Abstract
Despite its tumor suppressive role in normal mammary epithelial cells, TGFβ has been reported to promote the migration, invasion and survival in breast cancer cells overexpressing the HER2 (ERBB2; neu) oncogene, and to accelerate the metastasis of neu-induced mammary tumors in mice. A clearer understanding of the molecular mechanisms underlying the crosstalk between TGFβ and HER2 has started to emerge. In recent studies reviewed here, the synergistic effect of TGFβ and HER2 on tumor progression has been shown to likely be a combined result of two distinct features: (1) loss of TGFβ's tumor suppressive effect through functional alterations in the anti-mitogenic effect of Smad-mediated transcription, and (2) gain of pro-survival and pro-migratory function through HER2-dependent mechanisms. In HER2-overexpressing breast cancer, this crosstalk results in increased cancer cell proliferation, survival and invasion, accelerated metastasis in animal models, and resistance to chemotherapy and HER2-targeted therapy. Thus, the transformed cellular context imparted by constitutively active HER2 signaling, as a consequence of HER2 gene amplification or overexpression, aborts the tumor suppressive role of TGFβ and facilitated the oncogenic role of this pathway. In turn, TGFβ potentiates oncogenic HER2 signaling by inducing shedding of the ERBB ligands and clustering of HER2 with integrins. Here we discuss recent studies examining Smad-dependent and -independent mechanisms of crosstalk between TGFβ and HER2. Therefore, blockade of TGFβ:HER2 crosstalk may suppress breast cancer progression and metastasis, and enhance the efficiency of conventional therapies in patients with HER2-overexpressing breast cancer.
Keywords: TGFβ, HER2 (ERBB2), Breast cancer, Drug resistance
Introduction
The cellular phenotypes regulated by transforming growth factor β (TGFβ) signaling are highly context-dependent, which underly the different and often opposite TGFβ functions in cancerous and normal cells. TGF-β family members signal through the heteromeric complex of trans-membrane serine/threonine kinases, the type I and type II receptors (TβRI and TβRII), which subsequently phosphor-ylate receptor-regulated Smads (R-Smads). R-Smads, usually together with a common mediator Smad, Smad4, then translocate to the nucleus where they regulate gene transcription via binding to the promoter of target genes [1]. Non-Smad pathways, including the phosphatidylinositol-3 kinase (PI3K), extracellular signal-regulated kinase (ERK, MAPK), c-Jun NH2-terminal kinase (JNK), p38MAPK, and Rho GTPases, have also been implicated in TGFβ action [2, 3]. Studies by our group and others indicate that one of the contextual factors for TGFβ function is HER2 (ERBB2; neu, the rat/mouse homologue of HER2), an oncogene frequently activated by gene amplification or overexpression in human breast cancer. In fact, TGFβ plays a tumor suppressive role in normal epithelia by inhibiting cell proliferation and inducing apoptosis, but accelerates progression of established cancers by autocrine and paracrine mechanisms [2, 4, 5]. The role of TGFβ in cancer appears to result from two actions: (1) loss of its tumor suppressive effects; and (2) gain of function as a cancer-promoting agent that synergizes with transforming oncogenes. In this review, we discuss recent findings from our group and others on the mechanisms through which HER2, as an example of oncogene-mediated alterations of cellular context, redirects TGFβ's function to facilitate cancer progression.
