The Arabidopsis thaliana root cell plasma membrane contains a calcium channel that is activated by oxidizing conditions and operates in cell growth. It was identified here as the most abundant member of the Arabidopsis annexins. These are soluble proteins that can undergo conditional attachment to or insertion into membranes.
Abstract
Plant cell growth and stress signaling require Ca2+ influx through plasma membrane transport proteins that are regulated by reactive oxygen species. In root cell growth, adaptation to salinity stress, and stomatal closure, such proteins operate downstream of the plasma membrane NADPH oxidases that produce extracellular superoxide anion, a reactive oxygen species that is readily converted to extracellular hydrogen peroxide and hydroxyl radicals, OH•. In root cells, extracellular OH• activates a plasma membrane Ca2+-permeable conductance that permits Ca2+ influx. In Arabidopsis thaliana, distribution of this conductance resembles that of annexin1 (ANN1). Annexins are membrane binding proteins that can form Ca2+-permeable conductances in vitro. Here, the Arabidopsis loss-of-function mutant for annexin1 (Atann1) was found to lack the root hair and epidermal OH•-activated Ca2+- and K+-permeable conductance. This manifests in both impaired root cell growth and ability to elevate root cell cytosolic free Ca2+ in response to OH•. An OH•-activated Ca2+ conductance is reconstituted by recombinant ANN1 in planar lipid bilayers. ANN1 therefore presents as a novel Ca2+-permeable transporter providing a molecular link between reactive oxygen species and cytosolic Ca2+ in plants.
INTRODUCTION
Plant cells use cytosolic free Ca2+ ([Ca2+]cyt) in signal transduction and growth, yet the molecular identities of the Ca2+-permeable transporters that permit Ca2+ influx remain largely undiscovered (reviewed in Dodd et al., 2010). Two Pore Channel1 releases vacuolar Ca2+ in Arabidopsis thaliana (Dodd et al., 2010). The Cyclic Nucleotide-Gated Channel (CNGC) family is implicated, with CNGC2 forming a plasma membrane (PM) Ca2+-permeable channel in Arabidopsis for defense and senescence (Dodd et al., 2010; Ma et al., 2010). Arabidopsis loss-of-function mutants implicate glutamate receptor–like channels in stress signaling and pollen tube growth (Qi et al., 2006; Dodd et al., 2010; Michard et al., 2011). As yet, the genetic identities of the PM Ca2+ influx transporters that are regulated by reactive oxygen species (ROS) to elevate [Ca2+]cyt are unknown. In root cell growth, adaptation to salinity stress, and stomatal closure, these operate downstream of the PM NADPH oxidases that produce extracellular superoxide anion, a ROS readily converted to extracellular hydrogen peroxide and hydroxyl radicals, OH• (Demidchik et al., 2003, 2010; Foreman et al., 2003; Kwak et al., 2003; Chung et al., 2008; Dodd et al., 2010; Laohavisit et al., 2010). In plants, extracellular OH• are thought to coordinate growth and stress responses (Foreman et al., 2003; Renew et al., 2005; Müller et al., 2009; Demidchik et al., 2010). The Arabidopsis root epidermal PM contains a Ca2+-permeable conductance (activated by extracellular OH•), operating in cell elongation and implicated in sodicity stress signaling (Demidchik et al., 2003; Foreman et al., 2003; Chung et al., 2008). In elongation, the conductance lies downstream of the Respiratory Burst Oxidase Homolog C (RBOHC) NADPH oxidase (Foreman et al., 2003) that is the initial source of ROS. The conductance resembles that formed by purified maize (Zea mays) annexins incorporated into artificial bilayers containing a lipid peroxidation product (Laohavisit et al., 2010). Hence, an Arabidopsis annexin could be responsible for forming the PM OH•-activated Ca2+ conductance.
Annexins are membrane binding proteins found in pro- and eukaryotes (Morgan et al., 2006). Several animal annexins have been reported to function in vitro as Ca2+ channels, including vertebrate annexins A1, 2, 5 to 7, and 12 (Burger et al., 1994; Liemann et al., 1996; Kourie and Wood, 2000). Loss-of-function mutants may have impaired ability to regulate [Ca2+]cyt, for example, A5 (−/−) chicken DT40 cells (Kubista et al., 1999), A7 (+/−) murine brain cells (Watson et al., 2004), and A7 (−/−) murine cardiomyocytes (Schrickel et al., 2007). Annexin A5 binds to peroxidized membranes (Balasubramanian et al., 2001) and contributes to peroxide-induced Ca2+ influx in chicken DT40 cells (Kubista et al., 1999). However, no studies have causally linked in vitro annexin transport activity to that in native membrane (Konopka-Postupolska et al., 2011). Annexins are abundant throughout the plant kingdom (reviewed in Laohavisit and Davies, 2011a), and some contain the charged residues found in the hydrophobic pore of channel-forming animal annexins (Kourie and Wood, 2000; Laohavisit et al., 2009; Laohavisit and Davies, 2011a). Of these, Capsicum annuum annexin32/24 (ANN32/24) mediates Ca2+ influx in liposomes (Hofmann et al., 2000), while maize annexins ANN33 and ANN35 support a Ca2+-permeable conductance in planar lipid bilayers (PLBs) (Laohavisit et al., 2009, 2010), and Arabidopsis ANN1 forms a K+-permeable channel in PLB (Gorecka et al., 2007). More compelling evidence for annexins as ion transport pathways requires electrophysiological analysis of annexin mutants (Laohavisit et al., 2009; Konopka-Postupolska et al., 2011).
Expression and abundance of Arabidopsis ANN1 matches the occurrence of the OH•-activated PM Ca2+ conductance in the root epidermis and at the apex of root hairs (Clark et al., 2001, 2005; Dinneny et al., 2008). ANN1 transcript abundance decreases by 24% as root epidermal cells mature (Dinneny et al., 2008), and the magnitude of the root epidermal PM OH•-activated conductance decreases by ∼75% (Demidchik et al., 2003). ANN1 (one of eight annexins; Clark et al., 2001) can exist as an integral PM protein (Alexandersson et al., 2004; Lee et al., 1998; Santoni et al., 2004; Benschop et al., 2007; Marmagne et al., 2007). This makes ANN1 a prime candidate for the root epidermal PM OH•-activated conductance. We tested ANN1 function by applying electrophysiological analyses to the homozygous ann1 knockout mutant and the complemented mutant ann1/ANN1 (Lee et al., 2004). Recombinant ANN1 was used to reconstitute an OH•-activated Ca2+-permeable conductance in PLBs. Cytosolic aequorin was deployed to assess the impact of ann1 on OH•-activated [Ca2+]cyt elevation. The results show that ANN1 supports the OH•-activated conductance of the root epidermal PM, contributes to regulation of [Ca2+]cyt, and plays a role in growth.
RESULTS
ann1 Lacks the PM OH•-Activated Conductance
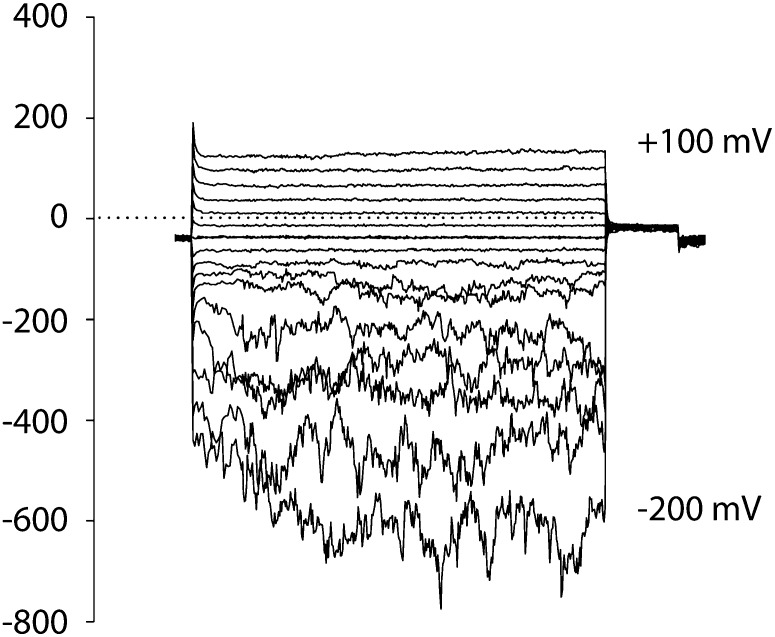
Spheroplasts were released from the apices of young wild-type root hairs and the ann1 homozygous single insert T-DNA knockout mutant (Lee et al., 2004). PM currents were obtained using the patch clamp whole-cell recording mode. In this, currents are generated as populations of channels open; negative current is influx of cations into the cytosol or efflux of anions. Ionic conditions were used previously to delineate OH•-activated currents in the wild type (Demidchik et al., 2003; Foreman et al., 2003). In controls, small inward and outward cation currents were observed at hyperpolarized and depolarized voltages, respectively, in both genotypes (Figures 1A and 1B). Mean values were not statistically significantly different between the wild type and ann1 (see Supplemental Table 1 online; n = 6). Wild-type apical PM supported increased inward and outward cation currents in response to the generation of extracellular OH• by 1 mM CuCl2 and 1 mM ascorbic acid (Cu-Asc; Demidchik et al., 2003) (Figure 1A; see Supplemental Table 1 online; n = 6). The conductance would be competent in Ca2+ influx across the PM at root hair apical voltages (Felle et al., 1992). OH•-activated currents did not occur in ann1 (Figure 1B; see Supplemental Table 1 online; n = 6). Root hair spheroplasts from the complemented ann1/ANN1 line were too fragile for use in patch clamp recordings.
