Abstract
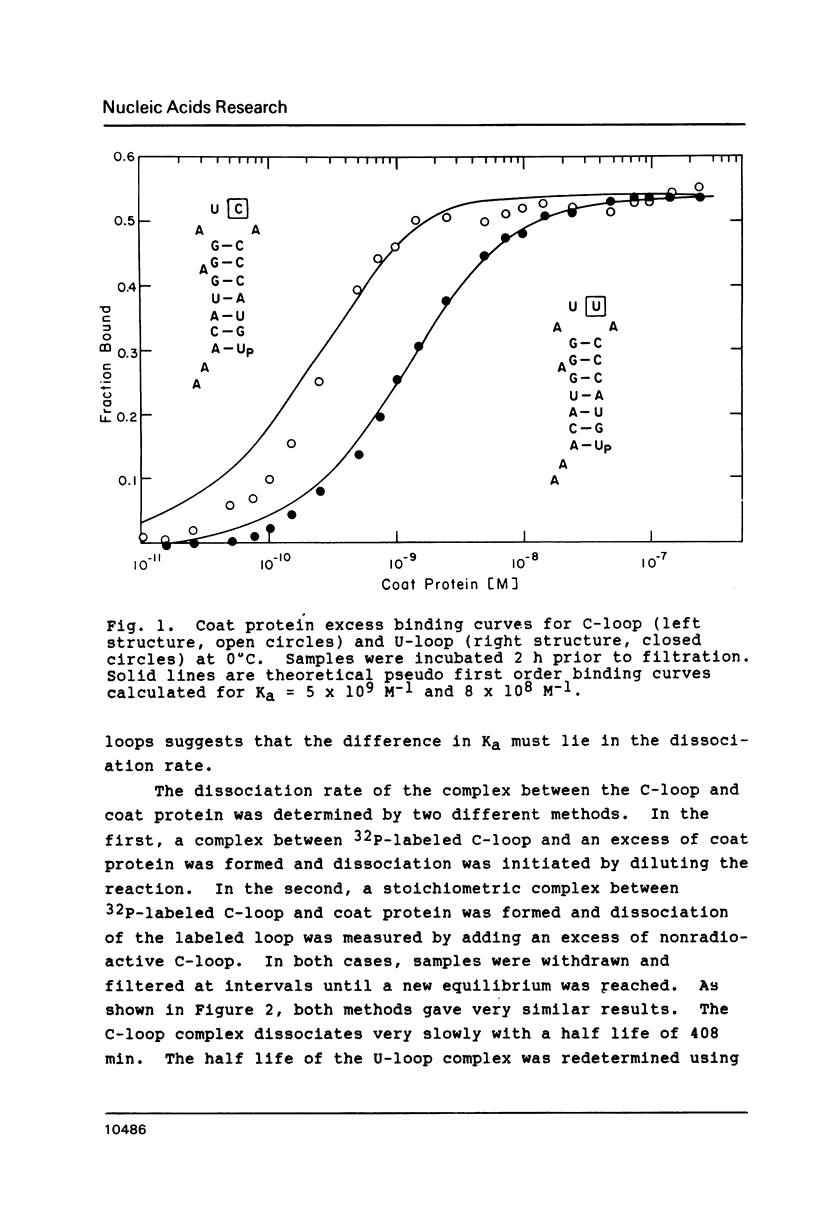