The HER2 (ERBB2) Oncogene and Breast Cancer
HER2 gene amplification or overexpression of its product, the receptor tyrosine kinase (RTK) HER2, occurs in approximately 25% of human breast cancers, where it is associated with drug resistance, metastatic behavior, and overall poor patient outcome [6, 7]. HER2 is a member of the ERBB (Erythroblastic Leukemia Viral Oncogene Homolog) receptor family, which also includes the epidermal growth factor receptor (EGFR, ERBB1), HER3 (ERBB3), and HER4 (ERBB4). Ligand binding to the ectodomains of EGFR, ERBB3, and ERBB4 results in the formation of catalytically active homo- and heterodimers to which HER2 is recruited as a preferred partner [8]. Although HER2 does not bind any ERBB ligand directly, its catalytic activity can potently amplify signaling by ERBB-containing heterodimers via increasing ligand binding affinity and/or receptor recycling and stability [9–12]. Activation of the ERBB network leads to receptor autophosphorylation of C-terminal tyrosines and recruitment to these sites of cytoplasmic signal transducers that regulate cellular processes such as proliferation, differentiation, motility, adhesion, protection from apoptosis, and malignant transformation [8]. Studies of HER2-overexpressing breast cancer cell lines and human tumors have shown constitutive HER2 phosphorylation and activation [13, 14]. Induced overexpression of HER2 is associated with mammary epithelial cell transformation [15, 16]. These studies indicate that HER2 is a potent oncogene in the mammary gland and a causative factor for breast cancer.
HER2-targeted Therapies
The humanized antibody trastuzumab and the ATP-mimetic tyrosine kinase inhibitor (TKI) lapatinib are FDA-approved anti-HER2 agents for the treatment of HER2-overexpressing (HER2+) breast cancers. As the first approved therapy for treating HER2+ breast cancers [17, 18], a large amount of clinical data on patient responses to trastuzumab has been obtained. Trastuzumab has been shown to induce tumor regression in 12~35% of heavily pretreated metastatic breast cancers with HER2 overexpression [19–21]. Nevertheless, most metastatic breast tumors with HER2 gene amplification and/or high levels of HER2 protein do not respond to trastuzumab; further, the majority of those cancer that initially respond eventually relapse, suggesting de novo and acquired mechanisms of therapeutic resistance. The mechanisms of resistance to trastuzumab are not fully understood. However, recent reports suggest that overexpression of the IGF-I receptor [22] or activated EGFR [23] as well as aberrant PI3K/AKT signaling [24] or PTEN deficiency [25] may all result in resistance to trastuzumab. Accumulating evidence suggests that combinations of agents targeted to the HER2 network or other pathways synergizing with HER2 may be beneficial for efficient treatment of HER2+ breast cancers (reviewed in [26]).
A Synergy Between TGFβ and HER2 in Mammary Tumor Progression
TGFβ Facilitates Metastasis of Neu-mediated Mammary Tumors
Synergy between TGFβ and HER2/ERBB2 (neu) was initially demonstrated by crossbreeding mice expressing the Neu oncogene in the mammary gland driven by the mouse mammary tumor virus (MMTV) promoter with either MMTV/ALK5T204D mice (expressing a constitutively active mutant of the type I TGFβ receptor or TβRI) [27, 28] or MMTV/TGFβ1S223/225 mice (expressing a constitutively active mutant of TGFβ1) [28, 29]. In both bi-transgenic models, overexpression of activated receptor or TGFβ ligand in the mammary gland of mice also expressing neu accelerates metastases from Neu-induced mammary tumors [28–30]. The Neu/ALK5T204D and Neu/TGFβ1S223/225 bigenic tumors exhibit less apoptosis and are more locally invasive and of higher histological grade compared to the neu tumors [27, 29]. The neu/TGFβ1S223/225 mice also appear to have more circulating tumor cells than Neu mice. At the molecular level, higher levels of phosphorylated AKT and mitogen-activated protein kinase (MAPK) are observed in tumors expressing both neu and ALK5T204D or TGFβ1S223/225 when compared to tumors expressing neu alone [27, 29].
Loss-of-function experiments have also supported the prooncogenic synergy between TGFβ and Neu signaling. For example, mice expressing soluble TβRII exhibit high levels of this TGFβ antagonist in circulation, leading to suppression of metastases from neu-induced mammary tumors [28, 31]. Collectively, these data suggest that TGFβ can accelerate the metastasis of neu-driven mammary tumors, possibly through the synergistic activation of PI3K/AKTand Ras/MAPK pathways with neu-dependent signaling. Moreover, the findings show that neu requires TGFβ signaling to maximally drive the metastatic progression of mammary tumors.
