Abstract
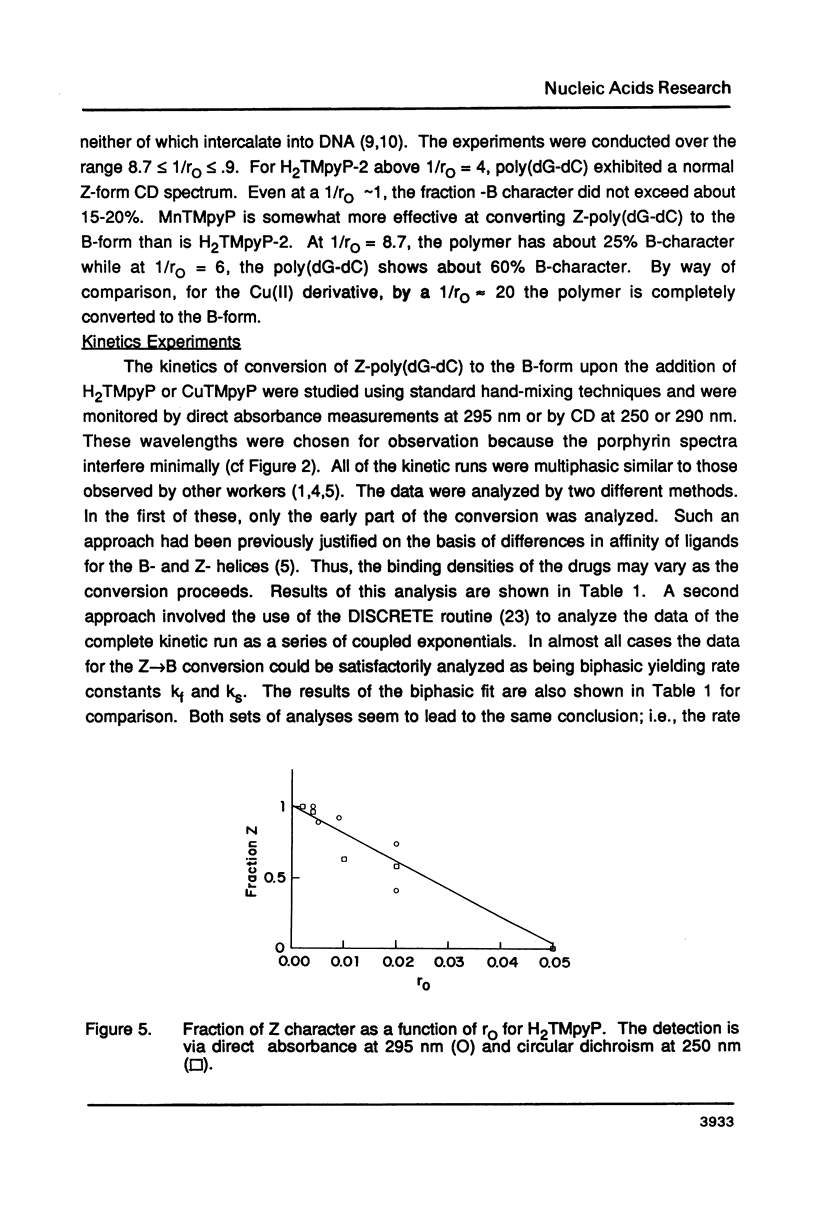
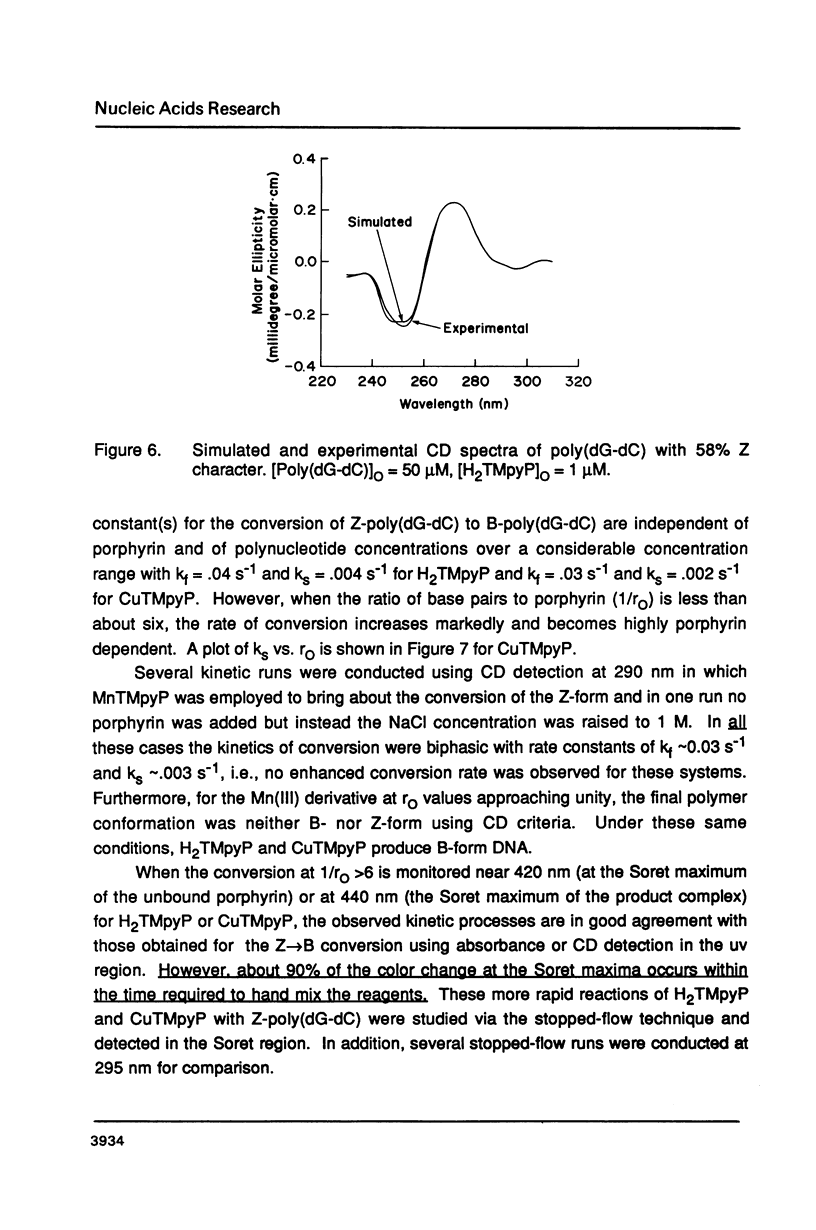
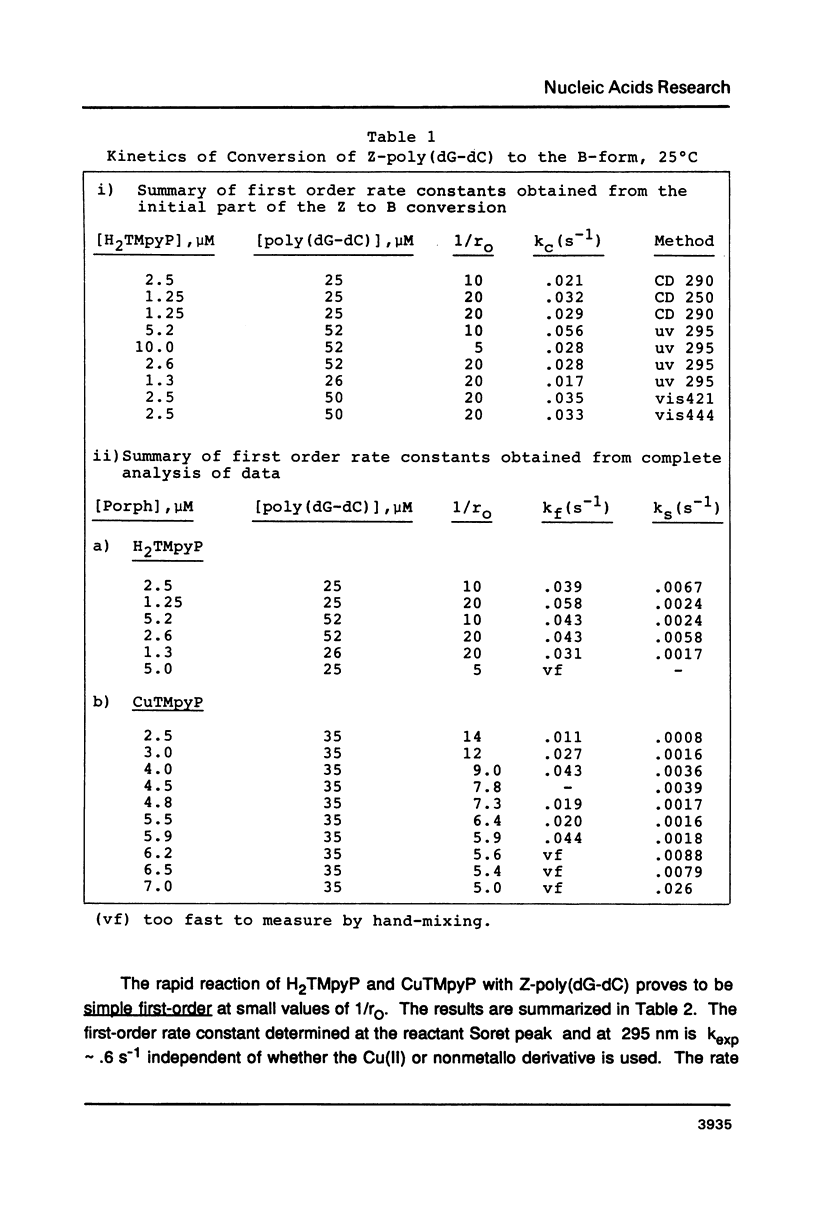
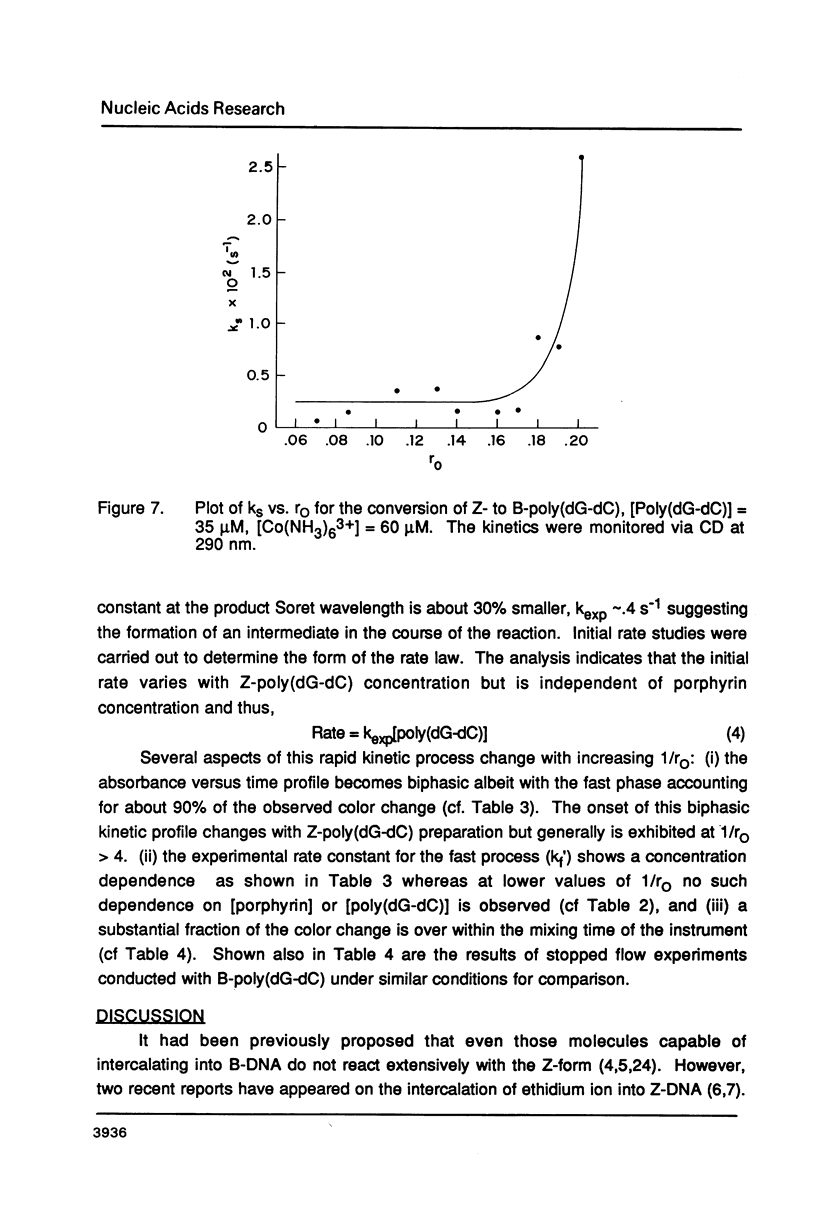
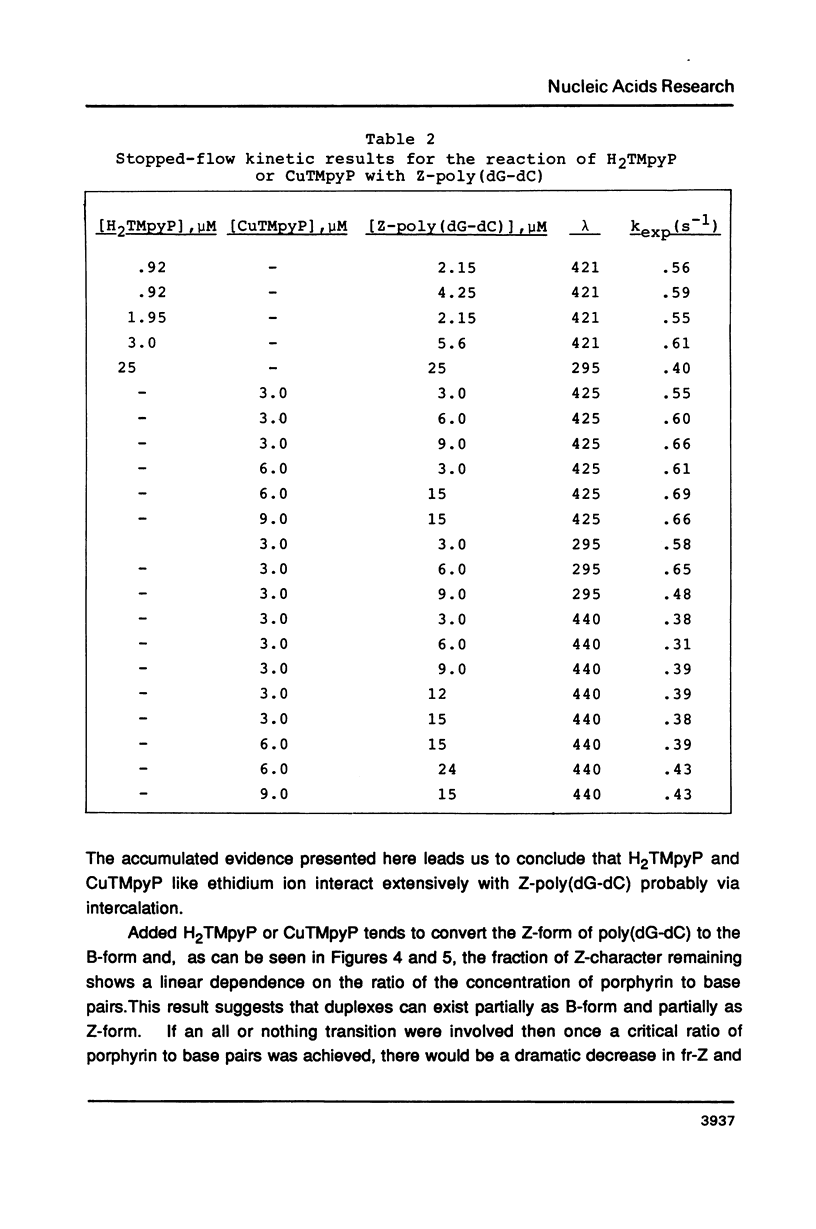
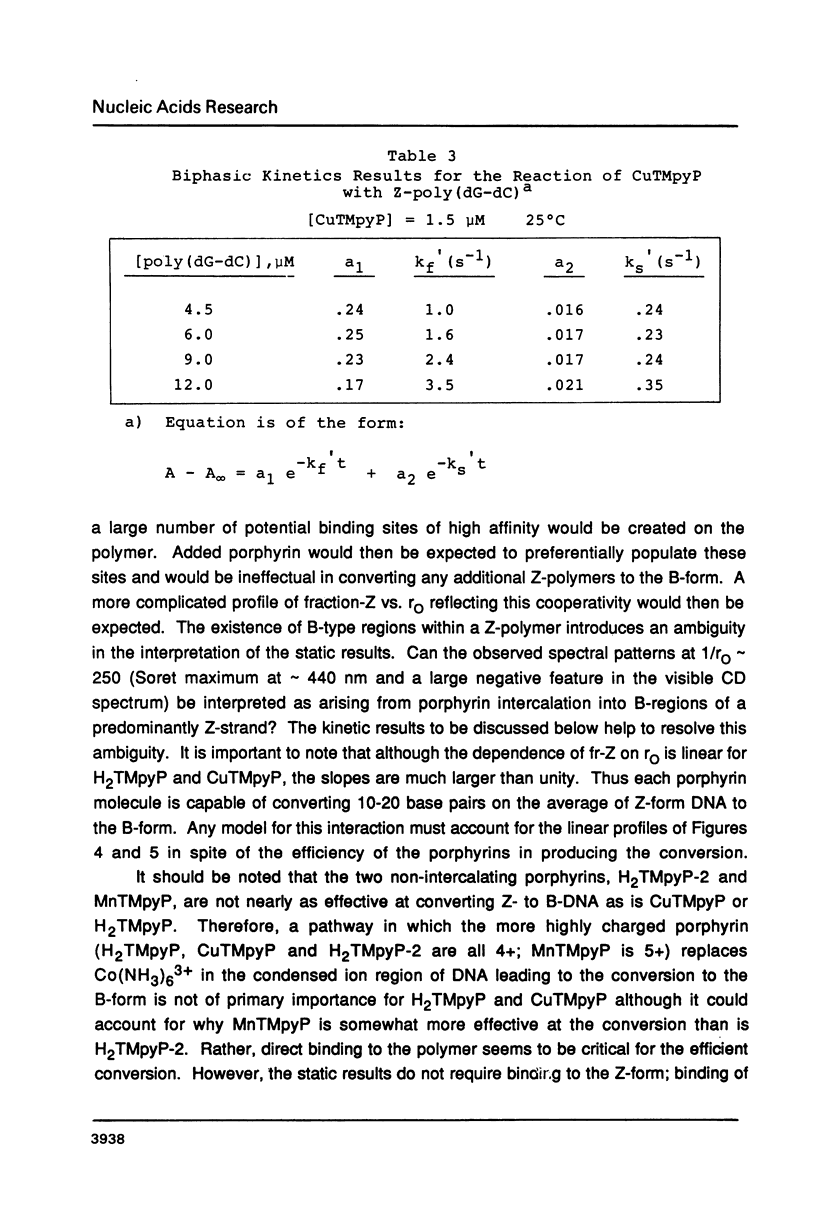
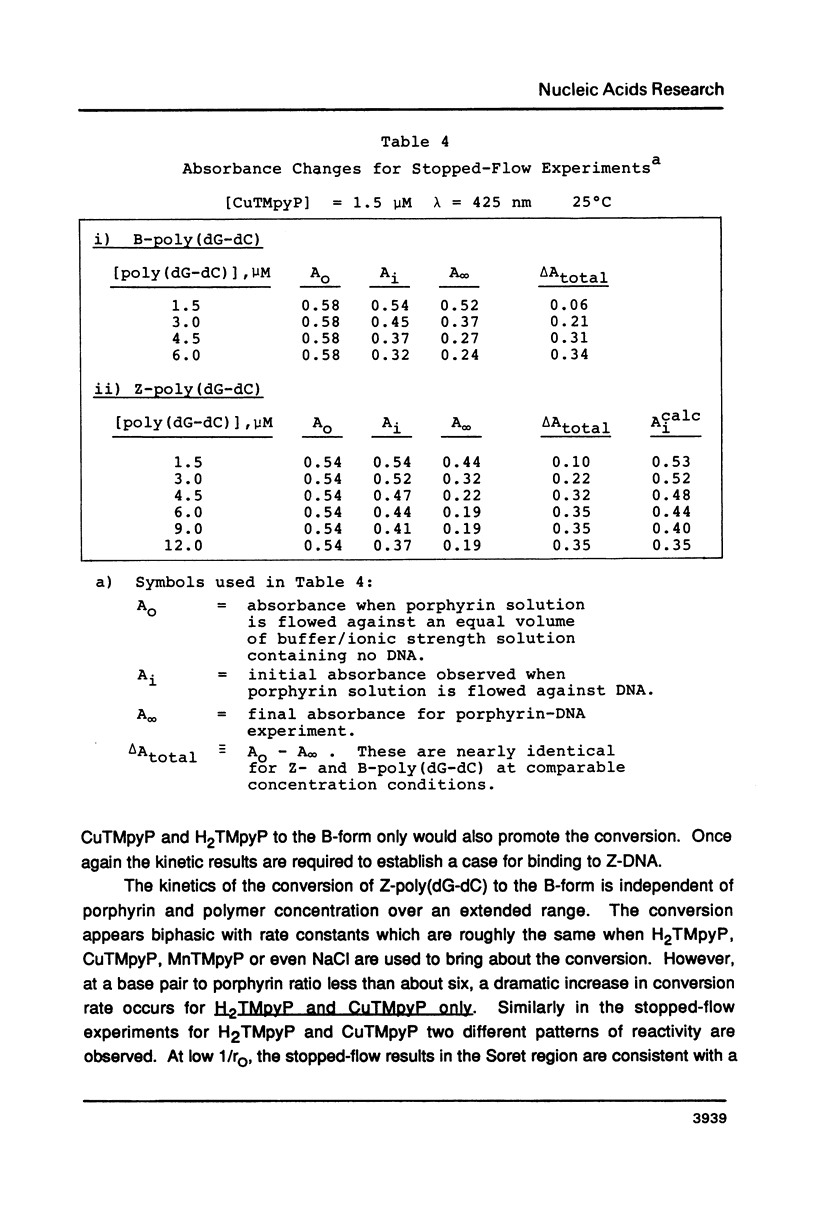
The water soluble porphyrin tetrakis(4-N-methylpyridyl)porphine (H2TMpyP) and its copper(II) derivative (CuTMpyP) convert Z-poly(dG-dC) to the B-form. For H2TMpyP, the fraction Z character (fr-Z) is given by fr-Z = 1.0 - 21 rO and for CuTMpyP, fr-Z = .94 - 12 rO where rO identical to [Porphyrin]O/[DNA]O. Neither the manganese(III) derivative of of this porphyrin (MnTMpyP) nor tetrakis(2-N-methylpyridyl)porphine (H2TMpyP-2) is nearly as effective at causing the conversion. The former two porphyrins have been shown to intercalate into B-poly(dG-dC) whereas the latter two porphyrins do not. The kinetics of the Z----B conversion are independent of porphyrin or poly(dG-dC) concentration for 1/rO greater than 6. At smaller values of 1/rO, the conversion rate is greatly increased for H2TMpyP and CuTMpyP. The interaction of these porphyrins with Z-poly(dG-dC) follows simple first order kinetics in this latter concentration range. It is proposed that for small values of 1/rO the sequence of events begins with a porphyrin-unassisted distortion of the Z-duplex (with a rate constant of 0.6 s-1) followed by a rapid uptake of porphyrin in what may be an intercalative mode. The porphyrin thus located in Z-regions brings about rapid conversion to the B-form. Binding of H2TMpyP or CuTMpyP to B-regions of a predominantly Z-strand leads to conversion of Z to B. However, this conversion process is considerably slower than when the porphyrins bind directly to Z-regions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashikawa I., Kinosita K., Jr, Ikegami A. Dynamics of Z-form DNA. Biochim Biophys Acta. 1984 May 15;782(1):87–93. doi: 10.1016/0167-4781(84)90109-x. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel P. Measurement of the helix opening rate in Z-DNA by 1H nuclear magnetic resonance relaxation spectroscopy. Biochem Biophys Res Commun. 