Abstract
Over the past two decades, research has identified extrasynaptic GABAA receptor populations that enable neurons to sense the low ambient GABA concentrations present in the extracellular space in order to generate a form of tonic inhibition not previously considered in studies of neuronal excitability. The importance of this tonic inhibition in regulating states of consciousness is highlighted by the fact that extrasynaptic GABAA receptors (GABAARs) are believed to be key targets for anaesthetics, sleep-promoting drugs, neurosteroids, and alcohol. The neurosteroid sensitivity of these extrasynaptic GABAARs may explain their importance in stress-, ovarian cycle- and pregnancy-related mood disorders. Moreover, disruptions in network dynamics associated with schizophrenia, epilepsy and Parkinson’s disease may well involve alterations in the tonic GABAAR-mediated conductance. Extrasynaptic GABAARs may therefore present a potential therapeutic target for treatment of these diseases, but also to enhance cognition and aid post-stroke functional recovery.
The GABAergic system of the mammalian brain consists of GABA-releasing cells and receptors that bind GABA. GABA-releasing cells are extraordinarily diverse and highly specialized (Freund and Buzsaki, 1996; Klausberger and Somogyi, 2008), controlling both the activity of local networks (e.g. interneurons), and forming the output of some brain areas and nuclei (e.g. striatal medium spiny neurons and cerebellar Purkinje cells). Receptors that bind GABA are present on virtually every neuron in the brain and represent a diverse array of receptor types (Mody and Pearce, 2004). This review focuses on GABAA receptors (GABAARs) that are excluded from synapses (see Figure 1). It has long been appreciated that ligand-gated ion channels that bind glutamate and GABA are found outside synapses in the somatic, dendritic and even axonal membranes of mammalian neurons (Brown et al., 1979; Soltesz et al., 1990). The first indication that a persistent, tonic conductance could result from activation of extrasynaptic GABAAR populations came from whole-cell voltage-clamp recordings made from developing neurons when synapses are being formed (Ben-Ari et al., 1994; Kaneda et al., 1995; Valeyev et al., 1993). In these experiments, the addition of GABAAR blockers reduced the standing holding current indicating that a tonic GABAAR-mediated conductance had to be present that was not associated with conventional IPSCs (Otis et al., 1991). It is believed that these early developmental forms of GABA signalling may play a role in controlling neuronal differentiation (LoTurco et al., 1995; Markwardt et al., 2011; Owens et al., 1999). This type of intercellular communication is fundamentally different from the “point-to-point” communication that underlies both synaptic transmission and gap junction-mediated electrical coupling. It is more similar to the volume and paracrine transmission associated with the actions of neuromodulators such as serotonin, histamine, dopamine, acetycholine and peptides in the brain (Agnati et al., 2010). Attention has subsequently focused on the molecular identity of the extrasynaptic GABAARs that generate the tonic conductance and on exploring their physiological relevance for the adult brain (Farrant and Nusser, 2005).
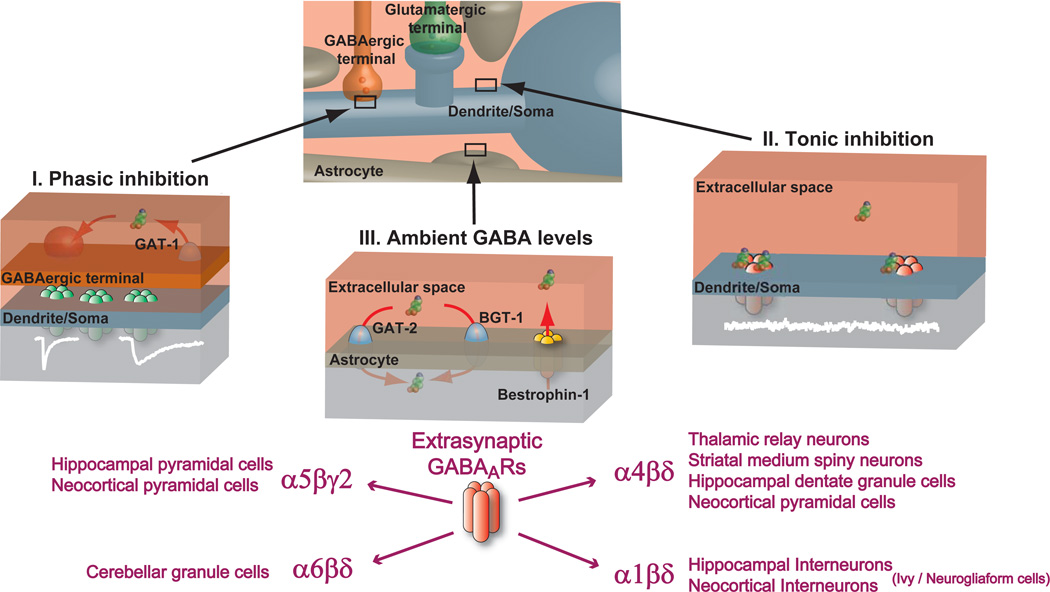
Figure 1. Tonic inhibition is mediated by extrasynaptic GABAA receptors.
The dendrite/soma of a neuron receives a constant barrage of synaptic drive from glutamatergic and GABAergic terminals. The astrocytes that closely intermingle with these structures sense the release of these neurotransmitters as well as modulating their levels within the extracellular space. Vesicular GABA release from GABAergic terminals as well as non-vesicular GABA release from other sources interacts with GABA uptake mechanisms to set the ambient GABA levels within the extracellular space. I. Phasic inhibition: GABA molecules are packaged into synaptic vesicles within the GABAergic terminal. Once released, GABA rapidly diffuses across the synaptic cleft to occupy synaptic GABAARs that can exist in various subunit compositions. The low affinity of synaptic receptors means that although the synaptic cleft concentration is high (1–10 mM) the GABA molecules only occupy the receptors for a very short duration. Brief GABAAR occupancy is further ensured by the rapid removal of GABA from the synaptic cleft (<1ms) due to diffusion and active binding and uptake by GABA transporter proteins (GAT-1) located in the presynaptic axon terminal. The resulting brief postsynaptic conductance change (white trace) is characterised by a fast rising and slow decaying waveform that can vary in duration depending upon the subunit composition of the synaptic GABAARs and the transmitter profile within the cleft. II. Tonic inhibition: The low resting ambient GABA levels present in the extracellular space are able to activate high-affinity extrasynaptic GABAARs to generate a persistent conductance (Cl- and to a lesser extent HCO3−) that is responsible for generating tonic inhibition (noisy white trace) in a number of neuronal types. III. Ambient GABA levels: The precise mechanisms for regulating ambient GABA levels within the brains extracellular space is beginning to be elucidated and involves an interplay between the level of vesicular GABA release, the stoichiometry of the GABA transporters (GAT-2 and BGT-1 in astrocytes and GAT-1 in axon terminals) and other forms of non-vesicular GABA release such as GABA permeation through bestrophin channels. Ultimately, it is the level of ambient GABA that leads to the activation of extrasynaptic GABAARs in the soma/dendrite and even axonal membrane that generate the tonic inhibition.