Enhanced In Vitro Migration and Invasion Mediated Through HER2 and TGFβ Synergy
The functional synergy between TGFβ and HER2 has been characterized using the MCF10A non-transformed human mammary epithelial cell (HMEC) model. A genetic modifier screen in MCF10A cells stably overexpressing transfected HER2 showed that TGFβ1 and TGFβ3 cDNAs cooperate with HER2 in inducing cell motility and invasion in both 2D and 3D basement membrane cultures [32]. This cooperation between HER2 and TGFβ correlates with sustained activation of AKT, ERK and p38 MAPK, and is abolished by pharmacological inhibition of PI3K, ERK, or p38 MAPK, as well as by trastuzumab or an integrin β1 blocking antibody [32, 33]. Taken together, these in vivo and in vitro data suggest that overexpression of HER2 is permissive for TGFβ-induced signals associated with tumor cell motility and, potentially, metastatic progression.
Molecular Mechanisms of the Crosstalk between TGFβ and HER2
Recent mechanistic studies reveal that the synergistic effect of TGFβ and HER2 on tumor progression is likely a combined result of loss of TGFβ's tumor suppressive function and gain of pro-survival and pro-migratory functions through HER2-dependent mechanisms. In these studies, the crosstalk between TGFβ and HER2 occurs at various levels, including (1) suppression of Smad-dependent transcriptional regulation, and (2) Smad-independent induction of ERBB ligands. A previous study indicates that the majority of human breast tumors exhibit intact Smad signaling indicated by the phosphorylation of Smad2, whereas lack of Smad2 phosphorylation is associated with poor patient outcome [34]. Thus, both Smad-dependent and -independent mechanisms may be employed by TGFβ to promote HER2+ breast cancer.
Silencing of TGFβ's Tumor Suppressive Effect
Smad transcription factors bind DNA with low affinity. Therefore, the contextual function of Smads relies on interactions with other transcriptional factors with more potent and specific DNA binding capacity. We have previously reported that TGFβ activates the promoter of the tumor suppressor maspin by inducing binding of Smads and p53 to an overlapping Smad binding element (SBE)-p53 site [35]. In fact, a number of genes are synergistically regulated by Smads and p53, and many of them are tumor suppressors. A common feature of the promoter regions of these genes is the presence of overlapping or adjacent p53 binding sites and SBEs, usually within a 100-bp segment [36]. This pattern is also observed in the promoter of the mutS homolog 2 (MSH2), a tumor suppressor and central component of the DNA mismatch repair (MMR) system [37]. Because efficient regulation of these genes by TGFβ and Smads requires intact p53 signaling, this TGFβ-mediated function is abolished in the context of loss of p53, a frequent alteration in multiple neoplasias, including breast cancer. Overexpression of HER2 has been reported to decrease p53 levels via activation of the PI3K pathway and induction of the nuclear translocation of MDM2, an E3 ubiquitin ligase targeting p53 [38]. Our study also indicates that HER2 overexpression impairs p53-dependent transcriptional regulation of MSH2 by TGFβ, which could be restored by nutlin-3, a small molecule that induces p53 stabilization by inhibiting MDM2-dependent degradation of p53 [37, 39]. Therefore, HER2 may alter Smad-dependent transcriptional regulation through modulating the functional status of p53 in transformed cells. In addition, overexpression of HER2 increases the level of miR-21, a TGFβ-inducible miRNA that targets and down-regulates MSH2 transcripts [37]. As a result, in HER2-transformed cells, TGFβ fails to activate the MSH2 promoter but instead decreases MSH2 expression through induction of miR-21 [37]. This downregulation of MSH2 by TGFβ also contributes to resistance to DNA-damaging chemotherapy agents in cancer cells, as MSH2 is required for the recognition of drug-induced DNA damage, which triggers apoptosis [37].