Figure 1.
ann1 Root Plasma Membrane Lacks the OH•-Activated Conductance.
(A) Whole-cell patch clamp recordings from wild-type (WT; Col-0) root hair apical spheroplast PM. Left: Control currents (I) elicited by step voltage (V) changes, from a representative spheroplast. Centre: I after exposure to extracellular OH• generated by 1 mM copper and 1 mM ascorbic acid (Cu-Asc). Holding potential was 0 mV. Right: mean ± se. I-V relationships for control (open circles) and OH• exposure (n = 6). I below the V axis is cation entry into the spheroplast.
(B) As in (A) but for ann1 (n = 6). Spheroplasts from the complemented mutant proved too fragile to patch clamp.
(C) As in (A) but for wild-type root epidermal protoplasts (n = 6).
(D) ann1 root epidermal protoplasts (n = 6).
(E) Complemented mutant ann1/ANN1 (n = 6). Bathing medium (mM): 20 CaCl2, 0.1 KCl, 0.02 NaCl, and 5 MES-Tris, pH 5.6, adjusted to 270 mOsM with d-sorbitol. Pipette solution: 40 K-gluconate, 10 KCl, 0.4 CaCl2, 1 mM BAPTA, and 2 MES-Tris, pH 7.2, adjusted to 270 mOsM with d-sorbitol.
Protoplasts were obtained from mature cells of wild-type and ann1 root epidermis. Control PM currents were not statistically significantly different between the wild type, ann1, and ann1/ANN1 (Figures 1C to 1E; see Supplemental Table 1 online; n = 6). In controls, Cu or Asc alone had no significant effect on currents (see Supplemental Figures 1A to 1D online; n = 3), in agreement with previous studies (Demidchik et al., 2003; Foreman et al., 2003). Rectifying inward and outward currents were evoked when OH• was generated by 1 mM Cu-Asc at the extracellular membrane face of the wild type (Figure 1C; see Supplemental Table 1 online; n = 6). Previously, removal of Ca2+ from the bath or K+ from the pipette abolished the inward current and outward current, respectively (Demidchik et al., 2003). Here, when Ba2+ replaced Ca2+ in the bathing solution, an inward current was still observed in response to OH• (see Supplemental Figure 1E online; n = 3), a response seen previously in root hair and epidermal PM (Demidchik et al., 2003; Foreman et al., 2003). As Ba2+ permeates Ca2+-permeable channels but not K+-inward rectifiers (Véry and Davies, 2000), these data confirm that an inward Ca2+ current was activated. At voltages more negative than the reversal potential for K+ (EK), it is feasible that K+ made a small contribution to the inward current (see Supplemental Figure 1E online). Time dependency was more pronounced than reported previously for this cell type (Demidchik et al., 2003). The conductance would be competent in PM Ca2+ influx at normal root epidermal voltages (Demidchik et al., 2002). Currents were effectively abolished in ann1 (even with increased observation time) but were restored in ann1/ANN1 (Figures 1D and 1E; see Supplemental Table 1 online; n = 6). That ann1/ANN1 currents were not greater than the wild type may indicate that ANN1 abundance or activity at the PM is tightly regulated in this cell type. Wild-type root hair apical PM and the root epidermal PM also contain a constitutive hyperpolarization-activated Ca2+ channel (HACC) that is involved in elongation (Véry and Davies, 2000; Demidchik et al., 2002). ann1 spheroplasts still retained HACC activity, demonstrating that the HACC has a distinct molecular identity (Figure 2; n = 3). HACC-mediated current at −200 mV was approximately double that of the OH•-activated Ca2+ conductance. Accordingly, ann1 root hairs still grew but were significantly shorter than the wild type on either low or replete nutrient medium. Growth was restored in ann1/ANN1 (mean mature length, μm ± sd; low nutrient: wild type 383 ± 114, ann1 249 ± 117 [P = 0.001, Student’s t test], ann1/ANN1 401 ± 129; replete medium: wild type 457 ± 128, ann1 404 ± 124 [P = 0.005], ann1/ANN1 435 ± 99; 100 root hairs per determination). These data are consistent with ANN1’s operation in root hair elongation and the previous observation of short ann1 primary roots (Clark et al., 2005).
Figure 2.
ann1 Root Hair Apical PM Retains the Constitutive HACC Conductance.
Representative currents recorded in the whole-cell patch clamp configuration from an ann1 root hair apical spheroplast PM showing the HACC conductance that is a characteristic of the root hair apex. No OH• were used in this experiment, and Ba2+ replaced Ca2+ in the bathing medium. Current flowing below the time axis is cation entry into the protoplast. Bathing solution comprised 10 mM BaCl2 and 2 mM MES-Tris, pH 6, adjusted to 275 mOsM with d-sorbitol. Pipette solution comprised 0.5 mM CaCl2, 8.5 mM Ca(OH)2, 2 mM MgATP, 0.5 mM Tris-ATP, 10 mM BAPTA (final free Ca2+, 1 μM), and 15 mM HEPES-Tris, pH 7.3, adjusted to 275 mOsM with d-sorbitol.
ann1 Is Impaired in [Ca2+]cyt Elevation
Extracellular OH• activate Ca2+ influx across the PM and cause elevation of [Ca2+]cyt in root epidermis and root hairs (Demidchik et al., 2003; Foreman et al., 2003). As the PM OH•-activated Ca2+-permeable conductance, ANN1 should be capable of elevating [Ca2+]cyt and its loss of function would be expected to manifest as an impaired OH•-induced [Ca2+]cyt increase. To test this, the ann1 mutant was transformed to express cytosolic (apo)aequorin constitutively. When single excised roots were challenged with extracellular OH• (generated by Cu-Asc), a transient increase in [Ca2+]cyt was observed in both genotypes, but there was no significant difference between maximum [Ca2+]cyt of the wild type (1.16 ± se 0.03 µM, n = 3) and mutant (1.16 ± 0.06 µM, n = 3; P = 0.999, Student’s t test). [Ca2+]cyt returned to its basal level (∼0.2 µM) after ∼70 s in both genotypes. However, mathematical simulations suggest that cell-specific changes in [Ca2+]cyt might not manifest at the whole-organ level (Dodd et al., 2006). Therefore, protoplasts were isolated from ann1 root epidermis to test for a lesion in [Ca2+]cyt regulation.
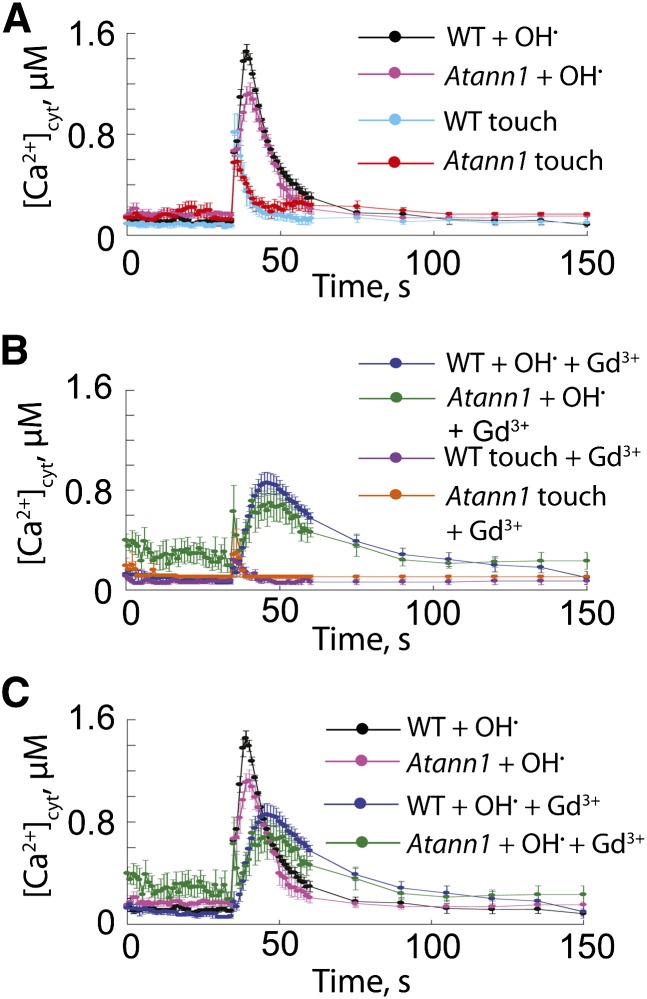
Buffer alone applied to root epidermal protoplasts elicited a transient [Ca2+]cyt increase (touch response) of 0.82 ± se 0.15 µM in the wild type (n = 4) and 0.58 ± 0.11 µM in ann1 (Figure 3A; n = 4). Differences were not statistically different. Basal [Ca2+]cyt was restored 10 s after the touch stimulus. The [Ca2+]cyt increase evoked by extracellular OH• was greater and more prolonged than the touch response in both genotypes. The maximum OH•-induced increase in [Ca2+]cyt occurred after the maximum touch response (Figure 3A): 1.45 ± 0.058 µM in the wild type (at 4 s after stimulus, n = 4) and 1.12 ± 0.084 µM in ann1 (at 5 s after stimulus, n = 4), a statistically significant 23% loss of signal in the mutant (P = 0.018). Basal [Ca2+]cyt was regained more quickly in the mutant than the wild type, and when the area under the curve was calculated (36 to 75 s), [Ca2+]cyt was statistically significantly lower in the mutant, consistent with a lesion in a Ca2+ influx pathway (wild type, 23.85 ± se 0.9 µM; ann1, 17.08 ± 1.51 µM, P = 0.0084). That genotypic differences could be resolved at the population level of a single cell type rather than organ upholds the modeling predictions of Dodd et al. (2006). The lesion in the [Ca2+]cyt response may be limited to the epidermis but does not appear to prevent propagation of the [Ca2+]cyt response at the whole-root level.
