Abstract
BACKGROUND & AIMS
The exocrine portion of the pancreas functions in digestion and preserves pancreatic homeostasis. Learning how this tissue forms during embryogenesis could improve our understanding of human pancreatic diseases. Expression of the homeo-box gene Prox1 in the exocrine pancreas changes throughout development in mice. We investigated the role of Prox1 in development of the exocrine pancreas in mice.
METHODS
Mice with pancreas-specific deletion of Prox1 (Prox1ΔPanc) were generated and their pancreatic tissues were analyzed using immunohistochemistry, transmission electron microscopy, histologic techniques, quantitative real-time polymerase chain reaction, immunoblotting, and morphometric analysis.
RESULTS
Loss of Prox1 from the pancreas led to multiple exocrine alterations, most notably premature acinar cell differentiation, increased ductal cell proliferation, altered duct morphogenesis, and imbalanced expression of claudin proteins. Prox1ΔPanc mice also had some minor alterations in islet cells, but beta-cell development was not affected. The exocrine congenital defects of Prox1ΔPanc pancreata appeared to initiate a gradual process of deterioration that resulted in extensive loss of acinar cells, lipomatosis, and damage to ductal tissue in adult mice.
CONCLUSIONS
Pancreas-specific deletion of Prox1 causes premature differentiation of acinar cells and poor elongation of epithelial branches; these defects indicate that Prox1 controls the expansion of tip progenitors in the early developing pancreas. During later stages of embryogenesis, Prox1 appears to regulate duct cell proliferation and morphogenesis. These findings identify Prox1 as an important regulator of pancreatic exocrine development.
Keywords: Transcription, Regulation, Organogenesis, Mouse Model
The exocrine compartment of the pancreas consists of a large mass of acinar cells that produce and secrete various enzyme precursors required for food digestion1 and an intricate system of ducts that collect and deliver those precursors to the duodenum.1 The pancreatic ductal tree comprises the main pancreatic duct that drains into the intestine, interlobular ducts that link the acinar lobules to the main duct, small intralobular ducts, and fine intercalated ducts that connect to acini.1 In addition to providing the framework that supports the acinar and endocrine tissues, the duct epithelium secretes both the fluid that carries the digestive enzymes and bicarbonate, which neutralizes gastric acids and adjusts a pH favorable for proenzyme activation in the duodenum. Although duct cells make up a small number of total pancreatic cells (approximately 5%–10%), their function is critical to maintain homeostasis in this organ. In fact, congenital alterations affecting the development or function of pancreatic ducts often lead to severe human diseases, including cystic fibrosis or pancreatitis.2
Studies mainly conducted in the past decade began to unravel the molecular mechanisms controlling the formation of pancreatic acinar cells.1 In contrast, duct development is a process that still remains poorly understood.3 In vertebrates, pancreatic duct morphogenesis initiates with the formation of microlumens that coalesce and expand into a continuous luminal network. This network gives rise to “primitive ducts,” consisting of a monolayered polarized epithelium, which subsequently remodels and matures into a tubular system. Genetic studies in both zebrafish and mice showed that pancreas ductal development requires Notch signaling,4,5 the activity of the transcription factors Pdx16 and HNF6,7,8 and primary cilia.9,10 Despite these limited advances, it is clear that a more comprehensive picture of pancreatic duct development requires identifying additional gene functions regulating this process.
Some years ago, we reported expression of the homeodomain transcription factor Prox1 in the developing pancreas of mice.11 Prox1 is a critical regulator of multiple processes during vertebrate organogenesis, including the development of the lymphatic system,12 liver (Seth et al, manuscript in preparation),13 eye,14,15 heart,16 and neurons.17 Prox1 also appears to regulate nuclear receptor activity in some cellular contexts.18 –20 Prox1 function has been implicated with tumor formation,21,22 and recently mutations in the PROX1 locus were found to be associated with fasting hyperglycemia and predisposition to diabetes in humans.23 To date, only a handful of Prox1 target genes have been identified in hepatocytes,18 endothelial cells,24 lens,25 hepatoblasts (Seth et al., manuscript in preparation), and cardiomyocytes.16
Prox1 is one of the earliest markers of vertebrate pancreas morphogenesis, and in mouse embryos the onset of its expression coincides with the emergence of the pancreatic buds (at approximately embryonic [E] day 9.0).26 Although Prox1 is broadly detected in multipotent progenitors of the early pancreas, its expression changes on segregation of the distinct epithelial cell lineages; it becomes extinguished in acinar cells but persists in the ductal and islet cells.11 Our previous characterization of mice with germline deletion of Prox1 (Prox1−/−) uncovered various abnormalities affecting early pancreas development, including reduced organ size, poor epithelial branching, premature exocrine cell differentiation, and decreased production of endocrine precursors.11 These results introduced Prox1 as a novel regulator of early pancreas organogenesis and predicted that the lack of its function could affect additional, late aspects of pancreatic development. However, this last possibility could not be explored in the pancreas of Prox1−/− mice because the multiorgan defects of these mutants preclude their survival beyond E14.5.15
This study conditionally inactivated Prox1 in pancreatic progenitors of mice. We report that the loss of Prox1 function perturbs morphogenesis of the pancreatic ductal tree, promotes massive acinar cell apoptosis at around weaning stages, and leads to a chronic pathology culminating with severe tissue damage and possibly compromised pancreatic physiology. These results indicate that the formation of a fully functional pancreas requires the activity of Prox1.
Materials and Methods
Animals
Prox1loxP/+ mice were maintained and genotyped as described.27 Pdx1-CreEARLY mice were obtained from the Mutant Mouse Regional Resource Centers (University of California, Davis, Davis, CA).28 Rosa26RLacZ mice were purchased from The Jackson Laboratory (Bar Harbor, ME).29
Cell Counting and Morphometric Analysis
Whole pancreata of control and Prox1ΔPanc embryos and newborns (n = 3) were sectioned (10 μm), and 3 sections from the largest area of the pancreata were selected for cell counting and morphometric analysis. Anti–E-cadherin antibodies were used to visualize the pancreatic epithelium, anti-elastase and anti-amylase for acini, anti-mucin for ducts, anti-synaptophysin for endocrine cells, and anti-PH3 for proliferating cells. Morphometric analysis was conducted using the ImageJ 1.37v program.
