Abstract
Objective
To determine the relationship between relative body composition and body mass to height, anterior knee pain, or patellofemoral pain (PFP) in adolescent female athletes.
Background
Patellofemoral pain is common in female athletes and has an undefined etiology. The purpose of this study was to examine whether there was an association among higher body mass index (BMI), BMI z-scores, and relative body fat percentage in the development of PFP in an adolescent female athlete population. We hypothesized that female athletes who developed PFP over the course of a competitive basketball season had higher relative body mass or body fat percentage compared with those who did not develop PFP.
Methods
Fifteen middle school basketball teams that consisted of 248 basketball players (mean age, 12.76 ± 1.13 years; height, 158.43 ± 7.78 cm; body mass, 52.35 ± 12.31 kg; BMI, 20.73 ± 3.88 kg/m2) agreed to participate in this study over the course of 2 basketball seasons, resulting in 262 athlete-seasons. Testing included the completion of the Anterior Knee Pain Scale (AKPS), International Knee Documentation Committee (IKDC) form, standardized history, physician-administered physical examination, maturational estimates, and anthropometrics.
Results
Of the 262 athlete-seasons monitored, 39 athletes developed PFP over the course of the study. The incidence rate of new PFP was 1.57 per 1000 athlete-exposures. The cumulative incidence of PFP was 14.9%. There was no difference in BMI between those who developed PFP (mean body mass, 20.2 kg/m2; 95% CI, 18.9–21.4) and those who did not develop PFP (mean body mass, 20.8 kg/m2; 95% CI, 20.3–21.3; P > 0.05). Body mass index z-scores were not different between those who developed PFP (mean, 0.3; 95% CI, 0.7–0.6) and those who did not develop PFP (mean, 0.4; 95% CI, 0.3–0.6; P > 0.05). A similar trend was noted in relative body fat percentage, with mean scores of similar ranges in those who developed PFP (mean body fat percentage, 22.2%; 95% CI, 19.4–24.9) to the referent group who did not (mean body fat percentage, 22.9%; 95% CI, 21.8–24.1; P > 0.05).
Conclusions
Our results do not indicate a relationship between relative body composition or relative body mass to height to the propensity to develop PFP in middle school–aged female basketball players. Although previous data indicate a relationship between higher relative body mass and overall knee injury, these data did not support this association with PFP specifically. These data suggest the underlying etiology of PFP may be neuromuscular in nature. Further research is needed to understand the predictors, etiology, and ultimate prevention of this condition.
Keywords: patellofemoral pain, anterior knee pain, biomechanics, body mass index, BMI z-score, anthropometrics, body fat, patellofemoral pain
Introduction
An estimated 30 million school-aged youths (aged 5–18) participate in sports in the United States annually.1 Of these 30 million youths, 34% of middle school–aged participants sustain an injury and seek medical treatment.1,2 The knee is the most commonly injured joint in young adolescents, with 54% experiencing knee pain yearly.1,3–5 Patellofemoral pain (PFP), also referred to as anterior knee pain, is the most common knee disorder in adolescents. It may cause up to 74% of knee pain in this age group, thereby limiting their ability to participate in sports.6–8
Patellofemoral pain syndrome is a multifaceted condition that includes idiopathic retropatellar or peripatellar pain, which can be acute or chronic.9–15 The incidence of PFP is increasing in adolescent adolescents; thus, it is important for both clinicians and researchers to be aware of the predictors, etiology, and ultimate prevention of this condition.16,17 Risk factors for all musculoskeletal lower-extremity conditions, including PFP syndrome, can be classified into 2 categories: nonmodifiable or modifiable.18 Nonmodifiable risk factors include biological predeterminations, such as intercondylar notch width, joint laxity, sex, or family history.18 Modifiable risk factors include exercise, training, and diet.18 For some lower-extremity conditions, such modifiable risk factors include strength or body mass index (BMI).18
Body mass index has been correlated with incidence of developing PFP or lower-extremity injury.19–31 There is contradictory evidence on whether BMI is related to PFP; however, the general consensus according to the World Health Organization is that any BMI that is greater than or less than the average BMI of the sample population is indicative of PFP syndrome.32 This ambiguity in the relationship between body composition to height may be the result of the measure. Subject-and population-specific measures, such as age-and sex-specific measures of relative body mass (BMI z-score) or relative body composition (body fat percentage) may help clarify the associations that lead to the development of PFP. The purpose of this study was to examine the relationship among BMI, BMI z-score, and body fat percentage and the development of PFP in an adolescent female athlete population. We hypothesized that female athletes who developed PFP have a higher BMI compared with those who do not develop PFP.