Figure 3.
OH•-Activated [Ca2+]cyt Increase in Root Epidermal Protoplasts Is Impaired in ann1.
(A) Root epidermal protoplast [Ca2+]cyt was measured with cytosolic aequorin. Control touch response (buffer only at 35 s) was similar in the wild type (WT) and ann1. OH• was generated by 0.5 mM Cu-Asc. Data are mean ± se (n = 4). Buffer comprised 10 mM CaCl2, 0.1 mM KCl, and 2 mM Tris/MES, pH 5.8, adjusted to 270 mOsM with d-sorbitol.
(B) As in (A) but protoplasts were incubated with 300 μM GdCl3 for 30 min prior to [Ca2+]cyt measurement (n = 3).
(C) Summary plot of experiments from (A) and (B) showing the effect of Gd3+ on OH•-induced [Ca2+]cyt responses of wild-type and ann1 protoplasts.
To test if [Ca2+]cyt increases came from PM Ca2+ influx, Gd3+ (300 µM) was applied prior to the stimulus to block influx channels. In Gd3+ controls, the touch response was 0.24 ± 0.083 µM in the wild type and 0.3 ± 0.91 µM in ann1 (Figure 3B; n = 3), suggesting ANN1 does not participate in the touch response. Aligning time courses (Figure 3B) indicated that the touch response would be completed when the maximum response to OH• occurred. Gd3+ delayed and weakened wild-type maximum response to OH• (0.86 ± 0.085 µM, 11 s after application; n = 3; P = 0.0019), indicating block of PM Ca2+ influx. Wild-type maximum [Ca2+]cyt response to OH• under Gd3+ block was not significantly different to that of ann1 without block (1.12 ± 0.084 µM), suggesting that Gd3+-sensitive influx in the wild type was accounted for by ANN1 (Figure 3C). However, the presence of non-ANN1 transport routes was apparent in the response to OH• of ann1 under Gd3+ block (Figures 3B and 3C). There, the maximum [Ca2+]cyt response was 0.71 ± 0.10 µM (12 s after application; n = 3), which was significantly lower than in ann1 without Gd3+ (1.12 ± 0.084 µM; P = 0.025). While consequences of non-ANN1 transporters are evident in both genotypes, overall, the data confirm ANN1’s acting in PM Ca2+ influx to mediate the [Ca2+]cyt response to OH• in a cell type known to support high levels of ANN1 expression and harbor the OH•-activated PM cation conductance.
ANN1-Mediated K+ Efflux Conductance Is Distinct to That of a Shaker-Like K+ Channel
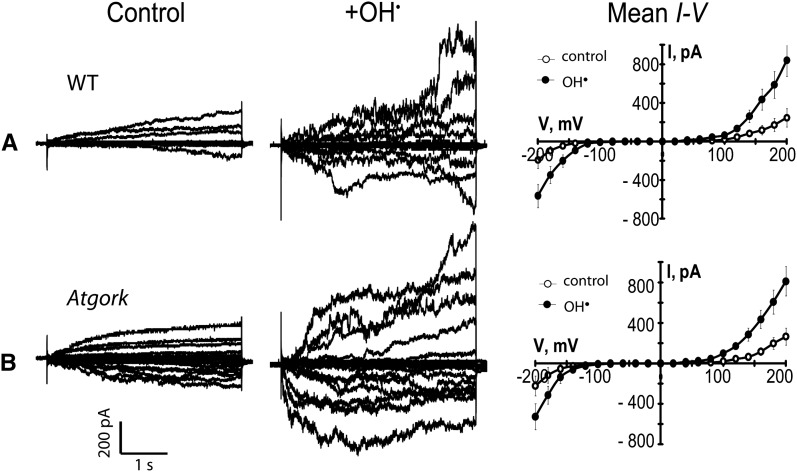
Root epidermal PM contains an OH•-activated Shaker-like K+ efflux channel, GORK (for guard cell outward rectifying K+ channel; Hosy et al., 2003), implicated in stress-induced cell death (Demidchik et al., 2010). Normal OH•-activated currents were observed in gork root epidermal PM, using the same conditions as for ann1 (Figure 4; see Supplemental Table 1 online; n = 6). The OH•-activated K+ efflux conductance seen in the wild type is most probably generated directly by ANN1; therefore, two distinct OH•-activated K+ efflux pathways operate in root epidermis. As gork root growth is normal (Hosy et al., 2003), these data support a specific role for ANN1 in root elongation.
Figure 4.
Atgork Root Epidermal PM Retains the OH•-Activated Conductance.
(A) Whole-cell recordings from wild-type (WT; Wassilewskija) root epidermal protoplasts. Left: Current traces under control conditions, elicited by step changes in voltage from a representative protoplast. Center: the same protoplast after exposure to extracellular OH• generated by 1 mM Cu/Asc. Right: Mean ± se current-voltage (I-V) relationships for control (open circles) conditions and OH• exposure (n = 6). Recording conditions are as in Figure 1.
(B) As in (A) but with data gathered from gork (n = 6).
K+ Efflux Is Impaired in Intact ann1 Cells
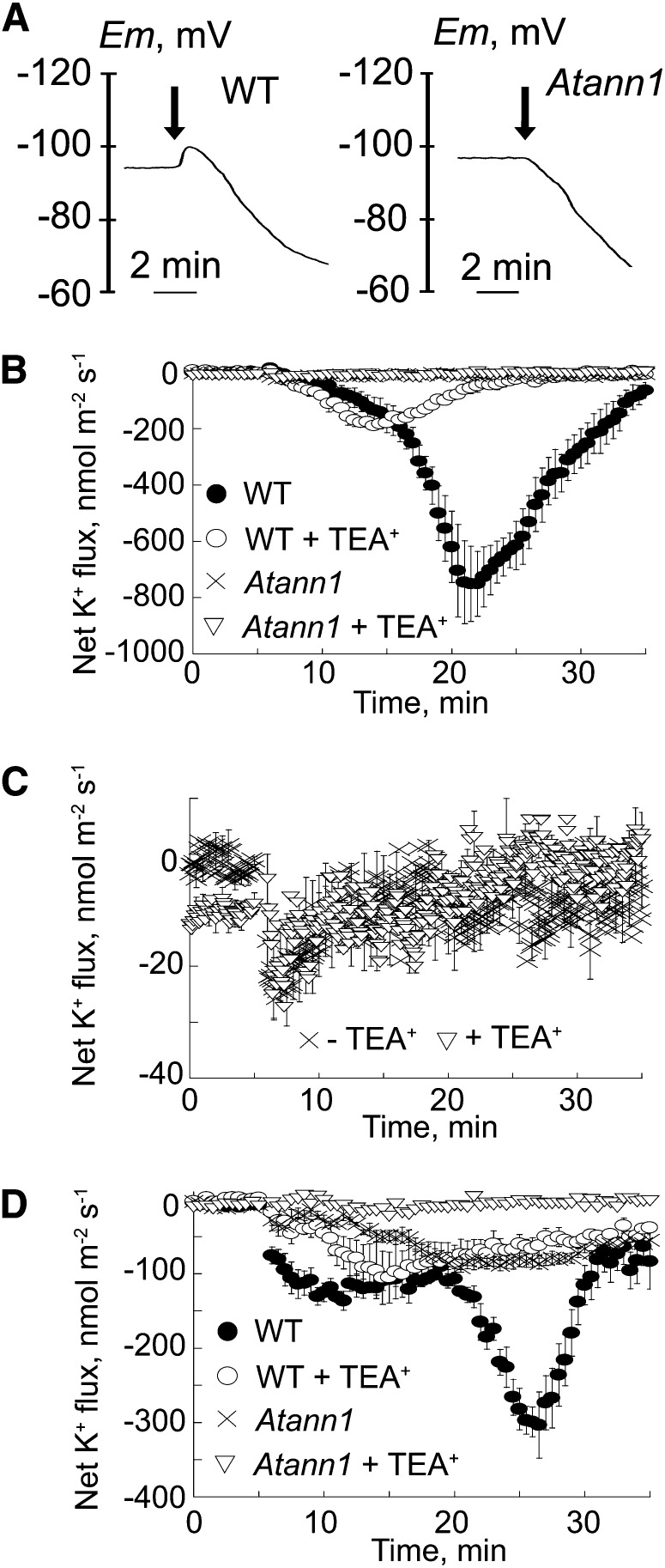
The PM voltage of single, intact root epidermal cells in the elongation zone (measured with an impalement microelectrode) did not differ significantly between genotypes: the wild type (−142 ± se 4 mV; n = 7), ann1 (−141 ± 4 mV; n = 7), and ann1/ANN1 (−134 ± 7 mV; n = 5). Respiratory inhibition with 0.1 mM NaCN and salicyl hydroxamic acid, to inhibit PM H+-ATPase–mediated H+ extrusion and reveal passive transport events (Leidi et al., 2010), evoked rapid membrane depolarization until the diffusion potential (ED) was attained. ED values were comparable between the wild type (−90 ± 4 mV; n = 7) and ann1/ANN1 (−87 ± 4 mV; n = 5) but were more negative in the ann1 mutant (−103 ± 3 mV; n = 7; P = 0.05). Extracellular OH• evoked a small and transient hyperpolarization followed by a depolarization in the wild type (Figure 5A; n = 3) and ann1/ANN1. Hyperpolarization was not observed in ann1 (Figure 5A; n = 3), and no differences were found in ED values 5 min after OH• treatment. Lack of arrest and reversal of the hyperpolarization in ann1 is consistent with ANN1-mediated K+ efflux (EK was estimated to be approximately −156 mV) in response to extracellular OH•.