Additional materials and methods are provided in Supplementary Materials and Methods.
Results
Early Pancreatic Defects of Prox1ΔPanc Embryos Phenocopy Those of Prox1−/− Embryos
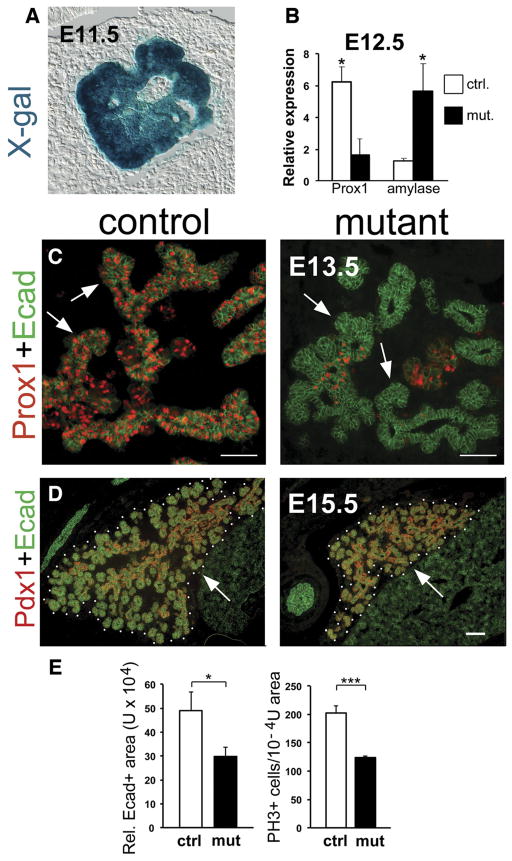
Pdx1-CreEARLY transgenic mice were used to delete Prox1 in pancreatic progenitors of Prox1loxP/loxPPdx1-Cre; mice (herein called Prox1ΔPanc).28 X-gal staining of E11.5 Pdx1-CreEARLY;ROSA26R embryos (Figure 1A) showed expression of the β-galactosidase (β-gal) reporter throughout the entire pancreas. Quantitative real-time polymerase chain reaction (qRT-PCR) results also revealed approximately 70% reduction of Prox1 transcripts in Prox1ΔPanc pancreata at E12.5 (Figure 1B). Likewise, immunohistochemistry results showed broad Prox1 expression in the E13.5 pancreatic epithelium of control mice (Figure 1C, left) but only a few Prox1+ cells in the pancreatic epithelium of Prox1ΔPanc littermates (Figure 1C, right). Together, these results verify the early activity of Cre recombinase in Prox1ΔPanc pancreata.
Figure 1.
Prox1 deletion in pancreatic progenitors reduces early pancreas growth. (A) Frozen section of an E11.5 Pdx1-CreEARLY; ROSA26R embryo stained with X-gal validates Cre recombinase activity in pancreatic progenitors. (B) qRT-PCR results at E12.5 (n = 3) show that a reduction in Prox1 expression is accompanied by an increment of amylase expression in Prox1ΔPanc pancreata. (C) Immunohistochemistry results show almost no Prox1 protein expression and reduced branches (arrows) in Prox1ΔPanc pancreata compared with control pancreata at E13.5. (D and E) Immunohistochemistry and morphometric results indicate that Prox1ΔPanc pancreata are smaller (Ecad+ area; D, dotted lines; E [left], n = 3) and have less proliferating cells (PH3+; E [right], n = 3) than control pancreata at E15.5. Scale bars = 50 μm (C), 100 μm (D). *P < .05, ***P < .001.
We previously reported that E13.5–E14.5 Prox1−/− pancreata were smaller and had shorter branches compared with control pancreata.11 These alterations were also noticed in E13.5–E15.5 Prox1ΔPanc embryos (Figure 1C–E). In addition, E15.5 Prox1ΔPanc pancreata had significantly reduced cell proliferation in comparison to similar control tissues (Figure 1E). These findings corroborate that Prox1 function controls early pancreatic growth.
Loss of Prox1 Function Affects the Onset of Differentiation but Not the Maturation of Pancreatic Acinar Cells
Our previous study showed that cells expressing various acinar markers were overabundant in E12.5–E13.5 Prox1−/− pancreata.11 Similar to those results, we detected a significant increment of amylase transcripts in the pancreas of E12.5 Prox1ΔPanc embryos (Figure 1B) and noticeably more amylase-positive cells in E13.5 Prox1ΔPanc pancreata compared with control tissues (Figure 2A). In contrast, we found that the acinar cell mass was smaller in Prox1ΔPanc pancreata than in control pancreata at E15.5 (Figure 2B). These opposite results could be the consequence of premature differentiation of acinar precursors, because this alteration is expected to reduce both the pool of acinar precursors and their progeny.
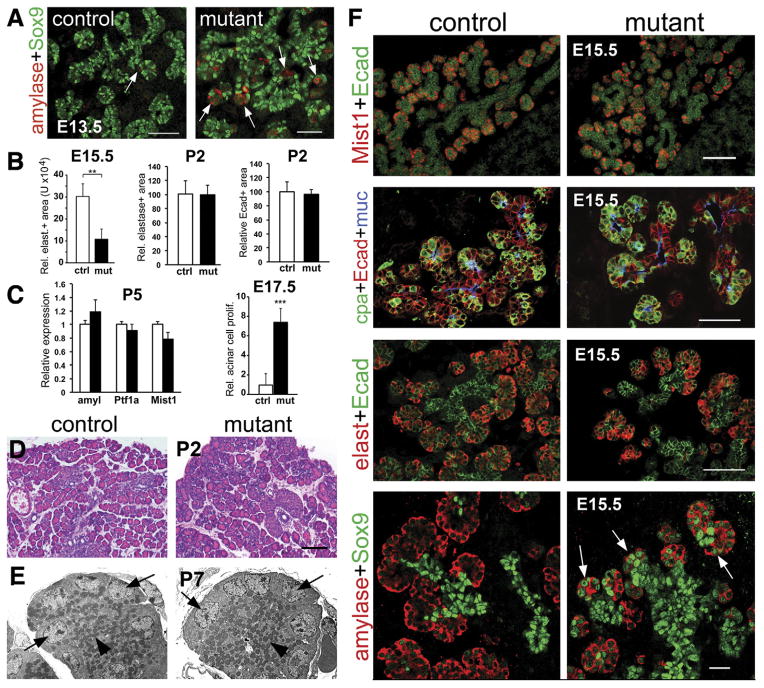
Figure 2.
