Abstract
Objective
Type 2 diabetes mellitus (T2DM) and related syndromes exhibit a deadly triad of dyslipoproteinemia, which leads to atherosclerosis; hyperglycemia, which causes microvascular disease; and hypertension. These features share a common, but unexplained, origin – namely, pathway-selective insulin resistance and responsiveness (SEIRR). Here, we undertook a comprehensive characterization of SEIRR in liver and hepatocytes by examining 18 downstream targets of the insulin receptor, surveying the AKT, ERK, and NAD(P)H oxidase 4 (NOX4) pathways.
Methods/Results
Injection of insulin into hyperphagic, obese T2DM db/db mice failed to inactivate hepatic protein tyrosine phosphatase gene family members, a crucial action of NOX4 previously thought to be required for all signaling through AKT and ERK. Insulin-stimulated T2DM livers unexpectedly produced an unusual form of AKT that was phosphorylated at Thr308 (pT308), with only weak insulin-stimulated phosphorylation at Ser473. Remarkably, knock-down or inhibition of NOX4 in cultured hepatocytes recapitulated the entire complicated pattern of SEIRR seen in vivo – namely, monophosphorylated pT308-AKT, impaired insulin-stimulated hypolipidemic and hypoglycemic pathways, but continued lipogenic pathways and robust ERK activation.
Conclusions
Functional disturbance of a single molecule, NOX4, is sufficient to induce the key harmful features of deranged insulin signaling in T2DM, obesity, and other conditions associated with hyperinsulinemia and SEIRR.
Keywords: diabetes, insulin resistance, obesity, NOX4, lipogenesis
Introduction
Accelerated macrovascular and microvascular disease remain significant, and growing, causes of morbidity and mortality in type 2 diabetes mellitus (T2DM) and related syndromes. The hallmark of these conditions is a distinctive pattern of pathway-selective insulin resistance and responsiveness (SEIRR).1–4 The result is the deadly triad of dyslipoproteinemia, which leads to atherosclerotic macrovascular disease;5–8 hyperglycemia, which causes microvascular disease;9–11 and hypertension, which worsens both large- and small-vessel complications.6, 12, 13
As an example of SEIRR, the T2DM liver upon stimulation with insulin fails to adequately take up glucose14 or suppress gluconeogenesis.15, 16 Yet the T2DM liver continues insulin-stimulated synthesis and storage of lipids;10, 14, 17 and it does not appropriately inhibit the secretion17–20 nor accelerate the clearance21–25 of atherogenic apolipoprotein (apo)-B–containing lipoproteins (Figure 1 and Supplemental Background).
Figure 1.
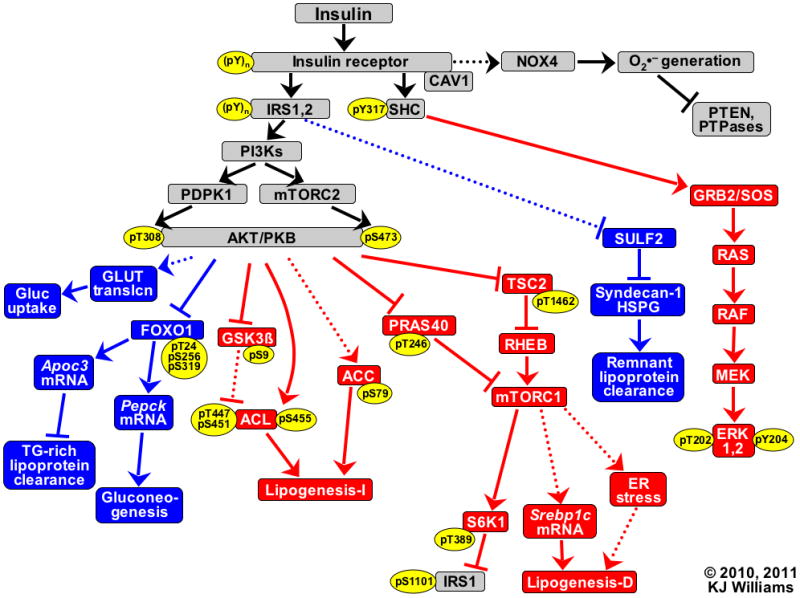
Key branches of the insulin signaling cascade. Hypolipidemic and hypoglycemic pathways that are normally triggered by insulin are indicated in blue, whereas insulin-stimulated pathways that activate lipogenesis and MAP kinases are shown in red. Solid lines represent recognized pathways; dotted lines are less well-characterized or putative. Pointed arrowheads indicate stimulation of the immediately downstream molecule or process; flat arrowheads indicate inhibition. Thus, endpoints that receive signals from the insulin receptor via pathways with an even number of flat arrowheads (0 or 2) are activated by insulin (Gluc uptake, TG-rich lipoprotein clearance, both mechanisms for Lipogenesis, Remnant lipoprotein clearance, and phosphorylations of ERK). Pathways with an odd number of flat arrowheads are inhibited by insulin (PTEN, PTPases, and Gluconeogenesis). Specific sites that become phosphorylated (p) upon insulin stimulation are indicated within yellow ovals, and numbers indicate residues in human sequences. Lipogenesis-I refers to lipogenesis that becomes activated independently from new protein synthesis; Lipogenesis-D depends on new protein synthesis.
Abbreviations: ACC, acetyl-CoA carboxylase-1; ACL, ATP citrate lyase; AKT/PKB, protein kinase B; Apoc3, apolipoprotein C-III; CAV1, caveolin-1; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; FOXO1, forkhead box O1A; Gluc, glucose; GLUT translcn, translocation of facilitated glucose transporters; GRB2, growth factor receptor-binding protein 2; GSK3β, glycogen synthase kinase 3β; HSPG, heparan sulfate proteoglycan; IRS, insulin receptor substrate; MEK, MAP/ERK kinase; mTORC, mammalian target of rapamycin complex; NOX4, NAD(P)H oxidase 4; PDPK1, 3′-phosphoinositide-dependent protein kinase 1; Pepck, phosphoenolpyruvate carboxykinase 1; PI3Ks, isoforms of phosphatidylinositol 3′-kinase; PRAS40, proline-rich AKT substrate of 40kDa; PTEN, phosphatase and tensin homolog deleted on chromosome 10; PTPases, protein-tyrosine phosphatases such as PTP1B; RAF, v-raf-1 murine leukemia viral oncogene homolog 1; RAS, rat sarcoma guanosine-nucleotide-binding protein; RHEB, RAS homolog enriched in brain; S6K1, ribosomal protein S6 kinase 1; SHC, Src homologous and collagen-like protein; SOS1, son of sevenless (Drosophila) homolog 1; Srebp1c, sterol regulatory element-binding protein-1c, which its discoverers originally named adipocyte determination- and differentiation-dependent factor 1 (ADD1); SULF2, heparan sulfate glucosamine-6-O-endosulfatase-2; TG, triglyceride; TSC2, tuberin.