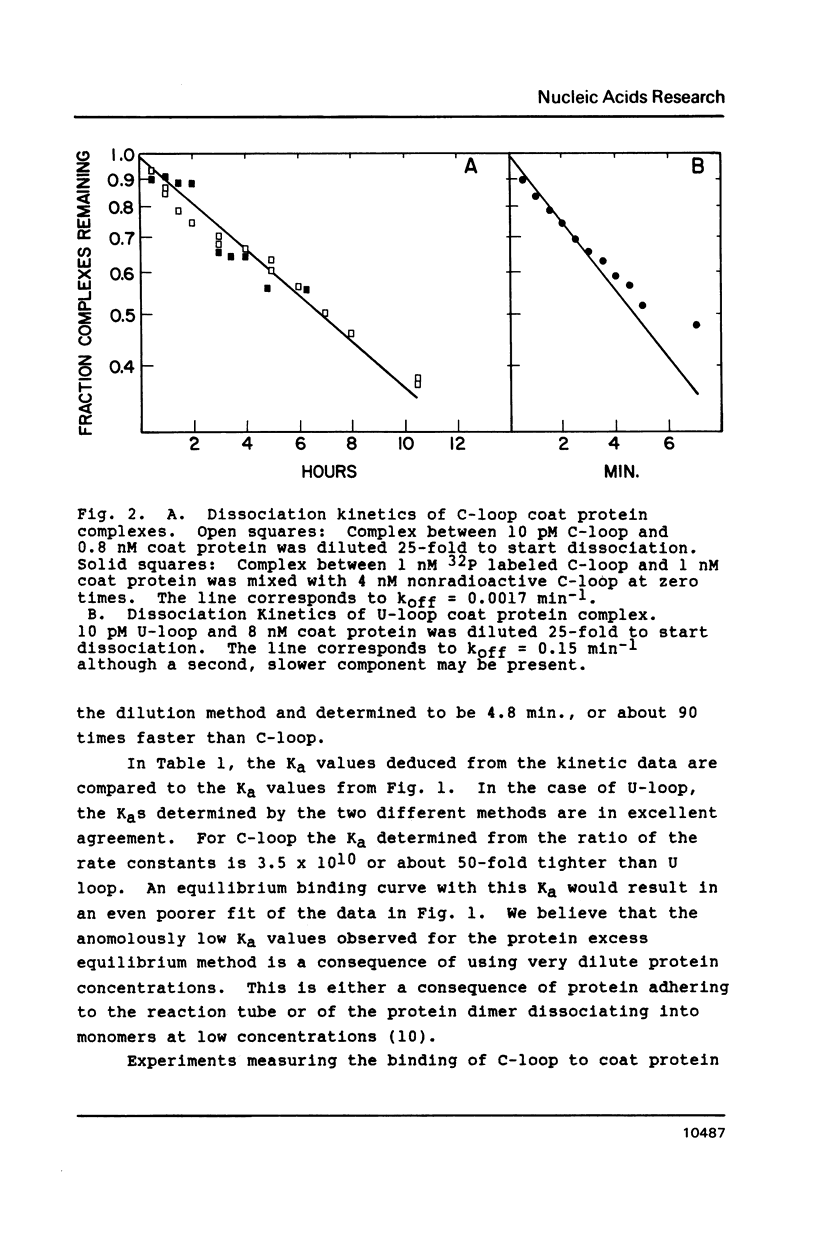
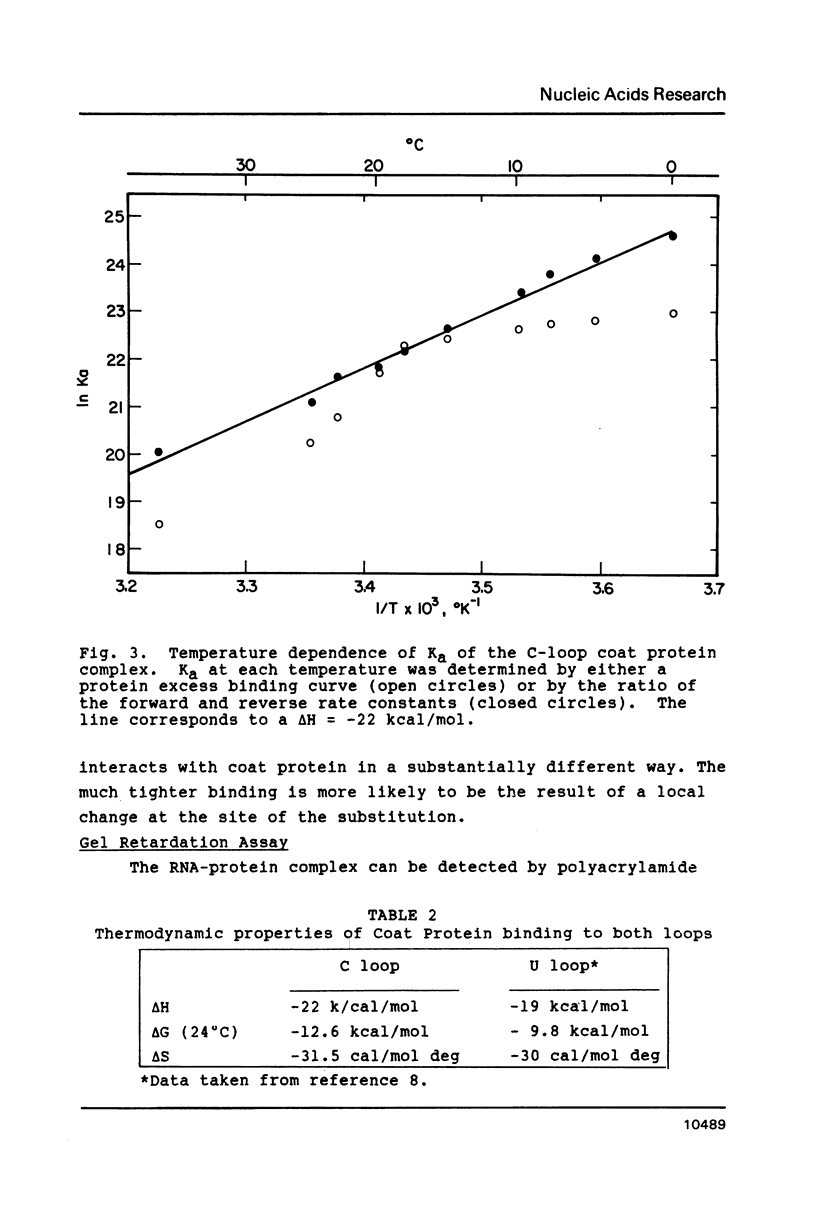