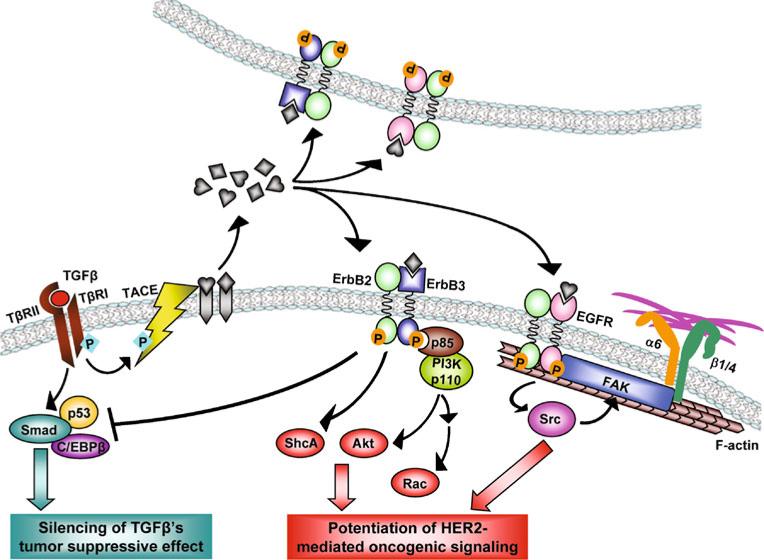
A recent study by Arnal-Estape et al. indicates that antagonism between two isoforms of the transcription factor C/EBPβ also alters Smad function in the context of HER2 overexpression [40]. In non-transformed epithelial cells, TGFβ induces formation of the C/EBPβ-Smad2/3-E2F4/5 transcriptional regulatory complex on the MYC promoter and inhibits gene transcription [41]. This tumor suppressive mechanism is abolished in HER2-overexpressing breast cancer cells. In this case, HER2 signaling increases the production of C/EBPβ isoform LIP, which antagonizes the TGFβ-induced assembly of transcriptional repressor complexes containing Smad and the active C/EBPβ isoform LAP on the MYC promoter [40]. Thus, the HER2-mediated functional switch between the two C/EBPβ isoforms, as well as its modulation of p53 activity, may both contribute to the silencing of TGFβ's tumor suppressive function, which is largely mediated by Smad-dependent transcriptional regulation (Fig. 1).
Figure 1.
Mechanisms of the pro-malignant crosstalk between TGFβ and HER2 in breast cancer (based on findings in [35, 37, 40, 43–46]). Signals from extracellular TGFβ are transduced into cells that express wild-type TGFβ receptors. Activated TβRI induces a set of Smad-dependent cytostatic gene responses, which are impaired by HER2-mediated alterations of p53 and C/EBPβ activities, as described in [35, 37, 40]. On the other hand, TβRI phosphorylates TACE, resulting in its translocation to the cell surface where it cleaves ERBB pro ligands [45]. ERBB ligands will initiate autocrine and paracrine ERBB signaling in adjacent cells. Ligand-induced ERBB signaling, especially in breast cancer cells overexpressing the ERBB signal amplifier HER2, can subsequently lead to activation of the ShcA, PI3K/Akt, and Src/FAK/integrin signaling cascades to promote cancer progression [43–46]. Thus, the crosstalk between TGFβ and HER2 is the functional sum of silenced TGFβ's tumor suppressive effect and enhanced HER2-mediated oncogenic signaling
In addition to the Smad cofactors p53 and C/EBPβ, HER2 has also been reported in breast cancer cells to collaborate with ETS transcription factor ER81 to activate the transcription of Smad7, an inhibitory Smad that suppresses Smad2/3-mediated gene regulation [42]. These events may also lead to a general suppression of the anti-proliferative Smad-dependent TGFβ action in HER2+ breast cancer cells.