GABAARs are pentameric assemblies usually made up from at least three different proteins selected from 19 different subunits (Olsen and Sieghart, 2008). These include α1-6, β1-3, γ1-3, δ, ε, θ, π and ρ1–3 (Olsen and Sieghart, 2008, 2009; Whiting, 2003). A receptors’ regional and developmental expression pattern, as well as their physiological and pharmacological properties are determined by differences in subunit gene expression and composition (Hevers and Luddens, 1998; Mody and Pearce, 2004) and the rules governing these relationships have received a great deal of attention in the search for highly specific drug targets in the CNS (Olsen and Sieghart, 2009; Whiting, 2003). The subunit identity of the final assembly also determines the synaptic or extrasynaptic localization of GABAARs within a neuron (Pirker et al., 2000) reflecting the existence of various subunit assembly rules and anchoring/trafficking mechanisms (Luscher et al., 2011; Vithlani et al., 2011). Following the original description of the GABAAR δ-subunit (Shivers et al., 1989) and its expression patterns in the brain (Wisden et al., 1992), it was first shown for mature cerebellar granule cells that extrasynaptic α6βδ subunit-containing GABAARs mediate a tonic form of inhibition both in vitro (Brickley et al., 2001; Hamann et al., 2002) and in vivo (Chadderton et al., 2004), while conventional synaptic γ2 subunit–containing GABAARs are involved in direct synaptic transmission (Farrant and Nusser, 2005). A tonic conductance mediated by α4βδ subunit-containing GABAARs has now also been reported in dentate gyrus granule cells, thalamic relay neurons, neocortical layer 2/3 pyramidal cells, and medium spiny neurons of the striatum (Ade et al., 2008; Drasbek and Jensen, 2006; Kirmse et al., 2008; Porcello et al., 2003; Salin and Prince, 1996; Santhakumar et al.; Stell et al., 2003). Additionally, a tonic conductance present in Ivy/neuorgliaform cells (Capogna and Pearce, 2010; Szabadics et al., 2007) is probably generated by the persistent activation of extrasynaptic α1βδ subunit-containing extrasynaptic GABAARs (Olah et al., 2009).
Given that persistently active δ-GABAAR openings make such a major contribution to the total charge that flows across the membrane (Belelli et al., 2005; Brickley et al., 1996; Nusser and Mody, 2002) it is not surprising that this type of conductance is capable of modulating both cell and network behavior (Farrant and Nusser, 2005). In thalamic relay neurons, for example, the membrane hyperpolarization associated with the persistent chloride flux through δ-GABAARs leads to burst firing (Cope et al., 2005) and slow thalamo-cortical oscillations (Winsky-Sommerer et al., 2007). However, the tonic conductance may not always result in membrane hyperpolarization. In cerebellar granule cells, the membrane shunt associated with tonic inhibition attenuates excitatory drive with little impact on the membrane potential (Brickley et al., 2001). It is also worth noting that a shunting inhibition associated with a tonic conductance could result in a small but persistent membrane depolarization (Farrant and Kaila, 2007). Another striking feature of the tonic conductance measured in adult neurons is that it represents the simultaneous opening of only a very small fraction of the available extrasynaptic GABAARs (Kasugai et al., 2010; Nusser et al., 1995) indicating that receptor occupancy is low and/or a large number of receptors are heavily desensitized. The δ-GABAARs recorded at room (Mortensen et al., 2010) and physiological temperatures (Bright et al., 2011) are predicted to be profoundly desensitized. Although tonic inhibition can be generated by a desensitized receptor population as long as receptor number is high, this feature could limit the ability of these receptors to operate as spillover detectors and other less desensitized extrasynaptic GABAARs could be better suited to this role. Slow-rising and slow-decaying IPSCs generated by GABA spillover is a significant feature of GABA release from Ivy/neuorgliaform cells (Capogna and Pearce, 2010; Szabadics et al., 2007) and has been reported in hippocampal neurons (Vargas-Caballero et al., 2009; Zarnowska et al., 2009). One challenge for the future is to establish whether the spillover currents observed in these and other cell types, reflects activation of distinct extrasynaptic GABAAR populations separate from those responsible for generating tonic inhibition (Farrant and Nusser, 2005).
It is now appreciated that in addition to δ-GABAARs, other GABAAR types are also capable of generating a tonic conductance in a number of adult brain regions. Most notably, α5βγ2 subunit-containing GABAARs (α5-GABAARs) generate a tonic conductance that regulates the excitability of pyramidal neurons in CA1 and CA3 regions of the hippocampus (Caraiscos et al., 2004; Glykys and Mody, 2006, 2007; Pavlov et al., 2009; Prenosil et al., 2006; Semyanov et al., 2004) and layer 5 cortical neurons (Yamada et al., 2007). High-affinity GABAARs made up of only αβ subunits are also a possibility (Mortensen and Smart, 2006), as are GABAARs that can open even in the absence of an agonist (Hadley and Amin, 2007), as reported in some immature neurons (Birnir et al., 2000). It is also possible, given the large number of γ2-GABAARs present in both the synaptic and extrasynaptic membrane (Kasugai et al., 2010; Nusser et al., 1995; Soltesz et al., 1990), that more conventional low-affinity GABAARs make a contribution to the steady-state conductance when ambient GABA concentrations are high (Farrant and Kaila, 2007). Nevertheless, it is now appreciated that specific high-affinity GABAAR populations, such as δ-GABAARs and α5-GABAARs, are predominantly responsible for generating the tonic conductance found in many brain regions under normal physiological conditions. The study of these extrasynaptic GABAAR populations is now entering a defining stage and this review focuses on new insights into the potential involvement of these receptors in the cellular and molecular abnormalities underlying neurological and psychiatric disorders including sleep disturbances, stress-related psychiatric conditions, and epilepsy. We also further discuss the potential role of these receptors in cognition, recovery from stroke, and in mediating the effects of alcohol.