Materials and Methods
Subjects
Female basketball players were recruited from a single county public school district in Kentucky that had 5 middle schools. A total of 15 middle school basketball teams were identified from these schools, and 248 basketball players (mean age, 12.76 ± 1.13 years; height, 158.43 ± 7.78 cm; weight, 52.35 ± 12.31 kg) agreed to participate in this study over the course of 2 basketball seasons, resulting in 262 athlete-seasons (Table 1). An “athlete-season” was defined as 1 athlete participating for 1 season.33 Because entire teams participated, there was decreased risk of selection bias, as those girls with knee injuries choose whether to participate. The participation rate was > 95%. Given the longitudinal nature of the study, subjects had the potential to be screened each of the 2 years if she was a returning athlete. This study was derived as part of a larger study investigating the underlying mechanisms for knee injuries. For the current study, we planned for a continuous response in the dependent variables from independent control and experimental subjects, with an expectation of 15 subjects with no injury per each subject with incident PFP per year.17,34 In a previous study, the response within each subject group was normally distributed, with a standard deviation of 2.65.27 If the true difference in the mean for the injured and uninjured subjects was 2.2, we needed to study a minimum of 12 new participants with PFP and 180 uninjured participants to be able to reject the null hypothesis that the population means of the experimental and control groups were equal, with a power of 0.827 and type 1 error of 0.05.
Table 1.
Descriptive Statistics of Participants
| Participants, n | Mean | SD | |
|---|---|---|---|
| Height, cm | 262 | 158.43 | 7.78 |
| Weight, kg | 262 | 52.35 | 12.31 |
| Age, years | 262 | 12.76 | 1.13 |
| BMI, kg/m2 | 262 | 20.73 | 3.88 |
| BMI z-score | 262 | 0.4 | 0.97 |
Abbreviations: BMI, body mass index; SD, standard deviation.
Procedures
The Institutional Review Board approved the data collection procedures and consent forms. Parental consent and athlete assent were obtained before data collection. Subjects were tested prior to the start of their competitive season. Testing consisted of completing of the Anterior Knee Pain Scale (AKPS), International Knee Documentation Committee (IKDC) form, standardized history and physician-administered physical examination, medical history, maturational estimates, and anthropometrics.
Knee Examination
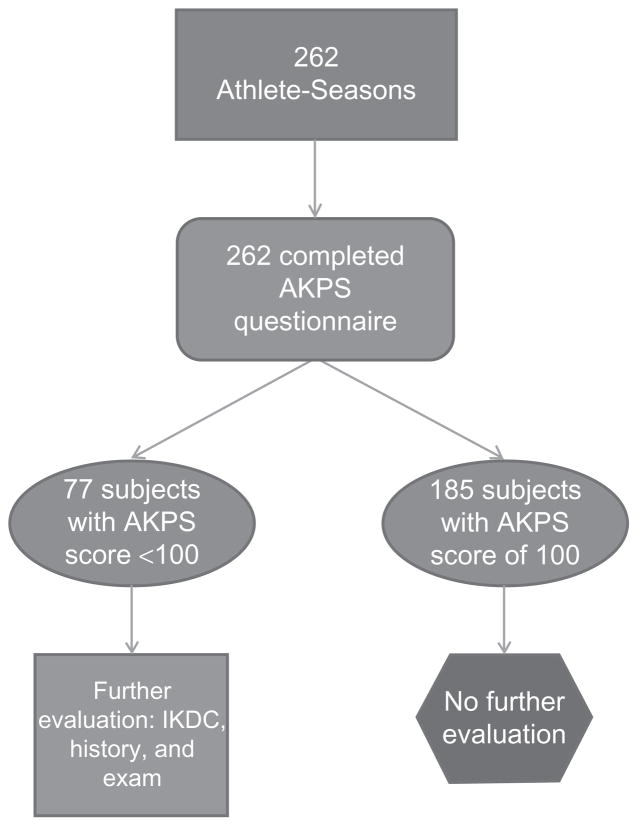
Of the 262 middle school–aged participants, all were initially screened for knee pain using the AKPS questionnaire. 35–37 The AKPS is a 13-item self-report questionnaire that is designed to evaluate subjective responses to specific activities and symptoms that are thought to correlate with PFP. The AKPS has a minimum score of 0 and a maximum score of 100 points, with 100 representing no pain. The AKPS has demonstrated high test-retest reliability and appears to be responsive to clinical changes in patients with PFP.37 Subjects with an AKPS score of 100 (n = 185) did not undergo further evaluation. However, all subjects with an AKPS score of < 100 (n = 77) underwent further assessment (Figure 1). This further assessment included a standard history form that documented current and prior knee symptoms and injury, completion of an IKDC score form, and a comprehensive knee physical examination conducted by a licensed physician. The IKDC is a reliable and valid instrument for use in a broad patient population concerning subjects’ pain, symptoms, function, and sports activity.38,39 Diagnoses included in the PFP category are displayed in Figure 2.