Figure 5.
ann1 Perturbs Diffusion Potentials and OH•-Activated Net K+ Efflux of the Root Epidermis.
(A) Representative recordings of PM potential from wild-type (WT; Col-0) and ann1 root elongation zone epidermis. Respiratory blockade was imposed by 0.1 mM NaCN-salicyl hydroxamic acid to reveal the diffusion potential (ED). Extracellular OH• were generated by 0.5 mM Cu-Asc addition, as indicated by the arrow, resulting in a hyperpolarization in the wild type (left) but not ann1 (right) followed by a similar depolarization in both lines (n = 3). Lack of arrest and reversal of the hyperpolarization in ann1 is consistent with ANN1-mediated K+ efflux (EK was estimated to be approximately −156 mV) in response to extracellular OH•.
(B) Net K+ efflux recorded from the wild-type (closed circles) and ann1 (crosses) elongation zone epidermis using an extracellular vibrating K+ electrode. Extracellular OH• were generated by 1 mM Cu-Asc addition at time = 0 min. TEA+ was 20 mM (wild type, open circles; Atann1, open triangles). Negative values signify net efflux. Data are mean ± se of five trials.
(C) ann1 elongation zone response from (B) at greater resolution.
(D) As in (B) but values were recorded at the mature epidermis (n = 5). Recordings in the first 60 s after this addition were discarded to allow for establishment of diffusion gradients.
Loss of AtANN1 effectively abolished the net K+ efflux from the root elongation zone in response to extracellular OH• (Figures 5B and 5C; n = 5; no respiratory inhibitors). Maximum net K+ efflux from Atann1 was –26 ± se 7 nmol⋅m−2⋅s−1 (n = 5) compared with the wild type’s –765 ± 149 nmol⋅m−2⋅s−1 (Figure 5B; n = 5). Wild-type K+ efflux was inhibited by 20 mM tetraethylammonium+ (TEA), leaving a residual net flux with a maximum of –198 ± 12 nmol⋅m−2⋅s−1 (n = 5) at 9.4 min after OH• generation. Given that Atann1 did not sustain a K+ efflux response, this incomplete inhibition by TEA+ in the wild type may represent incomplete penetration of this blocker. Complementation restored OH•-induced net K+ efflux to 72% of the wild-type maximum (n = 5) but altered the time course of the response so that the peak flux occurred 6.3 min after the OH• stimulus compared with the wild type at 16.8 min. Reasons for this are unclear at present. At the mature epidermis, kinetics and magnitude of net K+ efflux elicited by OH• from the wild type differed from that at the elongation zone (Figure 5D; n = 5). Maximum efflux was lower in the mature epidermis, a pattern observed previously (Demidchik et al., 2003). TEA+ abolished the maximum component of the wild-type efflux response (wild type, −310 ± 46 nmol⋅m−2⋅s−1 at 21.6 min; TEA+, −55 ± 15 nmol⋅m−2⋅s−1 at 21.6 min, n = 5), lowering it to the response shown by ann1 (−87 ± 8 nmol⋅m−2⋅s−1 at 21.6 min; Figure 5D; n = 5). Thus, the wild type’s maximum component can be attributed to ANN1. Appreciable OH•-induced net K+ efflux remained in ann1, but this was effectively abolished by TEA+ (−6 ± 3 nmol⋅m−2⋅s−1 at 21.6 min; Figure 5D; n = 5).
Purification of Recombinant ANN1
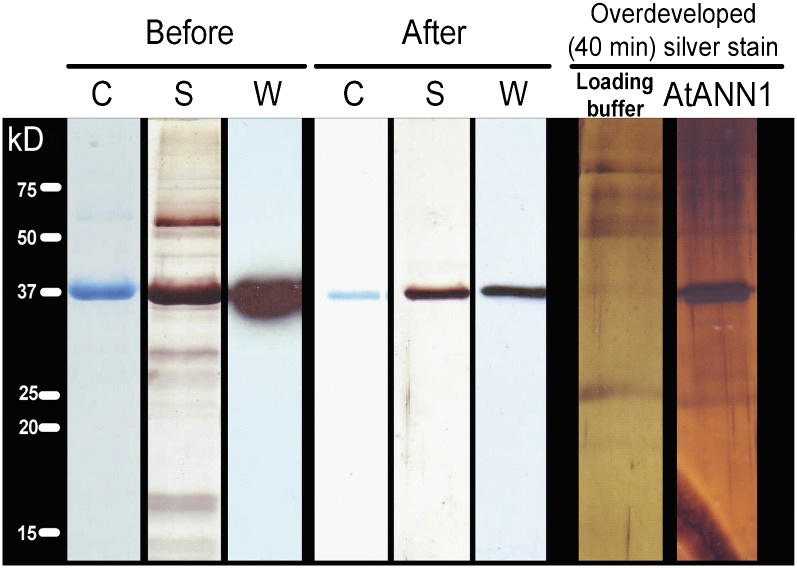
As a final test of the identity of the protein mediating the OH•-activated conductance, recombinant ANN1 was produced for incorporation in PLB. ANN1 was cloned from the wild type (Columbia-0 [Col-0]), and the predicted protein sequence matched that reported in the National Center for Biotechnology Information database for ANN1 (NP 174810.1). ANN1 was expressed in Saccharomyces cerevisiae (a null background for annexins; Creutz et al., 1992), and the protein was obtained by three cycles of Ca2+-dependent binding to asolectin liposomes (Laohavisit et al., 2009). ANN1 (37 kD, matching the predicted mass) was detected in the supernatant by Coomassie blue staining and confirmed by immunoblotting using an anti-ANN1 peptide antibody (Figure 6). Silver staining revealed several contaminant protein bands, including a predominant band at ∼60 kD, which was also found previously (Creutz et al., 1992) using S. cerevisiae to produce recombinant mammalian annexins. Analysis of the 60-kD yeast protein using matrix-assisted laser desorption/ionization (MALDI) peptide mass fingerprinting (searched against the yeast protein database) suggested three possible identities. These were Nuclear Signal Recognition1 (gi: 1323271), FK506-Sensitive Proline Rotamase3 (gi: 530998), and Survival Factor1 (gi: 6320553). ANN1 was not detected in preparations from an empty vector control.
Figure 6.
Purification of Recombinant ANN1.
ANN1 was expressed in S. cerevisiae, and the protein was purified by three cycles of Ca2+-dependent binding to asolectin liposomes (Before) followed by size exclusion chromatography (After). The gel was either colloidal Coomassie blue stained (C), silver stained (S), or used to perform immunoblot analysis (W) to probe for ANN1 using anti-ANN1 peptide antibody. ANN1 is 37 kD. Overdeveloping the silver-stained gel revealed low-level contaminants that were also present in the buffer, including a predominant band at ∼60 kD, which was also found when using S. cerevisiae to produce recombinant mammalian annexins. ANN1 was not detected in preparations from an empty vector control.
Size exclusion chromatography was used to remove contaminants, an approach used previously for maize annexin purification (Laohavisit et al., 2009). The resultant fraction contained only ANN1, as shown by Coomassie blue staining, silver staining for 30 min, and immunoblotting (Figure 6). MALDI fingerprinting and electrospray ionization mass spectroscopy confirmed that the protein was ANN1; peptide fragments yielded 72% coverage. Overdeveloping the silver-stained gel (40 min) revealed very-low-level contaminants that were mostly also present in the buffer (Figure 6). To exclude the possibility that these contaminants were contributing to transport activity in PLB, the highly purified ANN1 preparation was immunoprecipitated for use in control experiments (Laohavisit et al., 2009, 2010).