SLEEP DISORDERS
Adequate sleep is essential for our well being, and many neuropsychiatric conditions, such as depression and schizophrenia, are associated with severe disruptions in sleep patterns. It is thus dissapointing that we understand little about the mechanisms that control sleep and rely on limited repertoires of clinical interventions to treat sleep disorders (Wafford and Ebert, 2008). GABAARs play a pivotal role in the control of our sleep rhythms, and for many decades benzodiazepines and zolpidem, known for their ability to potentiate GABAAR currents, have remained the most widely prescribed treatment for insomnia, in spite of producing tolerance, addiction and withdrawal problems. In a search for more refined drug interventions, it has become clear that the hypnotic actions of the sleep promoting drug gaboxadol (Wafford and Ebert, 2006) (4,5,6,7-tetrahydroisothiazolo-[5,4-c]pyridin-3-ol; THIP) can be attributed to this drug’s selective action on δ-GABAARs (Brown et al., 2002). At concentrations of around 500 nM, this drug activates δ-GABAARs with little action on synaptic GABAAR types. This selectivity arises from gaboxadol’s lower apparent affinity at γ2-GABAARs compared to δ-GABAARs (Mortensen et al., 2010). Gaboxadol acts as a hypnotic in humans to increase sleep duration by promoting slow-wave or non rapid eye movement (non-REM) sleep (Faulhaber et al., 1997). When δ-GABAARs are removed by genetic manipulations in mice, gaboxadol-induced slow oscillations are absent from the EEG (Winsky-Sommerer et al., 2007) and the anaesthetic potency of gaboxadol is reduced (Boehm et al., 2006). Unfortunately, due to side-effects such as hallucinations and disorientation in a subset of patients, gaboxadol failed phase III clinical trials as an alternative to benzodiazepines but, more potent δ-GABAAR selective agonists are being developed (Wafford et al., 2009).
Alterations in the dynamics of the thalamo-striatal-cortical network likely underlie the sleep disturbances common to many neurological disorders and this may involve alterations in extrasynaptic GABAAR function. In the thalamus a tonic GABA conductance promotes burst firing of thalamic relay neurons (Bright et al., 2007; Cope et al., 2005), a key requirement in the generation of slow 1–4 Hz EEG rhythms during non-REM sleep. During non-REM sleep, ambient GABA levels are higher in the thalamus than during REM or waking states (Kekesi et al., 1997). δ-GABAARs are also found in the superficial neocortical layers 2/3 but there is currently little evidence to suggest that these neocortical δ-GABAARs contribute to the slow thalamo-cortical rhythms observed during sleep. However, neocortical circuits switch between periods of high activity and quiescence (Steriade et al., 1993). In Parkinson’s disease, sleep abnormalities are among the frequent non-motor symptoms that present during its early evolution prior to drug treatment (Chaudhuri and Naidu, 2008). The caudate-putamen of the striatum is a brain region that regulates motor planning and is, therefore, critically linked to Parkinson’s disease. This brain region also expresses high levels of extrasynaptic α4βδ subunit-containing GABAARs and dopamine D1 receptor-expressing medium spiny neurons display a tonic conductance mediated by δ-GABAAR populations (Ade et al., 2008; Kirmse et al., 2008). The loss of dopaminergic drive that characterises Parkinson’s disease explains the enhanced GABA concentrations found in the striatum (Kish et al., 1986) and it is intriguing to speculate that this change may underlie the sleep disruptions associated with Parkinson’s and alterations in ambient GABA levels may contribute to the sleep disturbances commonly associated with a number of neurological disorders including depression.
Drugs which modulate sleep and anaesthesia share common molecular targets (Franks, 2008). Raising ambient GABA levels alone, with GABA uptake blockers, will induce an anaesthesia-like state (Katayama et al., 2007) and neurosteroids (which are brain-synthesized metabolites of ovarian and adrenal cortical steroid hormones) act as anaesthetics through an action on δ-GABAARs (Stell et al., 2003). Indeed, the loss of δ-GABAARs is associated with an attenuated response to neurosteroid-induced anaesthesia (Mihalek et al., 1999). Other important general anaesthetics such as propofol and isoflurane enhance tonic inhibition in hippocampal neurons (Bai et al., 2001), thalamic relay neurons (Jia et al., 2008b), and neocortical neurons (Drasbek et al., 2007). However, the amnesia-inducing effect, but not the anaesthetic potency of isoflurane, is altered in α4 knockout mice which also lack δ- GABAARs on the cell surface (Rau et al., 2009) indicating that extrasynaptic GABAARs are not a primary site of action for all anaesthetics.
STRESS AND PSYCHIATRIC DISORDERS
Neurosteroids are among the most powerful regulators of GABAAR function in the CNS (Belelli and Lambert, 2005; Chisari et al., 2010; Mitchell et al., 2008; Reddy, 2010). The first example of this robust modulatory effect was discovered nearly 30 years ago (Harrison and Simmonds, 1984) for the synthetic steroid alphaxalone (5α-pregnan-3α-ol-11,20 dione). Shortly after, it was demonstrated that a metabolite of the ovarian steroid hormone progesterone (allopregnanolone, also called 3α-hydroxy-5α-pregnan-20-one, or 3α,5α-tetrahydroprogesterone, or 5α-pregnan-3α-ol-20-one, or 5α3α-THPROG) and a metabolite of the stress steroid deoxycorticosterone (aka 5α3α-THDOC) are potent barbiturate-like ligands of GABAARs (Majewska et al., 1986). Our first collaborative research (Stell et al., 2003) demonstrated δ-GABAARs are a preferred site of action for neurosteroids at low (nanomolar) concentrations. This preferred action likely reflects a simple property of these receptors: GABA is not an efficacious agonist at δ-GABAARs (Chisari et al., 2010), which means that the coupling of GABA binding to channel opening is not efficient. Because neurosteroids increase the likelihood that GABA will open the channel (Chisari et al., 2010), they can enhance the efficacy of GABA at δ-GABAARs and thus modulate receptor activity, while this is less likely at other GABAARs where GABA is already an efficacious agonist. Perhaps δ-GABAARs are the preferred site of action for paracrine neurosteroid signaling where the neurosteroids synthesized in another cell (e.g., astrocyte) must travel through the extracellular space to act on extrasynaptic δ-GABAARs. Neurosteroid synthesis in astrocytes is regulated by the mitochondrial 18 kD translocator protein TSPO (the peripheral benzodiazepine receptor by its former name) for which the drug XBD173 is an excellent non-sedative anxiolytic and antipanic agent (Rupprecht et al., 2009). The mitochondrial TSPO is also in CNS neurons where it may mediate autologous effects of neurosteroids on neuronal excitability in brain slices following benzodiazepine (Tokuda et al., 2010) or ethanol (Tokuda et al., 2011) administration.
Since neurosteroid levels in the brain will also mirror ovarian or stress steroid hormone levels, the tonic inhibition regulated by the neurosteroid metabolites of these hormones may contribute to CNS disorders associated with altered hormonal states. For example, the anxiety associated with premenstrual dysphoric disorder (PMDD) has been linked to neurosteroid regulation of tonic inhibition in animals (Maguire et al., 2005; Smith et al., 1998) and the discrepancy between extrasynaptic GABAAR number and post-partum levels of the progesterone metabolite allopregnanolone has been linked to post-partum depression (Maguire and Mody, 2008). During pregnancy, progesterone levels increase by over 100-fold, and the levels of allopregnanolone (produced in the brain from progesterone), which could potentially enhance inhibition through δ-GABAARs, are elevated accordingly. High neurosteroid levels in the brain are dangerous because, they might produce an anaesthetic-like effect by sedating expectant mothers. Most likely as a compensatory mechanism, the number of neurosteroid-sensitive δ-GABAARs decrease during pregnancy, so that the high levels of neurosteroids are offset by fewer δ-GABAARs. However, this balance in the mother’s brain recalibrates just after delivery, when progesterone and neurosteroid levels are restored. With the post-partum drop in neurosteroid levels, the reduced numbers of δ-GABAARs are no longer sufficient to maintain an optimal level of inhibitory tone. The result is a period of increased neuronal excitability until the number of δ-GABAARs is restored to pre-pregnancy levels. In our experiments, we found that delays in the process δ-GABAAR recovery, severe depression-like behavior ensues in mice, which results in mothers cannibalizing their offspring. This behavior is reduced by administering gaboxadol to activate the δ-GABAARs. The recently identified selective δ-GABAAR agonist (DS-1) and an allosteric enhancer (DS-2) of δ-GABAAR function (Wafford et al., 2009) may aid the design of specific treatments for post-partum depression. Analogous changes in δ-GABAAR expression have also been reported to occur during puberty (Shen et al., 2007), which could in part explain why this developmental period is associated with increased susceptibility to stress-related disorders.