Potentiation of HER2-mediated Oncogenic Signaling
Using the HER2-overexpressing MCF10A cell model (MCF10A/HER2), we have shown that addition of exogenous TGFβ or expression of constitutively active TβRI (ALK5T204D) induces motility of MCF10A/HER2 cells but not control MCF10A/vec cells [33]. This effect is mediated at least in part by actvation of PI3K and involves translocation of HER2 to cell membrane protrusions, where it co-localizes with Vav2, Rac1, Pak1 and the actin cytoskeleton, resulting in prolonged Rac1 activation and enhanced cell survival and invasiveness [43]. By anchoring HER2 to actin fibers, TGFβ also induces clustering of HER2 with integrin α6, β1 and β4; this clustering is mediated by focal adhesion kinase (FAK) and is required for TGFβ-induced motility and oncogenic signaling of HER2 in breast cancer cells (Fig. 1) [44]. We further investigated the mechanism through which TGFβ activates PI3K in HER2-overexpressing cells, and found that treatment with TGFβ or expression of ALK5TD induces phosphorylation of the TACE/ADAM17 sheddase and its translocation to cell surface, resulting in increased secretion of TGF-α, amphiregulin, and heregulin. In turn, these ERBB ligands enhance HER2-mediated signaling, such as the association of PI3K p85 subunit with the HER2:ERBB3 heterodimers, leading to sustained activation of the PI3K/ AKT signaling pathway (Fig. 1) [45]. Notably, activation of TGFβ signaling in HER2-overexpressing breast cancer cells also reduces their sensitivity to trastuzumab, likely as a result of PI3K hyperactivation [45].
Another study by Northey et al. indicates that signaling through phosphorylated tyrosine residues 1226/1227 and 1253 of HER2/neu, the sites mediating binding of the ShcA adaptor protein, is essential for the synergistic effect of TGFβ on the motility and invasion of HER2/neu-over-expressing cells [46]. Suppression of ShcA function using a dominant-negative mutant abrogates the TGFβ-mediated effect in breast cancer cells [46] suggesting that, similar to PI3K, ShcA is another key adaptor that mediates the crosstalk between TGFβ and HER2. Although it is not yet clear if TGFβ-mediated TACE activation and ERBB ligand shedding are the upstream events that cause HER2/neu phosphorylation at these ShcA-interacting tyrosines, this study also supports the model that TGFβ exerts the proinvasive, and potentially, pro-metastatic effect by modulating the amplitude of HER2/neu-dependent signaling.
In addition to TGFβ-induced shedding of ERBB ligands, changes in the tumor microenvironment occur by way of HER2-induced expression and secretion of TGFβ1 and TGFβ3 through a mechanism involving Rac1 activation and JNK-AP1-dependent transcription [47]. Moreover, vascular endothelial growth factor (VEGF), a target of the TGFβ-Smad transcriptional regulation, is synergistically induced by HER2 and TGFβ [47]. Thus, the crosstalk between HER2 and TGFβ not only alters intracellular signaling in cancer cells but also influences other components of the tumor microenvironment through the induction of several pro-invasive growth factors. Targeting these extracellular factors may provide novel therapeutic strategies directed at both cancer-driving oncogenes and the modified tumor microenvironment.