Figure 1.
Flowchart of subject evaluation.
Abbreviations: AKPS, Anterior Knee Pain Scale; IKDC, International Knee Documentation Committee.
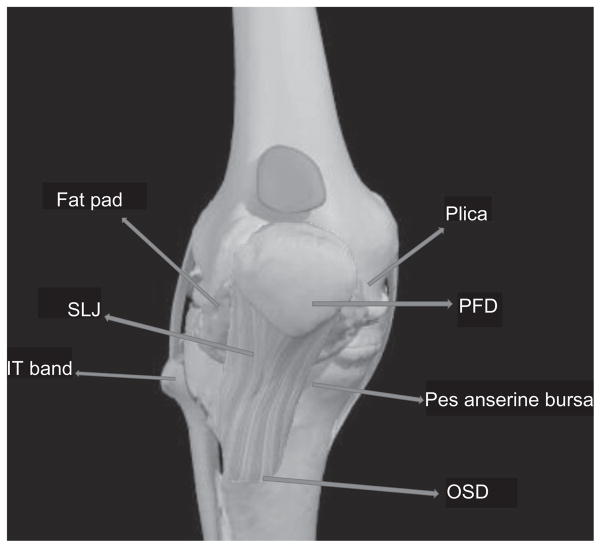
Figure 2.
Diagnoses included in the incidence of PFP.
Notes: Diagnoses contributory to PFP incidence calculation included PFD (n = 16), plica (n = 5), fat pad (n = 5), OSD (n = 4), SLJ/PT (n = 8), IT band (n = 1), and pes anserine bursitis (n = 0). Excluded from the calculations included the diagnosis of trauma.
Abbreviations: PFP, patellofemoral pain; IT, IT band; OSD, Osgood–Schattler’s disease; SLJ, Sinding-Larsen-Johansson; PT, patella tendon.
Image courtesy and copyright Primal Pictures Ltd.
Anthropometrics
Height was measured with a stadiometer with the participant in bare feet. Body mass was measured on a calibrated medical-grade scale. Body mass index z-score was calculated using SAS® (SAS Institute, Inc.).40 This program generates the number of standard deviation units from the mean; this is termed the z-score for BMI. These z-scores are based on data from the 2000 Centers for Disease Control and Prevention growth charts, according to height, weight, age (months), and sex of each child or adolescent between birth and age 20 years in the data set.40
Data Analyses
Athlete Tracking, Follow-up, Exclusionary Criteria, and Control Group
The athletes were monitored on a weekly basis by a certified athletic trainer for athletic exposures, new PFP, or any other lower-extremity injury with resultant time loss. Athletic exposures were defined as 1 game or practice session. The athletic exposures of subjects with preseason PFP were not included in the compilation of total athletic exposures. Postseason, the AKPS questionnaire was re-administered to all subjects. Athletes with pain identified by the AKPS as well as all athletes who had been examined preseason underwent further evaluation with a standardized personal interview and physical examination by the same physician. These data were compiled to calculate preseason prevalence and in-season incidence of PFP.33
Statistics
One-way univariate analysis of variance (ANOVA) group testing was performed on the dependent variables of BMI, BMI z-scores, and body fat percentage. Statistical analyses were conducted using SPSS® version 87.0. Statistical significance was set a priori at P < 0.05.