Stress induced by social isolation in rats leads to upregulation of extrasynaptic δ-GABAARs and correlates with an increase in hippocampal tonic inhibition (Serra et al., 2006). Hippocampal tonic inhibition counteracts the excitation of interneurons, and can regulate the frequency of gamma oscillations (Mann and Mody, 2010) that have been shown to be altered in schizophrenic patients (Uhlhaas and Singer, 2010). The observation that reductions in δ-GABAAR mRNA have been reported in post-mortem brains of patients with schizophrenia (Maldonado-Aviles et al., 2009), and the association between two polymorphisms in the GABRD gene and childhood onset mood disorders in males (Feng et al., 2010), potentially suggests that altered tonic conductance could explain the disturbances in network behaviour described in such disorders. Interestingly, in humans the GABAAR α5 subunit gene has also been identified as a susceptibility locus for schizophrenia (Maldonado-Aviles et al., 2009) and depression (Kato, 2007). Autopsy studies from individuals who have suffered from major depression exhibit marked changes in a number of genes involved in both glutamate and GABA signalling pathways, including alterations in the expression of α5-GABAARs and δ-GABAARs (Choudary et al., 2005; Sequeira et al., 2009). Although many genes, including those involved in synaptic GABAAR function, can be altered in neuropsychiatric disorders an emerging theme of these and many other studies is that the α5 and δ containing GABAARs are heavily regulated by stress hormones, and this feature is likely to explain why changes in extrasynaptic GABAA receptor expression are so often associated with stress-related disorders.
EPILEPSY
Disturbances in synaptic and extrasynaptic GABAAR function, including several point mutations (Macdonald et al., 2010), have been implicated in many forms of epilepsy. Given the importance of maintaining appropriate levels of tonic inhibition for the control of neuronal network behaviour (Vida et al., 2006) it is not surprising that δ-GABAARs are targets in the treatment of specific forms of epilepsy. Several of the drugs listed in Table I, which are already in clinical use as antiepileptics, modulate tonic inhibition by altering ambient GABA levels in the brain (see also Figure 2). Mutations in the δ subunit gene have also shown some degree of association with genetic forms of human epilepsy (Dibbens et al., 2004; Mulley et al., 2005) and mouse models of temporal lobe epilepsy (Peng et al., 2004) involve changes in tonic inhibition within the hippocampus (Maguire et al., 2005; Peng et al., 2004; Spigelman et al., 2002; Zhang et al., 2007). The neurosteroid analogue ganaxolone is in clinical trials for the treatment of catamenial epilepsy, a form of epilepsy in women that shows cyclic variations in the frequency and intensity of seizures depending on the phases of the menstrual cycle. δ-GABAAR-mediated tonic inhibition has been shown to change during the ovarian cycle (Maguire et al., 2005). As extrasynaptic δ-GABAARs are highly sensitive to modulation by neurosteroids such as progesterone (Stell et al., 2003), the ability of ganaxolone to enhance tonic inhibition (Belelli and Herd, 2003) could explain why this drug protects against seizure during these sensitive periods of the ovarian cycle. However, enhancing tonic inhibition is not a useful strategy for the treatment for all epilepsies. For example, slow wave discharges within the thalamo-cortical network are a defining feature of absence seizures. Paradoxically, this type of seizure is triggered by enhanced δ-GABAAR openings with the GABA agonist gaboxadol (Fariello and Golden, 1987). In rodents, a model of absence epilepsy correlates with increased levels of tonic inhibition on thalamic relay neurons (Cope et al., 2009) due to dysfunction of the GABA transporter (GAT-1) and the resulting elevated ambient GABA levels within the thalamus (Errington et al., 2011). The membrane hyperpolarisation that occurs following enhanced tonic conductance in thalamic relay neurons(Cope et al., 2005) alters the fine balance of the thalamo-cortical network(Bright et al., 2007), leading to slow wave discharges. These observations provide a plausible explanation why treatment of absence seizures in humans, with drugs like tiagabine and vigabatrin, exacerbates this particular form of epilepsy(Perucca et al., 1998).
TABLE 1.
Summary of some clinically relevant drugs that can alter tonic inhibition within the brain.
| Drug (trade names) | Mechanism of action | Current drug indications |
|---|---|---|
|
Gabapentin (Fanatrex, Gabarone, Gralise, Neurontin) |
Originally thought to be a GABA mimetic, but mechanism of action is now unclear. Possible enhancement of GABA synthesis could explain why ambient GABA levels in the brain are raised1. |
Partial-onset seizures in adults and the elderly2 Alcohol withdrawal as a combination therapy3 Sleep disorders4 |
|
Vigabatrin (Sabril) |
Irreversible block of GABA transaminase to interfere with GABA cetabolism and, therefore, raise ambient GABA levels. |
Refractory complex partial seizures and infantile spasms5. Not favoured due to visual field loss in some adults and children6. |
|
Tiagabine (Gabitril) |
Blockade of GABA transporters on nerve terminals (predominantly GAT-1) leads to raised ambient GABA levels. |
Partial seizures Generalized anxiety disorders/panic disorders7 |
|
Pregabalin (Lyrica) |
Enhances the activity of glutamic acid decarboxylase (GAD) leading to increased GABA synthesis and, therefore, raised ambient GABA levels. |
Partial seizures with or without secondary generalization8 Neuropathic pain in diabetese, post herpetic neuralgia and fibromyalgia8. Generalized anxiety disorder8. |
| Gaboxadol | Selective orthosteric agonist at δ-GABAARs leading to specific enhancement of the tonic conductance. |
Sleep enhancer, but withdrawn from Phase III clinical trials due to poor risk-to-benefit ratio9. |
| L-655,708 | High-affinity negative allosteric modulator of α5-GABAARs that will reduce tonic conductances. |
Cognitive enhancer but not thought to be suitable for human use due to anxiogenic properties10. |
| Ganaxolone | Positive allosteric modulator of most GABAARs with greater potency at δ-GABAARs leading to selective enhancement of the tonic conductance. |
Catemenial epilepsy11 |
|
Alphaxalone (Althesin, Saffan) |
Positive allosteric modulator of most GABAARs with greater potency at δ-GABAARs leading to selective enhancement of the tonic conductance. |
Anaesthetic12 and sedative in long-term intensive care patients13. Was withdrawn from clinical practice due to complications with the vehicle, Cremophor EL. Re-branded as Saffan and widely used as an anaesthetic in veterinary surgery. |
|
Propofol (Diprivan) |
Positive allosteric modulator of most GABAARs including α5 and δ-GABAARs leading to enhanced tonic conductance. |
Widely used as an intravenous anaesthetic. |
Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K, (2003) Cellular and molecular action of the putative GABAmimetic, gabapentin CMLS, Cell. Mol. Life Sci. 60; 742–750
Beghi E. Treating epilepsy across its different stages. (2010) Ther Adv Neurol Disord. 3(2):85–92.
Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. (2011) Gabapentin Combined With Naltrexone for the Treatment of Alcohol Dependence. Am J Psychiatry. Mar 31. Epub ahead of print.
Ehrenberg B. (2000) Importance of sleep restoration in co-morbid disease: effect of anticonvulsants. Neurology. 54(5 Suppl 1): S33.
Tolman JA, Faulkner MA. (2009) Vigabatrin: a comprehensive review of drug properties including clinical updates following recent FDA approval. Expert Opin Pharmacother. 10(18): 3077–89.
Chiron C, Dulac O. Epilepsy: (2011) Vigabatrin treatment and visual field loss. Nat Rev Neurol. 7(4):189–90.
Pollack MH, Roy-Byrne PP, Van Ameringen M, et al. (2005). The selective GABA reuptake inhibitor tiagabine for the treatment of generalized anxiety disorder: results of a placebo-controlled study. The Journal of clinical psychiatry 66 (11): 1401–8.
Tassone DM, Boyce E, Guyer J, Nuzum D. (2007) Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 29(1): 26–48.
Saul S (2007) Merck Cancels Work on a New Insomnia Medication. The New York Times. March 29
Navarro JF, Burón E, Martín-López M (2002). Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABA(A) receptors which contain the alpha-5 subunit, in the elevated plus-maze test". Progress in neuro-psychopharmacology & biological psychiatry 26 (7–8): 1389–92.
Biagini G, Panuccio G, Avoli M (2010) Neurosteroids and epilepsy. Curr Opin Neurol. 23(2):170–6.
Winter L, Nadeson R, Tucker AP, Goodchild CS. (2003) Antinociceptive properties of neurosteroids: a comparison of alphadolone and alphaxalone in potentiation of opioid antinociception, Anesth. Analg. 97 798–805.
Stewart GO, Dobb GJ, Craib IA. (1983) Clinical trial of continuous infusion of alphaxalone/alphadolone in intensive care patients. Anaesth Intensive Care. 11(2):107–12.
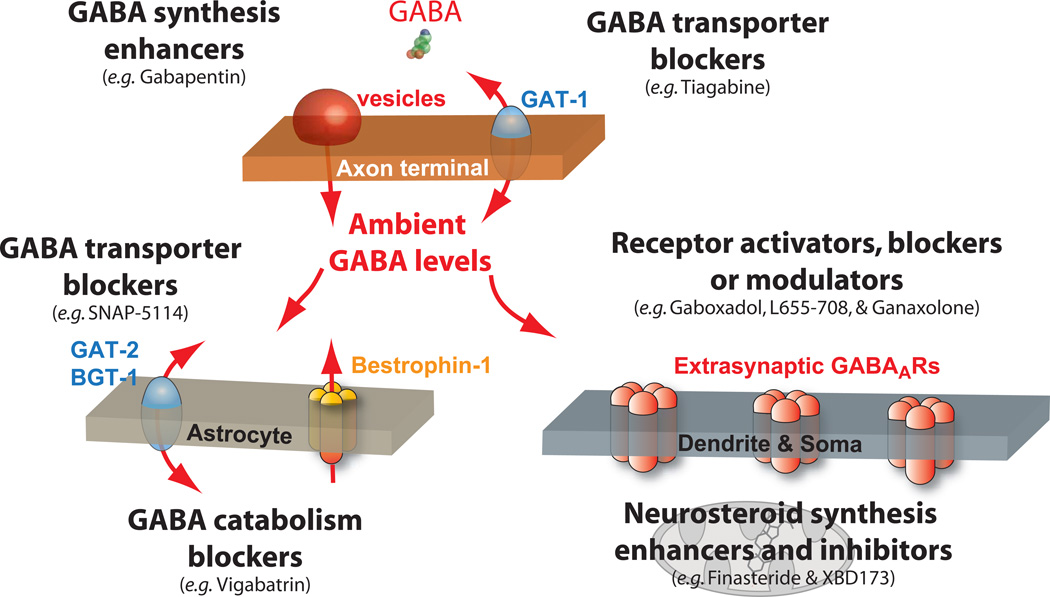
Figure 2. Pharmacological strategies for altering the tonic conductance.
A number of clinically relevant drugs are available that are known to alter the tonic conductance via a variety of direct and indirect targets. Here we illustrate a number of these targets situated within the principal neuronal and non-neuronal compartments of the brain. Although it was originally thought to be a GABA mimetic, the mechanism of action for Gabapentin is currently unclear, but the drugs ability to increase ambient GABA levels in the brain could reflect an alteration in GABA synthesis or release. Gabapentin is currently prescribed for the treatment of partial-onset seizures in adults and the elderly as well as a combination therapy for alcohol withdrawal and for sleep disorders. Tiagabine is a GABA transporter blocker acting predominantly on GAT-1 in nerve terminals leading to raised ambient GABA levels. This drug is prescribed for the treatment of partial seizures as well as generalized anxiety disorders/panic disorders. Other GABA transporter blockers such as SNAP-5114 are more selective blockers of GABA uptake in astrocytes, but these also lead to enhanced ambient GABA levels. Although bestrophin-1 channels could be an alternative nonvesicular source of GABA release, blockade of these channels by NPPB (5-nitro-2-(3-phenylpropylamino) benzoic acid) has been reported to both increase(Rossi et al., 2003) and decrease tonic inhibition(Lee et al., 2010) onto cerebellar granule cells. Irreversible block of GABA transaminase with the prescription drug Vigabatrin represents another strategy for raising ambient GABA levels. Vigabatrin has been used for the treatment of refractory complex partial seizures and infantile spasms but is currently not favoured due to visual field loss in some adults and children. More direct mechanisms for altering tonic inhibition involve orthosteric and allosteric interactions with extrasynaptic GABAARs. For example, the orthosteric agonist THIP or gaboxadol will selectively activate δ-GABAARs and, therefore, promote non-REM sleep. DS-1 is a newly developed agonist that has greater selectivity for δ-GABAARs than THIP, but its clinical benefit has yet to be established. Inverse agonists such as L-655,508 are currently being used to block the current generated by α5-GABAARs with the general objective to being used as cognitive enhancers. Allosteric modulators such as neurosteroids also offer a mechanism for more directly enhancing tonic inhibition. One such drug, Ganaxolone, is currently being developed for the treatment of drug resistant forms of catamenial epilepsy. It may also be possible to enhance or reduce tonic inhibition with Finasteride that blocks neurosteroid synthesis and XBD173 that enhances neurosteroid synthesis via the mitochondrial 18 kD translocator protein TSPO. It is also possible that the β subunit isoform identity may provide a means for selectively modulating tonic inhibition as the preferred β partner is the β2 subunit (Belelli and Lambert, 2005; Belelli et al., 2005; Herd et al., 2008) for α4βδ subunit-containing GABARs in the thalamus and dentate gyrus of the hippocampus. Any future development of β-subunit-dependent phosphorylation drugs could be useful in this regard.