ANN1 Reconstitutes an OH•-Activated Conductance
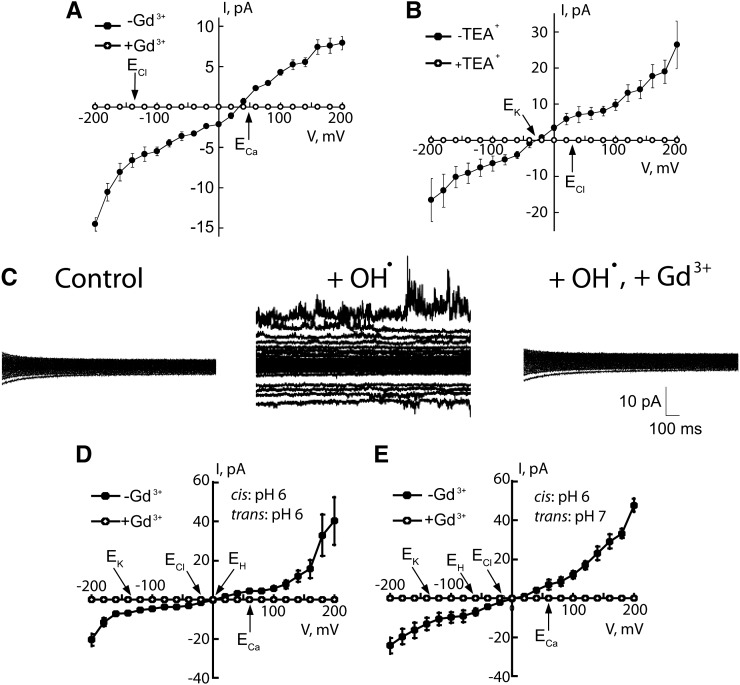
Recombinant ANN1 reconstituted a macroscopic OH•-activated Ca2+-permeable influx conductance in PLB designed as a PM mimetic. These experiments were designed to resemble the patch clamp trials, so negative current is equivalent to cation influx to the cytosol. PLBs comprised 1-palmitoyl 2-oleoyl phosphatidylethanolamine, cholesterol, and 1-palmitoyl 2-oleoyl phosphatidylserine in a 5:3:2 ratio, respectively (Laohavisit et al., 2009). Phosphatidylserine binding is a conserved characteristic of annexin, while cholesterol stabilized the PLBs. With Ca2+ as the major cation, OH• in the trans-chamber (equivalent to patch clamping with OH• at the extracellular PM face) resulted in a cation-permeable conductance (after 35 ± 5 min) in six out of 10 attempts with ANN1 (3 μg) in the cis-chamber (Figure 7A; difference currents, produced by subtracting the control from test, are reported). OH• are membrane-impermeable hence lipid peroxidation most likely promoted conductance formation. No conductance formed without OH• (2-h observation; n = 3). Current was inwardly rectifying at hyperpolarized voltage (−14.5 ± 1 pA at −200 mV, n = 6; Figure 7A), consistent with Ca2+ influx from the trans- to cis-chamber and the inward Ca2+ current across the PM. Mean ± se reversal voltage (Erev) was 33 ± 4 mV (n = 6). The permeability ratio (Véry and Davies, 2000) PCa:PCl was 34. The conductance was completely blocked by 50 μM Gd3+ (trans), confirming formation of a transport pathway by ANN1 (Figure 7A; n = 6). Thus, ANN1 reconstitutes an OH•-activated Ca2+-permeable conductance that is sensitive to a blocker that inhibits OH•-induced PM Ca2+ conductance and [Ca2+]cyt elevation in roots and protoplasts (Demidchik et al., 2003; Foreman et al., 2003).
Figure 7.
OH•-Activated Conductance Is Reconstituted by ANN1 in PLB.
(A) OH•-activated conductance mean ± se. I-V with Ca2+ as the major cation: cis-chamber (mM) 1 Ca2+, pH 6, ANN1 (3 µg); trans 200 Ca2+, 1 Cu-Asc, pH 6. Positive I is positive charge movement from cis to trans. Current was blocked by 50 μM Gd3+ trans (open symbols; n = 3).
(B) OH•-activated conductance I-V with K+ as the charge carrier: cis-chamber (mM) 200 KCl, pH 6, ANN1 (3 µg); trans 50 KCl, 1 Cu-Asc, pH 6.0 (n = 4). Current was blocked by trans 50 mM TEA+ (open symbols; n = 3).
(C) Representative traces from one of six experiments with cis (mM) 200 K+, 1 CaCl2, and trans 1 K+, 200 CaCl2 both pH 6. Left: ANN1 (3 µg) in the cis-chamber; V was changed in 20-mV steps. Middle: OH• (1 Cu-Asc) in trans evoked inward and outward currents. Right: Block by 50 μM Gd3+ trans.
(D) I-V relationships for the OH•-activated conductance of (C), n = 5 and Gd3+ block (open symbols; n = 6). Equilibria are indicated. Expanded current traces are shown in Supplemental Figure 2 online.
(E) OH•-activated conductance I-V with cis, pH 6, trans, pH 7.0, to change EH. All other conditions are as in (C); n = 4 without trans Gd3+; n = 6 with 50 μM Gd3+.
With K+ as the major cation, ANN1 supported an OH•-activated K+ conductance in PLB. A K+-permeable conductance was formed 53 ± 3 min after ANN1 addition (3 μg) under control conditions (four attempts out of six). OH• in the trans-chamber caused transport activity in 26 ± 2 min (six out of eight attempts). Current was completely blocked by application of trans 50 mM TEA+ (Figure 7B; difference currents reported, n = 6), consistent with block of At ANN1–mediated epidermal K+ efflux. A PK:PCl of 53 was estimated for the OH•-activated K+ conductance (Erev = −30 ± 5 mV, n = 6).
With both Ca2+ and K+ gradients present to reflect patch clamp recording conditions and pH 6.0 in both chambers, a rectifying OH•-activated conductance was observed 39 ± 6 min after OH• generation in nine out of 12 attempts (Figures 7C and 7D; difference currents reported). Even at lower voltages (± 20 mV), there was measurable current activation by OH• (see Supplemental Figure 2 online). The Erev was 4 ± 6 mV, close to the equilibrium potential (E) for H+ (EH 0 mV), suggesting that H+ could be permeating rather than Ca2+, K+, or Cl− (ECa +62 mV; EK −137 mV; ECl −15 mV). However, setting EH at −62 mV (with trans pH 7) had no significant effect on Erev (5 ± 5 mV, n = 5; P = 0.92; Figure 7E). Using the PCa:PCl and PK:PCl values obtained for the individual OH•-activated conductance, a PCa:PK of 0.6 was deduced (Véry and Davies, 2000) from the experiments with both K+ and Ca2+ present and symmetrical pH. This is lower than obtained for animal annexins in PLB, but the effects of ROS on animal annexin selectivity are unknown. Gd3+ (50 μM) in the trans-compartment blocked all current generated by ANN1, regardless of the trans pH (Figures 7C to 7E; n = 6). In all control experiments, heat-inactivated preparation was ineffective (n = 3), as was immunoprecipitated ANN1 (see Supplemental Figure 3 online; n = 4). This demonstrates that even in PLB (imperfect mimics of PM), ANN1 forms an OH•-activated Ca2+- and K+-permeable pathway.
DISCUSSION
ANN1 function has been examined here from protein to cellular level, demonstrating its transport activity at the root epidermal PM, ability to perturb both [Ca2+]cyt and K+ efflux, plus its involvement in growth. Even in the highly simplified PLB environment (which did not perfectly mimic the physico-chemical complexity of the PM), ANN1 was capable of reconstituting a rectifying OH•-activated Ca2+- and K+-permeable conductance. This strongly resembled the rectifying OH•-activated Ca2+- and K+-permeable conductance of the wild-type root epidermal and root hair apical PM that was absent in the ann1 mutant but restored by complementation. The longer lag time required for conductance formation in PLB compared with native membrane and cells might reflect the need for ANN1 to be lying in close proximity to the bilayer for OH•-activated transport events to occur or indicate that an interacting protein is required. Differences in ANN1 distribution between the PLB chamber and epidermal protoplast might also underpin the differences in the time dependency of the OH•-activated currents in PLB and patch clamp studies, respectively.
Recombinant ANN1 K+ current was blocked by Gd3+ and TEA+, which also block root epidermal OH•-activated K+ efflux (Cuin and Shabala, 2007). TEA+ brought OH•-activated net K+ efflux from wild-type mature zone root epidermis to values supported by the ann1 mutant, delineating the operation of ANN1 in intact cells. Moreover, OH•-activated net K+ efflux was lost completely from the elongation zone epidermis of ann1. The function of this OH•-activated net K+ efflux is at present unknown, but it could perhaps be involved in regulating membrane voltage. Results now point to two OH•-activated net K+ efflux pathways being present in the root epidermis: ANN1 and the previously characterized Arabidopsis GORK (Demidchik et al., 2010). The ANN1-mediated OH•-activated K+ efflux conductance was recorded in conditions that would suppress the Shaker-like GORK (low external K+), suggesting that external K+ may influence the operation of the two efflux pathways in vivo. For both, it can readily be envisaged that sustained production of OH• could lead to their participation in K+ loss leading to cell death (Demidchik et al., 2010).
Annexins have long been held to be a part of the plant cell’s growth machinery, being abundant at polar growth points and being able to stimulate exocytosis (Carroll et al., 1998; reviewed in Laohavisit and Davies, 2011a; Konopka-Postupolska et al., 2011). The ligon mutant of cotton (Gossypium hirsutum) is impaired in fiber elongation, and proteomic analysis revealed significant downregulation of five annexin isoforms when compared with the wild type, consistent with roles for annexins in growth (Zhao et al., 2010). ANN1’s OH•-activated Ca2+ transport activity in PLB was consistent with a capacity for PM Ca2+ influx that could stimulate exocytosis. Accordingly, the Ca2+ transport lesion in ann1 manifested in a significant impairment in epidermal cell PM-mediated OH•-activated [Ca2+]cyt elevation. ANN1 supported 23% of the peak [Ca2+]cyt response even though it is only one of over 20 possible channels in this cell type (Dinneny et al., 2008). Block of ANN1 by Gd3+ in PLB is consistent with Gd3+ inhibition of the wild type’s OH•-stimulated [Ca2+]cyt elevation in this and our previous study on root hairs (Foreman et al., 2003). Ca2+ influx at the root hair apex and elongation zone of the main root (where ANN1 is localized; Clark et al., 2001, 2005; Dinneny et al., 2008) is involved in growth and can be regulated by extracellular OH• (Foreman et al., 2003). ANN1 activation by OH• at acidic pH is consistent with its contributing to Ca2+ influx in root hair elongation, in which extracellular ROS are sourced by the RBOHC NADPH oxidase (Foreman et al., 2003) and apical cytosolic acidification oscillates (Monshausen et al., 2007). The short root and root hair phenotype of the ann1 mutant can thus be attributed to the absence of a PM OH•-activated Ca2+ influx conductance mediated by ANN1. That root and root hair growth could still advance highlights the importance of other root PM Ca2+ channels, such as the HACC, the molecular identity of which now need to be resolved. Additionally, the question now arises of whether annexins function as Ca2+ influx transporters in other tip-growing cells in which growth is linked to NADPH oxidase activity, such as pollen tubes and fungal hyphae (Cano-Domínguez et al., 2008; Liu et al., 2009).
