Abstract
Rationale
Bone marrow derived cells to treat myocardial injury improve cardiac function and support beneficial cardiac remodeling. However, survival of stem cells is limited due to low proliferation of transferred cells.
Objective
Demonstrate long-term potential of c-kit+ bone marrow stem cells (BMCs) enhanced with Pim-1 kinase to promote positive cardiac remodeling.
Methods and Results
Lentiviral modification of c-kit+ BMCs to express Pim-1 (BMCeP) increases proliferation and expression of pro-survival proteins relative to BMCs expressing GFP (BMCe). Intramyocardial delivery of BMCeP at time of infarction supports improvements in anterior wall dimensions and prevents left ventricle dilation compared to hearts treated with vehicle alone. Reduction of the akinetic left ventricular wall was observed in BMCeP treated hearts at 4 and 12 weeks after infarction. Early recovery of cardiac function in BMCeP-injected hearts facilitated modest improvements in hemodynamic function up to 12 weeks post infarction between cell treated groups. Persistence of BMCeP is improved relative to BMCe within the infarct together with increased recruitment of endogenous c-kit+ cells. Delivery of BMC populations promotes cellular hypertrophy in the border and infarcted regions coupled with an up regulation of hypertrophic genes. Thus, BMCeP treatment yields improved structural remodeling of infarcted myocardium compared to control BMCs.
Conclusions
Genetic modification of BMCs with Pim-1 may serve as a therapeutic approach to promote recovery of myocardial structure. Future approaches may take advantage of salutary BMC actions in conjunction with other stem cell types to increase efficacy of cellular therapy and improve myocardial performance in the injured myocardium.
Keywords: Pim-1, bone marrow cells, myocardial infarction, myocardial structure, hypertrophy
Introduction
Cardiovascular disease leading to myocardial infarction impacts a large population, with occurrence of acute coronary blockage causing irreversible myocardial damage, morbidity, and mortality. Due to the limited regenerative capacity of the heart after injury1, sustained cardiomyocyte damage and myocyte loss contribute to increases in infarct size, decreased cardiac function and the development of heart failure. In the past decade, cell-based therapeutic treatment to mitigate myocardial infarction (MI) injury has been investigated with a variety of adult stem cells. Bone marrow-derived cells (BMC) have been a popular choice due in part to easy availability, proven safety, and efficacy in the clinical setting. BMC promote repair of infarcted myocardium via mobilization of actively proliferating stem and progenitor cells following cytokine stimulation2. As a result, BMC therapy to mitigate heart failure has been attempted with unfractionated BMCs as well as selected constituent populations derived from bone marrow including mesenchymal stem cells3,4, endothelial progenitor cells5, very small embryonic-like stem cells6,7 and lineage negative c-kit positive hematopoietic stem cells (Lin−/c-kit+)8,9. Heterogeneous BMC preparations and individual subpopulations impart beneficial repair to the damaged myocardium10,11, although consensus as to the optimal cell population(s) to enact BMC-mediated repair remains elusive. Regardless of cell type employed, persistence is relatively poor and the majority of delivered cells are rapidly lost12. Mechanistically, promotion of endogenous repair is thought to involve paracrine dependent action of a secretome13,14 with transdifferentiation playing a very limited role in formation of functional myocardium. Although safety of BMC delivery has been validated in the clinic15–18, efficacy remains disparate among different studies and the salutary effects of BMC transplantation for the heart after injury are modest, falling short of what would be needed to restore normal contractility.
The serine/threonine kinase Pim-1 promotes cell proliferation and survival and regulates aspects of cellular metabolism and transcriptional activity19–21. Enhancement of stem cells to improve regenerative capacity is a proven successful strategy in experimental animal models. Molecular engineering to increase cell survival, proliferation, and augmentation of endogenous repair has been achieved with a number of interventional approaches22–24. Our group recently demonstrated that lentiviral mediated Pim-1 kinase overexpression in cardiac progenitor cells (CPCs) increases survival and promotes efficient differentiation into cardiogenic lineages needed to sustain improvements in myocardial structure and function25. Mechanistically, Pim-1 improves regeneration by enhancing resistance to apoptosis26 and increasing cell cycling27. Thus, expression of Pim-1 ex vivo is a promising approach to advance the application of BMC-based cell therapy in vivo.
Growth factor delivery after myocardial infarction promotes acute reparative responses by activation of endogenous stem cells, protection of cardiomyocytes and induction of protective signaling pathways in rodent models28,29. However, the limited success of growth factor delivery in human clinical trials has turned the focus to stem cell based therapies to perform similar paracrine effects within the myocardium30. Benefits from BMC delivery include improved metabolic activity31, reduced inflammation and fibrosis32 and reactivation of the fetal gene program to sustain live myocardium after myocardial damage33. Genetic modifications of stem cells enhance the therapeutic capabilities of BMCs by modulating transcription, changing paracrine profiles and directing cellular fate24,31. Pim-1 has been shown to promote similar effects on transcription in the heart as well as in distinct cell populations. Pim-1 augments cytokine production via the NF-κB pathway and induces expression of NFATc1, promoting cellular differentiation34. Therefore, it becomes important to investigate the differential effects of Pim-1 overexpression in BMCs to activate survival and hypertrophic pathways in heart tissue to mitigate myocardial damage relative to non-modified BMCs.
Genetic modification through use of lentiviral-based gene delivery systems has been employed in BMC-based studies25,35, yet the application of Pim-1 kinase for modification of a c-kit+ BMC has not been utilized as a modality of stem cell protection after myocardial delivery. Findings presented in this study reveal distinct properties of Pim-1 modified BMCs (BMCeP) to promote myocardial repair following delivery into the infarcted myocardium compared to BMCs modified with GFP alone (BMCe). BMCeP treated hearts display acute salvaging of the myocardium, which prevents scar formation and concomitant reduction in infarct size. Therefore, this study describes the therapeutic advantages of BMCeP to blunt the progression of pathological remodeling that inevitably accelerate the onset of heart failure.