Several explanations have been proposed for SEIRR,1, 3, 4, 26–29 but all have focused on the two canonical limbs of the insulin signaling cascade, meaning protein kinase B (AKT) with its downstream targets and mitogen-activated protein (MAP) kinases with their downstream targets (Figure 1; see reference30 for a comprehensive, timely review). The effect of SEIRR on the AKT limb is particularly puzzling, because AKT regulates hypolipidemic and hypoglycemic pathways that become insulin-resistant, but also lipogenic pathways that remain insulin-responsive. As shown in Figure 1, hypolipidemic and hypoglycemic pathways downstream of AKT include translocation of facilitated glucose transporters (GLUT) in extrahepatic tissues31 and AKT-mediated phosphorylations of forkhead box-1 (FOXO1) that suppress hepatic apoC-III expression20, 32 and gluconeogenesis.33 Pathways downstream of AKT that stimulate lipid biosynthesis include alterations in three site-specific phosphorylations of ATP-citrate lyase (ACL), in part through GSK3β,34–36 and mTORC1-mediated transcription of lipogenic genes37 via SREBP1c.28, 29, 38, 39 Obesity and related conditions impair FOXO1 phosphorylation in vivo, resulting in abnormally high hepatic Apoc320, 32 and Pepck40 mRNA levels, whereas AKT-mediated phosphorylation of GSK3 is barely blunted (see the data in figure 3 of reference41) and mTORC1 exhibits enhanced activity.42, 43 In addition, SEIRR leaves the MAP kinase limb responsive to insulin, as assessed by ERK1, 2 and JNK44 phosphorylation, despite a marked impairment in overall insulin-stimulated tyrosine phosphorylation of the insulin receptor.1, 2 The basis for differential dysregulation of major AKT targets and MAP kinases remains unclear.
There is, however, a non-canonical third limb in insulin signaling. When insulin binds to the insulin receptor, it activates the NAD(P)H oxidase homologue NOX4 to generate a transient burst of superoxide (O2•−) and its byproduct H2O2 that enhance signal propagation by disabling enzymes in the protein-tyrosine phosphatase (PTPase) gene family (Figure 1).45–50 Two such enzymes of note are, first, the phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which can terminate PI3K signaling by dephosphorylating its active lipid product, phosphatidylinositol-3,4,5-trisphosphate (PIP3); and second, the nonreceptor-type PTPase-1B (PTP1B), which blunts insulin signaling by dephosphorylating tyrosyl residues within the insulin receptor and its substrates (IRS1,2). Interfering with insulin-stimulated generation of superoxide and H2O2 was reported to cause abnormal persistence of PTEN51, 52 and PTP1B48, 49 activities and hence a global decrease in all pathways downstream of the insulin receptor, including insulin-stimulated phosphorylation of AKT and ERK and cellular uptake of glucose. These studies found no role for impaired NOX4 activation in pathway selectivity of the responses to insulin.
In the current study, we undertook a comprehensive examination of SEIRR in T2DM livers in vivo and in cultured hepatocytes by examining 18 downstream targets of the insulin receptor, including key members of the AKT, ERK, and NOX4 pathways (Figure 1). Unexpectedly, we found that functional derangement of a single molecule, NOX4, is sufficient to induce the key harmful features of SEIRR seen in vivo. This work was presented at the XVII Lipid Meeting Leipzig (10 December 2011).53
Material and Methods
Insulin stimulation of the livers of control and T2DM mice in vivo
To characterize SEIRR in vivo, we used hyperphagic mice, which mimic humans who overeat and thereby develop the same complications, including obesity, fatty liver, dyslipoproteinemia, hyperglycemia, hyperinsulinemia, and disturbed insulin signaling. Lean db/m mice (controls) and obese, T2DM db/db (Leprdb/db) littermates on the C57BLKS background (Jackson Laboratory, Bar Harbor, ME) were fed ad-libitum until age 14 weeks.23 We modified an insulin administration protocol41 to allow sampling of their livers in vivo. Animals were fasted overnight, weighed, and then anesthetized. The abdominal cavity was opened, a single lobe of liver was gently tied off, and a sample distal to the ligature was surgically removed and promptly snap-frozen (pre-insulin sample). Because of the ligature, the cut surface on the remaining liver did not bleed. Next, 10 units of bovine insulin (#I1882, Sigma Chemical Company, St. Louis, MO) per kg of body weight was administered into the vena cava, followed by staunching of the puncture site with the tip of a moistened cotton swab. To avoid acute hypoglycemia, we bathed the exposed peritoneum in a warm 10% dextrose solution. After 10 min, blood was obtained by heart puncture, and sections from other hepatic lobes were taken and snap-frozen (post-insulin sample). To verify diabetic status, hemoglobin A1c levels in blood were quantified by the Vanderbilt Mouse Metabolic Phenotyping Center (Nashville, TN), giving values of 3.9%±0.05% in db/m and 7.4%±0.56% in db/db (mean±SEM, n=3, P<0.005 by the unpaired Student’s t-test). All animal protocols were approved by the Institutional Animal Care and Use Committee.
Insulin stimulation of cultured liver cells, with and without active NOX4
For studies in vitro, we used two complementary systems. First, we previously reported that, upon exposure to physiologic concentrations of insulin, McArdle 7777 rat hepatoma cells exhibit robust insulin-induced suppression of SULF2, an inhibitor of remnant lipoprotein uptake,23 and our studies here indicated that these cells phosphorylate every downstream target of the insulin receptor that we examined (Figure 1 and Results). Moreover, McArdle cells can be maintained in culture for the three days typically required for siRNA knockdown.23 Thus, cells replete or deficient in NOX4 were prepared by transfection with 50 nM nontarget siRNA or rat Nox4 siRNA (#D-001810-01-05 and # L-080114-01-0010, respectively; Dharmacon, Lafayette, CO), using the siIMPORTER transfection reagent (#64-101, Millipore Corporation, Billerica, MA), for 4h in serum-free medium and then overnight in DMEM with 10% FBS, followed by an additional 48h at 37°C in DMEM/10% FBS without siRNA. In accordance with prior literature,1, 23, 48, 54 cells were switched to serum-free (DMEM/1% BSA) or low-serum (DMEM/2% FBS) medium for 2h before supplementation with 0 or 10nM insulin. Exposure to 0 or 10nM insulin lasted 10 min for studies of phosphorylations of pre-existing protein targets, 15 min to assess uptake of [3H]2-deoxy-D-glucose, a non-metabolizable glucose analogue, or 18 h for studies of SULF2 protein regulation. Serum-free medium was used only for short-term studies of phosphorylations or 2-deoxy-D-glucose uptake;1, 48, 54 the longer studies used low-serum medium to maintain cell health.23
Second, because McArdle cells appear to lack sufficient endogenous expression of LXR,55 a required factor for insulin to induce Srebp1c transcription,56 we relied on freshly isolated rat primary hepatocytes57 for our studies of mRNA regulation. Primary hepatocytes exhibit vigorous insulin-induced regulation of Pepck and Srebp1c,28, 38, 56 but lose many physiologic responses after prolonged culture, necessitating our use of a fast-acting chemical inhibitor of NOX4 (diphenyleneiodonium, DPI).58, 59 We applied DPI at a concentration, 1.0 μM, that provides ~90% inhibition.60 Owing to potential issues of selectivity, particularly at higher micromolar concentrations,61 we used DPI in only a limited fashion. To allow studies of the suppressive effect of insulin on Pepck mRNA levels, all culture media for primary hepatocytes included 100nM dexamethasone to elevate the basal expression of this target, as described.28, 62 Our computational analysis of the NOX4 sequence revealed two candidate caveolin-binding motifs (ΦXΦXXXXΦ, where Φ represents an aromatic amino acid), in residues 190FWYTHNLF197 and 570FEYNKESF577, similar to the single caveolin-binding motif in the insulin receptor (see references63–65 and Figure 1). Thus, we inferred that NOX4 would reside in cholesterol-rich caveolae, and that its superoxide burst could thereby contribute to the putative generation of oxysterol ligands for LXR after insulin stimulation. Activation of LXR appears to be an essential step in Srebp1c induction,56 in addition to the phosphorylation cascades leading to mTORC1 (reference28 and Figure 1). Consistent with this model, we found that global inhibition of NOX4 in primary hepatocytes blocked Srebp1c mRNA induction by insulin (not shown), even though phosphorylations upstream and downstream of mTORC1 remained responsive. Accordingly, primary cells also received 0.5-2nM TO901317, which is ≤10% of its IC50 for LXR activation.66 Primary hepatocytes replete or deficient in NOX4 were prepared by a 30-min pre-incubation at 37°C with DMSO vehicle or 1.0 μM DPI, respectively, followed by addition of 0 or 10nM insulin. These cells were then incubated for an additional 6h to allow mRNA levels to change.