Clinical Implications of HER2-dependent TGFβ Action
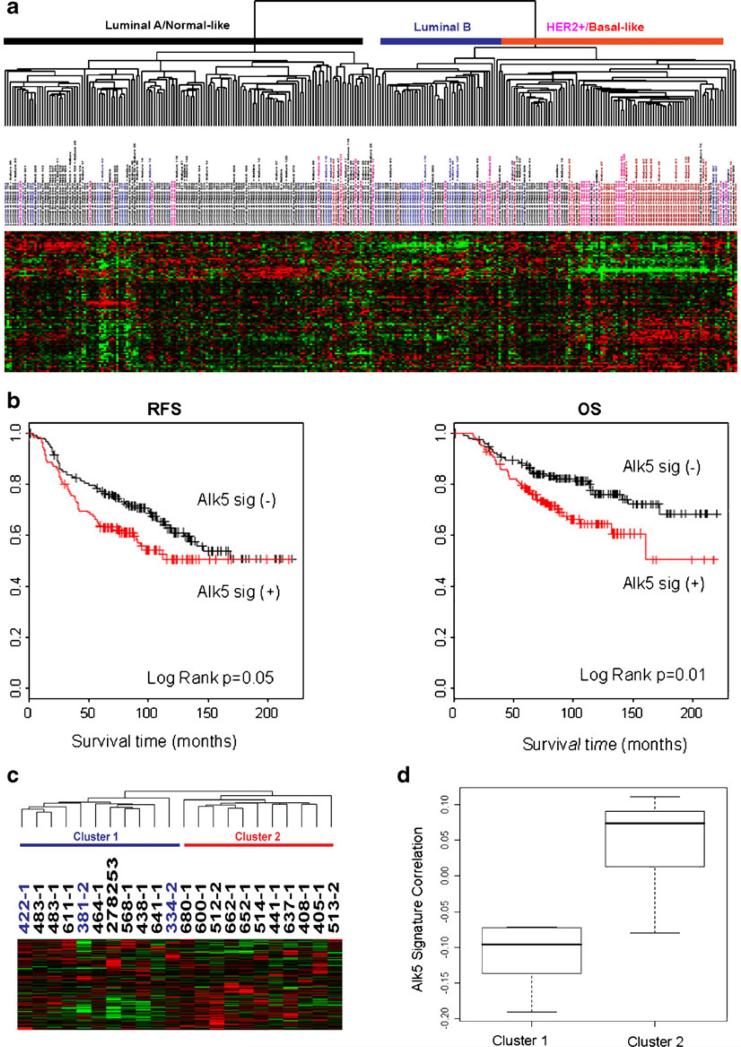
To understand the clinical relevance of the HER2:TGFβ crosstalk, we mapped an ALK5T204D-induced gene expression signature to an array dataset published by van de Vijver et al. [48] and Chang et al. [49]. The ignature correlated with biological and clinical differences among 295 primary breast tumors. Tumors whose gene expression correlated with the active TβRI (ALK5TD) signature are mostly HER2-positive or basal-like with some luminal B tumors, while the tumors with a negative correlation are predominantly luminal A and normal-like tumors (Fig. 2a) [45]. Patients with tumors expressing the ALK5TD signature exhibited a shorter survival than those with tumors that do not express the ALK5TD signature (Fig. 2b). We further explored possible correlations of the ALK5TD signature with resistance to trastuzumab by mapping this gene expression signature to an array data set reported by Harris et al. [50] that had been obtained from 22 patients with HER2-overexpressing breast cancer treated with neoadjuvant trastuzumab and vinorelbine. Hierarchical clustering analysis shows that all 3 patients achieving pathological complete response do not share a similar expression pattern with the TGFβ signature (Fig. 2c & d) [45]. These findings, therefore, support an association between TGFβ signaling and clinical resistance to trastuzumab. A causal association will require confirmation in clinical studies using combinations of HER2 and TGFβ antagonists.
Figure 2.
ALK5TD signature is associated with clinical outcome in women with breast cancer (figure adapted from [45]). a Hierarchical clustering of 295 breast tumors [48, 49] using 90 overlapping genes with the 271-gene ALK5TD signature. b Kaplan Meier plots for recurrence-free survival (RFS) and overall survival (OS) comparing the two groups of tumors with and without a correlation with the ALK5TD signature. c Hierarchical clustering of 22 breast tumors from patients who were treated with navelbine and trastuzumab [50] using 190 overlapping genes with the 271-gene ALK5TD signature. Cluster 2 shows a positive correlation with the ALK5TD signature. d Box-and-Whisker plot of Standard Pearson Correlation between the ALK5TD signature and Clusters determined in c
As indicated by the studies reviewed herein, the cellular phenotypes induced by TGFβ are context-dependent and largely edited by the overexpression of HER2, the major pathogenic oncogene in a significant cohort of breast cancers. In HER2-transformed cells, TGFβ further stimulates HER2 signaling to promote malignancy and may induce resistance to anti-HER2 therapy. Taken together, the documented evidence suggests that simultaneous blockade of the HER2:TGFβ axis may significantly enhance the efficiency of conventional therapies in patients with HER2-dependent breast cancers.
Acknowledgements
This review article was supported by NCI K99/ R00 CA125892 (SEW).
