Abstract
Objective:
To define the ictal cortical/subcortical network of reading-induced seizures.
Methods:
We analyzed ictal magnetoencephalography (MEG) and EEG-correlated fMRI (EEG-fMRI) data in a unique patient with reading epilepsy (RE) affected by frequent perioral reflex myocloni triggered by reading silently.
Results:
Ictal MEG corroborated EEG localization and revealed activity extending precentrally into Brodmann area (BA) 6. fMRI blood oxygen level−dependent (BOLD) signal changes in the left deep piriform cortex (PFC) and left BA6 preceded seizures and occurred before BOLD changes were observed in thalamus and right inferior frontal gyrus (BA44). Dynamic causal modeling provided evidence of a causal link between hemodynamic changes in the left PFC and reading-evoked seizures.
Conclusion:
Our findings support the important role of deep cortical and subcortical structures, in particular the frontal PFC, as key regions in initiating and modulating seizure activity. In our patient with RE, BA6 appeared to be the area linking cognitive activation and seizure activity.
In neuroimaging studies of human epilepsy, we make inferences about seizure mechanisms mainly by evaluating the interictal state. Reading epilepsy (RE), a localization-related reflex epilepsy with seizures precipitated by reading, is an ideal model to study the structures involved in generation of seizures.1 A previous case linked RE to subtle structural MRI abnormalities in the left precentral gyrus.2 Current understanding holds that the grapheme-to-phoneme transition is what commonly triggers epileptic activity.3 The dominant premotor cortex (Brodmann area [BA] 6) is considered to be the anatomic core of this epileptogenic network,4 but its exact role in generating seizures and the relationships with the others brain regions involved in reading remain controversial.
Here, we studied the interrelationships between cortical and subcortical structures and their role in seizure generation in a patient with RE using EEG-correlated fMRI (EEG-fMRI) and magnetoencephalography (MEG).
METHODS
We studied a right-handed 24-year-old man with frequent short-lasting oral reflex myocloni (ORMs) triggered by reading silently. Previously we reported EEG-fMRI data acquisition and standard general linear model (GLM) analysis in detail.5
EEG/fMRI study.
Preictal changes: Fourier analysis.
We investigated blood oxygen level−dependent (BOLD) signal changes in a period of 21 seconds, starting 9 seconds before and ending 12 seconds after each seizure, using a Fourier basis set with the number of Fourier basis functions determined by the term with the shortest wavelength set at 2TR (6 seconds). We used an F-contrast (p < 0.05 family-wise error-corrected) to assess BOLD changes corresponding to any linear combination of the Fourier basis set functions.
Effective connectivity analysis.
Four regions of interest (ROIs) were selected and extracted based on the results of the Fourier analysis: left BA6, thalamus, deep piriform frontal cortex (PFC), and right inferior frontal gyrus (BA44). For each ROI, we computed the first principal eigenvariate of the voxel time series.6 The regional responses were whitened, and nuisance effects were removed to obtain the corrected time course for each region. Using the dynamic causal modeling module in SPM8 (DCM10), we constructed 4 linear models (figure e-1A on the Neurology® Web site at www.neurology.org). After the parameters of each competing model were estimated, they were compared using Bayesian model comparison, in which the evidence for each model is quantified using the difference in their log evidence.6
MEG study.
With use of a 74-channel 2-sensor system (Magnes II; 4D-Neuroimaging, San Diego, CA) in a magnetically shielded room, MEG and 32-channel surface EEG were performed simultaneously under 3 conditions: eyes closed, scanning Greek letters, and reading silently. ORMs were visually identified during the inspection of the 3 recordings. A single dipole analysis assuming a spherical head model was performed with MSI software, and the solution was considered valid if it had at least a correlation coefficient of 0.97 and a confidence volume less than 3 cm3. Further source analysis was performed using CURRY software (version 4.6), and results were coregistered onto the patient's structural MRI scan.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the local ethics committee, and written consent was obtained.5
RESULTS
Reading fMRI session.