Results
Over the course of 2 basketball seasons, there was a total of 24 904 athlete exposures (17 377 practices and 7527 games). Of the 262 athlete-seasons, 39 developed PFP in either the first or second season. As a measure of PFP severity, the average score on the AKPS for this incident population was 86.8. The incidence rate of PFP is 1.566 per 1000 athlete exposures, with a cumulative incidence rate of 14.9%.
The mean data for both groups are presented in Table 2. Contrary to our hypothesis, there was no difference in BMI between athletes who developed PFP (mean BMI, 20.2 kg/m2; 95% CI, 18.9–21.4) and those who did not develop PFP (mean BMI, 20.8 kg/m2; 95% CI, 20.3–1.3; P > 0.05). Body mass index z-score was not different between those who developed PFP (mean, 0.3; 95% CI, −0.7 to 0.6) and those who did not develop PFP (mean, 0.4; 95% CI, 0.3–0.6; P > 0.05). A similar trend was noted in relative body fat percentage, with mean scores of similar ranges in those who developed PFP (mean, 22.2%; 95% CI, 19.4–24.9) compared with the referent group (mean, 22.9%; 95% CI, 21.8–24.1; P > 0.05). To further evaluate the potential association of PFP with relative body composition, we employed bivariate correlation analysis and multivariate logistic regression analysis. There were no significant associations between relative body fat percentage (r = 0.03), BMI (r = 0.06), or BMI z-score (r = 0.06) to PFP incidence status. In addition, the logistic regression model that included body fat percentage, BMI, and BMI z-score did not provide significant discrimination between those who developed PFP and those who did not develop PFP. Specifically, this regression model explained between 0.6% (Cox and Snell R2) and 1.1% (Nagelkerke R2) of the variance in PFP incidence in this population. A final multivariate linear regression analysis model that included fat percentage, BMI, and BMI z-score was employed to determine the potential association between the body composition measures to severity of symptoms, as evidenced in the AKPS. Similar to previous results, the linear regression model that included these relative body composition measures did not significantly predict severity of symptoms in the incident PFP group (adjusted R2, 0.04; P > 0.05).
Table 2.
Descriptives Between PFP Group and Controls
| Participants, n | Mean | SD | 95% CI | ||
|---|---|---|---|---|---|
| BMI z-score | No PFP | 223 | 0.43 | 0.969 | 0.3–0.55 |
| PFP | 39 | 0.26 | 0.993 | −0.7 to 0.58 | |
| Total | 262 | 0.4 | 0.973 | 0.28–0.52 | |
| BMI | No PFP | 223 | 20.82 | 3.882 | 20.31–21.34 |
| PFP | 39 | 20.16 | 3.84 | 18.91–21.4 | |
| Total | 262 | 20.73 | 3.876 | 20.25–21.2 | |
| Body fat % | No PFP | 223 | 22.94 | 8.523 | 21.81–24.06 |
| PFP | 39 | 22.15 | 8.397 | 19.43–24.88 | |
| Total | 262 | 22.82 | 8.493 | 21.79–23.85 |
Abbreviations: BMI, body mass index; PFP, patellofemoral pain; SD, standard deviation.
Discussion
The cumulative incidence and incidence rates for the development of new PFP in this population were 14.9% and 1.57 per 1000 athlete exposures, respectively. All new cases of PFP developed in middle school–aged female athletes. In similar populations, that also included high school–aged female athletes’ incidence risk; the rates were lower in this group, at 9.66 per 100 athletes and 1.09 per 1000 athlete exposures, respectively.17 These data suggest that PFP develops most frequently in early adolescence.
Patellofemoral pain is one of the most common disorders among young adolescent athletes.8,16,41,42 Patellofemoral pain often limits participation in sport activities and may lead to cessation of participation.8,41,43 Restricting participation in physical activities can result in a reduction in the physical and psychosocial health benefits that are gained from physical activity.44,45 We hypothesized that young adolescent female athletes who developed PFP would have higher BMIs compared with those who did not. Our results showed no difference in BMI or BMI z-scores or relative body fat percentage between athletes who developed PFP and those who did not develop PFP. Thus, the association previously found (ie, that athletes with increased BMI sustained a higher proportion of knee injuries than normal-weight counterparts) was not observed in the present study, which focused on PFP in a younger population than similar studies.30 Body mass index does appear to be a predictor of injury in adults and older adolescents.30
Previous research has been conflicting regarding the association between BMI and PFP. Some studies have found a correlation between higher BMI and clinically significant PFP, but others have not. Two studies reported a correlation between those with a lower BMI score and knee pain.9,11,22,26,29–31,46 Theories supporting a correlation between an increase in BMI and PFP include BMI and its relation to decreased joint space in the knee, and BMI and its relation to an increased q-angle in the lower extremity.23,47 Leppälä et al13 showed that BMI had no relation to decreased bone mineral density.
