Abstract
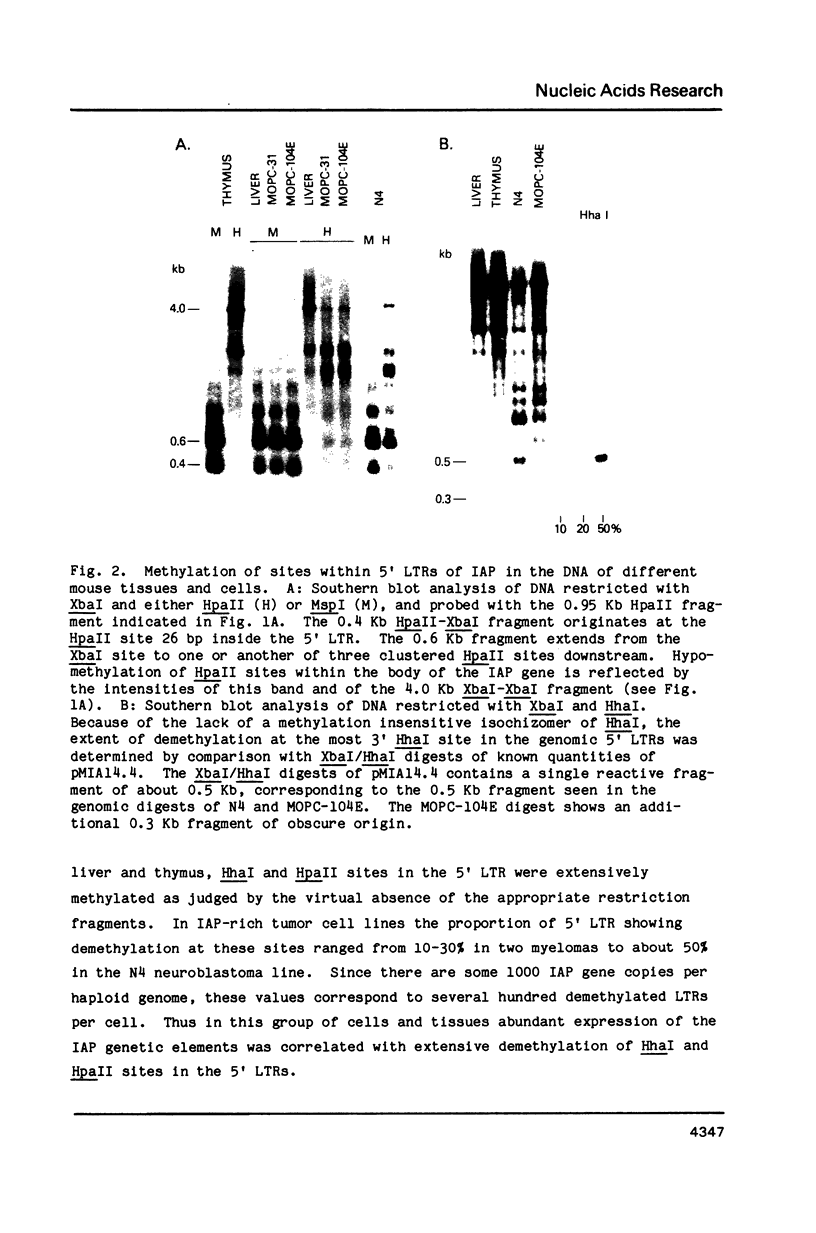
We studied the relation between LTR methylation and expression of the family of endogenous retrovirus-like elements related to mouse intracisternal A-particles (IAP). Comparative HpaII/MspI and HhaI restriction analysis of genomic DNA's showed that in cells and tissues with a low level of IAP gene expression, HpaII and HhaI sites within the 5' LTR were heavily methylated, while in cells abundantly expressing IAP's 20 to 30% of the 5' LTRs were demethylated at these sites. The effects of methylation on the promoter activity of a cloned IAP 5' LTR was studied directly, using the plasmid pMIA5' L-cat in which this LTR was linked to the chloramphenicol acetyl transferase (CAT) gene. In vitro methylation of three HhaI sites located between -137 and -205 bp from the RNA start site of this LTR completely inactivated the promoter activity of pMIA5' L-cat transfected into COS7 cells. Methylation of a HpaII site located 94 bp downstream from the RNA start site reduced the promoter activity by 75%. The results show that methylation at sites both upstream and downstream from the RNA start site profoundly effects the promoter activity of this LTR and suggest that methylation within the 5' LTR can serve to regulate IAP gene expression in vivo.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensimhon M., Gabarro-Arpa J., Ehrlich R., Reiss C. Physical characteristics in eucaryotic promoters. Nucleic Acids Res. 1983 Jul 11;11(13):4521–4540. doi: 10.1093/nar/11.13.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation--how important in gene control? Nature. 1984 Feb 9;307(5951):503–504. doi: 10.1038/307503a0. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Reith A. D., Brammar W. J. A retroviral provirus closely associated with the Ren-2 gene of DBA/2 mice. Nucleic Acids Res. 1984 Nov 26;12(22):8579–8593. doi: 10.1093/nar/12.22.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couez D., Deschamps J., Kettmann R., Stephens R. M., Gilden R. V., Burny A. Nucleotide sequence analysis of the long terminal repeat of integrated bovine leukemia provirus DNA and of adjacent viral and host sequences. J Virol. 1984 Feb;49(2):615–620. doi: 10.1128/jvi.49.2.615-620.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Caradonna S. J., Casey J. W. Bovine leukemia virus long terminal repeat: a cell type-specific promoter. Science. 1985 Jan 18;227(4684):317–320. doi: 10.1126/science.2981431. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Hozumi N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol Cell Biol. 1984 Dec;4(12):2565–2572. doi: 10.1128/mcb.4.12.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman-Montes de Oca F., Dianoux L., Peries J., Emanoil-Ravicovitch R. Intracisternal A particles: RNA expression and DNA methylation in murine teratocarcinoma cell lines. J Virol. 1983 Apr;46(1):307–310. doi: 10.1128/jvi.46.1.307-310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman-Montes de Oca F., Lasneret J., Dianoux L., Canivet M., Ravicovitch-Ravier R., Périès J. Regulation of intracisternal A particles in mouse teratocarcinoma cells: involvement of DNA methylation in transcriptional control. Biol Cell. 1984;52(3):199–204. doi: 10.1111/j.1768-322x.1985.