The combination of viral genetics and animal knockout technologies enables one to define precise molecular pathways and targets of specific viral virulence and/or host defense genes. The dual genetic knockout paper in this issue of PNAS (1) exemplifies this concept. Leib and coworkers provide convincing in vivo evidence supporting a previously proposed mechanistic basis by which herpes simplex virus type 1 (HSV-1) ICP34.5 gene product mediates neurovirulence: by antagonizing the host IFN-induced protein kinase, PKR. This finding not only supports but also extends the role of PKR as an important player in antiviral defense. How does the ability to modulate PKR contribute to HSV-1 neuropathogenesis? The answer will be forthcoming as we review the function and regulation of PKR, an enzyme originally identified more than 25 years ago for its inhibitory effects on protein synthesis in cell-free systems (2, 3).
PKR: A Member of an Expanding Family of Eukaryotic Initiation Factor 2α (eIF2α) Protein Kinases
Eukaryotic cells respond to stress conditions, including viral infection, in part by down-modulating the overall rate of protein synthesis. This translational control response to stress occurs largely through the modification of the translation initiation factor, eIF2 (4). eIF2 delivers the Met-tRNAi to the 40 S ribosome, a rate-limiting step in translation initiation when the α subunit of eIF2 (eIF2α) is phosphorylated on serine 51 by a family of structurally related Ser/Thr kinases. Phosphorylated eIF2α has a higher affinity for the eIF2B guanine nucleotide exchanger than does the nonphosphorylated eIF2 isoform. This increased affinity impedes eIF2B function, resulting in its sequestration within an inactive complex with eIF2 [S51-phospho]⋅GDP. This blocks the requisite recycling of GDP for GTP on eIF2 and prevents de novo eIF2⋅GTP⋅Met-tRNAi ternary complex formation. Thus, translation initiation is halted, ultimately leading to shutdown of global cellular protein synthesis.
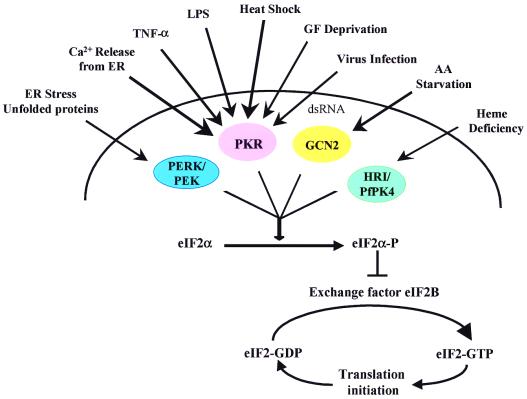
At least four distinct members of eIF2α protein kinases have been identified: GCN2, HRI/PfPK4, PERK/PEK, and PKR, each providing the cell a unique ability to modulate mRNA translation in response to specific cellular stresses (Fig. 1; ref. 4). For example, the HRI/PfPK4 protein kinase is expressed in mammalian reticulocytes and phosphorylates eIF2α in response to heme depletion. The PERK/PEK protein kinases reside within the endoplasmic reticulum (ER) where they mediate translational control in response to ER stress. The GCN2 enzyme presents an example for both global and specific control of mRNA translation by phosphorylating eIF2α upon amino acid starvation. This results in the specific stimulation of GCN4 translation and concomitant repression of global protein synthesis. GCN4 in turn stimulates amino acid production by inducing the expression of amino acid biosynthetic components.
Figure 1.
Stress-responsive eIF2α protein kinases. Consistent with its signal transducing role, PKR responds to a variety of different stimuli, including growth factor (GF), interleukin 3 (IL-3), tumor necrosis factor α (TNF-α), and altered calcium (Ca2+) levels, heat shock, and bacterial lipopolysaccharide (LPS). AA, amino acid; dsRNA, double-stranded RNA; ER, endoplasmic reticulum. See text for further details.
PKR is ubiquitously expressed in most mammalian tissues and belongs to an expanding double-stranded RNA (dsRNA)-binding protein family (4, 5). PKR normally exists as a latent form, but upon binding to dsRNA becomes autophosphorylated on multiple Ser and Thr residues and undergoes a conformational change (Fig. 2). The conformational change may promote kinase dimerization and thus trans-phosphorylation, which could in turn ensure maximal PKR activation and/or its substrate specificity. Although a mediator of a panoply of extracellular signals, PKR is best known for its ability to phosphorylate eIF2α and repress mRNA translation during virus infection (5, 6). Viral replication produces highly structured viral transcripts in the form of dsRNA that can bind to and activate PKR, which in turn phosphorylates eIF2α. As a result, the cellular translational machinery is incapacitated and viral protein synthesis and replication are restricted within the infected cell.