The risk of developing long-term degenerative changes, such as osteoarthritis (of which PFP may be a precursor) is strongly associated with BMI.48 In addition, higher BMI is associated with common orthopedic conditions in adults.49 Older adolescents also demonstrated a relationship between higher BMI and history of lower leg pain among cross-country runners.50 The present results, which indicated no difference in either relative body composition between those with and without incident PFP lead us to suspect an alternative underlying factor in the etiology of PFP in this age group, most notably lack of neuromuscular control. Lopes et al51 found an inverse relationship between motor coordination and BMI; subjects with higher BMIs had lower levels of motor coordination across ages 6 to 14 years. The obese children (BMI ≥ 30 kg/m2) showed markedly worse motor control levels than those with a normal-range BMI score.51 Motor coordination deficits do not appear to be transient and may even deteriorate further relative to age in overweight and obese children.52
In 2010, Myer et al17 found that athletes who developed PFP demonstrated increased knee abduction moment at initial contact when landing compared with uninjured controls.17 They hypothesized that dynamic knee abduction loading during a landing task contributed to the onset of PFP during the basketball season.17 Females with increased knee abduction moment and load during dynamic tasks also showed increased incidence of knee injury, such as PFP.53 This impaired neuromuscular control may be the modulator to the pathomechanics of PFP in this population, as opposed to the anthropometric measures that we assessed.
Based on our findings and previous research showing no association between PFP incidence and BMI,54 young adolescents with higher BMI should not be prohibited or restricted from participating in athletic activities due to the risk of developing PFP. Rather, young females should be encouraged to regularly participate in sports, because it can enhance their health and well-being.55,56 Participation in organized sports should evolve out of regular participation in a well-rounded preparatory conditioning program designed to reduce the neuromuscular deficits that increase the risk of PFP.57–59
There are potential limitations to the current study. The first is that the screening examinations were performed by different physicians each year. However, we standardized both the questionnaires and physician training for identification of PFP to limit potential for inter-rater diagnosis differences. Second, this study was limited to basketball players; therefore, inferences to athletes in other sports should be limited. This analysis does not differentiate between athletes who had unilateral or bilateral PFP. The final limitation concerns information about the PFP condition itself, including treatment that was undertaken, severity, and outcome measures, with all positive cases of PFP being treated equally. Not all diagnoses included under PFP, as shown in Figure 2, may be individually influenced by body composition measures. The authors acknowledge that PFP often occurs on a continuum and treatment is related to severity. Some athletes may not complain of PFP unless specifically asked, whereas others will openly acknowledge that it is disabling. Although more research is needed to determine both who should be treated and what interventions should be employed, the reported incidence should focus greater attention to screening and study of the natural history of this condition in youth.
Conclusion
The current study results indicate that there was no difference in BMI, BMI z-score, or body fat percentage between middle school–aged female basketball players who developed PFP and those who did not develop PFP. Rather, a different etiology, such as impaired neuromuscular control as the modulator to the pathomechanics of PFP, may be more important than the anthropometric measures assessed in this study. Future research is needed to understand the predictors, etiology, and ultimate prevention of PFP.
Acknowledgments
The authors would like to acknowledge funding support from National Institutes of Health/NIAMS Grants R01-AR049735, RO1-AR05563, and R01-AR056259. The authors would like to thank the Boone County Kentucky School District, especially School Superintendent Randy Poe, for participation in this study. The authors would also like to thank Mike Blevins, Ed Massey, Dr. Brian Blavattand, the Boone County public school district, and their athletes for their participation in this study. We would also like to thank the Cincinnati Children’s Hospital Department of Sports Medicine fellows for their participation in data collection. All authors are independent of any commercial funder, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest Statement
Kim D. Barber Foss MS, ATC; Myles Hornsby; Nicholas M. Edwards, MD; Gregory D. Myer, PhD, CSCS; and Timothy E. Hewett, PhD disclose no conflicts of interest.