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Luria S., Rechavi G., Givol D. Mechanism of activation of the mouse c-mos oncogene by the LTR of an intracisternal A-particle gene. EMBO J. 1984 Dec 1;3(12):2937–2941. doi: 10.1002/j.1460-2075.1984.tb02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D. Methylation, expression and chromosomal position of genes in mammals. Biochim Biophys Acta. 1984 May 15;782(1):1–9. doi: 10.1016/0167-4781(84)90099-x. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Activation of the SV40 late promoter: direct effects of T antigen in the absence of viral DNA replication. Cell. 1984 Feb;36(2):381–389. doi: 10.1016/0092-8674(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Kruczek I., Doerfler W. Expression of the chloramphenicol acetyltransferase gene in mammalian cells under the control of adenovirus type 12 promoters: effect of promoter methylation on gene expression. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7586–7590. doi: 10.1073/pnas.80.24.7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasneret J., Canivet M., Hojman-Montes de Oca F., Tobaly J., Emanoil-Ravicovitch R., Peries J. Activation of intracisternal a particles by 5-azacytidine in mouse Ki-BALB cell line. Virology. 1983 Jul 30;128(2):485–489. doi: 10.1016/0042-6822(83)90275-1. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Kuff E. L. Intracisternal Type A particles in murine pancreatic B cells. Immunocytochemical demonstration of increased antigen (p73) in genetically diabetic mice. Am J Pathol. 1984 Jan;114(1):46–55. [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Fewell J. W., Kuff E. L., Koch T. The long terminal repeat of an endogenous intracisternal A-particle gene functions as a promoter when introduced into eucaryotic cells by transfection. Mol Cell Biol. 1984 Oct;4(10):2128–2135. doi: 10.1128/mcb.4.10.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Mays-Hoopes L. L., Brown A., Huang R. C. Methylation and rearrangement of mouse intracisternal a particle genes in development, aging, and myeloma. Mol Cell Biol. 1983 Aug;3(8):1371–1380. doi: 10.1128/mcb.3.8.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Huang R. C. Correlation of undermethylation of intracisternal A-particle genes with expression in murine plasmacytomas but not in NIH/3T3 embryo fibroblasts. Cancer Res. 1984 Nov;44(11):5234–5241. [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Ono M., Ohishi H. Long terminal repeat sequences of intracisternal A particle genes in the Syrian hamster genome: identification of tRNAPhe as a putative primer tRNA. Nucleic Acids Res. 1983 Oct 25;11(20):7169–7179. doi: 10.1093/nar/11.20.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Hammons M. D., Taylor K. D. Amounts, synthesis, and some properties of intracisternal A particle-related RNA in early mouse embryos. Proc Natl Acad Sci U S A. 1984 Jan;81(2):488–492. doi: 10.1073/pnas.81.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Szyf M. DNA methylation patterns. Formation and function. Biochim Biophys Acta. 1984 Sep 10;782(4):331–342. doi: 10.1016/0167-4781(84)90043-5. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]