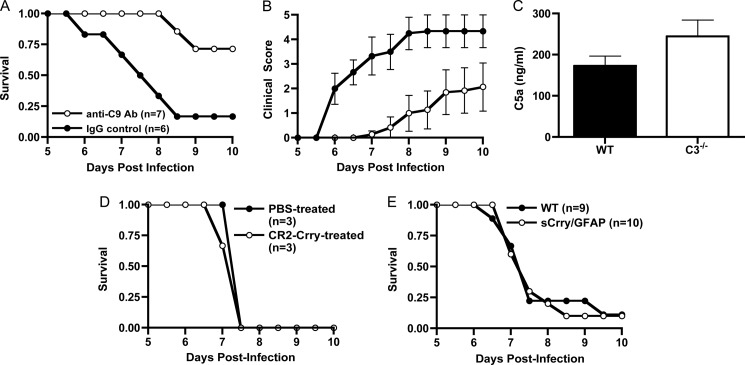
Previous studies from our laboratory have shown that inhibition of MAC formation through treatment with anti-C9 antibody reduces mortality and disease severity in wild type mice with ECM (6). Based on this, we reasoned that if C5 was cleaved in C3−/− mice with ECM, then a similar anti-C9 antibody treatment regimen would also provide C3−/− mice with protection from disease. Indeed we found that treatment of C3−/− mice with anti-C9 antibody significantly reduced mortality (p = 0.014, log rank test) and improved clinical scores (p < 0.05, Wilcoxon rank sum test) as compared with isotype control IgG-treated wild type mice (Fig. 2, A and B). To directly determine whether C5 is cleaved in ECM, we examined for the presence of C5a in serum of wild type and C3−/− mice at the peak of disease. We found that serum C5a levels in C3−/− mice were elevated, although not significantly, as compared with wild type mice (Fig. 2C). These results demonstrate that despite the absence of C5 convertases, C5 was cleaved and the MAC contributed to the progression and development of ECM in C3−/− mice comparable with that seen in wild type mice. We extended these observations by treating wild type mice with the complement inhibitor CR2-Crry, a chimeric recombinant protein that blocks the activity of both the C3 and the C5 convertases (18). Previous studies have shown that CR2-Crry is efficacious in reducing inflammation and cellular damage in a rodent model of spinal cord injury and experimental autoimmune encephalomyelitis, the animal model of multiple sclerosis (22).3 Wild type mice treated with CR2-Crry had an identical course of ECM as compared with PBS-treated controls (Fig. 2D). In addition, we performed ECM using sCrry/GFAP mice, which produce a CNS-specific, soluble form of the mouse complement convertase inhibitor Crry under the control of an astrocyte promoter (14). Soluble Crry production in these transgenic mice has been shown to be protective in numerous CNS disease settings (23–25). sCrry/GFAP mice succumbed to ECM identically to wild type mice (Fig. 2E). Together these data demonstrate that blocking C3 and C5 convertase activity, either in the cerebral vasculature or in the brain parenchyma, does not prevent disease development and provides additional experimental evidence for C5 convertase-independent activation of C5 in ECM.
FIGURE 2.
Terminal pathway activation occurs in C3−/− mice in ECM. Wild type mice were injected with 5 × 105 PbA-iRBC as described under “Experimental Procedures” A, anti-C9 antibody (Ab)-treated mice were significantly protected from ECM (p = 0.0001, log rank test) and had reduced clinical scores (B) as compared with mice treated with isotype control antibody (p < 0.05 on days 6–9, Wilcoxon rank sum test). C, wild type (n = 8) and C3−/− mice (n = 5) were injected with PbA-iRBC as in A. On day 6 after infection, mice were bled, and serum C5a levels were determined by ELISA. C5a levels in C3−/− mice were elevated, but not significantly, as compared with wild type mice. D, treatment of wild type mice with CR2-Crry (100 μg, n = 3) on days 5 and 6 did not significantly alter the course of ECM as compared with PBS-treated mice (n = 3). E, sCrry/GFAP mice (n = 10) were fully susceptible to disease-induced mortality as compared with wild type mice (n = 9).