References
- 1.Adirim TA, Cheng TL. Overview of injuries in the young athlete. Sports Med. 2003;33(1):75–81. doi: 10.2165/00007256-200333010-00006. [DOI] [PubMed] [Google Scholar]
- 2.McGuine T. Sports injuries in high school athletes: a review of injury-risk and injury-prevention research. Clin J Sport Med. 2006;16(6):488–499. doi: 10.1097/01.jsm.0000248848.62368.43. [DOI] [PubMed] [Google Scholar]
- 3.Knowles SB, Marshall SW, Bowling JM, et al. A prospective study of injury incidence among North Carolina high school athletes. Am J Epidemiol. 2006;164(12):1209–1221. doi: 10.1093/aje/kwj337. [DOI] [PubMed] [Google Scholar]
- 4.Louw QA, Manilall J, Grimmer KA. Epidemiology of knee injuries among adolescents: a systematic review. Br J Sports Med. 2008;42(1):2–10. doi: 10.1136/bjsm.2007.035360. [DOI] [PubMed] [Google Scholar]
- 5.Calmbach WL, Hutchens M. Evaluation of patients presenting with knee pain: part II. Differential diagnosis. Am Fam Physician. 2003;68(5):917–922. [PubMed] [Google Scholar]
- 6.Blønd L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7-year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393–400. [PubMed] [Google Scholar]
- 7.Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685–693. doi: 10.1302/0301-620X.66B5.6501361. [DOI] [PubMed] [Google Scholar]
- 8.Witvrouw E, Lysens R, Bellemans J, Cambier D, Vanderstraeten G. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two-year prospective study. Am J Sports Med. 2000;28(4):480–489. doi: 10.1177/03635465000280040701. [DOI] [PubMed] [Google Scholar]
- 9.Collins NJ, Crossley KM, Darnell R, Vicenzino B. Predictors of short and long term outcome in patellofemoral pain syndrome: a prospective longitudinal study. BMC Musculoskelet Disord. 2010;11:11. doi: 10.1186/1471-2474-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boling MC, Padua DA, Marshall SW, Guskiewicz K, Pyne S, Beutler A. A prospective investigation of biomechanical risk factors for patellofemoral pain syndrome: the Joint Undertaking to Monitor and Prevent ACL Injury (JUMP-ACL) cohort. Am J Sports Med. 2009;37(11):2108–2116. doi: 10.1177/0363546509337934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvigneaud N, Bernard E, Stevens V, Witvrouw E, Van Tiggelen D. Isokinetic assessment of patellofemoral pain syndrome: a prospective study in female recruits. Isokinet Exerc Sci. 2008;16(4):213–219. [Google Scholar]
- 12.Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447–456. doi: 10.1177/03635465020300032501. [DOI] [PubMed] [Google Scholar]
- 13.Leppälä J, Kannus P, Natri A, Sievänen H, Järvinen M, Vuori I. Bone mineral density in the chronic patellofemoral pain syndrome. Calcif Tissue Int. 1998;62(6):548–553. doi: 10.1007/s002239900477. [DOI] [PubMed] [Google Scholar]
- 14.Moss RI, Devita P, Dawson ML. A biomechanical analysis of patellofemoral stress syndrome. J Athl Train. 1992;27(1):64–69. [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson RL, Nee RJ. Analysis of hip strength in females seeking physical therapy treatment for unilateral patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2007;37(5):232–238. doi: 10.2519/jospt.2007.2439. [DOI] [PubMed] [Google Scholar]
- 16.Kannus P, Aho H, Järvinen M, Niittymäki S. Computerized recording of visits to an outpatient sports clinic. Am J Sports Med. 1987;15(1):79–85. doi: 10.1177/036354658701500112. [DOI] [PubMed] [Google Scholar]
- 17.Myer GD, Ford KR, Barber Foss KD, et al. The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clin Biomech (Bristol, Avon) 2010;25(7):700–707. doi: 10.1016/j.clinbiomech.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alentorn-Geli E, Myer GD, Silvers HJ, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 2: a review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee Surg Sports Traumatol Arthrosc. 2009;17(8):859–879. doi: 10.1007/s00167-009-0823-z. [DOI] [PubMed] [Google Scholar]