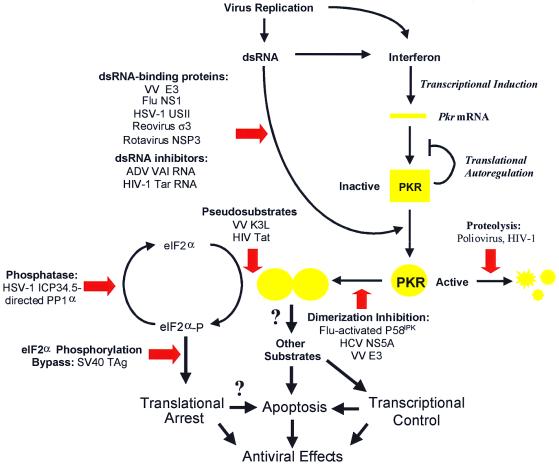
Figure 2.
Viral strategies against PKR-dependent translational block. PKR is subjected to elaborate regulatory mechanisms, including IFN-dependent transcriptional activation, translational autoregulation, and posttranslational control mechanisms (3). Virally encoded/induced mechanisms are denoted by red block arrows. ADV, adenovirus; Flu, influenza virus; HIV-1, HIV type 1; SV40, simian virus 40; VV, vaccinia virus. See text for further details.
Not to be outdone, viruses have evolved ways to counteract the PKR-mediated translational block (Fig. 2; refs. 3 and 6). These include directing inhibitors that: (i) interfere with the dsRNA-mediated activation of PKR; (ii) interfere with kinase dimerization; (iii) block the kinase catalytic site and PKR-substrate interactions; (iv) alter the physical levels of PKR; (v) regulate eIF2α phosphorylation directly; and (vi) affect components downstream from eIF2α. Not only are these viruses a diverse group, but some appear to use multiple strategies for inhibiting PKR (Fig. 2), underscoring the pivotal role played by the kinase within the IFN-induced antiviral response of the host cell.
HSV-1 Infection and Neuropathogenesis
The neurotropic DNA HSV-1 establishes latency in neuronal cells to allow viral persistence in the face of an active immune response (7). In response to certain stimuli, episodic reactivation of infectious or replicating virus can occur. In humans, the natural host, this can lead to a number of diseases, including gingivostomatitis and pharyngitis after primary oral-facial infection, recurrent herpes labialis, genital herpes, keratitis after eye infection, disseminated visceral infections in immunocompromised patients, hepatitis, and encephalitis after spread to the central nervous system (CNS) (8). To successfully penetrate and spread within the CNS, not only must HSV-1 deal with the unique aspects of neuroanotomy and cell biology, but it also has to evade the compartmentalized immune responses within the CNS to achieve viral persistence. This lifestyle has led to the evolution of elaborate control mechanisms that coordinately regulate HSV-1 gene expression during latent and productive infection (9).
The Role of ICP34.5 in HSV-1 Neurovirulence: Insights from the PKR Knockout Mice
The HSV-1 protein ICP34.5 is dispensable for viral growth in non-neuronal culture cells, but it is required for the virus to replicate in the mouse CNS (10). Furthermore, the ICP34.5 gene has been mapped to a region of the HSV-1 genome previously implicated in CNS replication (11). Infection of neuronal cells with ICP34.5-deficient mutant viruses resulted in premature shutoff of cellular protein synthesis and limitation of viral production in an apoptosis-like manner (12). Thus it was hypothesized that at least one function of ICP34.5 is to overcome the host cell resistance to viral infection. Because IFNs are key mediators of innate immune response and have been shown to control early acute HSV infection, possible targets for immunomodulation by ICP34.5 during infection may include components of the IFN response. As discussed earlier, a popular target for viruses to evade host IFN response is the PKR protein kinase, and HSV-1 is no exception in this regard.