The host defense mechanisms of the complement system make an as yet undefined contribution to protection against P. falciparum infection. It is well established from numerous animal and clinical studies that complement is activated through the classical and alternative pathways during malaria infection (4); however, the parasite-specific molecule or molecules that activate complement remain poorly defined with the exception of the digestive vacuole released on rupture of parasitized erythrocytes (26). Intracellular sequestration of the malaria parasite throughout the majority of its life cycle severely limits the opportunity for complement-mediated opsonization and/or lysis. In contrast, recent studies demonstrating that C5-deficient mice are resistant to ECM suggest that the complement system contributes to, rather than protects against, malarial pathogenesis (5, 6). The results we report here support and extend these observations and show that ECM develops in the absence of the early complement activation pathways and the canonical C5 convertases.
There is ample evidence showing activation of the classical and alternative pathways in malaria infection in humans and primate and rodent models of malaria (4, 27–32). In early animal model studies, depletion of complement in rats by cobra venom factor (CVF), which rapidly activates the alternative pathway, led to exacerbated disease, suggesting that complement may play a protective role in malaria infections (29). This observation supports our findings with C4−/− and factor B−/− mice; however, the data need to be interpreted with caution as it is unclear whether the reported increased mortality in CVF-treated rats in that study was due to higher parasitemia alone or to CVF-mediated inflammation combined with elevated parasitemia. Our results do not suggest that activation of complement through either pathway is irrelevant to the disease outcome, but simply that neither pathway is critical for the development of ECM, and therefore these pathways do not represent likely therapeutic targets for modulating the outcome of malaria infection.
The most intriguing finding we report here is the development of severe ECM in C3−/− mice. In the absence of C3, the C5 convertases (C4b2a3b for the classical pathway and C3bnBb for the alternative pathway) cannot be formed. Therefore if C5 is required for susceptibility to ECM, an alternative mechanism for C5 cleavage is required. Studies published almost three decades ago by Wetsel and Kolb (7) demonstrated that cleavage of C5 by trypsin, plasmin, thrombin, and elastase generated C5a-like fragments and that these fragments activated neutrophils and were chemotactic. Recent studies have confirmed and extended these initial observations and shown that coagulation enzymes including factors Xa, IXa, and XIa can cleave both C3 and C5 to generate C3a and C5a (8). Furthermore, the addition of factor Xa to serum generates the MAC, indicating that cleavage by what is now termed the extrinsic protease pathway provides a mechanism for activation of the terminal complement pathway in the absence of C5 convertases (10). Our data showing C5a production in C3−/− mice during ECM suggest utilization of this mechanism. Given that the coagulation system is activated during malaria infection (reviewed in Refs. 33 and 34) and that plasminogen can be activated on binding to C7 (35) and the MAC (36), it seems possible that a self-amplifying inflammatory complement/coagulation-based cascade contributes to cerebral malaria.
These data support our previously published observations indicating that the complement terminal pathway is an important contributor to the pathogenesis of ECM (6). This raises the possibility that selective pressure on terminal pathway genes (C6 through C9) could be a mechanism for surviving malaria and/or cerebral malaria. C6 and C9 deficiencies are common in humans (37), but C6 deficiency is highly prevalent in African Americans in the southeastern United States, with a frequency of almost 1 in 1600 (38). These individuals likely originated in West Africa and moved through the Caribbean and then into the southeastern United States (39) based on the tracking of two loss-of-function mutations in C6. Individuals deficient in terminal pathway components are susceptible to meningococcal infections (37, 40), a genotype with no apparent selective advantage. However, selective pressure in the face of malaria infection frequently maintains genetic mutations that can be life threatening (sickle cell anemia (41)) or are associated with autoimmune disease (systemic lupus erythematosus (42)) but provide a survival advantage in malaria-endemic regions. We are currently pursuing studies to address this possibility for C6 deficiency in malaria and cerebral malaria.