Methods
Bone Marrow Cell Culture and Lentiviral Transduction
C-kit+ cells were isolated from whole bone marrow extracted from young FVB male using magnetic activated cell sorting and plated in full growth media (see online supplement for full details). BMCs were transduced with lentiviruses as previously described for CPCs25 with additional details provided in the online supplement.
Trypan Blue Exclusion Assay, MTT assay and Cell Proliferation assay
Cell numbers were determined by trypan blue staining to exclude non-viable cells and immediately placed in 96-well microplate with 100 μL of full growth media. Additional methods for these assays are provided in the online supplement.
Cell Death Detection
Apoptotic cell death assay using a photometric enzyme-immunoassay (Cell Death Detection ELISAPLUS, Roche Applied Science) for detection of histone associated DNA fragments was performed on BMCs plated in duplicates of 10,000 cells in a 96-well microplate and treated with 10 μM and 20 μM tert-Butyl hydroperoxide solution (Sigma) in 200 μL of full growth media for 16 hours. Measurements and quantitation of the assay are provided in the online supplement.
Myocardial Infarction Surgery, Echocardiography and Hemodynamics
Animal model surgeries, performance of echocardiography, and in vivo hemodynamics were performed as previously described25 with further description in the online supplement.
Statistical Analysis
Statistical analysis was performed using Prism software. Graphical data is represented as the mean ± SEM. Student t-test was used when comparing two experimental groups and one-way Anova followed by a tukey post-hoc test was calculated when more than two groups were being analyzed. Echocardiography assessment was analyzed using repeated measures two-way Anova followed by a Bonferroni post-hoc test. A p-value <0.05 were considered statistically significant.
Animals
All animal experiments were performed in accordance with protocols approved by the SDSU IACUC.
Results
Characterization of c-kit+ BMCs for Pim-1 kinase, phenotypic properties and cytokine expression
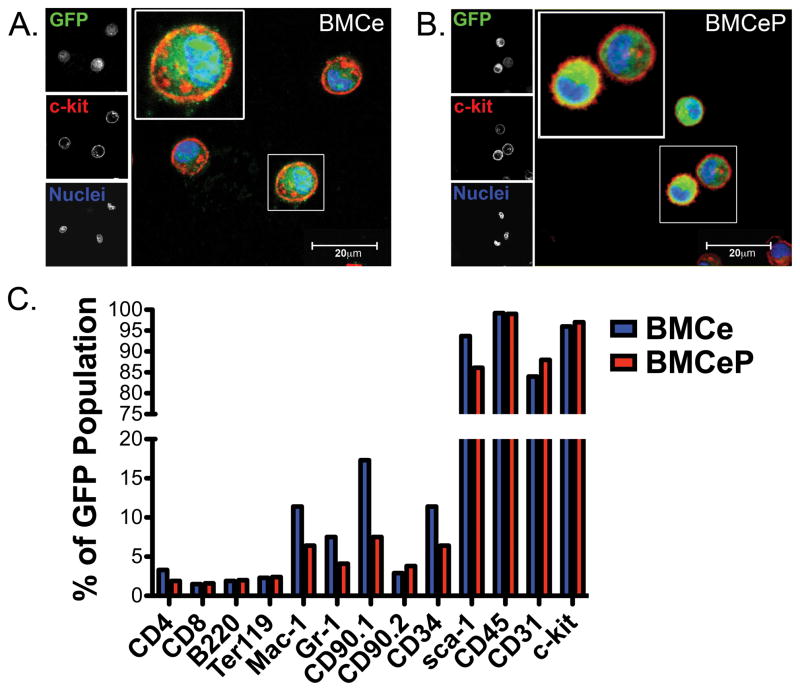
Bone marrow cells (BMCs) were transduced using bicistronic lentiviral constructs (Supplemental Figure IA) and passaged eight times in a 96-well microplate to efficiently integrate transgene(s) and create stable cell lines. BMCs expressing enhanced green fluorescent protein (eGFP) are referred to as BMCe, whereas cells overexpressing human Pim-1 kinase in combination with eGFP are designated as BMCeP. Expression of the exogenous Pim-1 transgene in BMCeP as well as the presence of eGFP in both BMCe and BMCeP populations was confirmed by immunoblot (Supplemental Figure IB). Expression of eGFP and the membrane associated stem cell marker c-kit were verified by flow cytometry (Supplemental Figure IC and ID) and immunohistochemistry (Figure 1A and 1B) in BMCe and BMCeP populations demonstrating that BMCs were effectively modified to express GFP and Pim-1 kinase.
Figure 1. Genetic Engineering of BMCs with Pim-1 kinase presents a unique population of stem cells from the bone marrow.
A. BMCe and B. BMCeP express GFP (green) and c-kit (red) after transduction. C. Percentage of BMCe and BMCeP cells positive for hematopoietic and stem cell markers. Scale bars=20 μm.
C-kit, sca-1, CD45 and CD31 are highly expressed in BMCs after flow cytometric analysis (Figure 1C). Mature hematopoietic markers for T cells, B cells, or erythrocytes were not prominently expressed in BMCe and BMCeP (Figure 1C). Consistent with increased acquisition of the myeloid progenitor marker CD34 in BMCe, the non-enhanced BMC population revealed increased expression of Mac-1, Gr-1 and MSC maker CD90.1 compared to BMCeP (Figure 1C). Collectively, these results indicate Pim-1 over expression in BMCs promotes an enriched stem cell population of the hematopoietic origin that is unadulterated by mesenchymal stem cell populations after long-term culture. Expression profile of cytokines was determined by a mouse specific cytokine and inflammation PCR array in engineered BMCs and CPCs (Supplemental Figures IIA–IIC and Supplemental Results for full description).