EFFECTS OF ALCOHOL ON THE BRAIN
Unlike other addictive drugs, that have well defined targets in the CNS (e.g. cannabis and cocaine), the intoxicating actions of alcohol have poorly defined molecular targets (Kumar et al., 2009). To demonstrate measurable and consistent effects on neuronal targets, past in vitro studies have used higher ethanol concentrations than those considered to be performance-imparing. In the US, for example, every state sets the legal threshold for blood alcohol concentration at 0.08%, which corresponds to ~17 mM ethanol in the blood. Thus, intoxicating alcohol concentrations within a physiologically relevant range should be used when searching for brain targets of ethanol. In expression systems, δ-GABAARs containing the α4, α6, α1, and β2 or β3 subunits, are all potentiated by ethanol at intoxicating concentrations (Sundstrom-Poromaa et al., 2002). Moreover, ethanol’s action on δ-GABAARs was demonstrated in native neurons (Fleming et al., 2007; Hanchar et al., 2005; Jia et al., 2008a; Liang et al., 2007; Santhakumar et al., 2007; Wei et al., 2004). However, a number of studies have failed to replicate these findings in heterologous expression systems (Baur et al., 2009; Borghese et al., 2006; Korpi et al., 2007; Yamashita et al., 2006) calling into question extrasynaptic δ-GABAARs as a molecular target for intoxicating ethanol concentrations. Indeed, antagonism of the putative alcohol binding site on the δ-GABAAR does not alter alcohol-related behavioural responses in vivo (Linden et al., 2011). It is of course possible that acute effects are due to indirect actions of alcohol on δ-GABAARs (Kumar et al., 2009), either by enhancing vesicular GABA release (Carta et al., 2004) or by enhancing neurosteroid synthesis (Sanna et al., 2004). Hopefully, it will not be too long before a consensus is reached on the acute actions of intoxicating levels of alcohol in the brain.
In the context of the underlying path physiology in alcohol dependence, δ-GABAARs may contribute to the effects of alcohol on the reward system of the brain responsible for reinforcing continued alcohol abuse. RNA interference (RNAi) to reduce the expression of α4 subunits (Rewal et al., 2009) or of δ subunits (Nie et al., 2011) in the nucleus accumbens dorsomedial shell decreased ethanol intake and alcohol preference in rats. Since this highly circumscribed region of the nucleus accumbens is the preferred site of self administration for alcohol and other drugs of abuse such as amphetamine, cocaine, or dopamine receptor agonists, novel mechanisms of acute and chronic ethanol actions on δ-GABAARs discovered over the past decade are beginning to form a cohesive picture, and constitute a first step in understanding the role of the GABAergic system in alcohol abuse, tolerance and dependence. Additionally, long-term alcohol abuse alters GABAAR expression patterns in both animal models and postmortem brain tissue(Kumar et al., 2009). Understanding how changes in extrasynaptic GABAAR function may impact upon addictive behaviour could lead to more rational strategies for the treatment of alcohol dependence and abuse.
LEARNING AND MEMORY/COGNITION ENHANCEMENT
After the discovery of long-term potentiation (LTP) (Bliss and Lomo, 1970) at glutamatergic synapses, a form of neuronal plasticity widely thought to underlie learning and memory, it was discovered that GABAergic inhibition obstructs this plasticity (Wigstrom and Gustafsson, 1983). Low doses of picrotoxin, a non-competitive antagonist that blocks synaptic and extrasynaptic GABAARs, alleviates learning and memory deficits in mouse models of Alzheimer’s disease (Yoshiike et al., 2008), neurofibromatosis (Cui et al., 2008) and Down syndrome (Fernandez et al., 2007). Specific blockers of tonic inhibition mediated by α5-GABAARs and knockout mice for the α5-GABAARs have also provided insights into how these receptors, and the tonic inhibition they mediate, impede learning and cognition (Atack, 2010; Martin et al., 2009). First, mice with a partial or full deficit of α5-GABAARs show improved performance in associative learning and memory tasks(Collinson et al., 2002; Crestani et al., 2002; Yee et al., 2004), with only a minimal deficit in memory for object location (Prut et al., 2010). Second, negative allosteric modulators (or BZD-site inverse agonists) selective for α5-GABAARs, such as α5IA, L-655,708 or RO-493851, all enhance learning and cognitive performance in rodents(Ballard et al., 2009; Chambers et al., 2004; Dawson et al., 2006; Navarro et al., 2002), while having no proconvulsant effects. Data in humans are scarce, but an ethanol-induced amnesia was reduced by administering α5IA to healthy volunteers (Nutt et al., 2007). In hippocampal pyramidal cells, the elevated numbers of δ-GABAARs and enhanced allopregnanolone levels during puberty reduce the probability of inducing LTP (Shen et al., 2010). Adolescent mice also exhibited deficits in an LTP-dependent spatial learning task, which are reversed in adolescent mice lacking δ-GABAARs. The continuing development and refinement of negative allosteric modulators specific for α5-GABAARs (Knust et al., 2009), and other drugs that modulate tonic inhibition mediated by δ-GABAARs, holds promise as novel treatments for Alzheimer’s disease or other neurological and psychiatric disorders characterized by deficits in learning, memory or cognition.