1985 Apr 16;128(1):352–359. doi: 10.1016/0006-291x(85)91686-9. [DOI] [PubMed] [Google Scholar]
- Butzow J. J., Shin Y. A., Eichhorn G. L. Effect of template conversion from the B to the Z conformation on RNA polymerase activity. Biochemistry. 1984 Oct 9;23(21):4837–4843. doi: 10.1021/bi00316a004. [DOI] [PubMed] [Google Scholar]
- Daune M. P., Westhof E., Koffel-Schwartz N., Fuchs R. P. Covalent binding of a carcinogen as a probe for the dynamics of deoxyribonucleic acid. Biochemistry. 1985 Apr 23;24(9):2275–2284. doi: 10.1021/bi00330a023. [DOI] [PubMed] [Google Scholar]
- Fiel R. J., Howard J. C., Mark E. H., Datta Gupta N. Interaction of DNA with a porphyrin ligand: evidence for intercalation. Nucleic Acids Res. 1979 Jul 11;6(9):3093–3118. doi: 10.1093/nar/6.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner R. V., Quigley G. J., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Structural basis for stabilization of Z-DNA by cobalt hexaammine and magnesium cations. Biochemistry. 1985 Jan 15;24(2):237–240. doi: 10.1021/bi00323a001. [DOI] [PubMed] [Google Scholar]
- Markovits J., Ramstein J., Roques B. P., Le Pecq J. B. Effect of B-Z transition and nucleic acid structure on the conformational dynamics of bound ethidium dimer measured by hydrogen deuterium exchange kinetics. Nucleic Acids Res. 1985 May 24;13(10):3773–3788. doi: 10.1093/nar/13.