Pim-1 increases proliferation and reduces apoptosis in BMCs
BMCeP cell number is significantly increased relative to BMCe at day 5 (p<0.01) and day 7 (p<0.01) in culture (Figure 2A). Furthermore, metabolic activity is increased in BMCeP compared to BMCe at day 3 (p<0.001) and day 6 (p<0.001) (Figure 2B). BMCeP proliferation is enhanced compared to BMCe at day 5 (p<0.01) and day 7 (p<0.001) as confirmed by a direct nuclear stain (Figure 2C). Anti-apoptotic protein Bcl2-α expression is 2.5 fold higher in BMCeP relative to BMCe (p<0.0001) (Figure 2D), consistent with previous results36. Bcl-2α expression is not significantly increased in BMCeP maintained in full growth media (Supplemental Figure IIIA). Therefore, prior to protein analysis, cells were subjected to growth factor withdrawal of cytokines for 72-hours to down-regulate endogenous Pim-1 in BMCe37,38 (Supplemental Figure IIIC). However, we recognize that cytokine independence in vitro does not recapitulate an acute MI, an environment that has the potential to supply cytokines to activate pro-survival signaling in delivered BMCs39,40. BMCeP display reduced apoptosis by measurement of fragmented nucleosomes in the cytoplasm compared to BMCe after treatment with oxidative stress agent hydrogen peroxide H2O2 at 10 μM (p=0.02) and 20 μM (p=0.04) (Figure 2E). However, basal apoptosis in non-treated BMCe and BMCeP was not significantly different (Supplemental Figure IIIB). This data indicates enhanced proliferation and reduction of apoptotic cell death in BMCeP.
Figure 2. Pim-1 increases proliferation and reduces apoptosis in BMCs.
A. Number of viable cells in BMCe and BMCeP assessed by trypan blue exclusion counting. B. Metabolic activity of BMCe and BMCeP. C. Cell proliferation in BMCe and BMCeP relative to day of plating (Day 0). D. Top, Bcl-2α expression in BMCe and BMCeP verified by immunoblot. Bottom, quantitative analysis of relative Bcl-2α expression normalized to GAPDH. E. Fold change of fragmented nucleosomes in the cytoplasm of H2O2 treated BMCe and BMCeP.
Myocardial Structure is improved in BMCeP hearts
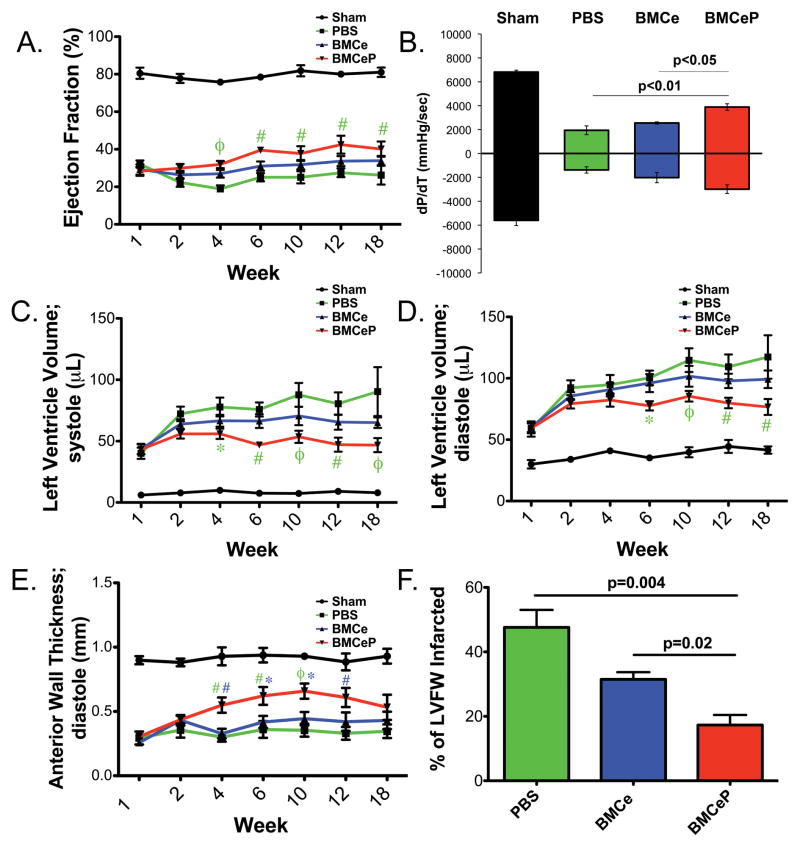
Cardiac structure and performance was assessed by echocardiography up to 18 weeks following injection of BMCs or vehicle into the border zone immediately after left anterior descending artery (LAD) ligation. Decreased ejection fraction (Figure 3A, EF), chamber volume dilation (Figure 3C and 3D; LVV;s and LVV;d), and anterior wall thinning (Figure 3E, Supplemental Figure IVB, and Supplemental Table I) were verified one week post-infarction. However, posterior wall thickness (PW;d) was not affected by LAD ligation or long-term infarction in sham operated and experimental groups (Supplemental Figure IVA). EF is significantly increased in hearts receiving BMCeP (p<0.001) (Figure 3A) compared to PBS treatment at four-weeks post injection. Hemodynamic performance was improved in BMCeP delivered hearts compared to both PBS (p<0.01) and BMCe (p<0.05) (Figure 3B) treated hearts 12 weeks post infarction. Additionally, prevention of left ventricle dilation up to 18 weeks post-surgery (Figure 3C and 3D) compared to PBS-injected groups was observed. Anterior wall thickness was not significantly different from treated heart groups (Supplemental Figure IVB) when measurements were taken directly in the area of the infarct. However, anterior wall (AW) thickness of the neighboring border zone of the infarct was increased in BMCeP delivered hearts compared to PBS (p<0.01) and BMCe (p<0.01) (Figure 3E) at four-weeks post infarction and maintained this effect up to 12 weeks post infarction compared to PBS alone. The relative percentage of akinetic left ventricular wall was decreased in BMCeP treated hearts at four and 12 weeks post infarction compared to PBS (p<0.001) and BMCe (p<0.001) treated groups (Supplemental Figure IVC). These results were corroborated at 12 weeks post infarction as the percentage of the left ventricular free wall was greatly reduced in BMCeP hearts compared to PBS treated groups (p=0.004) and BMCeP relative to BMCe injected hearts at 12 weeks post infarction (p=0.02) (Figure 3F). These results indicate that long term introduction of BMCeP supports modest improvements in EF and hemodynamic performance, increases in AW and enhanced preservation of the myocardium by reducing the formation of scar and infarct size (Values and sample sizes in Supplemental Table I.)