Assays of 18 downstream targets of the insulin receptor, including key members of the AKT, ERK, and NOX4 pathways
Pre- and post-insulin liver samples in which we assayed phosphatase activities were handled under strictly anaerobic conditions in an enclosed work station (model #1025, Thermo Fisher Scientific, Marietta, OH).48, 67 The liver samples were used to prepare homogenates, from which we immunoprecipitated PTEN and PTP1B in separate aliquots and then quantified their enzymatic activities. The substrate was para-nitrophenyl phosphate (pNPP), which PTEN68 and PTP1B48, 67 cleave into a product that absorbs 410-nm light. Activities were normalized to the amounts of recovered enzyme, which were assessed by densitometry of immunoblots by ImageJ software (http://rsbweb.nih.gov/ij/download.html) and expressed as the sum of gray values above baseline. Enzyme recovery did not significantly differ between db/m and db/db livers.
All other tissue and cellular extractions, immunoprecipitations, immunoblots, and qRT-PCR reactions were performed as we previously described.23 Antibodies against target proteins (total target as well as forms with site-specific phosphorylations) are listed in Supplemental Table I, following the nomenclature in Figure 1. Close reading of product inserts from Cell Signaling Technologies (Beverly, MA) indicated that catalogue #4376 was specific for pT202-ERK and catalogue #4377 was specific for pY204-ERK, instead of recognizing solely the doubly phosphorylated forms. Clean immunoblots of pT24-FOXO1 from liver homogenates required prior immunoprecipitation of total FOXO1.69 Primers and probes for qRT-PCR were synthesized by the Gene Expression Facility at the University of North Carolina (Chapel Hill, NC, Dr. Hyung Suk Kim, director), and the sequences are given in Supplemental Table II. In studies of labeled 2-deoxy-D-glucose uptake by cultured McArdle hepatocytes, this molecule was added during the final 4 min of incubation.48, 54
Statistical analyses
Normally distributed data are reported as means±SEMs. For comparisons of enzymatic activities in pre-insulin versus post-insulin liver samples, the paired t-test was used. For comparisons involving several groups of cultured hepatocytes, ANOVA was initially used, followed by pairwise comparisons using the Student-Newman-Keuls q statistic.
Results
Characterization of pathway-selective insulin resistance and responsiveness in the livers of hyperphagic T2DM mice
We began with a detailed characterization of SEIRR in T2DM db/db livers in vivo. Consistent with our preliminary report showing dysregulation of the hepatic NOX4 pathway in db/db mice,70 we found that insulin stimulation of T2DM livers in vivo failed to inhibit the activities of PTEN (Figure 2A) or PTP1B (Supplemental Figure I), in contrast to the responses of control db/m livers. Surprisingly, despite the inability to suppress PTEN or PTP1B activities, acute insulin administration in vivo provoked completely normal phosphorylation of AKT at Thr308 (pT308) in the livers of obese T2DM db/db mice compared to lean db/m controls (Figure 2B, upper immunoblots). The original report of SEIRR documented a significant defect in insulin-stimulated phosphorylation of AKT at Ser473 (pS473) in isolated microvessels from obese, hyperphagic rats, but did not examine Thr308.1 In line with that report, we found only weak insulin-stimulated pS473-AKT in db/db livers compared to db/m controls (Figure 2B, lower immunoblots). Thus, acute insulin stimulation of T2DM livers in vivo failed to inactivate PTPase gene family members, yet unexpectedly produced an unusual, monophosphorylated form of AKT, pT308-AKT.
Figure 2.
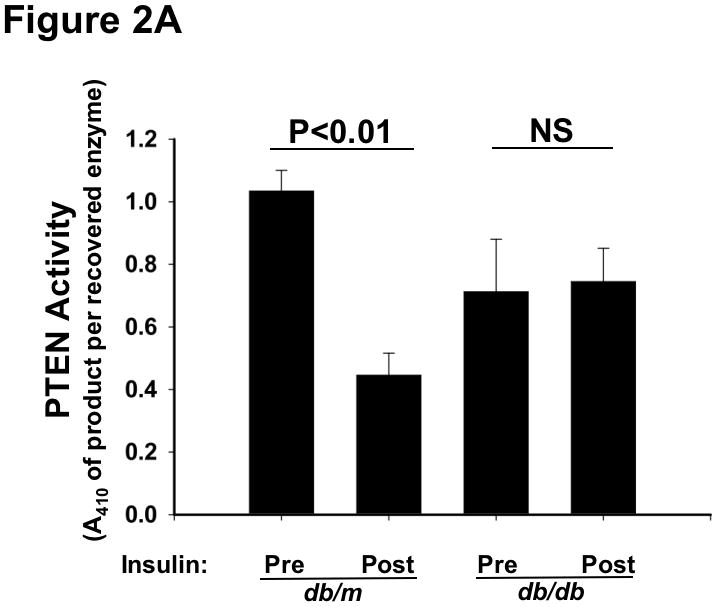
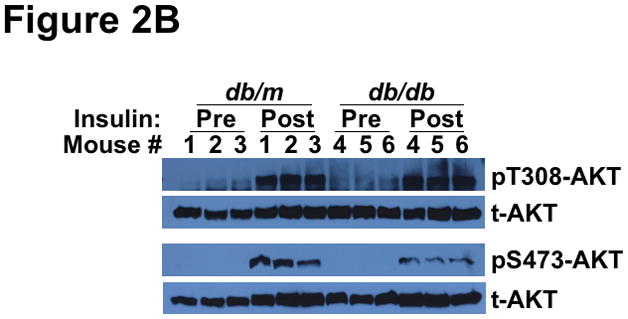
Type 2 diabetes renders the liver unable to inactivate PTEN in response to insulin, yet still able to phosphorylate AKT at Thr308. Liver samples were obtained just before (Pre) and 10min after (Post) an intravenous injection of insulin into 14-week-old lean db/m mice (controls) and their hyperphagic, obese T2DM db/db littermates, as indicated. Other nomenclature follows Figure 1. Panel A: PTEN activities in liver homogenates, assayed under strictly anaerobic conditions (mean±SEM, n=3). Statistical comparisons by the paired t-test are indicated. Panel B: Phosphorylation of AKT at Thr308 (pT308-AKT, upper immunoblots) and Ser473 (pS473-AKT, lower immunoblots). Immunoblots for total AKT (t-AKT, meaning phosphorylated plus unphosphorylated forms) is also shown for each sample. Numbers over the lanes refer to individual animals.