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirau P. A., Kearns D. R. The effect of intercalating drugs on the kinetics of the B to Z transition of poly(dG-dC). Nucleic Acids Res. 1983 Mar 25;11(6):1931–1941. doi: 10.1093/nar/11.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Pasternack R. F., Gibbs E. J., Villafranca J. J. Interactions of porphyrins with nucleic acids. Biochemistry. 1983 May 10;22(10):2406–2414. doi: 10.1021/bi00279a016. [DOI] [PubMed] [Google Scholar]
- Pasternack R. F., Gibbs E. J., Villafranca J. J. Interactions of porphyrins with nucleic acids. Biochemistry. 1983 Nov 8;22(23):5409–5417. doi: 10.1021/bi00292a024. [DOI] [PubMed] [Google Scholar]
- Pasternack R. F., Huber P. R., Boyd P., Engasser G., Francesconi L., Gibbs E., Fasella P., Venturo G. C., Hinds L. de C. On the aggregation of meso-substituted water-soluble porphyrins. J Am Chem Soc. 1972 Jun 28;94(13):4511–4517. doi: 10.1021/ja00768a016. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Porschke D. Dynamics of DNA condensation. Biochemistry. 1984 Oct 9;23(21):4821–4828. doi: 10.1021/bi00316a002. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Shafer R. H., Brown S. C., Delbarre A., Wade D. Binding of ethidium and bis(methidium)spermine to Z DNA by intercalation. Nucleic Acids Res. 1984 Jun 11;12(11):4679–4690. doi: 10.1093/nar/12.11.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]