Acknowledgments
We thank Dr. Hubert Tse and Lindsay Padgett (Department of Microbiology, UAB) for assistance with the cytokine analysis. The continuing inspiration of Max Barnum is gratefully acknowledged.
This work was supported, in whole or in part, by National Institutes of Health Grants T32 AI07051 and NS077811 (to T. N. R.), AI08382 (to S. R. B.), and HL82485 (to S. T.). This work was also supported by Veterans Affairs Grant NURC-051-10F (to S. T.) and a research fellowship from the German Research Foundation (to S. W.).
X. Hu, S. Tomlinson, and S. R. Barnum, manuscript in preparation.
- used: CM
- cerebral malaria
- ECM
- experimental cerebral malaria
- MAC
- membrane attack complex
- PbA
- P. berghei ANKA
- GFAP
- glial fibrillary acidic protein
- CVF
- cobra venom factor
- iRBC
- infected red blood cell
- Crry
- complement receptor-related protein y
- sCrry
- soluble Crry.
REFERENCES
- 1. Guerra C. A., Snow R. W., Hay S. I. (2006) Mapping the global extent of malaria in 2005. Trends Parasitol. 22, 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haldar K., Murphy S. C., Milner D. A., Taylor T. E. (2007) Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu. Rev. Pathol. 2, 217–249 [DOI] [PubMed] [Google Scholar]
- 3. Grau G. E., Craig A. G. (2012) Cerebral malaria pathogenesis: revisiting parasite and host contributions. Future Microbiol. 7, 291–302 [DOI] [PubMed] [Google Scholar]
- 4. Silver K. L., Higgins S. J., McDonald C. R., Kain K. C. (2010) Complement-driven innate immune response to malaria: fuelling severe malarial diseases. Cell Microbiol. 12, 1036–1045 [DOI] [PubMed] [Google Scholar]
- 5. Patel S. N., Berghout J., Lovegrove F. E., Ayi K., Conroy A., Serghides L., Min-oo G., Gowda D. C., Sarma J. V., Rittirsch D., Ward P. A., Liles W. C., Gros P., Kain K. C. (2008) C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J. Exp. Med. 205, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramos T. N., Darley M. M., Hu X., Billker O., Rayner J. C., Ahras M., Wohler J. E., Barnum S. R. (2011) Cutting edge: the membrane attack complex of complement is required for the development of murine experimental cerebral malaria. J. Immun. 186, 6657–6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wetsel R. A., Kolb W. P. (1983) Expression of C5a-like biological activities by the fifth component of human complement (C5) upon limited digestion with noncomplement enzymes without release of polypeptide fragments. J. Exp. Med. 157, 2029–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huber-Lang M., Sarma J. V., Zetoune F. S., Rittirsch D., Neff T. A., McGuire S. R., Lambris J. D., Warner R. L., Flierl M. A., Hoesel L. M., Gebhard F., Younger J. G., Drouin S. M., Wetsel R. A., Ward P. A. (2006) Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 12, 682–687 [DOI] [PubMed] [Google Scholar]
- 9. Markiewski M. M., Nilsson B., Ekdahl K. N., Mollnes T. E., Lambris J. D. (2007) Complement and coagulation: strangers or partners in crime? Trends Immunol. 28, 184–192 [DOI] [PubMed] [Google Scholar]
- 10. Amara U., Flierl M. A., Rittirsch D., Klos A., Chen H., Acker B., Brückner U. B., Nilsson B., Gebhard F., Lambris J. D., Huber-Lang M. (2010) Molecular intercommunication between the complement and coagulation systems. J. Immunol. 185, 5628–5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wessels M. R., Butko P., Ma M., Warren H. B., Lage A. L., Carroll M. C. (1995) Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. U.S.A. 92, 11490–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumoto M., Fukuda W., Circolo A., Goellner J., Strauss-Schoenberger J., Wang X., Fujita S., Hidvegi T., Chaplin D. D., Colten H. R. (1997) Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc. Natl. Acad. Sci. U.S.A. 94, 8720–8725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Circolo A., Garnier G., Fukuda W., Wang X., Hidvegi T., Szalai A. J., Briles D. E., Volanakis J. E., Wetsel R. A., Colten H. R. (1999) Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology 42, 135–149 [DOI] [PubMed] [Google Scholar]