Ca2+ influx mediated by ANN1 may also help explain the drought and salt sensitivity of the ann1 mutant (Konopka-Postupolska et al., 2009; Huh et al., 2010), particularly as plant annexins have been proposed to act as sensors of stress and stress-evoked ROS (Gorecka et al., 2007; Laohavisit et al., 2010; Laohavisit and Davies, 2011a). In roots of Arabidopsis under salt stress, preventing the formation of or scavenging OH• compromises the stability of the newly made mRNAs encoding for the PM Na+/H+ exporter Salt Overly Sensitive1 (SOS1; Chung et al., 2008). The ultimate source of the salt-induced ROS necessary for SOS1 mRNA stability is the NADPH oxidase RBOHC (Chung et al., 2008). This is also the NADPH oxidase that sources the ROS for the OH•-activated calcium conductance involved in root cell elongation (Foreman et al., 2003), shown here to be mediated by ANN1. Further studies are now required to ascertain whether ANN1 is involved in events leading to stabilizing SOS1 mRNA.
Animal annexins produce complex effects on ion transport across bilayers in vitro (reviewed in Konopka-Postupolska et al., 2011). In this study, ANN1 formed a macroscopic conductance, and single channel events were only rarely observed. Reasons for this are unclear. ANN1 was found previously to form single channel events in nonoxidized asolectin PLBs (Gorecka et al., 2007), and maize annexins ANN33/35 formed a 17 pS Ca2+-permeable channel in PLBs containing malondialdehyde (Laohavisit and Davies, 2011b). Plant Ca2+-permeable cation channel PCa:PK values range from 0.14 for nonselective cation channels (Demidchik and Maathuis, 2007) to orders of magnitude greater for HACCs (e.g., 15 for Arabidopsis root hair HACC (Véry and Davies, 2000). ANN1’s estimated PCa:PK (0.6) is of similar magnitude to the conductance formed by maize ANN33/ANN35 in PLB (0.36; Laohavisit et al., 2009). Such values are lower than determined for animal annexins in PLB, albeit in nonoxidized conditions (e.g., 4.34 for annexin A5; Burger et al., 1994; Liemann et al., 1996) and indicate that residues other than those of the salt bridges may affect transport activity. PLBs and patch clamping are highly reductionist systems, and the permeability of ANN1 in vivo may well differ from values found here. The molecular identities of ROS-regulated Ca2+-permeable channels in plants have remained obscure, despite their manifest significance in growth, adaptation, and defense (Dodd et al., 2010). The Ca2+-permeable Stelar K+ Outward K+ Rectifier PM channel (Gaymard et al., 1998) has been shown to be activated by hydrogen peroxide in a heterologous expression system (Garcia-Mata et al., 2010), but the effects of ROS on its Ca2+ permeation, ability to affect [Ca2+]cyt, and where it lies in relation to NADPH oxidase activity are unknown. Maize ANN33/ANN35 have previously been shown to form a hyperpolarization-activated macroscopic Ca2+ conductance in PLBs containing malondialdehyde (to mimic peroxidized PM; Laohavisit et al., 2010). A greater mechanistic understanding of how annexins form conductance pathways in membranes, particularly membranes subject to modification by ROS, is now required so that their contribution to Ca2+ signaling governed by ROS in development and adaptation (Mittler et al., 2011) can be better understood. With the demonstration that a Ca2+ influx pathway requires ANN1, annexins of crop plants (Laohavisit and Davies, 2011a) can now be addressed confidently as components of growth and signaling, alongside the more conventional CNGCs, glutamate receptor–like channels, and Ca2+-permeable high-affinity K+ transporters as Ca2+ influx routes (Qi et al., 2006; Dodd et al., 2010; Lan et al., 2010; Ma et al., 2010; Michard et al., 2011).
METHODS
Plant Culture
Arabidopsis thaliana Columbia (Col-0) and Wassilewskija were the wild-type parental lines for the ann1 and gork homozygous insertional T-DNA lines, respectively (Hosy et al., 2003; Lee et al., 2004). The 35S-complemented ANN1 mutant was generated and described previously (Lee et al., 2004). For patch clamp, luminometry, and vibrating microelectrode experiments, plants were grown on Murashige and Skoog medium (Duchefa Biochemie) with 1% (w/v) Suc and 0.3% (w/v) Phytagel (Sigma-Aldrich). For impalement recordings and growth determinations, growth was on replete medium comprising (in mM) 5 KNO3, 5 MgSO4⋅7H2O, 2 NaCl, 1 CaCl2⋅2H2O, 1 (NH4)2HPO4, plus 0.1 g⋅1−1 myo-inositol, 1 mg⋅L−1 thiamine, 0.5 mg⋅L−1 nicotinic acid, 0.5 mg⋅L−1 pyridoxine, 25 µM KCl, 25 µM Fe-Na EDTA, 17 µM H3BO3, 10 µM MnSO4⋅H2O, 10 µM ZnSO4⋅7H2O, 2.5 µM CuSO4⋅5H2O, 1% (w/v) Suc, 5 mM MES/Tris, pH 5.7, and 2% (w/v) agar. Nutrient-poor medium was as described previously (Véry and Davies, 2000): 0.1 mM CaCl2, 0.1 mM KCl, pH 5.7, and 2% (w/v) agar. Growth was for 4 to 10 d at 22°C in a 16-h day at 100 μmol⋅m−2⋅s−1 irradiance. Mature root hair lengths were determined at day 4.
Patch Clamp Electrophysiology
The protoplasting protocol was adapted from Demidchik et al. (2003). Spheroplasts were released from young root hair apices using laser ablation (Véry and Davies, 2000). Standard patch clamp procedures were applied (Véry and Davies, 2000). For studies on epidermal protoplasts and the effects of hydroxyl radicals on root hair spheroplasts, bathing solution comprised 20 mM CaCl2, 0.1 mM KCl, 20 μM NaCl, and 5 mM MES-Tris, pH 5.6, adjusted to 270 mOsM with d-sorbitol. The pipette solution comprised 40 mM K-gluconate, 10 mM KCl, 0.4 mM CaCl2, 1 mM bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and 2 mM MES-Tris, pH 7.2, adjusted to 270 mOsM with d-sorbitol. For trials on root hair spheroplast constitutive HACC, bathing solution comprised 10 mM BaCl2 and 2 mM MES-Tris, pH 6, adjusted to 275 mOsM with d-sorbitol. Pipette solution comprised 0.5 mM CaCl2, 8.5 mM Ca(OH)2, 2 mM MgATP, 0.5 mM Tris-ATP, 10 mM BAPTA (final free Ca2+, 1 μM), and 15 mM HEPES-Tris, pH 7.3, adjusted to 275 mOsM with d-sorbitol.
Impalement and Vibrating Microelectrode Recordings
Seedlings (6 to 10 d old) were bathed in (mM) 0.2 KCl, 0.1 CaCl2, 14 MES, and 5 Tris, pH 5.7. Membrane potential was measured in elongation zone epidermis using a 1-mm outer diameter microelectrode. Diffusion potential was achieved by superfusing with 0.1 mM NaCN and 0.1 mM salicyl hydroxamic acid (Leidi et al., 2010). Net fluxes of K+ were measured noninvasively using the MIFE technique (Cuin and Shabala, 2007) in (mM) 0.2 KCl, 0.1 CaCl2, pH 5.6, and ±20 TEA-Cl. Apical root segments (8 to 10 mm) were equilibrated in bathing solution for 50 min. Recordings were made 2 to 3 mm (mature epidermis) or 120 μm (elongation zone) from the apex. To test for OH•-induced changes in net K+ flux, CuCl2 and ascorbic acid were added simultaneously in bathing solution (1 mM final concentration). Recordings up to 60 s after this addition were discarded to allow for establishment of diffusion gradients. Significant differences in these and other trials were assessed with Student’s t tests.
Protoplast [Ca2+]cyt Determination
Col-0 and ann1 were transformed using floral dip with Agrobacterium tumefaciens to express (apo)aequorin under a 35S promoter (Dodd et al., 2006). T3 generations were verified as ann1. Two independently generated lines per genotype yielding total photon counts in excess of 105 in rosette leaf Ca2+ discharge assays were used. Protoplasts were incubated in aequorin buffer (mM: 10 CaCl2, 0.1 KCl, and 2 Tris/MES, pH 5.8, adjusted to 270 mOsM with d-sorbitol) containing 10 μM coelentrazine (Lux Biotechnology) for 4 h in the dark. Protoplasts were washed with osmotically adjusted aequorin buffer without coelentrazine and resuspended in this (70 per μL). Suspension (100 μL) was placed into the well of a white 96-well plate (Greiner Bio-One), transferred to a plate-reading luminometer (FLUOstar OPTIMA; BMG Labtech), and left in the dark for 30 min. After recording baseline luminescence for 30 s, 50 μL of buffer (with or without CuCl2) and 50 μL of buffer (with or without ascorbic acid) were injected (0.5 mM final concentration of CuCl2/ascorbic acid). Luminescence was recorded every second for 30 s and then every 15 s for a further 2085 s. Discharge solution was then injected (final concentration: 10% [v/v] ethanol, 1 M CaCl2), and luminescence was recorded for a further 120 s. Calibration to convert luminescent values to [Ca2+]cyt was performed as described previously (Dodd et al., 2006).