Financial Support: NCI K99/R00 CA125892 (SEW)
Abbreviations
- EGFR
Epidermal growth factor receptor
- ERBB
Erythroblastic leukemia viral oncogene homolog
- ERK
Extracellular signal-regulated kinase
- FAK
Focal adhesion kinase
- HER2
Human epidermal growth factor receptor 2
- JNK
C-Jun NH2-terminal kinase
- MAPK
Mitogen-activated protein kinase
- MMR
DNA mismatch repair
- MSH2
mutS homolog 2
- PI3K
Phosphatidylinositol-3 kinase
- RTK
Receptor tyrosine kinase
- SBE
Smad binding element
- TGFβ
Transforming growth factor β
- VEGF
Vascular endothelial growth factor
Contributor Information
Amy Chow, Division of Tumor Cell Biology, Department of Cancer Biology, Beckman Research Institute of City of Hope, Duarte, CA, USA.
Carlos L. Arteaga, Departments of Medicine and Cancer Biology, Vanderbilt University School of Medicine, Nashville, TN, USA
Shizhen Emily Wang, Division of Tumor Cell Biology, Department of Cancer Biology, Beckman Research Institute of City of Hope, Duarte, CA, USA; Cancer Biology Program, City of Hope Comprehensive Cancer Center, Duarte, CA, USA; Beckman Research Institute of City of Hope, KCRB Room 2007, 1500 East Duarte Road, Duarte, CA 91010, USA.
References
- 1.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 2.Ewan KB, Shyamala G, Ravani SA, et al. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160(6):2081–93. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 4.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 5.Dumont N, Arteaga CL. Targeting the TGF beta signaling network in human neoplasia. Cancer Cell. 2003;3(6):531–6. doi: 10.1016/s1535-6108(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 7.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Oncologist. 1998;3(4):237–52. [PubMed] [Google Scholar]
- 8.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 9.Pinkas-Kramarski R, Soussan L, Waterman H, et al. Diversification of neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. Embo J. 1996;15(10):2452–67. [PMC free article] [PubMed] [Google Scholar]
- 10.Graus-Porta D, Beerli RR, Daly JM, et al. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. Embo J. 1997;16(7):1647–55. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LM, Kuo A, Alimandi M, et al. ErbB2 expression increases the spectrum and potency of ligand-mediated signal transduction through ErbB4. Proc Natl Acad Sci USA. 1998;95(12):6809–14. doi: 10.1073/pnas.95.12.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274(13):8865–74. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- 13.Thor AD, Liu S, Edgerton S, et al. Activation (tyrosine phosphorylation) of ErbB-2 (HER-2/neu): a study of incidence and correlation with outcome in breast cancer. J Clin Oncol. 2000;18(18):3230–9. doi: 10.1200/JCO.2000.18.18.3230. [DOI] [PubMed] [Google Scholar]
- 14.Alimandi M, Romano A, Curia MC, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10(9):1813–21. [PubMed] [Google Scholar]
- 15.Pierce JH, Arnstein P, DiMarco E, et al. Oncogenic potential of erbB-2 in human mammary epithelial cells. Oncogene. 1991;6(7):1189–94. [PubMed] [Google Scholar]
- 16.Muthuswamy SK, Li D, Lelievre S, et al. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3(9):785–92. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89(10):4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roskoski R., Jr The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319(1):1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 19.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26(4 Suppl 12):78–83. [PubMed] [Google Scholar]
- 20.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 21.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst. 2001;93(24):1852–7. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 23.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62(11):3151–8. [PubMed] [Google Scholar]
- 24.Yakes FM, Chinratanalab W, Ritter CA, et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62(14):4132–41. [PubMed] [Google Scholar]
- 25.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Garrett JT, Arteaga CL. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: Mechanisms and clinical implications. Cancer Biol Ther. 2011;11(9) doi: 10.4161/cbt.11.9.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muraoka-Cook RS, Shin I, Yi JY, et al. Activated type I TGFbeta receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene. 2006;25(24):3408–23. doi: 10.1038/sj.onc.1208964. [DOI] [PubMed] [Google Scholar]