- 14. Davoust N., Nataf S., Reiman R., Holers M. V., Campbell I. L., Barnum S. R. (1999) Central nervous system-targeted expression of the complement inhibitor sCrry prevents experimental allergic encephalomyelitis. J. Immunol. 163, 6551–6556 [PubMed] [Google Scholar]
- 15. Sinden R. E., Butcher G. A., Beetsma A. L. (2002) Maintenance of the Plasmodium berghei life cycle. Methods Mol. Med. 72, 25–40 [DOI] [PubMed] [Google Scholar]
- 16. Leinhase I., Rozanski M., Harhausen D., Thurman J. M., Schmidt O. I., Hossini A. M., Taha M. E., Rittirsch D., Ward P. A., Holers V. M., Ertel W., Stahel P. F. (2007) Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J. Neuroinflammation 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wohler J. E., Smith S. S., Zinn K. R., Bullard D. C., Barnum S. R. (2009) γδ T cells in EAE: early trafficking events and cytokine requirements. Eur. J. Immunol. 39, 1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atkinson C., Song H., Lu B., Qiao F., Burns T. A., Holers V. M., Tsokos G. C., Tomlinson S. (2005) Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J. Clin. Invest. 115, 2444–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lou J., Lucas R., Grau G. E. (2001) Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin. Microbiol. Rev. 14, 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohno T., Nishimura M. (2004) Detection of a new cerebral malaria susceptibility locus, using CBA mice. Immunogenetics 56, 675–678 [DOI] [PubMed] [Google Scholar]
- 21. Delahaye N. F., Coltel N., Puthier D., Flori L., Houlgatte R., Iraqi F. A., Nguyen C., Grau G. E., Rihet P. (2006) Gene-expression profiling discriminates between cerebral malaria (CM)-susceptible mice and CM-resistant mice. J. Infect. Dis. 193, 312–321 [DOI] [PubMed] [Google Scholar]
- 22. Qiao F., Atkinson C., Song H., Pannu R., Singh I., Tomlinson S. (2006) Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. Am. J. Pathol. 169, 1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Read R. W., Szalai A. J., Vogt S. D., McGwin G., Barnum S. R. (2006) Genetic deficiency of C3 as well as CNS-targeted expression of the complement inhibitor sCrry ameliorates experimental autoimmune uveoretinitis. Exp. Eye Res. 82, 389–394 [DOI] [PubMed] [Google Scholar]
- 24. Barnum S. R., Szalai A. J. (2006) Complement and demyelinating disease: no MAC needed? Brain Res. Rev. 52, 58–68 [DOI] [PubMed] [Google Scholar]
- 25. Stahel P. F., Barnum S. R. (2006) The role of the complement system in CNS inflammatory diseases. Expert Rev. Clin. Immunol. 2, 445–456 [DOI] [PubMed] [Google Scholar]
- 26. Dasari P., Heber S. D., Beisele M., Torzewski M., Reifenberg K., Orning C., Fries A., Zapf A. L., Baumeister S., Lingelbach K., Udomsangpetch R., Bhakdi S. C., Reiss K., Bhakdi S. (2012) Digestive vacuole of Plasmodium falciparum released during erythrocyte rupture dually activates complement and coagulation. Blood 119, 4301–4310 [DOI] [PubMed] [Google Scholar]
- 27. Glew R. H., Atkinson J. P., Frank M. M., Collins W. E., Neva F. A. (1975) Serum complement and immunity in experimental simian malaria. I. Cyclical alterations in C4 related to schizont rupture. J. Infect Dis. 131, 17–25 [DOI] [PubMed] [Google Scholar]
