With the development in the early 1980s of technology to transfer genes to murine hematopoietic stem cells by using recombinant murine oncoretroviral vectors (1), the possibility of genetic therapy for a number of human disorders of the lympho-hematopoietic system seemed an attainable goal. Hemoglobin disorders were among the first diseases to be considered for gene therapy. Both sickle cell disease and the thalassemias are common monogenic disorders worldwide that cause serious morbidity and mortality. The pathophysiology of these disorders is amenable to correction by the addition of a functional globin gene to stem cells with subsequent high level expression in maturing erythroid cells (Fig. 1). Encouraging reports of successful transfer of a β-globin gene into the hematopoietic stem cells of mice using an oncoretroviral vector appeared in 1988 (2, 3). Erythroid lineage-specific expression of the globin gene was observed, but the levels of expression were much too low (<1% of mouse β-globin) for a potential therapeutic effect. Recent studies using murine models of both sickle cell disease and β-thalassemia and γ-globin expressing transgenic mice substantiate the clinical impression that sustained expression of a transferred globin gene in the range of 10–20% of the level of endogenous globin in a majority of developing erythroblasts will be required for a therapeutic benefit (4).†
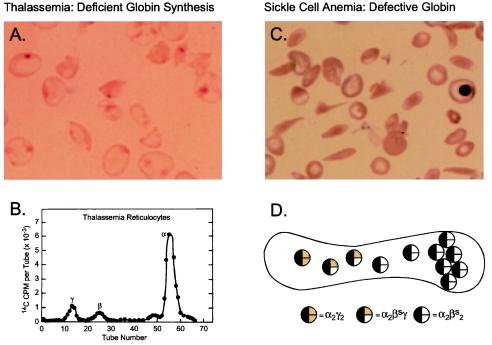
Figure 1.
Pathophysiology of hemoglobin disorders: Prospects for gene therapy. Normal human red cells contain 30 pg of hemoglobin, which in the adult is composed of two α and two β-globin chains (HbA = α2β2). The two globin chains are synthesized in nearly equal amounts in maturing erythroblasts. Before birth, γ-globin rather than β-globin is present in the hemoglobin tetramer. During the perinatal period, there is a switch from γ- to β-globin synthesis, resulting in the replacement of HbF with HbA in neonatal red blood cells. (A) Peripheral blood smear from a patient with severe homozygous β-thalassemia. The red blood cells are poorly hemoglobinized. The smear has been stained to depict the insoluble inclusions of α chains. (B) Biosynthesis of globin chains by erythroid cells from a patient with severe homozygous β-thalassemia. Incorporation of the radioactive amino acid into globin chains is characteristically abnormal with excess synthesis of α-globin and deficient synthesis of non-α- (γ- and β-) globin. Effective gene therapy could be achieved by increasing the synthesis of β-globin, the normal adult chain, or γ-globin, which is typically expressed during fetal life. (C) Blood cells from a patient with sickle cell anemia. The characteristic sickle shape reflects polymerization of hemoglobin, which occurs as a consequence of a single amino acid substitution, valine for glutamic acid, at position six on the surface of the hemoglobin molecule. (D) Expression of the naturally, anti-sickling fetal (γ-) globin in red cells from patients with sickle cell anemia reduces sickling propensity as a consequence of the formation of mixed tetramers (α2βsγ) in red blood cells. The mixed tetramers are unable to participate in polymerization, thereby effectively reducing the concentration of sickle hemoglobin (Hbα2βs2). Effective gene therapy could be achieved by introducing a functional γ-globin gene that expressed at least 20% the level of βs globin genes.
Over the past 12 years, progress toward obtaining a globin vector capable of achieving these therapeutic levels of expression in the erythroid progeny of hematopoietic stem cells has been painstakingly slow. Only four additional reports have presented data consistent with the expression of a transferred globin gene in the erythroid progeny of genetically modified murine stem cells (5–8). Unfortunately, despite inclusion of elements from the locus control region (see below), the levels of expression obtained were subtherapeutic and subject to decay over time, suggesting silencing of the globin gene. In this issue of PNAS, Kalberer et al. use an updated β-globin gene vector coupled with preselection and transplantation of vector-expressing bone marrow cells to achieve, for the first time, sustained vector-encoded globin gene expression over time in a cohort of long-term murine transplant recipients (9). Despite preselection of vector-expressing stem cells for transplantation, significant vector silencing and/or position effect variegation (PEV) of expression still occurred, and the overall levels of human β-globin gene expression averaged only 3% of endogenous globin in long-term recipients.