Figure 3. Improvement of myocardial structure after injection of BMCeP.
A. Ejection fraction (EF%) of Sham, PBS, BMCe and BMCeP treated groups. #p<0.01 and φp<0.001 (BMCeP vs. PBS). B. Developed pressure over time (dP/dT;mmHg/sec) at 12 weeks post infarction by in vivo hemodynamics. C. Left ventricle volume during systole (LVV;s, μL). *p<0.05, #p<0.01 and φp<0.001 (BMCeP vs. PBS) and D. during diastole (LVV;d, μL). *p<0.05, #p<0.01 and φp<0.001 (BMCeP vs. PBS). E. Anterior wall thickness (AW;d mm). #p<0.01 and φp<0.001 (BMCeP vs. PBS, green); *p<0.05 and #p<0.01 (BMCeP vs. BMCe, blue). F. Infarct size of the left ventricular free wall (LVFW) in treated heart groups.
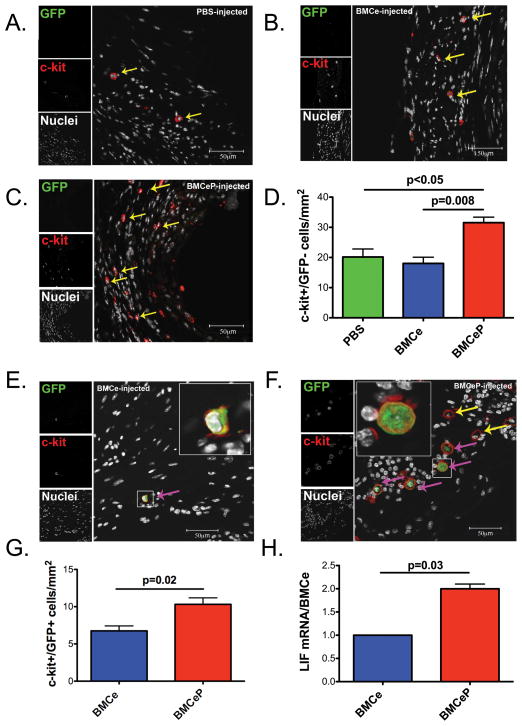
Enhanced persistence and recruitment of endogenous c-kit+ cells in BMCeP hearts
Endogenous c-kit+/GFP− cell recruitment is increased by BMCeP transfer to infarcted hearts relative to PBS injected controls (p<0.05) or BMCe treated hearts (p=0.008) at 12 weeks (Figures 4A#x02013;4D) paralleled by increases in donated BMCeP c-kit+/GFP+ cell number relative to BMCe (p=0.02) (Figures 4E–4G). Additionally at this time point, co-expression of the hematopoietic marker CD45 and GFP was detected in hearts delivered with BMCe and BMCeP (Supplemental Figure VB and VC). Similarly, endogenous and exogenous cell populations were increased in BMCeP delivered hearts compared to infarcted controls at 18 weeks post injection (Supplemental Figure VIA and VIB). Activation and recruitment of endogenous stem cells is mediated by LIF41, which shows a 2-fold induction in mRNA level in BMCeP compared to BMCe (p=0.03) (Figure 4H). In summary, BMCeP treated hearts exhibit the highest level of cellular persistence and recruitment of donated and endogenous populations respectively, which may be due to enhanced paracrine secretion of LIF.
Figure 4. Increased recruitment and persistence of c-kit+ cells in BMCeP injected hearts.
C-kit+ cells (yellow arrows) in A. PBS, B. BMCe and C. BMCeP injected hearts. D. Quantitation of c-kit+/GFP− cells observed in the infarct. E. BMCe and F. BMCeP (pink arrows) transplanted cells. G. Quantitation of c-kit+/GFP+ cells in BMCe and BMCeP injected hearts. Graphs present number of positive cells normalized to the total area of the infarct (mm2), N=3 hearts per group. H. LIF mRNA in BMCe and BMCeP cells in vitro. Values are represented as fold change of relative cycle threshold. 18s mRNA was the internal control. Scale bars=50 μm.
Protective effects of BMCeP as evidenced by increased cellular hypertrophy in the damaged region
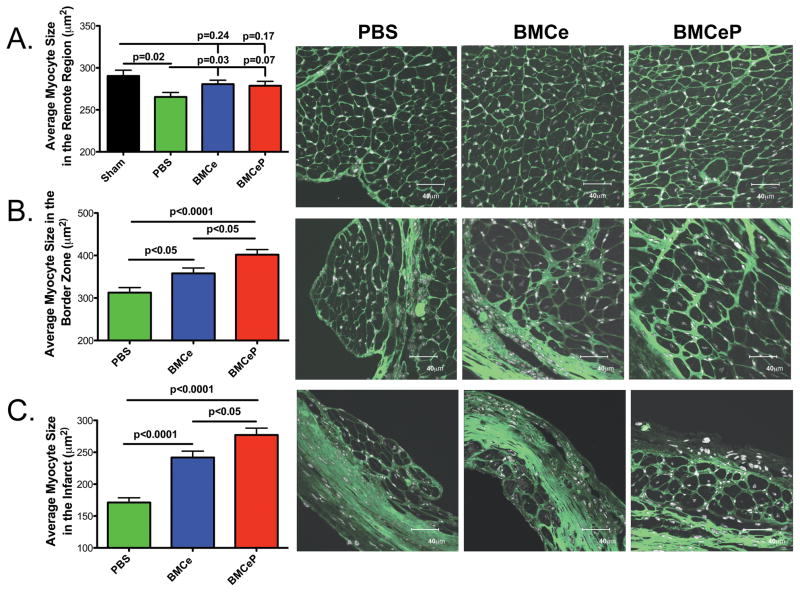
Cross-sectional myocyte size in the border zone (BZ) and infarcted (IZ) regions is increased in BMCeP (BZ p<0.0001, IZ p<0.0001) and BMCe (BZ p<0.05, IZ p<0.0001) injected hearts relative to the PBS group (Figure 5B and 5C). Myocyte hypertrophy in BMCeP injected hearts is significantly greater in the border zone region and the infarcted regions compared to BMCe (BZ p<0.05, IZ p<0.05) (Figure 5B and 5C), but cardiomyocyte size was not affected in BMC injected hearts relative to sham controls in the remote region (Figure 5A; BMCe p=0.24 and BMCeP p=0.17). Decreases in myocyte size in the remote regions of PBS injected hearts are likely a consequence of accelerated progression into heart failure (Figure 5A; Sham p=0.02, BMCe p=0.03 and BMCeP p=0.07). Localized cardiomyocyte hypertrophy resulting from BMCeP treatment is distinct from the protective effects mediated by Pim-1 over expressing CPCs (CPCeP) as treatment revealed increases in small myocytes in the damaged regions consistent with enhanced regenerative response up to 12 weeks without increased cardiomyocyte hypertrophy (Supplemental Figure VII)25. BMCeP treated hearts had increased heart weight to body weight ratios (HW/BW) from sham controls at 12 weeks (p=0.04) (Supplemental Figure VIII and Supplemental Table II). However, heart weight (HW) at 12 and 18 weeks and HW/BW ratios at 18 weeks in infarcted groups were significantly increased from sham animals comparably. In conclusion, increased localized cardiomyocyte size in BMC treated hearts does not significantly alter heart weight relative to PBS treated controls.