Prox1ΔPanc pancreata have relatively normal acinar phenotype after E15.5. (A) Amylase-positive cells (arrows) are noticeably more abundant in Prox1ΔPanc pancreata compared with control tissues at E13.5, presumably due to precocious acinar cell differentiation. This alteration probably reduces the acinar progenitor pool because E15.5 Prox1ΔPanc pancreata have proportionally less acinar area (elastase positive) than control pancreata (B, left). Morphometric analyses show no significant differences in relative acinar area (elastase positive; B, center) or total pancreatic area (Ecad+; B, right) at P2, and qRT-PCR results (n = 4–5) show no significant differences in acinar gene expression (C, left) between Prox1ΔPanc and control pancreata at P5. (C, right) The recovery of Prox1-deficient pancreata size is likely due to increased acinar cell proliferation (percentage of PH3-positive/amylase-positive cells) at E17.5. (D) H&E staining shows comparable gross appearance of acinar tissue between control and Prox1ΔPanc pancreata at P2. (E) TEM results at P7 indicate correct polarity of the mutant acinar cells (right), with basally located nuclei (arrows) and apically localized zymogen granules (arrowheads; original magnification 2000×). (F) Expression of various acinar cell markers (Mist1, carboxypeptidase A [cpa], elastase, and amylase) is indistinguishable between Prox1ΔPanc and control pancreata at E15.5. However, different from control tissues, some acini of Prox1-deficient pancreata retain low levels of Sox9 expression (arrows) at this stage. Scale bars = 25 μm (A, left; F, bottom), 50 μm (A, right; F, middle), and 100 μm (D; F, top). **P < .01, ***P < .001.
Acinar cells of E15.5 Prox1ΔPanc embryos expressed several differentiation markers, including Mist1, carboxypeptidase A, elastase, and amylase (Figure 2F). Numerous acinar cells of E15.5 Prox1ΔPanc embryos also expressed moderate levels of Sox9 (Figure 2F, bottom, arrows), a transcription factor normally detected in multipotent progenitors but not in acinar cells of the pancreas,30 although this alteration was no longer detected after E17.5 (Figure 3F).
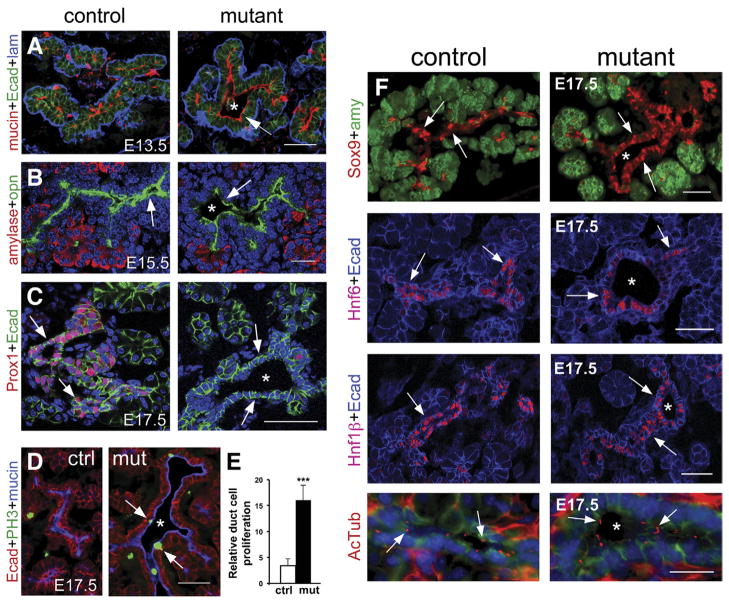
Figure 3.
Prox1 deficiency affects both morphogenesis and proliferation of the pancreatic ducts. The developing ducts of E13.5–E17.5 Prox1ΔPanc pancreata have enlarged lumens (asterisks) but correctly localize mucin (A [red, arrowhead], D [blue]) and osteopontin (B, green, arrows) on the apical side and laminin (A, blue) on the basal side. At E17.5, Prox1 (red) is highly expressed in control ducts (C, left, arrows) but absent in Prox1ΔPanc ducts (C, right, arrows). The E17.5 mutant duct epithelium has more cells undergoing proliferation (PH3 positive, n = 3) than the control ducts (D and E) but relatively normal expression of Sox9, Hnf6, and Hnf1 β (F) or abundance of cilia (anti-acetylated β-tubulin staining, arrows in F, bottom panels). The image in C was taken with a confocal microscope. Scale bars = 50 μm (A–D, F [Sox9, Hnf6, Hnf1β]) and 20 μm (F [AcTub]). ***P < .001.
Interestingly, both the total epithelial area and the acinar cell mass appeared to recover in Prox1ΔPanc pancreata at around birth (Figure 2B). In addition, transmission electron microscopy (TEM) and histology results showed comparable acinar cell morphology between control and Prox1ΔPanc pancreata at postnatal day (P)2 to P7 (Figure 2D and E), whereas qRT-PCR results revealed that the expression levels of various acinar transcripts were very similar between control and Prox1-deficient pancreata at P5 (Figure 2C). Quantitative results indicated that compensatory acinar cell proliferation most likely accounted for the size recovery of Prox1ΔPanc pancreata (Figure 2C). Therefore, we conclude that Prox1 loss of function affects the initiation of differentiation, but not maturation, in pancreatic acinar cells.
Specific Islet Cell Defects Are Observed in Prox1ΔPanc Pancreata
We previously reported deficient expression of the proendocrine genes Nkx6.1 and Ngn3 in the pancreas of E13.5–E14.5 Prox1−/− embryos,11 and these alterations were also observed in similar Prox1ΔPanc tissues (Supplementary Figure 1A and data not shown). However, we found that the formation of Ngn3+ cells began to recover in Prox1ΔPanc pancreata at approximately E15.5 (Supplementary Figure 1B). Moreover, islet genesis seemed to extend postnatally in these mutant tissues, because we noticed that Ngn3 transcripts were more abundant in Prox1ΔPanc pancreata than in control pancreata at P5 (Supplementary Figure 1E). These results indicate that Prox1 loss of function does not abrogate but only transiently delays islet genesis in the pancreas.