The first connection between ICP34.5 and PKR was suggested when an unknown 90-kDa phosphoprotein (p90) coprecipitated with anti-PKR antibody from lysates of cells infected with ICP34.5-deficient viruses (13). p90 phosphorylation correlated with the premature shutoff of protein synthesis. It was thought that the ICP34.5 protein might function to negatively modulate PKR by blocking the interaction between p90 and PKR. It turns out, however, that ICP34.5 operates through a different mode of action. Using the yeast two-hybrid system, He and coworkers (14) found that ICP34.5 associated with the catalytic subunit of the host protein phosphatase 1α (PP1α). ICP34.5 formed a complex with PP1α in HSV-1-infected cells, and fractions containing the complex were capable of dephosphorylating purified eIF2α (15). Furthermore, ICP34.5 contains the amino acid sequence motif common to other regulatory subunits of PP1α that is required for binding to the PP1α catalytic subunit. Thus ICP34.5 is likely to function as a viral regulatory/targeting subunit of PP1α, redirecting the phosphatase to dephosphorylate eIF2α during viral infection, therefore circumventing the translational block resulting from PKR activation.
Leib and coworkers (1) describe the first in vivo functional analysis of PKR as antiviral effector within the context of a pathogenic animal model. Specifically, they demonstrate that a virus that had been attenuated by removal of ICP34.5 exhibited wild-type replication and virulence in mice from which the PKR gene has been deleted. Loss of PKR, however, did not restore growth and virulence of HSV-1 viruses carrying mutations in genes unrelated to ICP34.5, demonstrating that deletion of PKR is specifically responsible for restoration of the attenuated phenotype of the ICP34.5 mutant virus. Further, ICP34.5-deficient virus remained nonvirulent in mice devoid of an IFN-regulated antiviral effector (RNase L) that is independent of the PKR pathway. However, it would be nice to see whether restoration of PKR in a PKR−/− background could inhibit replication of the ICP34.5-deficient virus. For example, one could test this by coinfecting embryonic neuronal cells derived from the PKR−/− mice with a recombinant PKR-expressing adenovirus and the ICP34.5 mutant virus.
We cannot yet conclude that ICP34.5 negates PKR through PP1α-mediated dephosphorylation of eIF2α as neither physical nor functional interaction between ICP34.5 and eIF2α has been demonstrated. Furthermore, PKR has been implicated as a signal transducer at both the transcriptional and translational levels, and accordingly is likely capable of phosphorylating additional targets (5). Moreover, other members of eIF2α protein kinases could phosphorylate eIF2α, a likely scenario considering eIF2α phosphorylation remained intact in the PKR knockout mice (16). Because transgenic mice expressing a nonphosphorylatable form (S51A) of eIF2α is available (17), it might be interesting to see how ICP34.5 mutant viruses fare in these animals.
The story becomes more complicated with studies describing the isolation of second-site suppressor mutant viruses that lack the ICP34.5 gene (18–20). These variant viruses, which contained additional mutations that affect distinct viral genetic elements, displayed reduced accumulation of phosphorylated eIF2α and regained the ability to grow on otherwise nonpermissive neuronal cells. One of these extragenic suppressor ICP34.5 alleles compensated for the loss of the ICP34.5 function by producing a viral RNA-binding, ribosome-associated protein (US11) early during viral infection that directly bound to PKR and reduced its activation (21, 22). Interestingly, US11 protein made late in infection did not block PKR activation, suggesting that in wild-type HSV-1 infection US11 may have other functions and may represent an ancient rather than modern mechanism to down-regulate PKR. Thus it appears that HSV-1, like many viruses, encodes at least two strategies to negate PKR function (Fig. 2).
Concluding Remarks and Future Perspectives
Historically, studies of the evolutionary battle between viruses and their host not only have helped elucidate mechanisms of viral pathogenesis, but they often also have revealed basic cellular mechanisms. The study of ICP34.5–PKR interaction also may help uncover previously unidentified pathways. ICP34.5 contains a region of significant homology to GADD34, a cellular protein that is induced in response to agents that promote cell growth arrest, DNA damage, and cell differentiation (14, 23, 24). Furthermore, GADD34 also could interact with PP1α and functionally replaced ICP34.5 in prolonging late protein synthesis in infected cells (25, 26). These observations suggest that signals that trigger cell differentiation, growth arrest, and DNA damage may be linked to PKR-dependent translational control, and thus warrant further studies.
PKR recently has been implicated in regulation of apoptosis (27). It would be important to determine whether and how the PKR-mediated translation shutoff and/or apoptosis in neuronal cells infected by ICP34.5 mutant viruses contributes to the host range phenotype. However, it should be mentioned that ICP34.5 is not highly conserved among HSV. It also remains to be seen whether the ICP34.5-deficient virus-PKR knockout mice study can translate to human neurological diseases, including those associated with other neurovirulent viruses, such as poliovirus, measles, and rubella viruses.