Figure 5. Cardiomyocyte size is increased in BMCeP hearts.
A. Average myocyte size in the remote region. B. Average myocyte size in the border zone of infarcted myocardium. C. Average myocyte size in the infarcted region. 150 cells were measured per group. Left, N=3 hearts per group for quantitation. Right, images of PBS, BMCe and BMCeP treated hearts. Myocyte size was visualized by wheat germ agglutinin-FITC (green). Scale bars=40 μm.
Cardiomyocyte hypertrophic signaling is induced by BMC delivery
Compensatory hypertrophy in cardiomyocytes in response to myocardial infarction has been reported in animal models as evidenced by induction of fetal hypertrophic markers33. Peri-nuclear expression of ANP is increased in border zone cardiomyocytes in hearts receiving BMCeP and BMCe whereas ANP expression is reduced in the perinuclear space following PBS treatment (Supplemental Figures IXA–IXC). Hypertrophic marker quantitation shows increased transcript levels of ANP (Supplemental Figure IXD), BNP (Supplemental Figure IXE), β-MHC (Supplemental Figure IXF), and SERCA2 (Supplemental Figure IXG) in BMCeP and BMCe hearts compared to PBS controls. Induction of the hypertrophic gene BNP was significantly different between BMCeP and BMCe (p=0.02) injected groups (Supplemental Figure IXE). Collectively, hypertrophic gene induction resulting from BMCeP treatment is consistent with earlier results showing decreased infarct size (Figure 3), increased myocyte size in the border zone and infarcted regions (Figure 5) and increased levels of LIF transcript (Figure 4), as LIF initiates transcription of early cardiac genes42 and genes to regulate hypertrophy43.
Discussion
Cellular therapy for treatment of acute myocardial infarction is gaining acceptance as a safe and efficacious approach to enhance hemodynamic function from experimental studies8,44 and clinical trials45. Spearheaded by adult stem cells derived from either cardiac or bone marrow sources, the field has begun the laborious task of defining the optimal cell type to promote cellular cardiomyoplasty and the technical refinements required to augment efficacy without compromising safety. However, substantial variations in preparation of cell type, implementation of treatment protocol design, assessments, and resultant outcomes have obscured straightforward conclusions of the biological superiority of bone marrow versus cardiac derived stem cells for treatment of infarction injury46. The stem cell marker c-kit was previously employed by our group as a selection marker to enrich for CPCs with regenerative properties enhanced by genetic engineering with Pim-1 kinase25. Therefore, an identical strategy was implemented to determine whether prior findings with CPC could be reproduced using BMCs. Genetic engineering with Pim-1 kinase remains a valid approach to improve the reparative potential of c-kit+ cells. Fundamentally distinct effects are mediated by BMC as presented in this study when compared to our previous CPC-based results25.
Evidence of hematopoietic cell transdifferentiation into specialized epithelial cells and skeletal myocytes supported interest in the bone marrow as a source of stem cells to repair physiological damage47. Studies of Lin−/c-kit+ BMCs in the heart were additionally reported to adopt cardiogenic fates upon cytokine stimulation and adoptive transfer after MI8, 9. Despite the initial optimism of BMC therapy to directly repair damaged tissue, contradictory findings have called into question the plasticity of hematopoietic cells48. We acknowledge that adequate differentiation of BMCs remains a challenge, and this may be due in part to differences in isolation, expansion and genetic priming of stem cells among research groups. In order to delineate conflicts among existing reports, BMCs were treated ex vivo similar to CPCs, yet they remained undifferentiated in vivo. Prudent characterization of BMCe and BMCeP revealed unique properties within hematopoietic cells that have not been previously reported. Genetically modified BMCs were subjected to long culture conditions, a comparatively rare practice before intramyocardial injection. This protocol allowed us to identify a distinct population influenced by Pim-1 over expression, decreasing the presence of contaminating mature hematopoietic markers and MSCs (Figure 1C). Therefore, our exclusive findings present that long-term introduction of Pim-1 in BMCs defines a novel cell population to enhance long-term structural benefits in the heart (Figure 3).