Globin Gene Therapy Vectors: 1989–1999.
In the mid 1980s, the locus control region (LCR) for the β-globin gene cluster was defined as a series of nuclease hypersensitivity (HS) sites in the 21 kb of DNA upstream from the β-globin locus (10, 11). Functional studies in transgenic mice demonstrated that the LCR possessed powerful erythroid-specific enhancer activity (12). In addition, the LCR was initially thought to confer integration position-independent expression by virtue of an ability to establish and maintain an open chromatin domain. These observations led to the hope that inclusion of relatively small (<1 kb) “core” HS fragments of the LCR might significantly increase globin vector expression because these elements were active in enhancing globin gene expression in transgenic mice. Unfortunately, inclusion of HS fragments in retroviral vectors proved problematic because they caused genetic instability of the vector genome and/or low titers of viral producer cell clones. Since the early 1990s, two approaches resulted in the development of stable globin gene vectors containing HS fragments. Sadelain et al. empirically tested multiple orientations of several LCR fragments and introduced a deletion of the second intron of the β-globin gene (13). This ultimately led to a configuration that allowed transmission of unrearranged proviral sequences and the ability to obtain viral producer cells capable of generating vector particles of high titer. However, in mouse transplant experiments, despite initial human β-globin expression averaging 5% of endogenous mouse globin at 10 weeks after transplant, the level of human β-globin expression was undetectable 4 months posttransplant, suggesting complete silencing of the vector-encoded globin gene (7). In studies by Leboulch et al., undesirable RNA processing signals within the β-globin gene and LCR fragments contained in a globin vector were defined, and site-directed mutagenesis was used to arrive at a stable configuration with a useful vector titer (14). Murine transplant studies with this vector yielded data also consistent with significant globin vector silencing: Of 12 mice positive for vector in peripheral blood leukocytes at 4 months posttransplant or longer, only 2 animals expressed human β-globin (6). These observations are consistent with the emerging perspective that sequences outside the HS sites are important for LCR function and that chromatin opening may depend on regulatory elements outside of the LCR (15).
Recently, several laboratories have investigated the utility of the HS40 core enhancer element from the α-globin locus LCR in γ-globin gene vectors, in place of the above described β-globin LCR HS fragments (16, 17). Stability and high titer have been documented with these vectors with relatively high levels of γ-globin expression observed in erythroid cell lines. However, no long-term studies in mice have been reported with respect to the transmission and expression characteristics of these vectors.
Position Effect Variegation (PEV) of Expression and Gene Silencing.
As summarized by Kalberer et al. (9), these two distinct but sometimes difficult to distinguish entities appear to be major obstacles in the quest for high levels of sustained expression of transferred globin genes in developing erythroblasts. Although most thoroughly studied in Drosophila, PEV in mammals has been studied in transgenic mice containing globin transgenes lacking an intact LCR (18, 19). In these instances, one can observe highly variable expression of the globin or reporter transgene in red blood cells, despite the fact that progeny red cells are derived from stem cells containing the transgene at one common chromosomal location. This phenomenon represents an epigenetic, stochastic process, likely attributable to fluctuating, local chromatin effects, which results in a variable probability of transgene expression in individual erythroblasts. Alternatively, gene silencing is more often thought of as an epigenetic process causing complete lack of expression in the progeny of a “silenced” stem cell. Silencing of retroviral vectors in hematopoietic cells has been attributed to particular sequences in the viral long-terminal repeat sequences and viral backbone, and recent modifications in these sequences have resulted in vectors more resistant to silencing of the viral promoter located within the long-terminal repeat (20, 21). Both PEV and silencing mechanisms may act on a transferred globin gene residing in chromatin outside of the normal globin locus during the important terminal phases of erythroblast development when globin transcripts normally accumulate rapidly despite heterochromatization and shutdown of the rest of the genome.