Cloning of ANN1
RNA was isolated from whole seedlings using the RNeasy mini kit (Qiagen) and used to synthesize cDNA using the Superscript III first-strand synthesis system (Invitrogen). cDNA was purified and cleaned using QIAquick (Qiagen). The PCR reaction consisted of 100 ng cDNA, 250 nM primers, 250 μM deoxynucleotide triphosphate, 2 mM MgCl2, 0.625 units of Biotaq DNA polymerase (Bioline), and 17 mM NH4SO4, pH 8.8. The primers were as follows: Ann 1-5-1, 5′-ATGGCGACTCTTAAGGTTTCTGATTC-3′; Ann 1-3-5, 5′-ACTATCATTAAGCATCATCTTCACCGAGAAGTGC-3′. The reaction was heated to 94°C for 2 min, followed by 30 cycles of denaturation (94°C, 20 s), annealing (50°C, 30 s), and extension (72°C, 2 min), followed by a final extension step (72°C, 10 min; iCycler, Bio-Rad). PCR products were analyzed by gel electrophoresis.
Heterologous Expression of ANN1
The pYES 2.1/V5-His Topo yeast expression vector (Invitrogen) was used for the expression of the Arabidopsis ANN1 construct under the control of a Gal-inducible promoter. Fast-growing diploid Saccharomyces cerevisiae strain INVSc1 (genotype Mat a, his 3D1, leu 2, trp1-289, ura3-52; Invitrogen) was transformed with yeast expression vector (pYES 2.1/ V5-His Topo; Invitrogen) containing ANN1. Empty vector was used as a negative control. A single yeast colony was grown in liquid SC-U medium for 20 h at 30°C. Washed cells were resuspended in yeast extract/peptone YP medium to an OD600 of 0.18 and grown at 30°C, until an OD600 of 2 to 3 had been reached (18 h).
Purification of Recombinant ANN1
Cells were resuspended in extraction buffer (10 mM HEPES, 5 mM EGTA, 150 mM NaCl, and 0.01% [v/v] protease inhibitor cocktail [Sigma-Aldrich], pH 7.5) and disrupted by two cycles in an EmulsiFlex C5 homogenizer at 15,000 p.s.i. (Avestin). Debris were removed by centrifugation, the supernatant was incubated with DNase (5 µg mL−1; Promega), RNase A (10 µg mL−1; Roche Applied Science), and MgCl2 (1 mM; 99.5% pure; Fisher Scientific) for 30 min at 180 rpm. Lysate was mixed with asolectin liposomes (200 mg soybean asolectin [Sigma-Aldrich] dissolved in (2:1) chloroform:methanol to give a final concentration of 13.3 mg/mL). CaCl2 was added to a final concentration of 10 mM, and the mixture was incubated for 1 h. After centrifugation (30,000g, 30 min), the lipid pellet was resuspended in filter-sterilized wash buffer (50 mM HEPES, 150 mM NaCl, 10 mM CaCl2, and 1 mM phenylmethanesulphonyl fluoride, pH 7.5). After a repeat centrifugation, the lipid pellet was resuspended in filter-sterilized wash buffer (50 mM HEPES and 10 mM CaCl2, pH 7.5) and centrifuged (30,000 g, 30 min). The lipid pellet was resuspended in filter-sterilized elution buffer (50 mM HEPES and 10 mM EGTA, pH 7.5). After centrifugation (125,000g, 1 h), the final supernatant was mixed with asolectin liposomes, and the purification process was repeated twice. The buffer was exchanged to 100 mM NaCl and 50 mM K-phosphate buffer, pH 7.5, and samples were concentrated using a Vivaspin20 concentrator (molecular mass cutoff 10 kD; VivaScience). All steps were performed at 4°C. Samples were loaded onto a gel filtration column (Superose 12; GE Healthcare; equilibrated with 100 mM NaCl and 50 mM phosphate buffer, pH 7.5) attached to a fast performance liquid chromatography system. ANN1 was eluted at a flow rate of 0.4 mL/min. Fractions (1 mL) were tested for ANN1 by SDS-PAGE and immunoblotting. All proteins were exchanged into buffer (10 mM K-phosphate buffer, pH 7.4) prior to use. In immunoprecipitation studies, the ANN1 preparation was incubated for 30 min at room temperature with anti–At ANN1 peptide antibody (against NRYQDDHGEEIL, residues 204 to 215; Lee et al., 2004) in a 2:1 ratio (Laohavisit et al., 2009). Antibody alone was used as a control.
Gel Electrophoresis, Immunoblotting, and Protein Verification
Samples were subjected to SDS-PAGE as described previously (Laohavisit et al., 2009). In silver staining, gels were fixed overnight to enhance detection, washed twice for 20 min, and then stained for up to 40 min. For immunoblotting, membranes were incubated with primary antibody (1:5000 dilution) for 1 h, at room temperature, with shaking and then after washing incubated for 1 h with secondary antibody (1:5000 peroxidase-linked anti-rabbit IgG; Invitrogen). Proteins were visualized using enhanced chemiluminesence (ECL-plus; GE Healthcare). Mass spectrometry was used to determine protein identity. Samples were prepared for in-gel digestion as described previously (Laohavisit et al., 2009) and analyzed by MALDI peptide mass fingerprinting and electrospray ionization mass spectroscopy. Identifications were based on multiple peptide matches.
PLBs
Lipids (25 mg mL−1; Avanti Polar Lipids) were used to form a bilayer (1-palmitoyl 2-oleoyl phosphatidylethanolamine:cholesterol:1-palmitoyl 2-oleoyl phosphatidylserine) in a 5:3:2 ratio, respectively, across a 200-µm diameter aperture at room temperature (20 to 24°C) (Laohavisit et al., 2009, 2010). In Ca2+-permeability experiments, cis- and trans-chambers contained 0.5 mL 10 mM MES/bis Tris propane (BTP) and 1 mM CaCl2, pH 6.0, and 2 mL 10 mM MES/BTP and 200 mM CaCl2, pH 6.0, respectively. In K+-permeability experiments, the cis-chamber comprised 200 mM KCl and 10 mM MES/BTP, pH 6.0, and the trans comprised 50 mM KCl and 10 mM MES/BTP, pH 6. In combined experiments, cis was 1 mM CaCl2, 200 mM KCl, pH 6; trans was 200 mM CaCl2, 1 mM KCl, pH 6.0 or 7. Annexin (3 µg) protein was added to the cis-chamber. The bilayer was held at −150 mV (cis negative) to aid insertion. Hydroxyl radicals were generated by adding 1 mM Cu-Asc to the trans-chamber. Conductance was monitored for annexin activity for up to 2 h. GdCl3 or TEA-Cl were also added to trans. Results were from at least three separate protein preparations.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank/National Center for Biotechnology Information RefSeq data library under accession number NP174810.1 for ANN1 (At1g35720; gb:AF083913).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Copper or Ascorbate Alone Do Not Elicit Plasma Membrane Currents; Copper and Ascorbate Together Elicit a Barium-Permeable Inward Conductance.
Supplemental Figure 2. Expanded Planar Lipid Bilayer Traces.
Supplemental Figure 3. Immunoprecipitated At ANN1 Does Not Support a Conductance in Planar Lipid Bilayers.
Supplemental Table 1. Root Plasma Membrane Currents.
Supplementary Material
Acknowledgments
We thank Carlos Hotta, Thomas Martin, Anthony Miller, Yingzhen Yang, and Julian Schroeder for advice and Len Packman for peptide analyses. This work was supported by the University of Cambridge, the Biotechnology and Biological Science Research Council, the Royal Society, the Fulbright Foundation, and the Australian Research Council.
AUTHOR CONTRIBUTIONS
All authors contributed to research design and commented on the article. A.L., Z.S., L.R., A.-A.V., T.A.C., J.C.M., N.M., and K.M.C. performed the research and analyzed the data. J.M.D. wrote the article.