Regardless of stem cell type employed for adoptive transfer therapy, substantial cell death after transplantation into the infarcted heart is a significant limitation to initiate repair49. Using the same lentiviral-based genetic engineering strategy that successfully improved CPC persistence and engraftment, BMCeP showed increased resistance to oxidative stress and increased proliferation (Figure 2) similar to CPCeP properties25. [Although intrinsic survival and expansion of the BMC population is improved by Pim-1 engineering, the survival of BMCeP in the ischemic myocardium required validation. In vivo persistence of BMCeP (Figure 4G) was increased relative to delivery of BMCe, subsequently confirmed by co-expression of CD45 and GFP (Supplemental Figure VB and VC), indicating that BMCe and BMCeP retain their hematopoietic marker despite long-term viability in the infarct. Increased cellular engraftment appears to be a direct consequence of preserved BMC survival as Pim-1 plays an integral role in mitochondrial integrity, contributing to decreased DNA damage and cellular death in the face of acute inflammation and oxidative stress. These attributes are integral to preserving BMC integrity, however our study is limited to an observation of long-term persistence following infarction, as heart samples were not analyzed during the acute stage post MI.] The increased presence of BMCeP augmented recruitment of endogenous c-kit cells in the infarcted region well after infarction challenge (Figure 4) similar to previous reports28,44,50–53. Prior analysis of CPCeP supported positive left ventricular remodeling without affecting the total number of c-kit+ cells25. This concludes that Pim-1 similarly influences the survival of BMCs and CPCs, yet the fate of CPCs is most consistent with cardiogenic differentiation25 and point to a potential paracrine role in BMCs to enhance myocardial repair.
Cardiac specific expression of Pim-1 antagonizes the adverse remodeling after pathological stress26, 54. Although Pim-1 expression has been observed to negate cardiomyocyte hypertrophic growth54, the role of Pim-1 in non-cardiac derived stem cells for adoptive transfer has not been investigated. Increased survival of delivered BMCeP is influential to determining a connection between paracrine secretion and observed myocardial structural remodeling. Noticeable physiological effects include localized cellular hypertrophy, a BMC-dependent mechanism as we analyzed 12-week CPC-injected hearts and observed no effect on cardiomyocyte hypertrophy in the border zone (Supplemental Figure VII). Similarly in a pressure overload model, hypertrophy was observed after delivery of BMCs from young mice, preventing progression into heart failure33,55. Protein expression of ANP was visualized in the perinuclear space of cardiomyocytes as previously reported56 and increasingly detected in BMCeP and BMCe treated hearts relative to PBS (Supplemental Figure IX). Attenuated myocardial hypertrophy was observed in transgenic mice expressing nuclear targeted Akt in cardiomyocytes due to increased ANP expression, yet BNP was not significantly altered56. Exogenous delivery of natriuretic peptides reduce hypertrophy57 and decrease adverse remodeling after MI58. These studies suggest that ANP does not promote hypertrophy but is expressed during hypertrophic development to inhibit pathological progression56. Although ANP is increased in our BMC treated hearts, cardiomyocyte hypertrophy may be regulated by induction of other hypertrophic genes such as BNP, which is increased significantly in BMCeP hearts (Supplemental Figure IXE).
As demonstrated in this report, 14 cytokine genes were differentially expressed in BMCeP compared to BMCe. Additionally, CPCeP were subjected to the exact array in order to determine contrasting production of cytokines relative to CPCe and BMCeP (Supplemental Figure IIC). For example, IL-6 family member of cytokines, LIF, CT-2 and IL-11 influence cardiomyocyte growth by activation of STAT3 (Supplemental Figure IIA and IIB, and Figure 4H)59 factors that were not increasingly detected in CPCeP. Additionally, BMP proteins (Supplemental Figure IIA and IIB), promote hypertrophy and myocardial remodeling by phosphorylation and activation of SMAD2 after MI60 a potential BMP dependent mechanism of observed cardiomyocyte hypertrophy in BMC treated hearts. Activation of STAT343 has been previously reported as a compensatory hypertrophic molecule in the instance of myocardial damage, however, combined chronic levels of LIF and IL-6 expression contribute to global hypertrophy and heart failure61. In our system, elevated levels of LIF were not detected in our injected heart groups (data not shown), and HW and HW/BW were not significantly altered among infarcted heart groups (Supplemental Figure VIII), suggesting the absence of adverse inflammation and pathological hypertrophy. In fact, we have data to suggest that BMCeP secretes anti-inflammatory factors IL-21 and decreased production of the pro-inflammatory factor IL-1α compared to BMCe (Supplemental Figure IIA and IIB). However, paracrine secretion observed in vitro is only correlative to induce cardiomyocyte hypertrophy after adoptive transfer. In order to explain reduced akinesis (Supplemental Figure IV) and increased AW;d (Figure 3E) as early as four weeks post infarction, it is postulated that pro-hypertrophic factors secreted by BMCs in the border zone and infarcted regions in the acute phase of MI were driving forces of localized cardiomyocyte hypertrophy by activation of SMADs and STAT3 signaling cascades, but can not be confirmed within the long-term time frame of our experiment.
Pim-1 genetic modification of BMCs to improve survival and resistance to apoptosis in the myocardial infarct is a revolutionary concept to treat the acute adverse effects of cardiovascular disease. Direct comparison of BMCeP versus CPCeP after myocardial delivery in the pathological heart reveals unique BMC-dependent mechanisms of myocardial repair. Previously from our group, we demonstrated the enhanced regenerative potential of CPCeP to directly replace cellular components of the damaged myocardium and significantly improve cardiac function relative to non-engineered cells25. Distinct properties of BMC to effect infarct reduction, recruitment of endogenous stem cells and hypertrophy suggest that complementary synergistic actions may result from combined dual stem cell treatment with BMCeP and CPCeP. The new era of cell therapy to treat heart damage may benefit from the new mechanistic insights that Pim-1 modified BMCs have produced and presents a clinical option to effectively improve myocardial structure and prevent heart failure.
Supplementary Material
Novelty and Significance.
What is Known?
The delivery of bone marrow cell (BMCs) after myocardial infarction (MI) confers modest improvements in cardiac function and supports beneficial cardiac remodeling.
The applications of stem cell therapy is hindered by poor survival of transferred cells, limiting beneficial repair.
Bone marrow-derived cells secrete paracrine factors that promote the survival and the differentiation of cardiac cells in the myocardium.