Morphometric results also showed that the islet masses of Prox1ΔPanc mice and control mice were similar at birth (Supplementary Figure 1D), although the Prox1-deficient islets tended to be smaller than wild-type islets (Supplementary Figure 1D). On the other hand, Prox1ΔPanc pancreata had significantly less glucagon (alpha cells) transcripts and, conversely, significantly more somatostatin (delta cells), ghrelin (epsilon cells), and cholecystokinin transcripts (Supplementary Figure 1E) than control pancreata at P5. These results coincide with our previous finding that E12.5 Prox1−/− pancreata had increased cholecystokinin expression and decreased glucagon expression.11
The combinatorial expression pattern of the previous hormones in islet cells of control and Prox1-deficient pancreata could not be determined because of the lack of appropriate antibodies. However, our results suggest that Prox1 function controls proper hormone expression in pancreatic endocrine cells or is necessary to correctly allocate certain islet cell types. On the other hand, loss of pancreatic Prox1 did not affect the expression of insulin1 or insulin2 transcripts (Supplementary Figure 1E), insulin, or other beta-cell proteins (Supplementary Figure 1C and data not shown) or beta-cell mass (data not shown). Therefore, we conclude that Prox1 function is dispensable for beta-cell development.
Pancreatic Ductal Development Requires Prox1
Tubular structures representing primitive ducts were observed in the pancreata of both control and Prox1ΔPanc embryos dissected at E13.5–E15.5 (Figure 3A).31 We noticed that the mutant primitive ducts almost entirely lacking Prox1 expression (data not shown) had abnormally large lumens (Figure 3A and B) and correct epithelial cell polarity, as indicated by the expression of mucin (Figure 3A) or osteopontin (Opn, Figure 3B) proteins on their apical side and laminin (Figure 3A) on the basal side. Immunohistochemistry results showed more prominent Prox1 expression deficiency in the Prox1ΔPanc duct epithelium at E17.5 (Figure 3C). At this stage, the mutant ducts not only appeared dilated but also had significantly more cells undergoing proliferation (Figure 3D and E) than control pancreatic ducts. These data indicate that Prox1 function restricts the proliferation of duct epithelial cells in the developing pancreas.
Pancreatic ductal cells have a single cilium, and mutations affecting cilia development promote a dilated ductal phenotype or cyst formation in this organ.7–10 Likewise, dilated ducts and cysts were also reported in pancreata devoid of the transcription factor Hnf6.7,8 Immunohistochemistry analyses conducted at E17.5 (Figure 3F) or at birth (data not shown) showed both normal expression of Hnf6 and the presence of primary cilia in duct epithelial cells of Prox1ΔPanc pancreata (Figure 3F). Therefore, the dilated phenotype of Prox1-deficient duct epithelial cells could not result from cilia deficiency or lack of Hnf6 activity. We also noticed similar expression of the transcription factors Sox930 and Hnf1β32 in pancreatic ducts of Prox1ΔPanc and control mice at both E17.5 and birth (Figure 3F and data not shown). These results rule out Sox9 or Hnf1β deficiencies as components of the Prox1ΔPanc ductal phenotype.
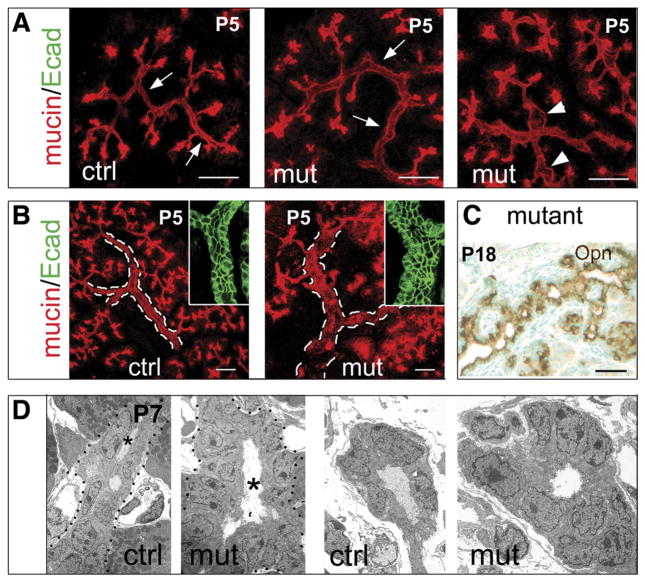
Immunostaining of thick (60 μm) P5 pancreatic sections with anti-mucin antibodies showed that both the small intralobular (Figure 4A) and the large interlobular (Figure 4B) ducts of Prox1ΔPanc pancreata were unusually thick and had dilations (Figure 4A, arrows) that were absent in similar control tissues. Also, E-cadherin immunostaining of P5 pancreata revealed that the mutant duct epithelium was slightly hyperplastic (Figure 4B, insets), whereas Opn immunostaining of P18 pancreata showed that the surface of the Prox1ΔPanc ducts was rugged and not smooth (Figure 4C and Supplementary Figure 3A). TEM analyses corroborated increased luminal size and mild hyperplasia in the pancreatic ducts of P7 Prox1ΔPanc mice (Figure 4D). Therefore, the Prox1ΔPanc embryonic ductal phenotype persists beyond birth.
Figure 4.
Prox1 deficiency promotes the formation of a mildly hyperplastic pancreatic ductal tree. Multiphoton confocal images from 60-μm frozen P5 pancreatic sections stained with anti-mucin antibodies reveal that both the small intralobular ducts (A, arrows) and the large interlobular (B, broken lines) ducts of Prox1ΔPanc pancreata are slightly thicker compared with control pancreatic ducts. Dilations in the mutant intralobular ducts are also frequently observed (A, arrowheads). Anti–E-cadherin staining (green, insets in B) shows that the mutant large ducts have more epithelial cells than similar control ducts. (C) Anti-osteopontin staining reveals the rugged surface of a P18 Prox1-deficient large duct. (D) TEM analysis shows that both intralobular (left) and interlobular (right) P7 Prox1ΔPanc pancreatic ducts (mut) have increased luminal size (asterisks) and more epithelial cells than control (ctrl) ducts (original magnification 3000×). Scale bars = 25 μm (A–C).