NEUROPROTECTION AND RECOVERY OF FUNCTION AFTER STROKE OR OTHER BRAIN INJURIES
It has been suggested that recovery of function following acute injury to the sensorimotor cortex may be controlled by the availability of GABA (Levy et al., 2002). Enhanced tonic inhibition has an acute neuroprotective quality. For example, medium spiny neurons (MSNs) of the striatum are protected against quinolinic acid or NMDA receptor-mediated toxicity by tonic inhibition (Santhakumar et al., 2010). Compared to wild-type, MSNs from adult mice lacking δ-GABAARs had both decreased tonic GABA currents and reduced MSN survival following an in vitro excitotoxic challenge with quinolinic acid. Furthermore, following acute exposure of MSNs to NMDA in WT, but not mice lacking δ-GABAARs, muscimol-induced tonic GABA currents reduced the acute swelling of the neurons. In a cortical stroke model, the increased size of the cortical lesion observed when the tonic conductance was reduced with an inverse agonist immediately after an experimental photothrombotic stroke also indicates an acute neuroprotective role for tonic inhibition in cortical neurons(Clarkson et al., 2010). These findings suggest targeting of extrasynaptic GABAARs that mediate tonic inhibition could potentially be developed as novel strategies to aid post stroke recovery. The adult brain possesses a remarkable structural and functional plasticity, but some barriers may impede its plasticity once a developmental window is closed (Bavelier et al., 2010). The plasticity of the brain that occurs after an injury is particularly important as it may either facilitate or hinder recovery of function. Plasticity can occur after stroke, particularly in the peri-infarct zone that is adjacent to the region devastated by the stroke(Murphy and Corbett, 2009). As our recent findings(Clarkson et al., 2010) indicate, mechanisms involving an enhanced tonic inhibition that impede the functional plasticity of the adult brain in learning and memory, such as those found in mice lacking α5-GABAARs or animals treated with a negative allosteric modulator of α5-GABAAR, might also be operational during post stroke recovery. Therefore, α5-GABAAR BZD-site inverse agonists developed for treating cognitive disorders may equally be useful as the first clinical treatment to enhance functional recovery after stroke or possibly other devastating brain injuries.
CONCLUSIONS
Our motivation for this review was to highlight an emerging link between changes in tonic inhibition and pathological brain states. There has been considerable progress in understanding the functional significance of extrasynaptic GABAARs in the adult brain and how the tonic conductance they generate can alter network behaviour in a number of ways. Manipulating ambient GABA levels and/or altering extrasynaptic GABAAR function may offer novel strategies for the treatment of a diverse array of neurological and psychiatric disorders. Nonetheless, the development of drugs to alter the function of extrasynaptic GABAARs has seen remarkable progress (see Figure 2). A number of drugs designed to modulate α5-GABAARs may turn out to be useful as cognition enhancers as well as removing some of the “brakes” in the path of adult plasticity necessary for functional recovery after neuronal injury. Several classes of drugs are also becoming available to enhance the function of δ-GABAARs, but the discovery of compounds that are able to specifically antagonize tonic inhibition mediated by δ-GABAARs is still needed. The diversity of the GABAergic system in general, and of GABAARs in particular (Mody and Pearce, 2004), will ensure that further advances in GABA pharmacology will provide a more targeted treatment of these diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci. 2008;28:1185–1197. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, Gasser R, Moreau JL, Wettstein JG, Buettelmann B, et al. RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–223. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- Baur R, Kaur KH, Sigel E. Structure of alpha6 beta3 delta GABA(A) receptors and their lack of ethanol sensitivity. J Neurochem. 2009;111:1172–1181. doi: 10.1111/j.1471-4159.2009.06387.x. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J. Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J. Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. gamma-Aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Birnir B, Everitt AB, Lim MS, Gage PW. Spontaneously opening GABA(A) channels in CA1 pyramidal neurones of rat hippocampus. J Membr Biol. 2000;174:21–29. doi: 10.1007/s002320001028. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Plasticity in a monosynaptic cortical pathway. J Physiol. 1970;207:61P. [PubMed] [Google Scholar]
- Boehm SL, 2nd, Homanics GE, Blednov YA, Harris RA. delta-Subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol. Eur J Pharmacol. 2006;541:158–162. doi: 10.1016/j.ejphar.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Werner DF, Topf N, Baron NV, Henderson LA, Boehm SL, 2nd, Blednov YA, Saad A, Dai S, Pearce RA, et al. An isoflurane- and alcohol-insensitive mutant GABA(A) receptor alpha(1) subunit with near-normal apparent affinity for GABA: characterization in heterologous systems and production of knockin mice. J Pharmacol Exp Ther. 2006;319:208–218. doi: 10.1124/jpet.106.104406. [DOI] [PubMed] [Google Scholar]
- Brickley SG, CullCandy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABA(A) receptors. Journal of Physiology-London. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABA(A) receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J. Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKenzie G, Hosie AM, Farrant M, Brickley SG. Profound desensitization by ambient GABA limits activation of delta-containing GABAA receptors during spillover. J. Neurosci. 2011;31:753–763. doi: 10.1523/JNEUROSCI.2996-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR, Higgins AJ, Marsh S. Distribution of gaba-receptors and gaba-carriers in the mammalian nervous system. J Physiol (Paris) 1979;75:667–671. [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogna M, Pearce RA. GABA A, slow: causes and consequences. Trends Neurosci. 2010;34:101–112. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, Ferris P, Hobbs SC, O'Connor D, Marshall G, et al. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–5832. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Naidu Y. Early Parkinson's disease and non-motor issues. J Neurol. 2008;255(Suppl 5):33–38. doi: 10.1007/s00415-008-5006-1. [DOI] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABA(A) receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, et al. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, et al. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. Journal of neurophysiology. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Errington AC, Cope DW, Crunelli V. Augmentation of Tonic GABA(A) Inhibition in Absence Epilepsy: Therapeutic Value of Inverse Agonists at Extrasynaptic GABA(A) Receptors. Adv Pharmacol Sci. 2011;2011:790590. doi: 10.1155/2011/790590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariello RG, Golden GT. The THIP-induced model of bilateral synchronous spike and wave in rodents. Neuropharmacology. 1987;26:161–165. doi: 10.1016/0028-3908(87)90204-8. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog. Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Faulhaber J, Steiger A, Lancel M. The GABAA agonist THIP produces slow wave sleep and reduces spindling activity in NREM sleep in humans. Psychopharmacology (Berl) 1997;130:285–291. doi: 10.1007/s002130050241. [DOI] [PubMed] [Google Scholar]
- Feng Y, Kapornai K, Kiss E, Tamas Z, Mayer L, Baji I, Daroczi G, Benak I, Kothencne VO, Dombovari E, et al. Association of the GABRD gene and childhood-onset mood disorders. Genes Brain Behav. 2010;9:668–672. doi: 10.1111/j.1601-183X.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice. J Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley SH, Amin J. Rat alpha6beta2delta GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol. 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008a;326:475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Homanics GE, Goldstein PA, Harrison NL. Isoflurane is a potent modulator of extrasynaptic GABA(A) receptors in the thalamus. J Pharmacol Exp Ther. 2008b;324:1127–1135. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995;485(Pt 2):419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur. J. Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama S, Irifune M, Kikuchi N, Takarada T, Shimizu Y, Endo C, Takata T, Dohi T, Sato T, Kawahara M. Increased gamma-aminobutyric acid levels in mouse brain induce loss of righting reflex, but not immobility, in response to noxious stimulation. Anesth Analg. 2007;104:1422–1429. doi: 10.1213/01.ane.0000261519.04083.3e. table of contents. [DOI] [PubMed] [Google Scholar]