Gene transfer of Pim-1 to cardiac progenitor cells (CPCs) increases cell cycling and asymmetric division in vivo, providing a mechanism of enhanced regeneration after MI.
What New Information Does this Article Contribute?
Pim-1 over-expression in BMCs (BMCeP) supports a unique stem cell population that exhibits enhanced proliferation and protection from oxidative stress.
BMCeP delivery preserves myocardial structure for 12 weeks post infarction injury, contributing to the repair of the anterior wall and prevention of left ventricular dilation.
Transfer of BMCeP cells into the heart contributes to increases in cardiomyocyte size and induction of hypertrophic genes.
Treatment of myocardial damage using adult stem cells from the bone marrow is a safe and efficacious choice validated in experimental models and clinical trials. However, BMC based interventional approaches are limited due to poor survival, proliferation and engraftment of delivered cells. Therefore, we established a genetic modification strategy to enhance BMC properties by overexpressingthe pro-survival molecule Pim-1. BMCeP presented a unique cellular profile by enhancing stem cell phenotypic characteristics, beneficial paracrine secretion and survival and proliferation in vitro. Adoptive transfer of BMCeP during acute MI highlighted the superior effects of these cells in preserving the structural integrity of the myocardium; and reducing the formation of fibrotic scarring as early as 4 weeks post-MI. The BMCeP stimulated an increase in cardiomyocyte size in the border zone and the infarcted regions in order to prevent left ventricular dilation. These results support the therapeutic efficiency of Pim-1 in adult stem cells, and contribute to the progress of future applications of BMCs to prevent heart failure.
Acknowledgments
Sources of Funding
M.A.S was supported by National Institutes of Health grants R21HL102714, R01HL067245, R37HL091102, P01HL085577, RC1HL100891, R21HL102613, R21 HL104544, and R01HL105759. P.Q. is supported by the San Diego ARCS foundation.
We thank all members of the Sussman laboratory for their helpful discussions and technicalsupport. We would also like to thank Brett Hilton and the SDSU Flow Cytometry Core.
Non-Standard Abbreviations and Acronyms
- AW;d
Anterior Wall thickness; diastole
- BMC
Bone marrow cells isolated based on c-kit expression
- BMCe
BMC expressing enhanced green fluorescent protein
- BMCeP
BMC expressing eGFP and Pim-1
- CPC
Cardiac Progenitor Cell
- CPCeP
CPC expressing eGFP and Pim-1
- LVV
Left Ventricular Volume
- PW;d
Posterior Wall thickness; diastole
Footnotes
Disclosures
None.
References
- 1.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 6.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuba-Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, Kucia M, Abdel-Latif A, Tang XL, Ratajczak MZ, Dawn B, Bolli R. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med. 15:1319–1328. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 10.Mazo M, Gavira JJ, Abizanda G, Moreno C, Ecay M, Soriano M, Aranda P, Collantes M, Alegria E, Merino J, Penuelas I, Garcia Verdugo JM, Pelacho B, Prosper F. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 19:313–328. doi: 10.3727/096368909X480323. [DOI] [PubMed] [Google Scholar]
- 11.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 12.Dow J, Simkhovich BZ, Kedes L, Kloner RA. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67:301–307. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbots L, D’Hooge J, Eroglu E, Thijs D, Ganame J, Claus P, Dubois C, Theunissen K, Bogaert J, Dens J, Kalantzi M, Dymarkowski S, Bijnens B, Belmans A, Boogaerts M, Sutherland G, Van de Werf F, Rademakers F, Janssens S. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J. 2009;30:662–670. doi: 10.1093/eurheartj/ehn532. [DOI] [PubMed] [Google Scholar]
- 16.Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, Assmus B, Dimmeler S, Zeiher AM, Hamm C. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–547. doi: 10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 18.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 161:487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Beharry Z, Mahajan S, Zemskova M, Lin YW, Tholanikunnel BG, Xia Z, Smith CD, Kraft AS. The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci U S A. 108:528–533. doi: 10.1073/pnas.1013214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta. 2002;1593:45–55. doi: 10.1016/s0167-4889(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27:4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 23.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 24.Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3beta-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 108:478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 27.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, Sussman MA. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao T, Zhang D, Millard RW, Ashraf M, Wang Y. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1alpha delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol. 2009;296:H976–986. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torella D, Ellison GM, Karakikes I, Nadal-Ginard B. Growth-factor-mediated cardiac stem cell activation in myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2007;4:S46–51. doi: 10.1038/ncpcardio0772. [DOI] [PubMed] [Google Scholar]
- 30.Shim W, Mehta A, Lim SY, Zhang G, Lim CH, Chua T, Wong P. G-CSF for stem cell therapy in acute myocardial infarction: friend or foe? Cardiovasc Res. 89:20–30. doi: 10.1093/cvr/cvq301. [DOI] [PubMed] [Google Scholar]
- 31.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–979. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldenberg RC, Jelicks LA, Fortes FS, Weiss LM, Rocha LL, Zhao D, Carvalho AC, Spray DC, Tanowitz HB. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J Infect Dis. 2008;197:544–547. doi: 10.1086/526793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sopko NA, Turturice BA, Becker ME, Brown CR, Dong F, Popovic ZB, Penn MS. Bone marrow support of the heart in pressure overload is lost with aging. PLoS One. 5:e15187. doi: 10.1371/journal.pone.0015187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Kim JH, Youn BU, Jin HM, Kim N. Pim-1 regulates RANKL-induced osteoclastogenesis via NF-kappaB activation and NFATc1 induction. J Immunol. 185:7460–7466. doi: 10.4049/jimmunol.1000885. [DOI] [PubMed] [Google Scholar]