We further investigated whether Prox1 loss of function affected the epithelial properties of pancreatic ductal cells using TEM and immunohistochemistry. We found that the distribution of E-cadherin proteins in the lateral membrane (Supplementary Figure 2A), the expression of ZO-1 proteins in tight junctions (Supplementary Figure 2A), and the ultrastructure of tight junctions (Supplementary Figure 2C) were comparable between control and Prox1ΔPanc duct epithelia at P5–P7. Interestingly, qRT-PCR results uncovered significantly increased expression of claudin-1 and claudin-2 transcripts, but normal expression of claudin-3 and claudin-7 in P5 Prox1ΔPanc pancreata (Supplementary Figure 2B). Results of immunohistochemistry corroborated a substantial increment of claudin-2 proteins, but normal expression of claudin-7 and claudin-3 in Prox1-deficient pancreata at P5 (Supplementary Figure 2A). Immunohistochemistry data also showed that in control duct epithelia claudin-2 proteins were distributed on the apical surface and largely colocalized with ZO-1 in tight junctions (Supplementary Figure 2A, left, inset). In contrast, claudin-2 proteins were both overabundant and aberrantly distributed in the lateral membrane of the Prox1ΔPanc ductal epithelium, although some of these proteins also localized correctly at the tight junction (Supplementary Figure 2A). In cultured cells, claudin-2 expression was shown to affect paracellular permeability by promoting the formation of “large pores” in epithelia.33 Furthermore, claudin-2 expression was found abnormally elevated in the intestine of patients with inflammatory bowel disease or Crohn’s disease,34 in preneoplastic pancreatic lesions (Westmoreland et al, manuscript in preparation), and in pancreatic tumors.35 However, whether aberrant claudin-2 expression affects ductal development or impairs the function of duct epithelia in the pancreas is currently unknown.
In summary, our data conclusively showed that Prox1 function is necessary to establish the structural properties of pancreatic duct epithelia.
Extensive Loss of Acinar Cells Occurs in Postnatal Prox1ΔPanc Pancreata
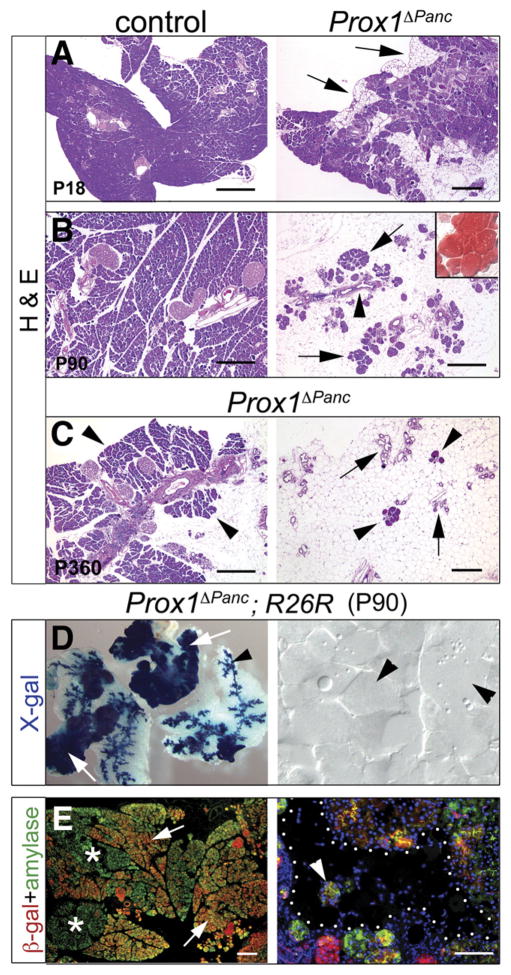
Prox1ΔPanc mice were viable and many had a life span comparable to control littermates. However, pancreas homeostasis gradually deteriorated in all Prox1ΔPanc mice and, in some instances, these mutants died spontaneously or were killed because of poor health. One major alteration observed in Prox1ΔPanc adult pancreata was an extensive lack of acinar tissue, a defect that was first obvious at approximately P18 (Figure 5A). We noticed that some lobes of Prox1-deficient pancreata were entirely devoid of acinar tissue and merely consisted of a few isolated ducts and islets embedded within a large mass of fat (Figure 5B and C). Staining with oil red O corroborated infiltration of adipocytes (inset in Figure 5B). Hence, Prox1 loss of function promotes considerable loss of acinar cells and lipomatosis in the pancreas.
Figure 5.
Loss of acinar tissue and lipomatosis in Prox1-deficient pancreata. (A) Prox1ΔPanc pancreata experience gradual loss of acinar tissue (arrows) starting at approximately P15–P18. (B) By P90, an extensive mass of adipocytes (oil red O+; inset) appears, engulfing some ducts (arrowhead) and a few small acini (arrows) in the mutant pancreas. Similar features are also observed in the pancreas of older Prox1ΔPanc mice (C, right), although these animals always retain a few intact acinar lobes (C, arrowheads, left). Lineage tracing results of P90 Prox1ΔPanc;R26R pancreata stained with X-gal (D) or with anti–β-gal and anti-amylase antibodies (E, asterisks show acini devoid of β-gal) support that both the remnants of acinar tissue (arrows, left) and the ducts (arrowhead, left) are derived from cells in which Prox1 was deleted. In contrast, X-gal staining (D, right, arrowheads) or immunohistochemistry results (E, right, dotted area; arrowhead shows an acinus) rule out that the adipocyte infiltrates of Prox1-deficient pancreata originate from pancreatic epithelial cells. Scale bar = 50 μm (A–C), 100 μm (E, right), and 200 μm (E, left).