Kalberer et al. clearly demonstrate sustained, long-term expression of a vector-encoded green fluorescent protein marker and a cis-linked β-globin expression cassette in a cohort of animals transplanted exclusively with bone marrow cells initially expressing the green fluorescent protein marker gene (9). This represents a clear advance for the field over the previously described studies even though expression of the transferred globin gene (3% of mouse βmaj) was well below the potentially therapeutic range. However, almost half of the mice in the current study lack expression of either transgene in a significant fraction of the red cell and leukocyte progeny at the earliest time points posttransplant, suggesting that significant posttransplant stem cell or more mature cell silencing and/or PEV continues to be problematic. Further work will be required to distinguish between these two possibilities: e.g., examination of clonal spleen hematopoietic colonies, derived from primitive marrow cells from primary recipients, for a “silenced” versus “variegated” pattern of vector expression. Thus, it is not clear whether the preselection strategy using flow cytometry resulted in the infusion of stem cell clones resistant to silencing but still susceptible to PEV or whether silencing occurred at a significant rate along with PEV. It is also possible that factors other than preselection, namely the specific vector design that was not tested in a cohort of control animals transplanted with nonselected cells, may have contributed to long-term expression. Despite these caveats, the concept of using selection methodology to increase the number of stem cell clones containing vector integration sites favorable to stable long-term expression in developing erythroid precursors will likely be an important one for globin gene therapy.
In Vivo Selection of Vector-Expressing Stem Cells and Progeny.
The work of Kalberer et al. (9) is consistent with earlier studies that showed that pretransplant flow cytometric cell sorting for a vector-expressing population of repopulating hematopoietic cells and their subsequent infusion into irradiated recipient mice will result in reconstitution of the hematopoietic system with high levels of vector-expressing peripheral blood cells (22, 23). However, this approach may not be practical in larger animal models and humans because the successfully transduced and recovered population may be too small to reproducibly reconstitute the recipient.
An alternative and perhaps more applicable strategy to selectively increase vector-expressing hematopoietic cells containing integration sites more favorable to long-term expression is to use a posttransplant in vivo selection system based on the expression of cis-linked drug-resistance genes. Recent work in a murine transplantation model demonstrated amplification of vector-expressing peripheral blood cells of multiple lineages from less than 10% to 70% or greater using a vector containing a mutant dihydrofolate reductase antifolate resistance gene and an antifolate treatment regimen (24). Secondary transplant experiments documented selection at the level of vector-expressing stem cells. Similarly, variants of the DNA repair enzyme methylguanine methyltransferase that are resistant to O6-benzylguanine, a pharmacological potentiator of alkylating agent toxicity, have been used for selection of vector-expressing hematopoietic cells using alkylator therapy and O6-benzylguanine (25). Other strategies for selection are also being explored (26). Incorporation of drug-resistance genes into globin gene therapy vectors and selection strategies based on their expression merit careful evaluation as a potential vehicle to improved levels and maintenance of expression of transferred globin genes.
Improving Gene Transfer Efficiency into Repopulating Hematopoietic Cells.
Another major obstacle to successful gene therapy for the hemoglobin disorders, as well as for other hematopoietic disorders, is the generally low levels of gene transfer into stem cells that have been achieved in vector-marking trials in both human adults and non-human primates (<1%, despite ablative marrow conditioning) (27–29). Two major barriers to efficient gene transfer have emerged: (i) lack of defined ex vivo conditions to induce a majority of stem cells to cycle (a requisite for oncoretroviral vector integration) without compromising repopulating activity, and (ii) low levels of the retroviral vector receptor on repopulating cells, which is requisite for viral vector binding and cell entry. Several recent studies in non-human primate models have demonstrated significantly higher levels of gene-marked blood cells in vivo than previously achieved (up to 5–10% several months after transplant) using cytokine-mobilized peripheral blood repopulating cells for transplantation after ex vivo gene transfer (30–33). This improvement is likely caused by use of more optimal ex vivo culture conditions containing complex cytokine combinations and the use of recombinant fibronectin fragments that facilitate gene transfer through colocalization of target cells with vector particles (34).