- 19.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 20.McHugh MP, Tyler TF, Mirabella MR, Mullaney MJ, Nicholas SJ. The effectiveness of a balance training intervention in reducing the incidence of noncontact ankle sprains in high school football players. Am J Sports Med. 2007;35(8):1289–1294. doi: 10.1177/0363546507300059. [DOI] [PubMed] [Google Scholar]
- 21.Myer GD, Ford KR, Divine JG, Wall EJ, Kahanov L, Hewett TE. Longitudinal assessment of noncontact anterior cruciate ligament injury risk factors during maturation in a female athlete: a case report. J Athl Train. 2009;44(1):101–109. doi: 10.4085/1062-6050-44.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neely FG. Intrinsic risk factors for exercise-related lower limb injuries. Sports Med. 1998;26(4):253–263. doi: 10.2165/00007256-199826040-00004. [DOI] [PubMed] [Google Scholar]
- 23.Pefanis N, Papaharalampous X, Tsiganos G, Papadakou E, Baltopoulos P. The effect of Q angle on ankle sprain occurrence. Foot Ankle Spec. 2009;2(1):22–26. doi: 10.1177/1938640008330769. [DOI] [PubMed] [Google Scholar]
- 24.Restrepo R, Reed MH. Impact of obesity in the diagnosis of SCFE and knee problems in obese children. Pediatr Radiol. 2009;39 (suppl 2):S220–S225. doi: 10.1007/s00247-008-1123-3. [DOI] [PubMed] [Google Scholar]
- 25.Spaine LA, Bollen SR. ‘The bigger they come …’: the relationship between body mass index and severity of ankle fractures. Injury. 1996;27(10):687–689. doi: 10.1016/s0020-1383(96)00136-2. [DOI] [PubMed] [Google Scholar]
- 26.Turbeville SD, Cowan LD, Owen WL, Asal NR, Anderson MA. Risk factors for injury in high school football players. Am J Sports Med. 2003;31(6):974–980. doi: 10.1177/03635465030310063801. [DOI] [PubMed] [Google Scholar]
- 27.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 28.Van Tiggelen D, Witvrouw E, Coorevits P, Croisier JL, Roget P. Analysis of isokinetic parameters in the development of anterior knee pain syndrome: a prospective study in a military setting. Isokinet Exerc Sci. 2004;12(4):223–228. [Google Scholar]
- 29.Webb R, Brammah T, Lunt M, Urwin M, Allison T, Symmons D. Opportunities for prevention of ‘clinically significant’ knee pain: results from a population-based cross sectional survey. J Public Health (Oxf ) 2004;26(3):277–284. doi: 10.1093/pubmed/fdh162. [DOI] [PubMed] [Google Scholar]
- 30.Yard E, Comstock D. Injury patterns by body mass index in US high school athletes. J Phys Act Health. 2011;8(2):182–191. doi: 10.1123/jpah.8.2.182. [DOI] [PubMed] [Google Scholar]
- 31.Zhai G, Cicuttini F, Ding C, Scott F, Garnero P, Jones G. Correlates of knee pain in younger subjects. Clin Rheumatol. 2007;26(1):75–80. doi: 10.1007/s10067-006-0248-8. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO Obesity Technical Report Series No. 894: Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 33.Knowles SB, Marshall SW, Guskiewicz KM. Issues in estimating risks and rates in sports injury research. J Athl Train. 2006;41(2):207–215. [PMC free article] [PubMed] [Google Scholar]
- 34.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 35.Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Arch Phys Med Rehabil. 2004;85(5):815–822. doi: 10.1016/s0003-9993(03)00613-0. [DOI] [PubMed] [Google Scholar]
- 36.Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159–163. doi: 10.1016/s0749-8063(05)80366-4. [DOI] [PubMed] [Google Scholar]
- 37.Watson CJ, Propps M, Ratner J, Zeigler DL, Horton P, Smith SS. Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35(3):136–146. doi: 10.2519/jospt.2005.35.3.136. [DOI] [PubMed] [Google Scholar]
- 38.Higgins LD, Taylor MK, Park D, et al. International Knee Documentation Committee. Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine. 2007;74(6):594–599. doi: 10.1016/j.jbspin.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. SAS Program for the CDC Growth Charts. Department of Health and Human Services Center for Disease Control and Prevention; [Accessed February 10, 2009]. http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm. [Google Scholar]