We noticed that the pancreas of Prox1ΔPanc old adult mice (>1 year old) always retained a variable portion of acinar tissue (Figure 5C). We used Pdx1-CreEARLY; ROSA26R mice to investigate if this acinar remnant was derived from cells spared of Cre-mediated Prox1 deletion (in these animals, β-gal reporter expression should be activated and maintained in the progeny of all cells expressing Cre).29 Analysis of 3-month-old Prox1ΔPanc; R26R pancreata stained with X-gal or anti–β-gal antibodies showed acini that were positive for these markers (arrows in Figure 5D and E) and acini that did not stain for β-gal proteins (asterisks in Figure 5E). These data indicate that some acini in which Prox1 was deleted were preserved in Prox1ΔPanc pancreatic tissues, although it remains plausible that the β-gal–positive acinar tissue of Prox1ΔPanc; R26R pancreata derived from progenitors in which only one floxed-Prox1 allele was deleted. Importantly, we found that all adipocytes of Prox1ΔPanc; R26R pancreata were negative for X-gal staining (Figure 5D, right) or devoid of β-gal immunoreactivity (Figure 5E, right), an indication that these cells did not originate from trans-differentiated pancreatic acinar cells.
Prox1ΔPanc Mice Gradually Develop a Long-lasting Pancreatic Pathology
In the pancreas of the oldest Prox1ΔPanc mice analyzed (8 –14 months old), we observed numerous ductal features that were absent in the pancreas of control mice. These abnormalities included acinar-to-ductal metaplasias and extensive cellular debris in the lumen of large ducts (Supplementary Figure 3B), cysts and ductal structures with abundant cytoplasm (Supplementary Figure 3C), ductal cells resembling intestinal goblet cells (periodic acid–Schiff positive) or expressing insulin (Supplementary Figure 3D), and ducts expressing phosphorylated Erk1/2 proteins or the proliferation marker Ki67+ (Supplementary Figure 3E). Some of the previous ductal defects of Prox1ΔPanc adult mice have been reported in the pancreas of rodents or humans with pancreatitis.4,8,36
Pancreatitis is initiated by intrapancreatic activation of digestive enzymes, leading to autodigestion of the organ and ensuing tissue damage.36 Acinar cell death, fibrosis, immune cell infiltration, and intrapancreatic activation of digestive enzymes are all features associated with this disease. Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) results indicated that the acinar cell loss in Prox1ΔPanc pancreata was due to apoptotic cell death (Figure 6A). H&E staining and trichrome staining also revealed mild fibrosis and increased stroma in Prox1ΔPanc adult pancreata (Figure 6B). In addition, immune infiltrates consisting of macrophages and neutrophils were noticed throughout the mutant pancreas (Figure 6C). Similar features were absent or only occasional in the corresponding control pancreatic tissues (data not shown). On the other hand, Prox1ΔPanc mice did not display the extensive fibrosis and massive infiltrates of B and T cells characteristic of pancreatitis.
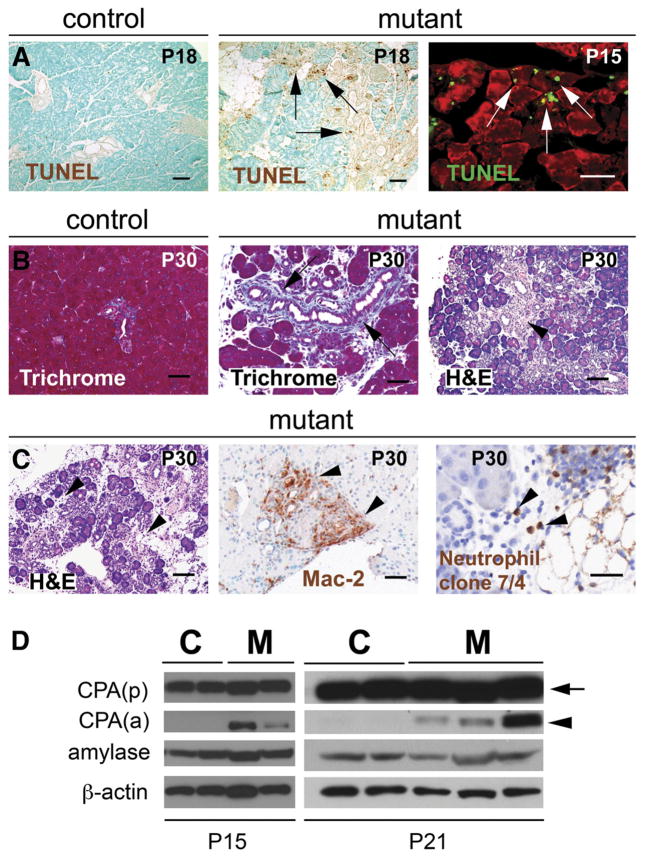
Figure 6.
Prox1ΔPanc pancreata have features indicative of tissue damage. (A) Results of TUNEL assay show apoptotic acinar cells (arrows) in P15–P18 Prox1ΔPanc pancreata but not in control tissues. Other tissue damage features observed in P30 Prox1ΔPanc pancreata but not in control pancreata include periductal fibrosis (B, center, arrows; trichrome staining), increased stromal cells (B, H&E staining, arrowhead), immune infiltrates (C, H&E staining, arrowheads), macrophage (C, center, arrowheads; anti–Mac-2 staining) and neutrophil infiltrates (C, right, arrowheads; anti-neutrophil clone 7/4 staining), and intrapancreatic activation of CPA (D). (D) Results of Western blot analysis of total protein from individual control (C) or Prox1ΔPanc (M) pancreata dissected at P15 or P21. CPA(p) (arrow) and CPA(a) (arrowhead) indicate the precursor or active forms of CPA, respectively. Scale bars = 50 μm (A, right; B, left and center; C, center and right) and 100 μm (A, left and center; B, right; C, left).
Western blot analyses were used to investigate whether inappropriate activation of digestive enzymes occurred in Prox1ΔPanc pancreata. The CPA exocrine enzyme is produced as a precursor protein (~45 kilodaltons), which undergoes proteolytic processing to generate an active form of ~35 kilodaltons. We detected equivalent levels of the precursor form of CPA in extracts of both control and Prox1ΔPanc pancreata dissected at P15–P21 (arrow in Figure 6D). Although the extent of activation was variable among individual specimens, the 35-kilodalton active form of CPA was detected in every Prox1ΔPanc pancreatic sample analyzed (arrowhead in Figure 6D). In contrast, activated CPA was absent in all control pancreatic extracts (Figure 6D). Intrapancreatic activation of CPA was also detected in P10 Prox1ΔPanc extracts, coinciding with the observed onset of exocrine cell apoptosis (data not shown).