The use of vector particles pseudotyped with alternative envelope proteins has been evaluated as a strategy to overcome the barrier imposed by the low level of amphotropic receptor expression on primitive hematopoietic cells with some success (35–37). Recent studies using the feline endogenous retrovirus (RD114) envelope demonstrate a very high rate of gene transfer into primitive umbilical cord blood human hematopoietic cells capable of establishing multilineage hematopoiesis in immunodeficient mice (38). Thus, major progress has been made toward overcoming obstacles to efficient stem cell targeted gene transfer in the past few years.
Globin Gene Therapy: Future Directions.
Vector design is emerging as the last major barrier to successful gene therapy for the hemoglobin disorders. Improvement in globin gene expression may eventually result from (i) the use of genetic insulator and chromatin “opening” elements that might decrease silencing and/or PEV; (ii) inclusion of larger fragments of the globin LCR in the globin vector; (iii) further alteration of viral backbone sequences; and (iv) the above mentioned drug-selection strategies to retrieve and amplify transduced cells containing integration sites resistant to silencing and PEV in erythroblasts. Such vectors must be tested in relevant murine models of sickle cell disease and β-thalassemia (39, 40). With focused and sustained effect, progress seems likely and ultimate success a reasonable possibility.
Footnotes
See companion article on page 5411.
Persons, D. A., Allay, E., Kelly, P., Sabatino, D. E., Bodine, D. M. & Nienhuis, A. W. (1999) Blood 94, 582 (abstr.).
References
- 1.Williams D A, Lemischka I R, Nathan D G, Mulligan R C. Nature (London) 1984;310:476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- 2.Dzierzak E A, Papayannopoulou T, Mulligan R C. Nature (London) 1988;331:35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson S, Bodine D M, Perry L, Papayannopoulou T, Nienhuis A W. Proc Natl Acad Sci USA. 1988;85:6062–6066. doi: 10.1073/pnas.85.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blouin M-J, Beauchemin H, Wright A, De Paepe M, Sorette M, Bleau A-M, Nakamoto B, Ou C-N, Stamatoyannopoulos G, Trudel M. Nat Med. 2000;6:177–182. doi: 10.1038/72279. [DOI] [PubMed] [Google Scholar]
- 5.Bender M A, Gelinas R E, Miller A D. Mol Cell Biol. 1989;9:1426–1434. doi: 10.1128/mcb.9.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raftopoulos H, Ward M, Leboulch P, Bank A. Blood. 1997;90:3414–3422. [PubMed] [Google Scholar]
- 7.Rivella S, Sadelain M. Semin Hematol. 1998;35:112–125. [PubMed] [Google Scholar]
- 8.Plavec I, Papayannopoulou T, Maury C, Meyer F. Blood. 1993;81:1384–1392. [PubMed] [Google Scholar]
- 9.Kalberer C P, Pawliuk R, Imren S, Bachelot T, Takekoshi K J, Fabry M, Eaves C J, London I M, Humphries R K, Leboulch P. Proc Natl Acad Sci USA. 2000;97:5411–5415. doi: 10.1073/pnas.100082597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuan D, Solomon W, Li Q, London I M. Proc Natl Acad Sci USA. 1985;82:63884–63888. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester W C, Takagawa S, Papayannopoulou T, Stamatoyannopoulos G, Groudine M. Nucleic Acids Res. 1987;15:10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosveld F, van Assendelft G B, Greaves D R, Kollias G. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 13.Sadelain M, Wang C H J, Antoniou M, Grosveld F, Mulligan R C. Proc Natl Acad Sci USA. 1995;92:6728–6732. doi: 10.1073/pnas.92.15.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leboulch P, Huang G M, Humphries R K, Oh Y H, Eaves C J, Tuan D Y, London I M. EMBO J. 1994;13:3065–3076. doi: 10.1002/j.1460-2075.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epner E, Reik A, Cimbora D, Telling A, Bender M A, Fiering S, Enver T, Martin D I, Kennedy M, Keller G, Groudine M. Mol Cell. 1998;2:447–455. doi: 10.1016/s1097-2765(00)80144-6. [DOI] [PubMed] [Google Scholar]