Understanding how ICP34.5 enhances neurovirulence may have implications in the use of HSV-1 as a potential tumoricidal agent for destroying malignant cells in the CNS. ICP34.5 mutant viruses can cytolytically discriminate between normal and malignant cells (28, 29). However, these variant viruses grow poorly on neuronal tumors, imposing a major limitation on their effectiveness in destroying neuronal tumors. The suppressor mutants of the ICP34.5 allele, which have regained the ability to grow on neoplastic cells, but retained the neuroattenuated phenotype of the ICP34.5 parent virus, may help address this dilemma. The recently described HSV-1 mutant whose transcription of ICP34.5 is under the control of the cell cycle-regulated B-myb promoter also may offer an alternative avenue to target HSV-1 virulence toward malignant neurons (30).
Before the study by Leib and coworkers (1), there was no evidence linking a viral virulence factor to the antiviral function of PKR in an animal model. There are clinical data supporting the antiviral function of PKR from studies of IFN resistance by hepatitis C virus (HCV) (6). The nonstructural protein NS5A from certain IFN-resistant, but not IFN-sensitive, HCV strains has been shown to bind and inhibit PKR activity, thereby suggesting a potential mechanism by which HCV induces or sustains resistance to IFN therapy. These studies should provide both the foundation and impetus for future work aimed at understanding the pathophysiological role of PKR in viral diseases.
Footnotes
See companion article on page 6097.
References
- 1.Leib D A, Machalek M A, Williams B R G, Silverman R H, Virgin H W. Proc Natl Acad Sci USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrell P J, Balkow K, Hunt T, Jackson R J, Trachsel H. Cell. 1977;11:187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- 3.Clemens M J, Elia A. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 4.Dever T E. Trends Biochem Sci. 1999;24:398–340. doi: 10.1016/s0968-0004(99)01457-7. [DOI] [PubMed] [Google Scholar]
- 5.Williams B R. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 6.Gale M J, Jr, Katze M G. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 7.Stevens J G. Microbiol Rev. 1989;53:318–333. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey L, Spear P G. N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 9.Gale, M. J., Jr., Tan, S.-L. & Katze, M. G. (2000) Microbiol Mol. Biol. Rev., in press. [DOI] [PMC free article] [PubMed]
- 10.Chou J, Roizman B. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou J, Roizman B. J Virol. 1986;57:629–637. doi: 10.1128/jvi.57.2.629-637.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou J, Chen J J, Gross M, Roizman B. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He B, Chou J, Liebermann D A, Hoffman B, Roizman B. J Virol. 1996;70:84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He B, Gross M, Roizman B. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Gross M, Roizman B. J Biol Chem. 1998;273:20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y L, Reis L F L, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R G, Aguet M, Weissmann C M. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donz'e O, Jagus R, Koromilas A E, Hershey J W, Sonenberg N. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohr I, Gluzman Y. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- 19.He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. J Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassady K A, Gross M, Roizman B. J Virol. 1998;72:7005–7011. doi: 10.1128/jvi.72.9.7005-7011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassady K A, Gross M, Roizman B. J Virol. 1998;72:8620–8626. doi: 10.1128/jvi.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulvey M, Poppers J, Ladd A, Mohr I. J Virol. 1999;73:3375–3385. doi: 10.1128/jvi.73.4.3375-3385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGeoch D J, Barnett B C. Nature (London) 1991;353:609. doi: 10.1038/353609b0. [DOI] [PubMed] [Google Scholar]
- 24.Zhan Q, Lord K A, Alamo I, Jr, Hollander M C, Carrier F, Ron D, Kohn K W, Hoffman B, Liebermann D A, Fornace A J., Jr Mol Cell Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He B, Chou J, Liebermann D A, Hoffman B, Roizman B. J Virol. 1996;70:84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown S M, MacLean A R, McKie E A, Harland J. J Virol. 1997;71:9442–9449. doi: 10.1128/jvi.71.12.9442-9449.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman R J. Proc Natl Acad Sci USA. 1999;96:11693–11695. doi: 10.1073/pnas.96.21.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffin R S, MacLean A R, Latchman D S, Brown S M. Gene Ther. 1996;10:886–891. [PubMed] [Google Scholar]
- 29.Andreansky S, Soroceanu L, Flotte E R, Chou J, Markert J M, Gillespie G Y, Roizman B, Whitley R J. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 30.Chung R Y, Saeki Y, Chiocca E A. J Virol. 1999;73:7556–7564. doi: 10.1128/jvi.73.9.7556-7564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]