In summary, we discovered that Prox1ΔPanc mice gradually develop a long-lasting pancreatic pathology, with potential unfavorable functional effects.
Discussion
Does Prox1 Control the Expansion of “Tip” Progenitors?
Our previous analyses of Prox1-nullizygous embryos underscored the participation of Prox1 function in various aspects of early pancreas organogenesis, including tissue growth, branching morphogenesis, and early endocrine differentiation. Using a conditional knockout approach to delete Prox1 in pancreatic progenitors, this study extended those results by corroborating that Prox1 function is required for early pancreatic growth and showing that the lack of Prox1 activity transiently delays islet cell genesis.
We found that Prox1 deficiency prematurely activates the expression of acinar genes and also abrogates the formation of branches in the early developing pancreas. It is conceivable that these 2 phenotypes are interrelated because a recent study showed that the tip of the pancreatic branches harbors a specific class of precursors called “tip” cells, which produce 2 types of progeny: tip cells and “trunk” precursors.37 According to this model, as tip cells expand, they deploy numerous trunk precursors that gradually form the pancreatic branches. Likewise, tip cells supply the precursors of acinar cells that normally initiate differentiation at approximately E12.5.37 We hypothesize that in the absence of Prox1 function, tip cells undergo acinar differentiation prematurely, which lessens the production of trunk precursors and, consequently, branch formation. A depletion of trunk precursors could also explain the Ngn3+ cell deficiency observed at E13.5 in Prox1-deficient pancreata, because trunk cells are believed to give rise to both endocrine and ductal progeny.37 Our finding that islet genesis was restored in Prox1ΔPanc pancreata after E15.5 is interesting, but the mechanism responsible for this effect has not yet been identified.
The specific role of Prox1 in tip cells/acinar precursors also remains to be determined. However, a recent study showed that the nuclear receptor Lrh-1 controls the expression of multiple acinar genes in the developing pancreas,38 and Prox1 was shown to act as an Lrh-1 corepressor in cultured hepatic cells.19 Therefore, one provocative possibility is that Prox1 prevents acinar gene expression by opposing Lrh-1 activity in tip cells/acinar precursors.
We found that the premature differentiation of acinar cells does not significantly impact this cell lineage, because no obvious alterations in morphology, ultrastructure, or gene expression were observed in the Prox1-deficient acinar tissue after E15.5. The lack of a noticeable postnatal acinar phenotype was perplexing because all Prox1ΔPanc adult mice displayed substantial acinar cell apoptosis after weaning stages. To conclusively determine whether this abnormality represents an intrinsic acinar defect or is a consequence of the ductal alterations will require inactivating Prox1 function specifically in those lineages.
Prox1 Is a Novel Regulator of Pancreatic Ductal Development
The morphology of the pancreatic duct epithelium varies at different levels of the ductal tree, and these variations include changes in the luminal area, which is large at the level of the main pancreatic duct and very small in the terminal intercalated ducts.1 These structural distinctions are established during embryogenesis and presumably are necessary to facilitate the flow of pancreatic secretions toward the duodenum. Prox1ΔPanc mice were born with various morphologic ductal defects that persisted through adulthood. In particular, we discovered that Prox1 loss of function augmented the luminal area of both small and large pancreatic ducts, probably because it enhanced the proliferation of duct epithelial cells. Uncovering the molecular bases of how Prox1 regulates the proper caliber of pancreatic ducts will be the focus of our future efforts.
Abnormalities in the development of pancreatic ducts were also reported in Hnf6-nullizygous mice7 or in mice with pancreas-specific inactivation of Hnf6 (Hnf6ΔPANC).8 However, loss of Hnf6 function perturbed primary cilia development, a defect not observed in the pancreas of Prox1ΔPanc mice. Also, Hnf6ΔPANC mice became afflicted with pancreatitis and their pancreatic tissues displayed more severe inflammation and fibrosis, and less fat infiltration, than Prox1ΔPanc pancreata. Interestingly, the study by Zhang et al reported deficient expression of Prox1 in the dilated pancreatic ducts of Hnf6ΔPANC mice,8 whereas our study found that in Prox1ΔPanc pancreatic ducts Hnf6 expression was normal. These data support the notion that Hnf6 acts upstream of Prox1 during pancreatic duct development.8
Are Pancreatic Ductal Defects the Primary Cause of Acinar Cell Death in Prox1ΔPanc Mice?
Pancreatic ductal cells secrete fluid necessary to transport digestive enzymes and bicarbonate.3,39 Defective anion secretion reduces water fluid flow, increases protein and solute concentration, thickens the pancreatic secretions, and leads to ductal obstruction.3 Importantly, impaired ductal secretion can predispose to chronic pancreatitis.2 Our analysis of Prox1ΔPanc adult pancreata uncovered numerous features indicative of ensuing, chronic tissue damage, such as intrapancreatic activation of digestive enzymes, prominent acinar apoptosis, macrophage infiltration, mild fibrosis, and extensive lipomatosis. We also found that the luminal area of the Prox1-deficient pancreatic ducts was larger than normal, especially in the terminal segments of the ductal tree. We argue that these 2 defects are possibly connected because an enlarged ductal lumen could retard the normal flow of pancreatic secretions and, consequently, expose the acinar epithelium to elevated concentrations of digestive enzymes that could cause tissue damage and activate apoptosis.
It is worth mentioning that the recently published Lrh-1 study found that this nuclear receptor also regulates secretion in the pancreatic ducts.38 Based on this result and the proposed role of Prox1 as an Lrh-1 transcriptional corepressor,19 one could hypothesize that loss of Prox1 function impairs the secretory properties of pancreatic ductal cells through the absence of Lrh-1 antagonism. Our future efforts also will attempt to explore this possibility.
In conclusion, we identified Prox1 as a novel regulator of pancreatic exocrine development and possibly ductal physiology. Dissecting the molecular pathway(s) controlled by Prox1 in developing exocrine cells of the pancreas remains a priority and should help disclose the etiology of some forms of pancreatic exocrine disease in humans.