To determine the consequences of these disturbances in insulin signaling in vivo, we next examined a set of downstream targets of AKT in the same insulin-stimulated db/m and db/db livers. Supplemental Figure II-A–B shows that T2DM impaired insulin-induced phosphorylation of hepatic FOXO1, consistent with prior reports of apoC-III overexpression and persistent gluconeogenesis, yet phosphorylations of GSK3β, PRAS40, and S6K1 remained robust, consistent with ongoing hepatic lipogenesis (see Figure 1 and Supplemental Background). We also found that insulin-stimulation of T2DM db/db livers in vivo provoked vigorous phosphorylation of both the Thr202 and Tyr204 sites on ERK1 (Supplemental Figure II-C).
Cell-culture models of SEIRR from deficiency or inhibition of NOX4
To explain the complicated pattern of insulin resistance and responsiveness in T2DM db/db livers in vivo, we hypothesized that disturbed NOX4 function might play a causal role. As our initial model, we used siRNA to knock-down this enzyme in cultured rat McArdle hepatoma cells (Figure 3A). Remarkably, knock-down of NOX4 preserved insulin-stimulated phosphorylation of AKT at Thr308, but blocked insulin-stimulated phosphorylation at Ser473 (Figure 3B), thereby recapitulating the production of monophosphorylated pT308-AKT that we saw in T2DM livers in vivo. To examine the other canonical limb of insulin signaling, we found that NOX4 knock-down still permitted vigorous insulin-stimulated phosphorylations of ERK1 at Thr204 and Tyr204 (Figure 3C), replicating the ERK response that we saw in T2DM livers in vivo. Thus, impairment of NOX4 does not produce the global decrease in insulin-stimulated signaling that had been previously reported, but instead causes an unexpected pattern of site-specific blockages or continued phosphorylations of AKT and ERK that mimics the upstream elements of SEIRR that we saw in the livers of hyperphagic T2DM db/db mice in vivo.
Figure 3.
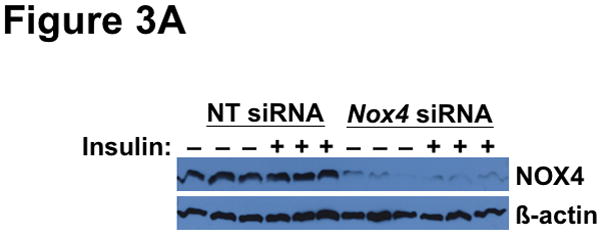
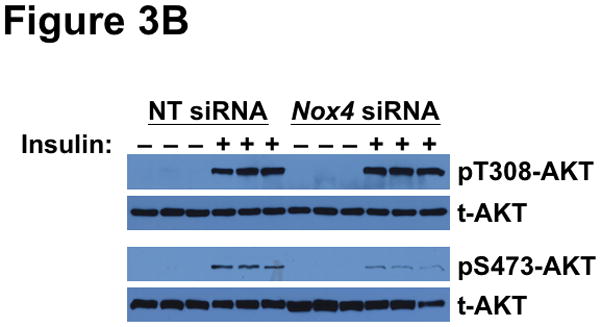
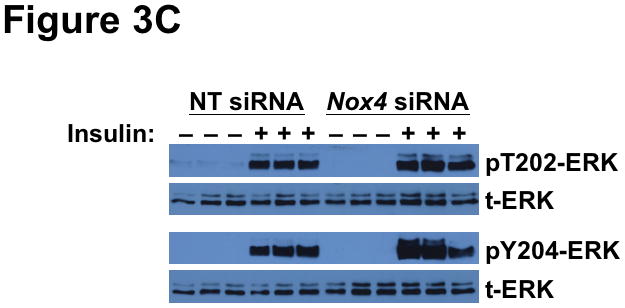
Deficiency of NOX4 in cultured liver cells mimics the effects of type 2 diabetes on the two canonical mediators of insulin signaling, AKT and ERK. As indicated, McArdle hepatocytes were pretreated with nontarget (NT) control siRNA or Nox4 siRNA, exposed to 0 or 10nM insulin for 10 min, and then harvested. This figure displays immunoblots in triplicate from a single set of cultured cells. Each lane represents a separate culture well. Panel A: Anti-NOX4 immunoblot. Panel B: Insulin-stimulated phosphorylations of AKT (pT308-AKT, pS473-AKT, and total AKT). Panel C: Insulin-stimulated phosphorylations of ERK (pT202-ERK, pY204-ERK, and total ERK). The data from these cultured cells are representative of a total of three independent insulin signaling experiments.
We next examined the effect of NOX4 deficiency on key insulin-stimulated phosphorylations downstream of AKT. We found that NOX4 knock-down abolished insulin-stimulated phosphorylation of FOXO1 at Thr24 (Figure 4A). Strikingly, the same NOX4-deficient hepatocytes showed unimpaired insulin-stimulated phosphorylations of GSK3β and ACC (Figure 4A). The two AKT-dependent inputs into mTORC1 – namely, PRAS40 and TSC2, showed continued responsiveness to insulin in these cells (Figure 4B). Likewise, in NOX4-deficient hepatocytes, insulin still stimulated brisk phosphorylation of the mTORC1 substrate S6K1 (85-kDa isoform), indicating unimpaired activation of mTORC1 (Figure 4B). The 70-kDa S6K1 isoform exhibited high basal and post-insulin phosphorylations (Figure 4B), exactly as in db/db livers (Supplemental Figure II-B). The S6K1 is of particular interest, because it desensitizes IRS1 in states of overnutrition and obesity (reference71 and Figure 1). Taken together, Figures 3 and 4 indicate that interference with just a single molecule, NOX4, causes the same complicated pattern of disturbed insulin-stimulated phosphorylations that we saw in T2DM livers in vivo in Figure 2 and Supplemental Figure II.
Figure 4.
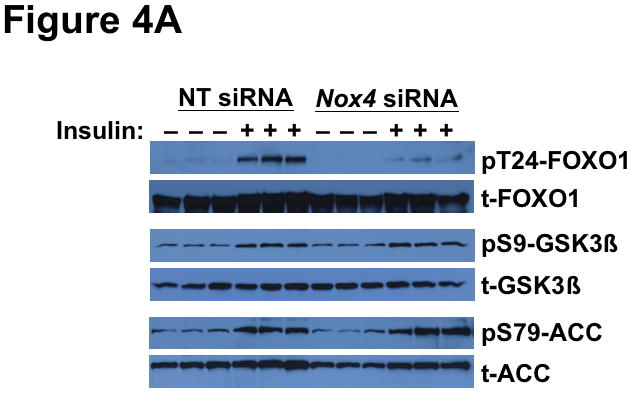
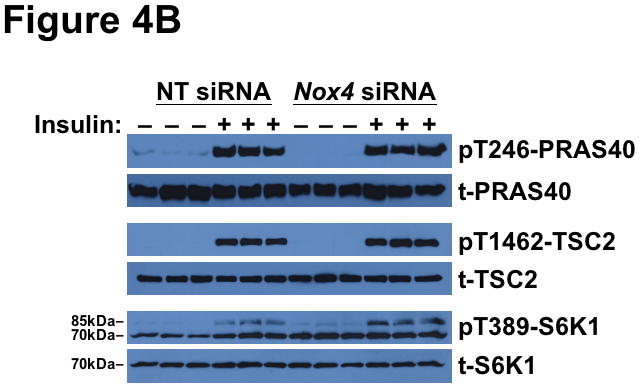
Deficiency of NOX4 in cultured liver cells mimics the effects of type 2 diabetes on phosphorylations downstream of AKT, thereby rendering hypolipidemic and hypoglycemic pathways resistant to insulin, while leaving lipogenic pathways insulin-responsive.