- 41.Natri A, Kannus P, Järvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30(11):1572–1577. doi: 10.1097/00005768-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Heintjes EM, Berger MY, Koes BW, Bierma-Zeinstra SM. Knee disorders in primary care: design and patient selection of the HONEUR knee cohort. BMC Musculoskelet Disord. 2005;6:45. doi: 10.1186/1471-2474-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utting MR, Davies G, Newman JH. Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? Knee. 2005;12(5):362–365. doi: 10.1016/j.knee.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Christou EA. Patellar taping increases vastus medialis oblique activity in the presence of patellofemoral pain. J Electromyogr Kinesiol. 2004;14(4):495–504. doi: 10.1016/j.jelekin.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Crossley KM, Cowan SM, McConnell J, Bennell KL. Physical therapy improves knee flexion during stair ambulation in patellofemoral pain. Med Sci Sports Exerc. 2005;37(2):176–183. doi: 10.1249/01.mss.0000152676.13197.49. [DOI] [PubMed] [Google Scholar]
- 46.Knapik JJ, Sharp MA, Canham-Chervak M, Hauret K, Patton JF, Jones BH. Risk factors for training-related injuries among men and women in basic combat training. Med Sci Sports Exerc. 2001;33(6):946–954. doi: 10.1097/00005768-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Duren DL, Sherwood RJ, Chumlea WC, Siervogel RM, Towne B. Radiographic joint space of the knee in healthy young adults. Hum Biol. 2006;78(3):353–364. doi: 10.1353/hub.2006.0042. [DOI] [PubMed] [Google Scholar]
- 48.Toivanen AT, Heliovaara M, Impivaara O, et al. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis—a population-based study with a follow-up of 22 years. Rheumatology (Oxford) 2010;49(2):308–314. doi: 10.1093/rheumatology/kep388. [DOI] [PubMed] [Google Scholar]
- 49.Bergkvist D, Hekmat K, Svensson T, Dahlberg L. Obesity in orthopedic patients. Surg Obes Relat Dis. 2009;5(6):670–672. doi: 10.1016/j.soard.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Reinking MF, Austin TM, Hayes AM. Risk factors for self-reported exercise-related leg pain in high school cross-country athletes. J Athl Train. 2010;45(1):51–57. doi: 10.4085/1062-6050-45.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopes VP, Stodden DF, Bianchi MM, Maia JA, Rodrigues LP. Correlation between BMI and motor coordination in children. J Sci Med Sport. 2012;15(1):3843. doi: 10.1016/j.jsams.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 52.D’Hondt E, Deforche B, Vaeyens R, et al. Gross motor coordination in relation to weight status and age in 5- to 12-year-old boys and girls: a cross-sectional study. Int J Pediatr Obes. 2011;6(2–2):e556–e54. doi: 10.3109/17477166.2010.500388. [DOI] [PubMed] [Google Scholar]
- 53.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 54.Warsh J, Pickett W, Janssen I. Are overweight and obese youth at increased risk for physical activity injuries? Obes Facts. 2010;3(4):225–230. doi: 10.1159/000319322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myer GD, Faigenbaum AD, Ford KR, Best TM, Bergeron MF, Hewett TE. When to initiate integrative neuromuscular training to reduce sportsrelated injuries and enhance health in youth? Curr Sports Med Rep. 2011;10(3):157–166. doi: 10.1249/JSR.0b013e31821b1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myer GD, Faigenbaum AD, Chu DA, et al. Integrative training for children and adolescents: techniques and practices for reducing sports-related injuries and enhancing athletic performance. Phys Sportsmed. 2011;39(1):74–84. doi: 10.3810/psm.2011.02.1854. [DOI] [PubMed] [Google Scholar]
- 57.Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. 2005;19(1):51–60. doi: 10.1519/13643.1. [DOI] [PubMed] [Google Scholar]
- 58.Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490–498. doi: 10.1177/0363546505282619. [DOI] [PubMed] [Google Scholar]
- 59.Myer GD, Ford KR, Brent JL, Hewett TE. Differential neuromuscular training effects on ACL injury risk factors in “high-risk” versus “low-risk” athletes. BMC Musculoskelet Disord. 2007;8:39. doi: 10.1186/1471-2474-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]