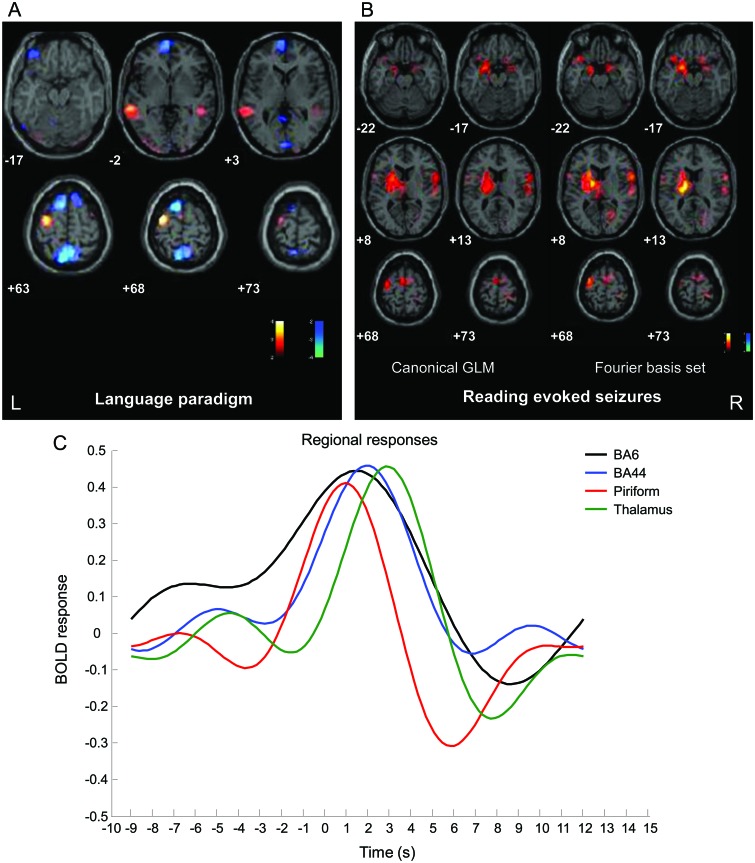
No myclonic jerks were noted by the patient during this session.5 Activation clusters were observed over the left BA6 and the middle temporal gyrus and deactivation in the left precuneus, left mesial frontal cortex, and bilateral superior frontal gyrus (figure 1A). No changes were found in subcortical structures.
Figure 1. EEG-fMRI results.
(A) Significant blood oxygen level−dependent (BOLD) changes during reading without seizures (red, activation; blue, deactivation) (p < 0.05 corrected for family-wise error [FWE]) overlaid onto the subject's T1 MRI scans. (B) SPM{t} (red, activation; blue, deactivation) (p < 0.05 corrected for FWE) for ictal fMRI, both general linear model (GLM) analyses, canonical hemodynamic response function plus temporal derivatives and Fourier basis set. All fMRI data were analyzed and preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). (C) Estimated time courses of event-related BOLD signal change plotted for the most significant clusters. x-axis represents time in seconds relative to event onset (between −10 seconds before and +15 seconds after ictal seizure onset; 0 seconds corresponds to the ictal seizure onset); y-axis represents the BOLD signal changes relative to baseline. See text for details. BA = Brodmann area.
Ictal fMRI.
Canonical GLM.
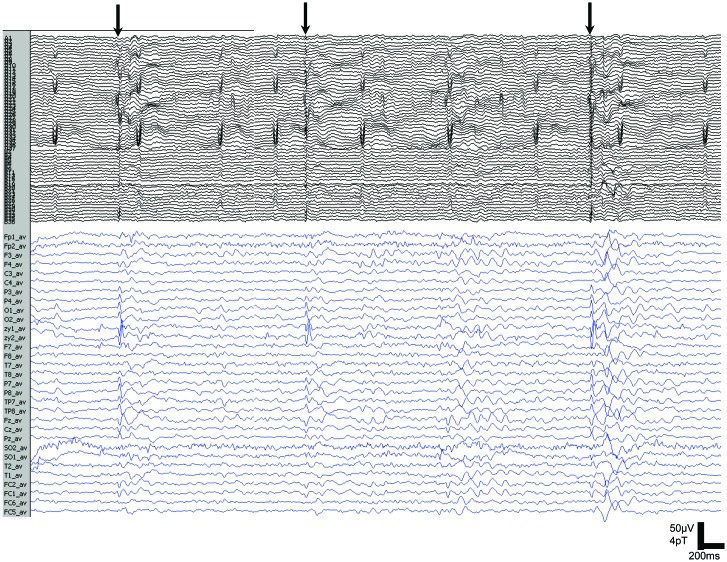
During 2 fMRI sessions, a total of 243 ORMs were recorded; the simultaneous ictal EEG pattern showed brief (<1 second) diffuse spike-wave activity with the maximum frontally (figure 2). Conventional analysis of fMRI data5 showed a reproducible BOLD increase centered in the left precentral gyrus (BA6), left thalamus, left PFC, and right inferior frontal gyrus (BA44) (figure 1B); a BOLD decrease was seen in the left PFC.