Displayed are immunoblots from the same single set of cultured McArdle hepatocytes as in Figure 3. Panel A: Resistance of FOXO1 to insulin-stimulated phosphorylation in NOX4-deficient hepatocytes (TG-rich lipoprotein clearance and Gluconeogenesis pathways from Figure 1), yet continued responsiveness of GSK3β and ACC (Lipogenesis-I pathway from Figure 1). Panel B: Continued activation of molecules upstream (PRAS40, TSC2) and downstream (S6K1) of mTORC1 in NOX4-deficient hepatocytes (Lipogenesis-D pathway from Figure 1). Both the 70- and 85-kDa isoforms of S6K1 can be seen on the immunblot for pT389-S6K1, as indicated. The data from these cultured cells are representative of a total of three independent experiments.
To assess metabolic effects caused by disturbances in NOX4, we focused on several functional endpoints, beginning with mRNA regulation. Consistent with Figure 4A, chemical inhibition of NOX4 blunted major functional effects of AKT-mediated phosphorylation of FOXO1 – namely, downstream suppression of the mRNAs encoding Apoc3 (Figure 5A) and Pepck (Figure 5B). These results indicate that deficiency or inhibition of NOX4 in hepatocytes renders these cells resistant to key hypolipidemic and hypoglycemic actions of insulin that are downstream of AKT, similar to SEIRR in vivo. Likewise, Irs2 mRNA, a factor regulated similarly to Apoc3 and Pepck,72 became resistant to suppression by insulin in the absence of functional NOX4 (Supplemental Figure III). Consistent with our finding of continued mTORC1 activation (Figure 4B), NOX4 inhibition still allowed high insulin-induced levels of Srebp1c mRNA, which encodes a major transcription factor in lipogenesis (Figure 5C).28, 38, 39, 73, 74
Figure 5.
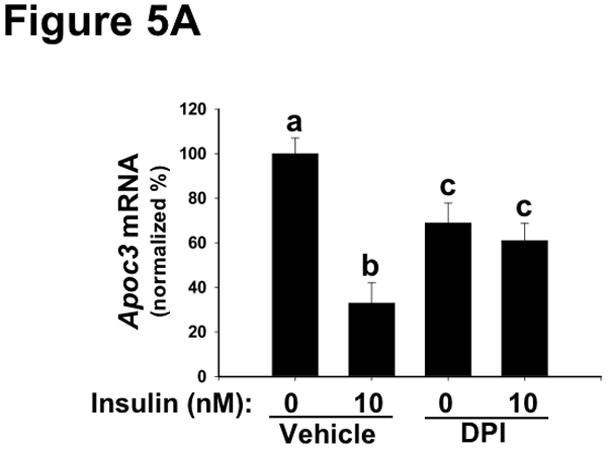
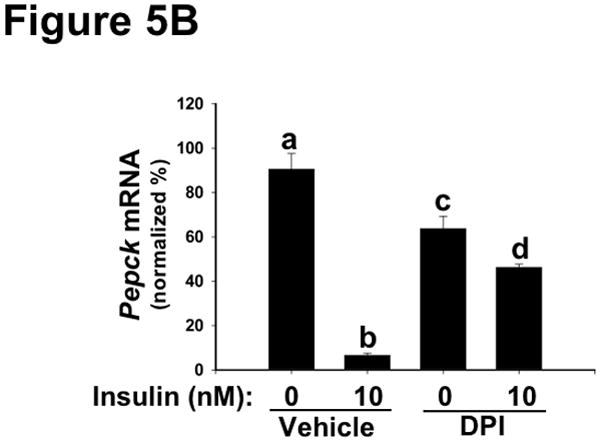
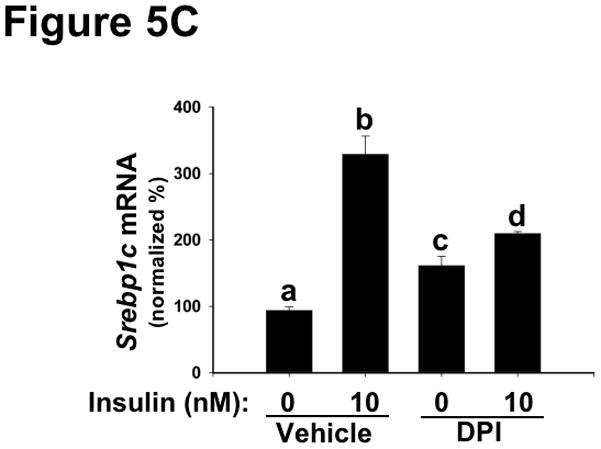
Inhibition of NOX4 in primary hepatocytes dysregulates key mRNAs in lipid and glucose control, thereby recapitulating harmful features of hepatic SEIRR.
As indicated, rat primary hepatocytes were placed in media supplemented with 0 (Vehicle) or 1.0 μM DPI (an inhibitor of NOX4) and then incubated for 30 min at 37ºC, after which the media were further supplemented to achieve insulin concentrations of 0 or 10nM. The cells were incubated for an additional 6h, to allow mRNA levels to change, and then harvested. Levels of Apoc3 (Panel A), Pepck (Panel B), and Srebp1c (Panel C) mRNA were assessed by way of qRT-PCR, normalized to β-actin mRNA levels ( Ct), and then expressed relative to the mean value from the cells that had been incubated with neither DPI nor insulin (2−ΔΔCt; mean±SEM, n=4). Displayed are quantifications of mRNA levels in a single set of cells. P<0.005 by ANOVA; columns labeled with different lowercase letters (a, b, c, d) are statistically different by the Student-Newman-Keuls test (P<0.01).
Lastly, we examined the effects of disturbed NOX4 function on glucose handling and regulation of remnant lipoprotein clearance. Under stimulation by insulin and other factors, the normal liver takes up one-third of each oral glucose load,75 but the process is defective in T2DM.14 Figure 6A shows that NOX4 deficiency nearly abolished the ability of insulin to stimulate glucose uptake into McArdle hepatocytes. Our current results show impairments in both basal and insulin-stimulated glucose uptake by hepatocytes, consistent with the finding that activated mTORC1 impairs glucose uptake by the liver (reference76 and Figure 4B). Regarding remnant lipoprotein clearance, we recently demonstrated that T2DM livers overexpress the heparan sulfate glucosamine-6-O-endosulfatase-2 (SULF2), a novel factor that complexes with the syndecan-1 heparan sulfate proteoglycan and impairs hepatocyte uptake of atherogenic remnant lipoproteins (references23, 24 and Figure 1). Overexpression of SULF2 is mediated in part through an impaired response to insulin.23 Figure 6B shows that NOX4 deficiency abolishes the ability of insulin to suppress SULF2 protein expression in cultured hepatocytes, again mimicking T2DM liver.