Glossary
- [Ca2+]cyt
cytosolic free Ca2+
- PM
plasma membrane
- ROS
reactive oxygen species
- PLB
planar lipid bilayer
- HACC
hyperpolarization-activated Ca2+ channel
- ED
diffusion potential
- Col-0
Columbia-0
- MALDI
matrix-assisted laser desorption/ionization
- Erev
reversal voltage
- BAPTA
bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BTP
bis Tris propane
References
- Alexandersson E., Saalbach G., Larsson C., Kjellbom P. (2004). Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol. 45: 1543–1556 [DOI] [PubMed] [Google Scholar]
- Balasubramanian K., Bevers E.M., Willems G.M., Schroit A.J. (2001). Binding of annexin V to membrane products of lipid peroxidation. Biochemistry 40: 8672–8676 [DOI] [PubMed] [Google Scholar]
- Benschop J.J., Mohammed S., O’Flaherty M., Heck A.J.R., Slijper M., Menke F.L.H. (2007). Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol. Cell. Proteomics 6: 1198–1214 [DOI] [PubMed] [Google Scholar]
- Burger A., Voges D., Demange P., Perez C.R., Huber R., Berendes R. (1994). Structural and electrophysiological analysis of annexin V mutants. Mutagenesis of human annexin V, an in vitro voltage-gated calcium channel, provides information about the structural features of the ion pathway, the voltage sensor and the ion selectivity filter. J. Mol. Biol. 237: 479–499 [DOI] [PubMed] [Google Scholar]
- Cano-Domínguez N., Alvarez-Delfin K., Hansberg W., Aguirre J. (2008). NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot. Cell 7: 1352–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A.D., Moyen C., Van Kesteren P., Tooke F., Battey N.H., Brownlee C. (1998). Ca2+, annexins, and GTP modulate exocytosis from maize root cap protoplasts. Plant Cell 10: 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.S., Zhu J.K., Bressan R.A., Hasegawa P.M., Shi H. (2008). Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 53: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G.B., Cantero-Garcia A., Butterfield T., Dauwalder M., Roux S.J. (2005). Secretion as a key component of gravitropic growth: Implications for annexin involvement in differential growth. Gravit. Space Biol. Bull. 18: 113–114 [PubMed] [Google Scholar]
- Clark G.B., Sessions A., Eastburn D.J., Roux S.J. (2001). Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiol. 126: 1072–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C.E., Kambouris N.G., Snyder S.L., Hamman H.C., Nelson M.R., Liu W., Rock P. (1992). Effects of the expression of mammalian annexins in yeast secretory mutants. J. Cell Sci. 103: 1177–1192 [DOI] [PubMed] [Google Scholar]
- Cuin T.A., Shabala S. (2007). Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 30: 875–885 [DOI] [PubMed] [Google Scholar]
- Demidchik V., Bowen H.C., Maathuis F.J.M., Shabala S.N., Tester M.A., White P.J., Davies J.M. (2002). Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J. 32: 799–808 [DOI] [PubMed] [Google Scholar]
- Demidchik V., Cuin T.A., Svistunenko D., Smith S.J., Miller A.J., Shabala S., Sokolik A., Yurin V. (2010). Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 123: 1468–1479 [DOI] [PubMed] [Google Scholar]
- Demidchik V., Maathuis F.J.M. (2007). Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 175: 387–404 [DOI] [PubMed] [Google Scholar]
- Demidchik V., Shabala S.N., Coutts K.B., Tester M.A., Davies J.M. (2003). Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J. Cell Sci. 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Dinneny J.R., Long T.A., Wang J.Y., Jung J.W., Mace D., Pointer S., Barron C., Brady S.M., Schiefelbein J., Benfey P.N. (2008). Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945 [DOI] [PubMed] [Google Scholar]
- Dodd A.N., Kudla J., Sanders D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Dodd A.N., Kyed Jakobsen M., Baker A.J., Telzerow A., Hou S.W., Laplaze L., Barrot L., Poethig R.S., Haseloff J.P., Webb A.A.R. (2006). Time of day modulates Ca2+ signals in Arabidopsis. Plant J. 48: 962–973 [DOI] [PubMed] [Google Scholar]
- Felle H.H., Tretyn A., Wagner G. (1992). The role of plasma membrane Ca2+-ATPase in Ca2+ homeostasis in Sinapsis alba root hairs. Planta 188: 306–313 [DOI] [PubMed] [Google Scholar]
- Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D., Davies J.M., Dolan L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C., Wang J., Gajdanowicz P., Gonzalez W., Hills A., Donald N., Riedelsberger J., Amtmann A., Dreyer I., Blatt M.R. (2010). A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J. Biol. Chem. 285: 29286–29294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F., Pilot G., Lacombe B., Bouchez D., Bruneau D., Boucherez J., Michaux-Ferrière N., Thibaud J.B., Sentenac H. (1998). Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94: 647–655 [DOI] [PubMed] [Google Scholar]
- Gorecka K.M., Thouverey C., Buchet R., Pikula S. (2007). Potential role of annexin AnnAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli. Plant Cell Physiol. 48: 792–803 [DOI] [PubMed] [Google Scholar]
- Hofmann A., Proust J., Dorowski A., Schantz R., Huber R. (2000). Annexin 24 from Capsicum annuum. X-ray structure and biochemical characterization. J. Biol. Chem. 275: 8072–8082 [DOI] [PubMed] [Google Scholar]
- Hosy E., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh S.M., Noh E.K., Kim H.G., Jeon B.W., Bae K., Hu H.C., Kwak J.M., Park O.K. (2010). Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant Cell Physiol. 51: 1499–1514 [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D., Clark G., Goch G., Debski J., Floras K., Cantero A., Fijolek B., Roux S., Hennig J. (2009). The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol. 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka-Postupolska D., Clark G., Hofmann A. (2011). Structure, function and membrane interactions of plant annexins: An update. Plant Sci. 181: 230–241 [DOI] [PubMed] [Google Scholar]
- Kourie J.I., Wood H.B. (2000). Biophysical and molecular properties of annexin-formed channels. Prog. Biophys. Mol. Biol. 73: 91–134 [DOI] [PubMed] [Google Scholar]
- Kubista H., Hawkins T.E., Patel D.R., Haigler H.T., Moss S.E. (1999). Annexin 5 mediates a peroxide-induced Ca(2+) influx in B cells. Curr. Biol. 9: 1403–1406 [DOI] [PubMed] [Google Scholar]
- Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D.G., Schroeder J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signalling in Arabidopsis. EMBO J. 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W.Z., Wang W., Wang S.M., Li L.G., Buchanan B.B., Lin H.-X., Gao J.-P., Luan S. (2010). A rice high-affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proc. Natl. Acad. Sci. USA 107: 7089–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A., Davies J.M. (2011a). Annexins. New Phytol. 189: 40–53 [DOI] [PubMed] [Google Scholar]
- Laohavisit A., Davies J.M. (2011b) Annexins. In Coding and Decoding of Calcium Signals in Plants, S. Luan, ed (Berlin, Heidelberg: Springer), pp. 111–128
- Laohavisit A., Brown A.T., Cicuta P., Davies J.M. (2010). Annexins: Components of the calcium and reactive oxygen signaling network. Plant Physiol. 152: 1824–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A., Mortimer J.C., Demidchik V., Coxon K.M., Stancombe M.A., Macpherson N., Brownlee C., Hofmann A., Webb A.A.R., Miedema H., Battey N.H., Davies J.M. (2009). Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 21: 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee E.J., Yang E.J., Lee J.E., Park A.R., Song W.H., Park O.K. (2004). Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell 16: 1378–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidi E.O., Barragán V., Rubio L., El-Hamdaoui A., Ruiz M.T., Cubero B., Fernández J.A., Bressan R.A., Hasegawa P.M., Quintero F.J., Pardo J.M. (2010). The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 61: 495–506 [DOI] [PubMed] [Google Scholar]
- Liemann S., Benz J., Burger A., Voges D., Hofmann A., Huber R., Göttig P. (1996). Structural and functional characterisation of the voltage sensor in the ion channel human annexin V. J. Mol. Biol. 258: 555–561 [DOI] [PubMed] [Google Scholar]
- Liu P., Li R.L., Zhang L., Wang Q.L., Niehaus K., Baluska F., Samaj J., Lin J.X. (2009). Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 60: 303–313 [DOI] [PubMed] [Google Scholar]
- Ma W., Smigel A., Walker R.K., Moeder W., Yoshioka K., Berkowitz G.A. (2010). Leaf senescence signaling: The Ca2+-conducting Arabidopsis cyclic nucleotide gated channel2 acts through nitric oxide to repress senescence programming. Plant Physiol. 154: 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmagne A., Ferro M., Meinnel T., Bruley C., Kuhn L., Garin J., Barbier-Brygoo H., Ephritikhine G. (2007). A high content in lipid-modified peripheral proteins and integral receptor kinases features in the Arabidopsis plasma membrane proteome. Mol. Cell. Proteomics 6: 1980–1996 [DOI] [PubMed] [Google Scholar]
- Michard E., Lima P.T., Borges F., Silva A.C., Portes M.T., Carvalho J.E., Gilliham M., Liu L.H., Obermeyer G., Feijó J.A. (2011). Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. (2011). ROS signaling: The new wave? Trends Plant Sci. 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Monshausen G.B., Bibikova T.N., Messerli M.A., Shi C., Gilroy S. (2007). Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 104: 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.O., Martin-Almedina S., Garcia M., Jhoncon-Kooyip J., Fernandez M.P. (2006). Deciphering function and mechanism of calcium-binding proteins from their evolutionary imprints. Biochim. Biophys. Acta 1763: 1238–1249 [DOI] [PubMed] [Google Scholar]
- Müller K., Linkies A., Vreeburg R.A.M., Fry S.C., Krieger-Liszkay A., Leubner-Metzger G. (2009). In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 150: 1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Stephens N.R., Spalding E.P. (2006). Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 142: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renew S., Heyno E., Schopfer P., Liszkay A. (2005). Sensitive detection and localization of hydroxyl radical production in cucumber roots and Arabidopsis seedlings by spin trapping electron paramagnetic resonance spectroscopy. Plant J. 44: 342–347 [DOI] [PubMed] [Google Scholar]
- Santoni V., et al. (1998). Use of a proteome strategy for tagging proteins present at the plasma membrane. Plant J. 16: 633–641 [DOI] [PubMed] [Google Scholar]
- Schrickel J.W., et al. (2007). Enhanced heterogeneity of myocardial conduction and severe cardiac electrical instability in annexin A7-deficient mice. Cardiovasc. Res. 76: 257–268 [DOI] [PubMed] [Google Scholar]
- Véry A.A., Davies J.M. (2000). Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W.D., Srivastava M., Leighton X., Glasman M., Faraday M., Fossam L.H., Pollard H.B., Verma A. (2004). Annexin 7 mobilizes calcium from endoplasmic reticulum stores in brain. Biochim. Biophys. Acta 1742: 151–160 [DOI] [PubMed] [Google Scholar]
- Zhao P.-M., Wang L.-L., Han L.-B., Wang J., Yao Y., Wang H.-Y., Du X.-M., Luo Y.-M., Xia G.-X. (2010). Proteomic identification of differentially expressed proteins in the ligon lintless mutant of upland cotton (Gossypium hirsutum L.). J. Proteome Res. 9: 1076–1087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.