- 28. Atkinson J. P., Glew R. H., Neva F. A., Frank M. M. (1975) Serum complement and immunity in experimental simian malaria. II. Preferential activation of early components and failure of depletion of late components to inhibit protective immunity. J. Infect Dis. 131, 26–33 [DOI] [PubMed] [Google Scholar]
- 29. Ward P. A., Sterzel R. B., Lucia H. L., Campbell G. H., Jack R. M. (1981) Complement does not facilitate plasmodial infections. J. Immunol. 126, 1826–1828 [PubMed] [Google Scholar]
- 30. Gabriel J., Berzins K. (1983) Specific lysis of Plasmodium yoelii-infected mouse erythrocytes with antibody and complement. Clin. Exp. Immunol. 52, 129–134 [PMC free article] [PubMed] [Google Scholar]
- 31. Healer J., McGuinness D., Hopcroft P., Haley S., Carter R., Riley E. (1997) Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect. Immun. 65, 3017–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pang X. L., Horii T. (1998) Complement-mediated killing of Plasmodium falciparum erythrocytic schizont with antibodies to the recombinant serine repeat antigen (SERA). Vaccine 16, 1299–1305 [DOI] [PubMed] [Google Scholar]
- 33. Ghosh K., Shetty S. (2008) Blood coagulation in falciparum malaria: a review. Parasitol. Res. 102, 571–576 [DOI] [PubMed] [Google Scholar]
- 34. Moxon C. A., Heyderman R. S., Wassmer S. C. (2009) Dysregulation of coagulation in cerebral malaria. Mol. Biochem. Parasitol. 166, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reinartz J., Hänsch G. M., Kramer M. D. (1995) Complement component C7 is a plasminogen-binding protein. J. Immunol. 154, 844–850 [PubMed] [Google Scholar]
- 36. Christiansen V. J., Sims P. J., Hamilton K. K. (1997) Complement C5b-9 increases plasminogen binding and activation on human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 17, 164–171 [DOI] [PubMed] [Google Scholar]
- 37. Skattum L., van Deuren M., van der Poll T., Truedsson L. (2011) Complement deficiency states and associated infections. Mol. Immunol. 48, 1643–1655 [DOI] [PubMed] [Google Scholar]
- 38. Zhu Z., Atkinson T. P., Hovanky K. T., Boppana S. B., Dai Y. L., Densen P., Go R. C., Jablecki J. S., Volanakis J. E. (2000) High prevalence of complement component C6 deficiency among African Americans in the southeastern USA. Clin. Exp. Immunol. 119, 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hobart M. J., Fernie B. A., Fijen K. A., Orren A. (1998) The molecular basis of C6 deficiency in the western Cape, South Africa. Hum. Genet. 103, 506–512 [DOI] [PubMed] [Google Scholar]
- 40. Würzner R. (2003) Deficiencies of the complement MAC II gene cluster (C6, C7, C9): is subtotal C6 deficiency of particular evolutionary benefit? Clin. Exp. Immunol. 133, 156–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malowany J. I., Butany J. (2012) Pathology of sickle cell disease. Semin. Diagn. Pathol. 29, 49–55 [DOI] [PubMed] [Google Scholar]
- 42. Waisberg M., Tarasenko T., Vickers B. K., Scott B. L., Willcocks L. C., Molina-Cruz A., Pierce M. A., Huang C. Y., Torres-Velez F. J., Smith K. G., Barillas-Mury C., Miller L. H., Pierce S. K., Bolland S. (2011) Genetic susceptibility to systemic lupus erythematosus protects against cerebral malaria in mice. Proc. Natl. Acad. Sci. U.S.A. 108, 1122–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramos T. N., Wohler J. E., Barnum S. R. (2009) Deletion of both the C3a and the C5a receptors fails to protect against experimental autoimmune encephalomyelitis. Neurosci. Lett. 467, 234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Health Organization (2011) World Malaria Report 2011, World Health Organization, Geneva, Switzerland [Google Scholar]