Figure 6.
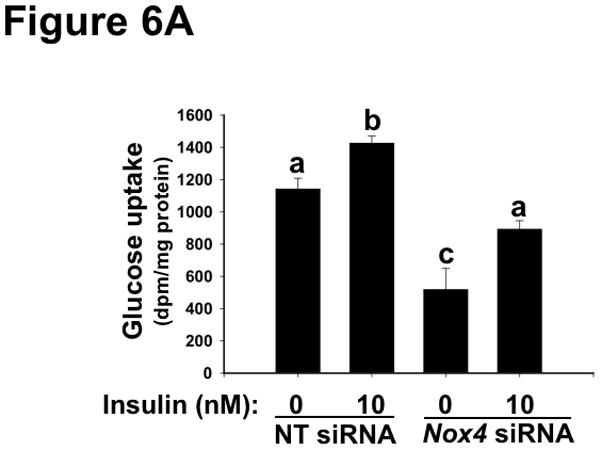
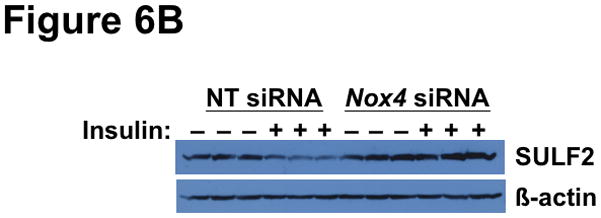
Deficiency of NOX4 in cultured liver cells impairs hypolipidemic and hypoglycemic functions, thereby recapitulating key harmful features of SEIRR in vivo.
Panel A: NOX4 deficiency impairs glucose uptake by McArdle hepatocytes. Cells were pre-treated with non-target (NT) or Nox4 siRNA, supplemented with 0 or 10nM insulin, as indicated, and then exposed to [3H]2-deoxy-D-glucose for the final 4 min. Displayed are cellular tritium dpms per mg of cellular protein (mean±SEM, n=3). P<0.001 by ANOVA; columns labeled with different lowercase letters (a, b, c) are statistically different by the Student-Newman-Keuls test (P<0.01). Panel B: NOX4 deficiency impairs the ability of insulin to suppress SULF2 expression (Remnant lipoprotein clearance from Figure 1). McArdle cells were treated with non-target (NT) or Nox4 siRNA, without or with insulin, as indicated. Cells were harvested 18h after the addition of insulin, to allow time to degrade pre-existing SULF2 protein. Displayed are immunoblots in triplicate for SULF2 and, as a loading control, β-actin.
Discussion
Our results indicate that T2DM liver exhibits defects in specific NOX4 functions, and that impairment of NOX4 in cultured hepatocytes produces a distinctive pattern of selective insulin resistance/responsiveness that recapitulates all known features of hepatic SEIRR in vivo. These include inhibition of key hypolipidemic and hypoglycemic effects of insulin, but continued activation of several pathways mediating lipogenesis, ongoing responsiveness of ERK, and loss of the ability of insulin to suppress SULF2 expression. Strikingly, in Figure 1, all pathways shown in blue become resistant, while all pathways in red remain responsive. From the standpoint of human health, it is the worst possible combination of effects.
Several other abnormalities have been proposed as underlying causes for impaired insulin signaling, such as an increased flux of non-esterified fatty acids, insulin-independent activation of mTOR by nutrient excess, infiltration of key tissues by immune cells, and ER stress. Unfortunately, many studies of these factors have focused largely or exclusively on their effects on glucose metabolism. Given our current understanding of SEIRR, however, it is essential to examine all key insulin signaling pathways, to determine if a proposed cause can faithfully produce the entire pattern of SEIRR that arises from chronic, flagrantly positive caloric balance.
Fragmentary information in the literature hints at problems with these other hypotheses. For example, an animal model of obesity induced by a high-fat, high-sucrose diet failed to show robust insulin-stimulated phosphorylation of hepatic AKT at Thr308.43 In another study, rapidly elevated plasma concentrations of non-esterified fatty acids in vivo blocked insulin-stimulated phosphorylation of aortic ERK.44 These disturbances in insulin-stimulated phosphorylations differ significantly from what we (Figure 2) and others1 have described in simple chronic hyperphagia. Doubts about a dominant role for fatty acid flux were re-enforced by work showing that drastic lowering of plasma concentrations of non-esterified fatty acids in obese, glucose-intolerant individuals only partially corrected their ability to handle carbohydrates in response to insulin.77 Regarding other proposed causes of selective insulin resistance, our finding of continued insulin-stimulated phosphorylation of a subset of the downstream targets of AKT in vivo (Supplemental Figure II-A–B) contradicts both the mTOR hypothesis and the ER stress hypothesis, as depicted in figure 3c,d of reference30. More detailed investigations will be needed to fully evaluate the roles of these well-known prior models.
The mechanism for NOX4 dysfunction in T2DM liver remains unknown, but we are particularly interested in the possibility that striking overexpression of protein phosphatase 5 (PPP5C), a novel binding partner we found for NOX4,70 may derange insulin-stimulated phosphorylations, while still allowing insulin-induction of a key target of mTORC1 and LXR – namely, Srebp-1c mRNA. A regulatory role for PPP5C might also reconcile our current results with earlier work showing that overexpression of enzymatically inactive NOX4 mutants in adipocytes blunts the canonical AKT and ERK limbs of insulin signaling.48 We now speculate that these mutants would have sequestered PPP5C so that it was no longer available to interact with the endogenous, active NOX4, thereby mimicking an unusual phenotype that we had reported from knocking down PPP5C, i.e., impaired insulin-stimulated formation of both pS473-AKT and pY204-ERK.70 Cell type-specific effects might also have played a role. If confirmed by additional studies, derangements in insulin signaling from either too much or too little PPP5C will imply a need for caution in manipulating this molecule therapeutically.
There are several possibilities for how disturbances in NOX4 function lead to the extensive manifestations of SEIRR that we report here. For example, the two sites on AKT are acted on by different kinases, PDPK1 and mTORC2 (references78, 79 and Figure 1), from which we now infer differential dependence on NOX4, perhaps via PTEN (references50, 52 and Figure 2A). One model would be metabolic channeling of PIP3 from PI3K directly to PDPK1, bypassing PTEN, whereas PIP3 that is destined for mTORC2 might have to contact PTEN beforehand (see Figure 1). Another possibility is that, despite its name, PDPK1 has been reported to be constitutively active,80, 81 and so any process that brings it and its substrate together may be sufficient to produce pT308-AKT.82, 83 In contrast, mTORC2 requires both intrinsic activation and physical contact with substrate.79 A third explanation could be that site-specific underphosphorylation of IRS1,2 in SEIRR causes the recruitment or activation of an unusual set of PI3K isoforms that trigger PDPK1 to act on AKT, but fail to signal to mTORC2 (compare with figure 7 in reference26 and the current Figure 1). Fourth, NOX4, possibly through PTEN and PIP3, might differentially regulate the enzymes that catalyze site-specific dephosphorylations of AKT (see references81, 84). Of note, monophosphorylation of AKT caused by artificial disruption of mTORC2 in non-diabetic animals impairs insulin-stimulated glucose uptake and the formation of pT24-FOXO1, yet still allows insulin, IGF-1, and PDGF to provoke phosphorylations of GSK3β and TSC2.85–89 These prior results add credence to the widely deranged substrate selectivity and other aberrant actions of pT308-AKT that we found in db/db livers and in our hepatocyte models of SEIRR. Robust activation of the ERK pathway in T2DM despite low total tyrosine phosphorylation of the insulin receptor has been a long-standing mystery.1, 2 The phenomenon may indicate reliance on a small but key subset of tyrosyl targets that we speculate would continue to undergo insulin-stimulated phosphorylation despite NOX4 dysfunction, thereby allowing activation of SHC and its downstream targets (Figure 1).