- Kato T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci. 2007;61:3–19. doi: 10.1111/j.1440-1819.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- Kekesi KA, Dobolyi A, Salfay O, Nyitrai G, Juhasz G. Slow wave sleep is accompanied by release of certain amino acids in the thalamus of cats. Neuroreport. 1997;8:1183–1186. doi: 10.1097/00001756-199703240-00025. [DOI] [PubMed] [Google Scholar]
- Kirmse K, Dvorzhak A, Kirischuk S, Grantyn R. GABA transporter 1 tunes GABAergic synaptic transmission at output neurons of the mouse neostriatum. J Physiol. 2008;586:5665–5678. doi: 10.1113/jphysiol.2008.161943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Rajput A, Gilbert J, Rozdilsky B, Chang LJ, Shannak K, Hornykiewicz O. Elevated gamma-aminobutyric acid level in striatal but not extrastriatal brain regions in Parkinson's disease: correlation with striatal dopamine loss. Ann Neurol. 1986;20:26–31. doi: 10.1002/ana.410200106. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust H, Achermann G, Ballard T, Buettelmann B, Gasser R, Fischer H, Hernandez MC, Knoflach F, Koblet A, Stadler H, et al. The discovery and unique pharmacological profile of RO4938581 and RO4882224 as potent and selective GABAA alpha5 inverse agonists for the treatment of cognitive dysfunction. Bioorg Med Chem Lett. 2009;19:5940–5944. doi: 10.1016/j.bmcl.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, Rabe H, Bohme I, Aller MI, Wisden W, Luddens H. Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol. 2007;41:163–176. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Levy LM, Ziemann U, Chen R, Cohen LG. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol. 2002;52:755–761. doi: 10.1002/ana.10372. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Schmitt U, Leppa E, Wulff P, Wisden W, Luddens H, Korpi ER. Ro 15-4513 Antagonizes Alcohol-Induced Sedation in Mice Through alphabetagamma2-type GABA(A) Receptors. Front Neurosci. 2011;5:3. doi: 10.3389/fnins.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. 2010;588:1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O'Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci. 2010;13:205–212. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat. Neurosci. 2011;14:1407–1409. doi: 10.1038/nn.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Bonin RP, Orser BA. The physiological properties and therapeutic potential of alpha5-GABAA receptors. Biochem Soc Trans. 2009;37:1334–1337. doi: 10.1042/BST0371334. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol. 2010;588:1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulley JC, Scheffer IE, Harkin LA, Berkovic SF, Dibbens LM. Susceptibility genes for complex epilepsy. Hum Mol Genet. 2005;14(Spec No. 2):R243–R249. doi: 10.1093/hmg/ddi355. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Buron E, Martin-Lopez M. Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABA(A) receptors which contain the alpha-5 subunit, in the elevated plus-maze test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1389–1392. doi: 10.1016/s0278-5846(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci U S A. 2011;108:4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J. Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR. Blockade of alcohol's amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology. 2007;53:810–820. doi: 10.1016/j.neuropharm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci. 2009;29:15341–15350. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E, Gram L, Avanzini G, Dulac O. Antiepileptic drugs as a cause of worsening seizures. Epilepsia. 1998;39:5–17. doi: 10.1111/j.1528-1157.1998.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking delta subunit. J. Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006 doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Prut L, Prenosil G, Willadt S, Vogt K, Fritschy JM, Crestani F. A reduction in hippocampal GABAA receptor alpha5 subunits disrupts the memory for location of objects in mice. Genes Brain Behav. 2010;9:478–488. doi: 10.1111/j.1601-183X.2010.00575.x. [DOI] [PubMed] [Google Scholar]
- Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI, 2nd, Fanselow MS, Homanics GE, Sonner JM. Gamma-aminobutyric acid type A receptor alpha 4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg. 2009;109:1816–1822. doi: 10.1213/ANE.0b013e3181bf6ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Salin PA, Prince DA. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. J Neurophysiol. 1996;75:1573–1588. doi: 10.1152/jn.1996.75.4.1573. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Jones RT, Mody I. Developmental regulation and neuroprotective effects of striatal tonic GABAA currents. Neuroscience. 167:644–655. doi: 10.1016/j.neuroscience.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Jones RT, Mody I. Developmental regulation and neuroprotective effects of striatal tonic GABAA currents. Neuroscience. 2010;167:644–655. doi: 10.1016/j.neuroscience.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41:211–221. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, Floris I, Maciocco E, Sanna E, Biggio G. Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98:122–133. doi: 10.1111/j.1471-4159.2006.03850.x. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat. Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers BD, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Roberts JD, Takagi H, Richards JG, Mohler H, Somogyi P. Synaptic and Nonsynaptic Localization of Benzodiazepine/GABAA Receptor/Cl- Channel Complex Using Monoclonal Antibodies in the Dorsal Lateral Geniculate Nucleus of the Cat. Eur. J. Neurosci. 1990;2:414–429. doi: 10.1111/j.1460-9568.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor delta subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA, slow and GABAA, fast. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14831–14836. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol Enhances Neurosteroidogenesis in Hippocampal Pyramidal Neurons by Paradoxical NMDA Receptor Activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O'Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Valeyev AY, Cruciani RA, Lange GD, Smallwood VS, Barker JL. Cl- channels are randomly activated by continuous GABA secretion in cultured embryonic rat hippocampal neurons. Neurosci Lett. 1993;155:199–203. doi: 10.1016/0304-3940(93)90707-r. [DOI] [PubMed] [Google Scholar]
- Vargas-Caballero M, Martin LJ, Salter MW, Orser BA, Paulsen O. alpha5 Subunit-containing GABA(A) receptors mediate a slowly decaying inhibitory synaptic current in CA1 pyramidal neurons following Schaffer collateral activation. Neuropharmacology. 2009;58:668–675. doi: 10.1016/j.neuropharm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–117. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The Dynamic Modulation of GABAA Receptor Trafficking and Its Role in Regulating the Plasticity of Inhibitory Synapses. Physiol Rev. 2011;91:1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Gaboxadol--a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Emerging anti-insomnia drugs: tackling sleeplessness and the quality of wake time. Nat Rev Drug Discov. 2008;7:530–540. doi: 10.1038/nrd2464. [DOI] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance delta subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABA(A)delta-subunit-containing receptors. Eur J Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Furukawa T, Ueno S, Yamamoto S, Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb Cortex. 2007;17:1782–1787. doi: 10.1093/cercor/bhl087. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABA(A) receptors. J Pharmacol Exp Ther. 2006;319:431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Mohler H, Rudolph U, Feldon J. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Yoshiike Y, Kimura T, Yamashita S, Furudate H, Mizoroki T, Murayama M, Takashima A. GABA(A) receptor-mediated acceleration of aging-associated memory decline in APP/PS1 mice and its pharmacological treatment by picrotoxin. PLoS One. 2008;3:e3029. doi: 10.1371/journal.pone.0003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor alpha5 subunits contribute to GABAA, slow synaptic inhibition in mouse hippocampus. J Neurophysiol. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]