- 35.Radyukhina NV, Rutkevich PN, Aref’yeva TI, Gurskaya T, Rybalkin IN, Shevelyov AY, Slinkin MA, Vlasik TN, Il’yinskaya OP, Tararak EM. Lentivirus transduction of bone marrow hemopoietic precursor cells with Lin-c-kit+ phenotype ex vivo using a genetic construct containing green fluorescent protein gene. Bull Exp Biol Med. 2007;143:723–726. doi: 10.1007/s10517-007-0224-6. [DOI] [PubMed] [Google Scholar]
- 36.Kim KT, Levis M, Small D. Constitutively activated FLT3 phosphorylates BAD partially through pim-1. Br J Haematol. 2006;134:500–509. doi: 10.1111/j.1365-2141.2006.06225.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim KT, Baird K, Ahn JY, Meltzer P, Lilly M, Levis M, Small D. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood. 2005;105:1759–1767. doi: 10.1182/blood-2004-05-2006. [DOI] [PubMed] [Google Scholar]
- 38.Pircher TJ, Zhao S, Geiger JN, Joneja B, Wojchowski DM. Pim-1 kinase protects hematopoietic FDC cells from genotoxin-induced death. Oncogene. 2000;19:3684–3692. doi: 10.1038/sj.onc.1203684. [DOI] [PubMed] [Google Scholar]
- 39.Aoyama T, Takimoto Y, Pennica D, Inoue R, Shinoda E, Hattori R, Yui Y, Sasayama S. Augmented expression of cardiotrophin-1 and its receptor component, gp130, in both left and right ventricles after myocardial infarction in the rat. J Mol Cell Cardiol. 2000;32:1821–1830. doi: 10.1006/jmcc.2000.1218. [DOI] [PubMed] [Google Scholar]
- 40.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 41.Mohri T, Fujio Y, Maeda M, Ito T, Iwakura T, Oshima Y, Uozumi Y, Segawa M, Yamamoto H, Kishimoto T, Azuma J. Leukemia inhibitory factor induces endothelial differentiation in cardiac stem cells. J Biol Chem. 2006;281:6442–6447. doi: 10.1074/jbc.M508969200. [DOI] [PubMed] [Google Scholar]
- 42.Snyder M, Huang XY, Zhang JJ. Stat3 directly controls the expression of Tbx5, Nkx2.5, and GATA4 and is essential for cardiomyocyte differentiation of P19CL6 cells. J Biol Chem. 285:23639–23646. doi: 10.1074/jbc.M110.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haghikia A, Stapel B, Hoch M, Hilfiker-Kleiner D. STAT3 and cardiac remodeling. Heart Fail Rev. 16:35–47. doi: 10.1007/s10741-010-9170-x. [DOI] [PubMed] [Google Scholar]
- 44.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erbs S, Linke A, Schachinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, Dimmeler S, Tonn T, Hambrecht R, Zeiher AM, Schuler G. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 46.Gambini E, Pompilio G, Biondi A, Alamanni F, Capogrossi MC, Agrifoglio M, Pesce M. C-kit+ cardiac progenitors exhibit mesenchymal markers and preferential cardiovascular commitment. Cardiovasc Res. 89:362–373. doi: 10.1093/cvr/cvq292. [DOI] [PubMed] [Google Scholar]
- 47.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 48.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 49.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Hiroi Y, Ngoy S, Okamoto R, Noma K, Wang CY, Wang HW, Zhou Q, Radtke F, Liao R, Liao JK. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 123:866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ii M, Horii M, Yokoyama A, Shoji T, Mifune Y, Kawamoto A, Asahi M, Asahara T. Synergistic effect of adipose-derived stem cell therapy and bone marrow progenitor recruitment in ischemic heart. Lab Invest. 91:539–552. doi: 10.1038/labinvest.2010.191. [DOI] [PubMed] [Google Scholar]
- 52.Lee BC, Hsu HC, Tseng WY, Chen CY, Lin HJ, Ho YL, Su MJ, Chen MF. Cell therapy generates a favourable chemokine gradient for stem cell recruitment into the infarcted heart in rabbits. Eur J Heart Fail. 2009;11:238–245. doi: 10.1093/eurjhf/hfn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai VK, Afzal MR, Ashraf M, Jiang S, Haider HK. Non-hypoxic stabilization of HIF-Ialpha during coordinated interaction between Akt and angiopoietin-1 enhances endothelial commitment of bone marrow stem cells. J Mol Med (Berl) doi: 10.1007/s00109-011-0852-1. [DOI] [PubMed] [Google Scholar]
- 54.Muraski JA, Fischer KM, Wu W, Cottage CT, Quijada P, Mason M, Din S, Gude N, Alvarez R, Jr, Rota M, Kajstura J, Wang Z, Schaefer E, Chen X, MacDonnel S, Magnuson N, Houser SR, Anversa P, Sussman MA. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci U S A. 2008;105:13889–13894. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Endo J, Sano M, Fujita J, Hayashida K, Yuasa S, Aoyama N, Takehara Y, Kato O, Makino S, Ogawa S, Fukuda K. Bone marrow derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation. 2007;116:1176–1184. doi: 10.1161/CIRCULATIONAHA.106.650903. [DOI] [PubMed] [Google Scholar]
- 56.Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, Sussman MA. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horikawa YT, Panneerselvam M, Kawaraguchi Y, Tsutsumi YM, Ali SS, Balijepalli RC, Murray F, Head BP, Niesman IR, Rieg T, Vallon V, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J Am Coll Cardiol. 57:2273–2283. doi: 10.1016/j.jacc.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuga H, Ogawa K, Oida A, Taguchi I, Nakatsugawa M, Hoshi T, Sugimura H, Abe S, Kaneko N. Administration of atrial natriuretic peptide attenuates reperfusion phenomena and preserves left ventricular regional wall motion after direct coronary angioplasty for acute myocardial infarction. Circ J. 2003;67:443–448. doi: 10.1253/circj.67.443. [DOI] [PubMed] [Google Scholar]
- 59.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 60.Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999;31:667–678. doi: 10.1006/jmcc.1998.0902. [DOI] [PubMed] [Google Scholar]
- 61.Kurdi M, Randon J, Cerutti C, Bricca G. Increased expression of IL-6 and LIF in the hypertrophied left ventricle of TGR(mRen2)27 and SHR rats. Mol Cell Biochem. 2005;269:95–101. doi: 10.1007/s11010-005-3085-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.