Overall, our findings suggest a unified molecular basis for fatty liver, atherogenic dyslipoproteinemia, hyperglycemia, and hence accelerated atherosclerosis and microvascular disease in T2DM, obesity, and related syndromes. Dysregulation of the NOX4 pathway in other insulin-responsive cell types and organs may further contribute to lipoprotein and glucose abnormalities, hypertension,4, 90–95 hypercoagulability,6, 96–98 destabilization of atheromata through overexpression of matrix metalloproteinases,44 and other vascular and non-vascular complications. Although by no means imminent, either NOX4 or its binding partner may become suitable targets for manipulation in vivo, to correct SEIRR and its devastating sequelae. In the meantime, improvements in lifestyle are likely to remain the best therapeutic approach, whenever they can be achieved.
Supplementary Material
Acknowledgments
Sources of funding
This work was supported by generous start-up funds from the Temple University School of Medicine, Philadelphia, PA, USA; and fortuitously by National Institutes of Health (USA) grants DK43396 and HL94277.
Footnotes
Disclosure
No conflicts of interest to report (XW, KJW).
References
- 1.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 4.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 5.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 7.Williams KJ. Molecular processes that handle – and mishandle – dietary lipids. J Clin Invest. 2008;118:3247–3259. doi: 10.1172/JCI35206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7:369–375. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 10.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am. 2004;33:283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 12.Standards of medical care in diabetes–2010. Diabetes Care. 2010;33 (Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson PM. ACCORD and risk-factor control in type 2 diabetes. N Engl J Med. 2010;362:1628–1630. doi: 10.1056/NEJMe1002498. [DOI] [PubMed] [Google Scholar]
- 14.Borra R, Lautamaki R, Parkkola R, Komu M, Sijens PE, Hallsten K, Bergman J, Iozzo P, Nuutila P. Inverse association between liver fat content and hepatic glucose uptake in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1445–1451. doi: 10.1016/j.metabol.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quinones-Galvan A, Sironi AM, Natali A, Ferrannini E. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50:1807–1812. doi: 10.2337/diabetes.50.8.1807. [DOI] [PubMed] [Google Scholar]
- 16.Boden G. Effects of free fatty acids on gluconeogenesis and glycogenolysis. Life Sci. 2003;72:977–988. doi: 10.1016/s0024-3205(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 17.Adiels M, Olofsson S-O, Taskinen M-R, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 18.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular apoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000;275:8416–8425. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 19.Fisher EA, Williams KJ. Autophagy of an oxidized, aggregated protein beyond the ER: a pathway for remarkably late-stage quality control. Autophagy. 2008;4:721–723. doi: 10.4161/auto.6346. [DOI] [PubMed] [Google Scholar]
- 20.Sparks JD, Dong HH. FoxO1 and hepatic lipid metabolism. Curr Opin Lipidol. 2009;20:217–226. doi: 10.1097/MOL.0b013e32832b3f4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dane-Stewart CA, Watts GF, Barrett PH, Stuckey BG, Mamo JC, Martins IJ, Redgrave TG. Chylomicron remnant metabolism studied with a new breath test in postmenopausal women with and without type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2003;58:415–420. doi: 10.1046/j.1365-2265.2003.01731.x. [DOI] [PubMed] [Google Scholar]
- 22.Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagne C, Couture P. Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J Lipid Res. 2007;48:1336–1342. doi: 10.1194/jlr.M600548-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Liu M-L, Schaffer L, Li M, Boden G, Wu X, Williams KJ. Type 2 diabetes in mice induces hepatic overexpression of sulfatase 2, a novel factor that suppresses uptake of remnant lipoproteins. Hepatology. 2010;52:1957–1967. doi: 10.1002/hep.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams KJ, Chen K. Recent insights into factors affecting remnant lipoprotein uptake. Curr Opin Lipidol. 2010;21:218–228. doi: 10.1097/MOL.0b013e328338cabc. [DOI] [PubMed] [Google Scholar]
- 25.Taskinen MR, Adiels M, Westerbacka J, Söderlund S, Kahri J, Lundbom N, Lundbom J, Hakkarainen A, Olofsson SO, Orho-Melander M, Borén J. Dual metabolic defects are required to produce hypertriglyceridemia in obese subjects. Arterioscler Thromb Vasc Biol. 2011;31:2144–2150. doi: 10.1161/ATVBAHA.111.224808. [DOI] [PubMed] [Google Scholar]
- 26.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 27.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635–646. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante M, Sabatini DM. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3281–3282. doi: 10.1073/pnas.1000323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12 (Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 31.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 32.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. 2004;114:1493–1503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 34.Hughes K, Ramakrishna S, Benjamin WB, Woodgett JR. Identification of multifunctional ATP-citrate lyase kinase as the α-isoform of glycogen synthase kinase-3. Biochem J. 1992;288 (Pt 1):309–314. doi: 10.1042/bj2880309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potapova IA, El-Maghrabi MR, Doronin SV, Benjamin WB. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry. 2000;39:1169–1179. doi: 10.1021/bi992159y. [DOI] [PubMed] [Google Scholar]
- 36.Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, Le Lièpvre X, Berthelier-Lubrano C, Spiegelman B, Kim JB, Ferré P, Foufelle F. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol. 1999;19:3760–3768. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valenti L, Rametta R, Dongiovanni P, Maggioni M, Fracanzani AL, Zappa M, Lattuada E, Roviaro G, Fargion S. Increased expression and activity of the transcription factor FOXO1 in nonalcoholic steatohepatitis. Diabetes. 2008;57:1355–1362. doi: 10.2337/db07-0714. [DOI] [PubMed] [Google Scholar]
- 41.Shao J, Yamashita H, Qiao L, Friedman JE. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J Endocrinol. 2000;167:107–115. doi: 10.1677/joe.0.1670107. [DOI] [PubMed] [Google Scholar]
- 42.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 43.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 44.Boden G, Song W, Pashko L, Kresge K. In vivo effects of insulin and free fatty acids on matrix metalloproteinases in rat aorta. Diabetes. 2008;57:476–483. doi: 10.2337/db07-1261. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee SP, Lane RH, Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- 46.May JM, de Haen C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979;254:2214–2220. [PubMed] [Google Scholar]
- 47.Krieger-Brauer HI, Kather H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J Clin Invest. 1992;89:1006–1013. doi: 10.1172/JCI115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–357. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu X, Williams KJ. The NOX4 pathway as a source of selective insulin resistance and responsiveness. XVII Lipid Meeting Leipzig. 2011 doi: 10.1161/ATVBAHA.111.244525. http://www.lipidmeeting.de/program.php. [DOI] [PMC free article] [PubMed]