- 28.Siegel PM, Shu W, Cardiff RD, et al. Transforming growth factor beta signaling impairs neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA. 2003;100(14):8430–5. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muraoka RS, Koh Y, Roebuck LR, et al. Increased malignancy of neu-induced mammary tumors overexpressing active transforming growth factor beta1. Mol Cell Biol. 2003;23(23):8691–703. doi: 10.1128/MCB.23.23.8691-8703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muraoka-Cook RS, Dumont N, Arteaga CL. Dual role of transforming growth factor beta in mammary tumorigenesis and metastatic progression. Clin Cancer Res. 2005;11(2 Pt 2):937s–43. [PubMed] [Google Scholar]
- 31.Bandyopadhyay A, Lopez-Casillas F, Malik SN, et al. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002;62(16):4690–5. [PubMed] [Google Scholar]
- 32.Seton-Rogers SE, Lu Y, Hines LM, et al. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci USA. 2004;101(5):1257–62. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda Y, Wang S, Dumont N, et al. Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility. J Biol Chem. 2004;279(23):24505–13. doi: 10.1074/jbc.M400081200. [DOI] [PubMed] [Google Scholar]
- 34.Xie W, Mertens JC, Reiss DJ, et al. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res. 2002;62(2):497–505. [PubMed] [Google Scholar]
- 35.Wang SE, Narasanna A, Whitell CW, et al. Convergence of p53 and transforming growth factor beta (TGFbeta) signaling on activating expression of the tumor suppressor gene maspin in mammary epithelial cells. J Biol Chem. 2007;282(8):5661–9. doi: 10.1074/jbc.M608499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordenonsi M, Dupont S, Maretto S, et al. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 2003;113(3):301–14. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Wang Y, Ren X, et al. Context-dependent bidirectional regulation of the mutS homolog 2 by transforming growth factor beta contributes to chemoresistance in breast cancer cells. Mol Cancer Res. 2010;8(12):1633–42. doi: 10.1158/1541-7786.MCR-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng L, Ren JQ, Li H, et al. Downregulation of wild-type p53 protein by HER-2/neu mediated PI3K pathway activation in human breast cancer cells: its effect on cell proliferation and implication for therapy. Cell Res. 2004;14(6):497–506. doi: 10.1038/sj.cr.7290253. [DOI] [PubMed] [Google Scholar]
- 39.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 40.Arnal-Estape A, Tarragona M, Morales M, et al. HER2 silences tumor suppression in breast cancer cells by switching expression of C/EBPss isoforms. Cancer Res. 2010;70(23):9927–36. doi: 10.1158/0008-5472.CAN-10-0869. [DOI] [PubMed] [Google Scholar]
- 41.Gomis RR, Alarcon C, Nadal C, et al. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell. 2006;10(3):203–14. doi: 10.1016/j.ccr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Dowdy SC, Mariani A, Janknecht R. HER2/neu- and TAK1-mediated up-regulation of the transforming growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol Chem. 2003;278(45):44377–84. doi: 10.1074/jbc.M307202200. [DOI] [PubMed] [Google Scholar]
- 43.Wang SE, Shin I, Wu FY, et al. HER2/neu (ErbB2) Signaling to Rac1-Pak1 Is Temporally and Spatially Modulated by Transforming Growth Factor {beta}. Cancer Res. 2006;66(19):9591–600. doi: 10.1158/0008-5472.CAN-06-2071. [DOI] [PubMed] [Google Scholar]
- 44.Wang SE, Xiang B, Zent R, et al. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69(2):475–82. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang SE, Xiang B, Guix M, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28(18):5605–20. doi: 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Northey JJ, Chmielecki J, Ngan E, et al. Signaling through ShcA is required for transforming growth factor beta- and neu/ErbB-2-induced breast cancer cell motility and invasion. Mol Cell Biol. 2008;28(10):3162–76. doi: 10.1128/MCB.01734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang SE, Yu Y, Criswell TL, et al. Oncogenic mutations regulate tumor microenvironment through induction of growth factors and angiogenic mediators. Oncogene. 2010;29(23):3335–48. doi: 10.1038/onc.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 49.Chang HY, Nuyten DS, Sneddon JB, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci U S A. 2005;102(10):3738–43. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris LN, You F, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13(4):1198–207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]