Supplementary Material
Acknowledgments
N.H.’s current affiliation is: Division of Haematology, Centre for Cancer Biology, SA Pathology, Adelaide, Australia.
The authors thank L. Bachaboina and Nanjia Yu for excellent technical assistance; K.G. Murti and J. Peters for help with the confocal/multiphoton microscopy; L. Mann and S. Fraser for help with TEM analyses; M. Gannon, A. Means, and R. McDonald for valuable advice; G. Oliver for providing Prox1loxP/loxP mice; D. Melton, G. Gonqiang, and the Mutant Mouse Regional Resource Centers for providing Pdx1-CreEARLY mice; and C. Wright, M. German, and S. Konyeczny for generously providing antibodies.
Funding
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (5 R01DK060542), and American Lebanese Syrian Associated Charities.
Abbreviations used
- β-gal
β-galactosidase
- E
embryonic day
- P
postnatal day
- qRT-PCR
quantitative real-time polymerase chain reaction
- TEM
transmission electron microscopy
- TUNEL
terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.12.007.
References
- 1.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132:745–762. doi: 10.1053/j.gastro.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Grapin-Botton A. Ductal cells of the pancreas. Int J Biochem Cell Biol. 2005;37:504–510. doi: 10.1016/j.biocel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Golson ML, Loomes KM, Oakey R, et al. Ductal malformation and pancreatitis in mice caused by conditional Jag1 deletion. Gastroenterology. 2009;136:1761–1771. e1. doi: 10.1053/j.gastro.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Yee NS, Lorent K, Pack M. Exocrine pancreas development in zebrafish. Dev Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Hale MA, Kagami H, Shi L, et al. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev Biol. 2005;286:225–237. doi: 10.1016/j.ydbio.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Pierreux CE, Poll AV, Kemp CR, et al. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology. 2006;130:532–541. doi: 10.1053/j.gastro.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Ables ET, Pope CF, et al. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech Dev. 2009;126:958–973. doi: 10.1016/j.mod.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano DA, Sekine S, Hebrok M. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology. 2006;131:1856–1869. doi: 10.1053/j.gastro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Davenport JR, Croyle MJ, et al. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Lab Invest. 2005;85:45–64. doi: 10.1038/labinvest.3700207. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Kilic G, Aydin M, et al. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev Biol. 2005;286:182–194. doi: 10.1016/j.ydbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24:2115–2126. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 14.Dyer MA, Livesey FJ, Cepko CL, et al. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- 15.Wigle JT, Chowdhury K, Gruss P, et al. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 16.Risebro CA, Searles RG, Melville AA, et al. Prox1 maintains muscle structure and growth in the developing heart. Development. 2009;136:495–505. doi: 10.1242/dev.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavado A, Lagutin OV, Chow LM, et al. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8:e1000460. doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charest-Marcotte A, Dufour CR, Wilson BJ, et al. The homeobox protein Prox1 is a negative modulator of ERR{alpha}/PGC-1{alpha} bioenergetic functions. Genes Dev. 2010;24:537–542. doi: 10.1101/gad.1871610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin J, Gao DM, Jiang QF, et al. Prospero-related homeobox (Prox1) is a corepressor of human liver receptor homolog-1 and suppresses the transcription of the cholesterol 7-alpha-hydroxylase gene. Mol Endocrinol. 2004;18:2424–2439. doi: 10.1210/me.2004-0009. [DOI] [PubMed] [Google Scholar]
- 20.Song KH, Li T, Chiang JY. A Prospero-related homeodomain protein is a novel co-regulator of hepatocyte nuclear factor 4alpha that regulates the cholesterol 7alpha-hydroxylase gene. J Biol Chem. 2006;281:10081–10088. doi: 10.1074/jbc.M513420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrova TV, Nykanen A, Norrmen C, et al. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell. 2008;13:407–419. doi: 10.1016/j.ccr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Yoshimoto T, Shimoda M, et al. Loss of function of the candidate tumor suppressor prox1 by RNA mutation in human cancer cells. Neoplasia. 2006;8:1003–1010. doi: 10.1593/neo.06595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Li H, Qi L, et al. Variants in GLIS3 and CRY2 are associated with type 2 diabetes and impaired fasting glucose in Chinese Hans. PLoS One. 2011;6:e21464. doi: 10.1371/journal.pone.0021464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan MR, Chang TM, Chang HC, et al. Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. J Cell Sci. 2009;122:3358–3364. doi: 10.1242/jcs.050005. [DOI] [PubMed] [Google Scholar]
- 25.Cui W, Tomarev SI, Piatigorsky J, et al. Mafs, Prox1, and Pax6 can regulate chicken betaB1-crystallin gene expression. J Biol Chem. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- 26.Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147–155. doi: 10.1016/s0925-4773(02)00240-x. [DOI] [PubMed] [Google Scholar]
- 27.Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 28.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 29.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 30.Seymour PA, Freude KK, Tran MN, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesavan G, Sand FW, Greiner TU, et al. Cdc42-mediated tubulogenesis controls cell specification. Cell. 2009;139:791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 32.Solar M, Cardalda C, Houbracken I, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Amasheh S, Meiri N, Gitter AH, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 34.Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borka K, Kaliszky P, Szabo E, et al. Claudin expression in pancreatic endocrine tumors as compared with ductal adenocarcinomas. Virchows Arch. 2007;450:549–557. doi: 10.1007/s00428-007-0406-7. [DOI] [PubMed] [Google Scholar]
- 36.Bhanot UK, Moller P. Mechanisms of parenchymal injury and signaling pathways in ectatic ducts of chronic pancreatitis: implications for pancreatic carcinogenesis. Lab Invest. 2009;89:489–497. doi: 10.1038/labinvest.2009.19. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Law AC, Rajagopal J, et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Holmstrom SR, Deering T, Swift GH, et al. LRH-1 and PTF1-L coregulate an exocrine pancreas-specific transcriptional network for digestive function. Genes Dev. 2011;25:1674–1679. doi: 10.1101/gad.16860911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MG, Muallem S. Physiology of duct cell secretion. In: Beger HG, Warshaw AL, Buchler MW, et al., editors. The pancreas: an integrated textbook of basic science, medicine, and surgery. Malden, MA: Blackwell; 2008. pp. 78–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.