Figure 2. Magnetoencephalography (MEG) and EEG recording of reading-evoked seizures.
MEG and simultaneous 32-channel EEG recording show bilateral discharges (arrows) that are maximal over the frontal regions during reading-elicited orofacial reflex myocloni.
Fourier basis set.
BOLD signal changes were highly concordant with the results from the canonical model (figure 1B). We extracted the temporal pattern of the BOLD signal for the main brain clusters, as revealed by Fourier analysis: left BA6, thalamus, PFC, and right BA44. The left PFC BOLD response peaked before that of the other brain regions, followed (∼1 second later) by BA6, by BA44, and then by the thalamus (figure 1C). The PFC hemodynamic response fell more rapidly and crossed the baseline 3 seconds after the electrographic event with an undershoot, which explains its negative response within the standard analysis.
Effective connectivity analysis.
Bayesian model comparison identified model 1 (i.e., PFC neuronal activity drives the changes in other ROIs) as the most likely model (figure e-1B), being significantly better than model 3 (BA6). Models 1 and 3 were significantly more likely than the other 2 models.
MEG analysis.
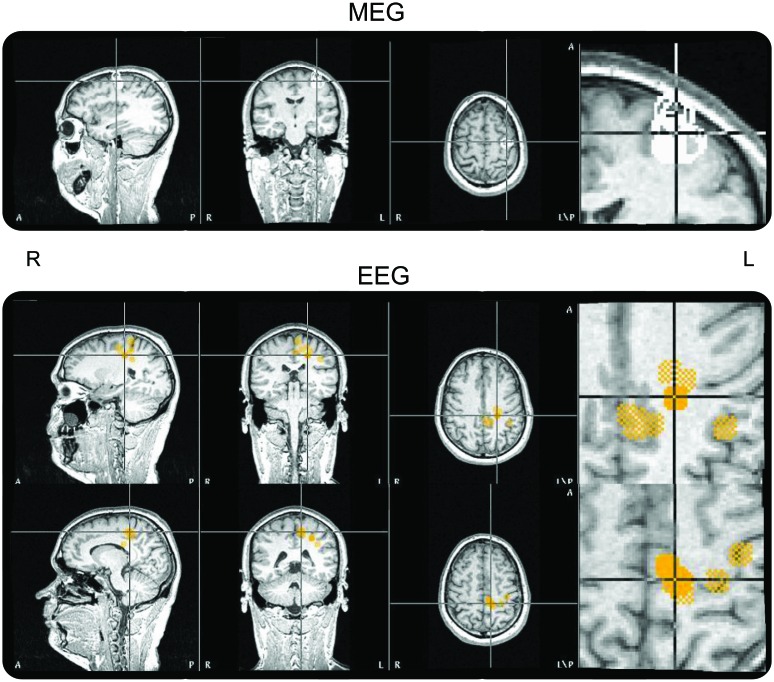
Ictal activity (56 seizures) in MEG and EEG was only recorded under the text scanning condition (figure 2). A single moving dipole source analysis was successful mainly for MEG spikes with solutions in the left precentral region, overlapping the motor representation of the upper mouth. EEG confirmed the MEG results, but localizations were more scattered, extending precentrally into BA6 (figure 3).
Figure 3. Simultaneous EEG and magnetoencephalography (MEG) results.
MEG source analysis (upper panel) overlaid on the subject's T1 image shows monofocal generation in the left precentral region. EEG source analysis (lower panel) overlaid on the subject's T1 image shows Brodmann area 6 sources extended more mesially.
DISCUSSION
Our connectivity studies based on simultaneous EEG-fMRI and EEG-MEG suggest that an area in the dominant premotor cortex (BA6) is the primary source of the epileptic activity in RE but also revealed an area in the left deep PFC closely linked to the initiation of seizures.
Cortical-subcortical circuitries are known to contribute to seizure initiation and propagation in both generalized and focal epilepsies.7 In our study, BOLD changes in subcortical structures, such as thalamus, do not seem to be the earliest change in relation to ictal onset. Our analysis captured the hemodynamic changes in the PFC that preceded those in the thalamus and in cortical regions. We deliberately used a flexible Fourier analysis to account for a range of possible hemodynamic shapes in a time window around the seizures. This allows for inferences to be made regarding effective connectivity during seizure initiation.