- 54.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 55.Gbaguidi GF, Agellon LB. The inhibition of the human cholesterol 7α-hydroxylase gene (CYP7A1) promoter by fibrates in cultured cells is mediated via the liver x receptor α and peroxisome proliferator-activated receptor α heterodimer. Nucleic Acids Res. 2004;32:1113–1121. doi: 10.1093/nar/gkh260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein-B: evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 58.Cross AR, Jones OT. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones OT, Cross AR, Hancock JT, Henderson LM, O’Donnell VB. Inhibitors of NADPH oxidase as guides to its mechanism. Biochem Soc Trans. 1991;19:70–72. doi: 10.1042/bst0190070. [DOI] [PubMed] [Google Scholar]
- 60.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambert AJ, Buckingham JA, Boysen HM, Brand MD. Diphenyleneiodonium acutely inhibits reactive oxygen species production by mitochondrial complex I during reverse, but not forward electron transport. Biochim Biophys Acta. 2008;1777:397–403. doi: 10.1016/j.bbabio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 62.O’Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA. Insulin-regulated gene expression. Biochem Soc Trans. 2001;29:552–558. doi: 10.1042/bst0290552. [DOI] [PubMed] [Google Scholar]
- 63.Cohen AW, Combs TP, Scherer PE, Lisanti MP. Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1151–1160. doi: 10.1152/ajpendo.00324.2003. [DOI] [PubMed] [Google Scholar]
- 64.Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2007;104:1242–1247. doi: 10.1073/pnas.0610523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 66.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, Hardy VE, Joseph JI, Jabbour S, Mahadev K, Zhu L, Goldstein BJ. Protein-tyrosine phosphatase activity in human adipocytes is strongly correlated with insulin-stimulated glucose uptake and is a target of insulin-induced oxidative inhibition. Metabolism. 2003;52:705–712. doi: 10.1016/s0026-0495(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Ernsting BR, Wishart MJ, Lohse DL, Dixon JE. A family of putative tumor suppressors is structurally and functionally conserved in humans and yeast. J Biol Chem. 1997;272:29403–29406. doi: 10.1074/jbc.272.47.29403. [DOI] [PubMed] [Google Scholar]
- 69.Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15:1307–1311. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu X, Li M, Chen K, Liu M-L, Williams KJ. Protein phosphatase 5, a novel member of the insulin signaling cascade, promotes insulin-induced phosphorylation of a highly specific subset of activation sites on ERK1 and Akt. Diabetes. 2010;59 (suppl 1):A13. [Google Scholar]
- 71.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, Ou J, Bashmakov Y, Horton JD, Brown MS, Goldstein JL. Insulin inhibits transcription of IRS-2 gene in rat liver through an insulin response element (IRE) that resembles IREs of other insulin-repressed genes. Proc Natl Acad Sci U S A. 2001;98:3756–3761. doi: 10.1073/pnas.071054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 76.Jiang X, Kenerson H, Aicher L, Miyaoka R, Eary J, Bissler J, Yeung RS. The tuberous sclerosis complex regulates trafficking of glucose transporters and glucose uptake. Am J Pathol. 2008;172:1748–1756. doi: 10.2353/ajpath.2008.070958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santomauro ATMG, Boden G, Silva MER, Rocha DM, Santos RF, Ursich MJM, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 78.Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Colworth Medal Lecture. Biochem Soc Trans. 2001;29:1–14. doi: 10.1042/0300-5127:0290001. [DOI] [PubMed] [Google Scholar]
- 79.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 80.Anderson KE, Coadwell J, Stephens LR, Hawkins PT. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8:684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 81.Freeley M, Park J, Yang KJ, Wange RL, Volkov Y, Kelleher D, Long A. Loss of PTEN expression does not contribute to PDK-1 activity and PKC activation-loop phosphorylation in Jurkat leukaemic T cells. Cell Signal. 2007;19:2444–2457. doi: 10.1016/j.cellsig.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 82.Komander D, Fairservice A, Deak M, Kular GS, Prescott AR, Downes CP, Safrany ST, Alessi DR, van Aalten DM. Structural insights into the regulation of PDK1 by phosphoinositides and inositol phosphates. EMBO J. 2004;23:3918–3928. doi: 10.1038/sj.emboj.7600379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding Z, Liang J, Li J, Lu Y, Ariyaratna V, Lu Z, Davies MA, Westwick JK, Mills GB. Physical association of PDK1 with AKT1 is sufficient for pathway activation independent of membrane localization and phosphatidylinositol 3 kinase. PLoS One. 2010;5:e9910. doi: 10.1371/journal.pone.0009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andjelkovic M, Maira SM, Cron P, Parker PJ, Hemmings BA. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol Cell Biol. 1999;19:5061–5072. doi: 10.1128/mcb.19.7.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 86.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 87.Polak P, Hall MN. mTORC2 Caught in a SINful Akt. Dev Cell. 2006;11:433–434. doi: 10.1016/j.devcel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 88.Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC., Jr Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances basal glycogen synthase activity. Mol Cell Biol. 2008;28:61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar A, Lawrence JC, Jr, Jung DY, Ko HJ, Keller SR, Kim JK, Magnuson MA, Harris TE. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 2010;59:1397–1406. doi: 10.2337/db09-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atchley DW, Loeb RF, Richards DW, Benedict EM, Driscoll ME. On diabetic acidosis: A detailed study of electrolyte balances following the withdrawal and reestablishment of insulin therapy. J Clin Invest. 1933;12:297–326. doi: 10.1172/JCI100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller JH, Bogdonoff MD. Antidiuresis associated with administration of insulin. J Appl Physiol. 1954;6:509–512. doi: 10.1152/jappl.1954.6.8.509. [DOI] [PubMed] [Google Scholar]
- 92.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brands MW, Lee WF, Keen HL, Alonso-Galicia M, Zappe DH, Hall JE. Cardiac output and renal function during insulin hypertension in Sprague-Dawley rats. Am J Physiol. 1996;271:R276–281. doi: 10.1152/ajpregu.1996.271.1.R276. [DOI] [PubMed] [Google Scholar]
- 94.Al-Khalili L, Kotova O, Tsuchida H, Ehrén I, Féraille E, Krook A, Chibalin AV. ERK1/2 mediates insulin stimulation of Na,K-ATPase by phosphorylation of the α-subunit in human skeletal muscle cells. J Biol Chem. 2004;279:25211–25218. doi: 10.1074/jbc.M402152200. [DOI] [PubMed] [Google Scholar]
- 95.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guha M, O’Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Stern D, Mackman N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor α expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- 97.Boden G, Vaidyula VR, Homko C, Cheung P, Rao AK. Circulating tissue factor procoagulant activity and thrombin generation in patients with type 2 diabetes: effects of insulin and glucose. J Clin Endocrinol Metab. 2007;92:4352–4358. doi: 10.1210/jc.2007-0933. [DOI] [PubMed] [Google Scholar]
- 98.Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler Thromb Vasc Biol. 2010;30:1818–1824. doi: 10.1161/ATVBAHA.110.209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