- 16.Ren S, Wong B Y, Li J, Luo X-N, Wong P M C, Atweh G F. Blood. 1996;87:2518–2524. [PubMed] [Google Scholar]
- 17.Emery D W, Morrish F, Li Q, Stamatoyannopoulos G. Hum Gene Ther. 1999;10:877–888. doi: 10.1089/10430349950018283. [DOI] [PubMed] [Google Scholar]
- 18.Enver T, Ebens A J, Forrester W C, Stamatoyannopoulos G. Proc Natl Acad Sci USA. 1989;86:7033–7037. doi: 10.1073/pnas.86.18.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland H G E, Martin D I K, Whitelaw E. Mol Cell Biol. 1997;17:1607–1614. doi: 10.1128/mcb.17.3.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grez M, Akgun E, Hilberg F, Ostertag W. Proc Natl Acad Sci USA. 1990;87:9202–9206. doi: 10.1073/pnas.87.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challita P M, Skelton D, el-Khoueiry A, Yu X J, Weinberg K, Kohn D B. J Virol. 1995;69:748–755. doi: 10.1128/jvi.69.2.748-755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawliuk R, Eaves C J, Humphries R K. Hum Gene Ther. 1997;8:1595–1604. doi: 10.1089/hum.1997.8.13-1595. [DOI] [PubMed] [Google Scholar]
- 23.Persons D A, Allay J A, Riberdy J M, Wersto R P, Donahue R E, Sorrentino B P, Nienhuis A W. Nat Med. 1998;4:1201–1205. doi: 10.1038/2704. [DOI] [PubMed] [Google Scholar]
- 24.Allay J A, Persons D A, Galipeau J, Riberdy J M, Ashmun R A, Blakley R L, Sorrentino B P. Nat Med. 1998;4:1136–1143. doi: 10.1038/2632. [DOI] [PubMed] [Google Scholar]
- 25.Davis B M, Reese J S, Koc O N, Lee K, Schupp J E, Gerson S L. Cancer Res. 1997;57:5093–5099. [PubMed] [Google Scholar]
- 26.Blau C A, Peterson K R, Drachman J G, Spencer D M. Proc Natl Acad Sci USA. 1997;94:3076–3081. doi: 10.1073/pnas.94.7.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunbar C E, Cottler-Fox M, O'Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M, et al. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 28.Bodine D M, Karlsson S, Nienhuis A W. Blood. 1993;82:1975–1980. [PubMed] [Google Scholar]
- 29.van Beusechem V W, Kukler A, Heidt P J, Valerio D. Proc Natl Acad Sci USA. 1992;89:7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiem H P, Andrews R G, Morris J A, Peterson L, Heyward S, Allen J M, Rasko J E, Potter J, Miller A D. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 31.Tisdale J F, Hanazono Y, Sellers S E, Agricola B A, Metzger M E, Donahue R E, Dunbar C E. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 32.Rosenzweig M, MacVittie T J, Harper D, Hempel D, Glickman R L, Johnson R P, Farese A M, Whiting-Theobald N, Linton G F, Yamasaki G, et al. Blood. 1999;94:2271–2286. [PubMed] [Google Scholar]
- 33.Donahue R E, Wersto R P, Allay J A, Metzger M E, Nienhuis A W, Persons D A, Sorrentino B P. Blood. 2000;95:445–452. [PubMed] [Google Scholar]
- 34.Hanenberg H, Xiao X L, Dilloo D, Hashino K, Kato I, Williams D A. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 35.Orlic D, Girard L J, Jordan C T, Anderson S M, Cline A P, Bodine D M. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiem H P, Heyward S, Winkler A, Potter J, Allen J M, Miller A D, Andrews R G. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 37.Rebel V I, Tanaka M, Lee J S, Hartnett S, Pulsipher M, Nathan D G, Mulligan R C, Sieff C A. Blood. 1999;93:2217–2224. [PubMed] [Google Scholar]
- 38.Kelly, P. F., Vandergriff, J., Nathwani, A., Nienhuis, A. W. & Vanin, E. F. (2000) Blood, in press. [PubMed]
- 39.Ryan T M, Ciavatta D J, Townes T M. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 40.Ciavatta D J, Ryan T M, Farmer S C, Townes T M. Proc Natl Acad Sci USA. 1995;92:9259–9263. doi: 10.1073/pnas.92.20.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]