The deep PFC closely corresponds to a discrete site within the primary olfactory cortex that has been suggested to control initiation or propagation of focal and generalized epileptic activity in animal models.8 In this site, unilateral microinjection of a γ-aminobutyric acid (GABA) receptor antagonist or glutamate receptor agonists elicited limbic motor seizures analogous to complex partial seizures in humans. In humans, we found evidence that the deep PFC was involved in modulation of seizure activity by showing a correlation between interictal spike-related BOLD responses, GABAA receptor binding, and seizure frequency in patients with focal epilepsies arising from different cortical areas.9
Besides deep cortical structure involvement, MEG confirmed that the left precentral cortex (BA6) was part of the core ictal network involved in reading-evoked seizures. This finding supports the view of the current classification that RE should be considered a localization-related epilepsy. The lack of deep sources identified by the MEG study might be due to the multifocal location of the changes, especially because these changes preceded electrographic onset as demonstrated by advanced fMRI analysis. In our case, fMRI data analysis corroborated premotor cortex involvement in the reading task without observable seizures, confirming the hypothesis that the cerebral networks subserving epileptic activity in RE comprise areas of the brain involved in normal reading processes2,4 and hence providing evidence that RE should be considered a model of “system epilepsy” as recently hypothesized.10 Our results from advanced fMRI analysis support this view further, postulating an important causal role of the frontal PFC in initiating reading-evoked seizures, by leading and potentially facilitating the epileptogenic cortex, i.e., the dominant premotor cortex.
We cannot generalize on the basis of this individual case, but further studies on larger cohorts of patients should help to confirm our hypothesis about the role of the structures and networks described.
Supplementary Material
GLOSSARY
- BA
Brodmann area
- BOLD
blood oxygen level−dependent
- GLM
general linear model
- MEG
magnetoencephalography
- ORM
oral reflex myoclonus
- PFC
piriform cortex
- RE
reading epilepsy
- ROI
region of interest
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
A.E.V., A.S.H., and S.R. contributed to data acquisition and data analysis. A.E.V. and D.W.C. performed statistical analysis. A.E.V., D.W.C., and S.R. wrote the article. L.L., M.J.K., and H.S. were involved in conception, analysis, and interpretation of the data presented as well as writing of the article.
DISCLOSURE
A.E. Vaudano reports no relevant disclosures. D. Carmichael reports no relevant disclosures. A. Haddadi reports no relevant disclosures. S. Rampp reports no relevant disclosures. H. Stefan has received honoraria and study support for serving on scientific advisory boards from 4D-Imaging, Medtronic, and Elekta. Professor Lemieux reports no relevant disclosures. M. Koepp served on scientific advisory boards for GE. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Koutromanidis M, Koepp MJ, Richardson MP, et al. The variants of reading epilepsy: a clinical and video-EEG study of 17 patients with reading-induced seizures. Brain 1998; 121: 1409– 1427 [DOI] [PubMed] [Google Scholar]
- 2. Archer JS, Briellmann RS, Syngeniotis A, Abbot DF, Jackson GD. Spike-triggered fMRI in reading epilepsy: involvement of left frontal cortex working memory area. Neurology 2003; 60: 415– 421 [DOI] [PubMed] [Google Scholar]
- 3. Wolf P. Reading epilepsy. In: Roger J, Bureau M, Dravet C, Dreifuss FE, Perret A, Wolf P. eds. Epileptic Syndromes in Infancy, Childhood and Adolescence. London: John Libbey; 1992: 281– 298 [Google Scholar]
- 4. Omura K, Tsukamoto T, Kotani Y, Ohgami Y, Yoshikawa K. Neural correlates of phoneme-to-grapheme conversion. Neuroreport 2003; 5: 949– 953 [DOI] [PubMed] [Google Scholar]
- 5. Salek-Haddadi A, Mayer T, Hamandi K, et al. Imaging seizure activity: a combined EEG/EMG-fMRI study in reading epilepsy. Epilepsia 2009; 50: 256– 264 [DOI] [PubMed] [Google Scholar]
- 6. Kiebel SJ, Garrido MI, Moran RJ, Friston KJ. Dynamic causal modelling for EEG and MEG. Cogn Neurodyn 2008; 2: 121– 136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex 2004; 14: 892– 902 [DOI] [PubMed] [Google Scholar]
- 8. Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature 1984; 317: 623– 625 [DOI] [PubMed] [Google Scholar]
- 9. Laufs H, Richardson MP, Salek-Haddadi A, et al. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology 2011; 77: 904– 910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Striano P, Striano S. Reading epilepsy and its variants: a model for system epilepsy. Epilepsy Behav 2011; 20: 591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.