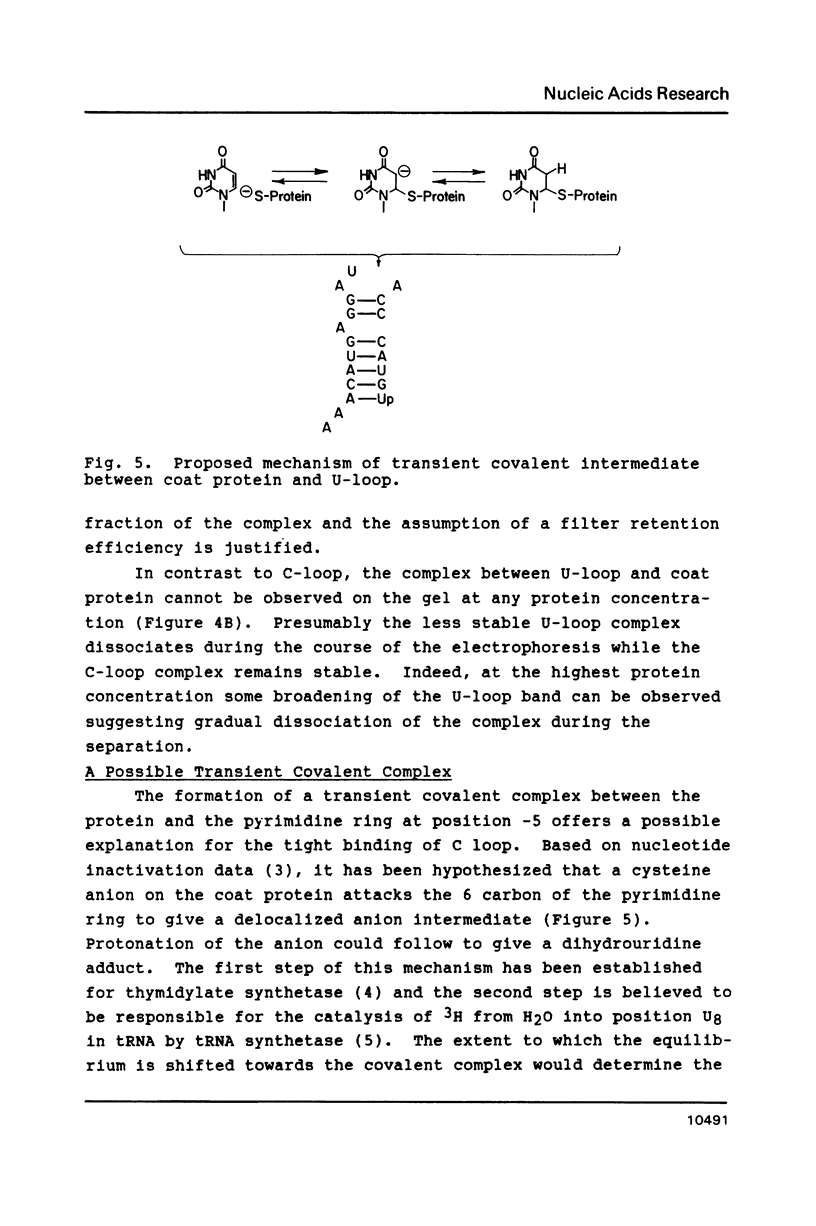
The introduction of a cytidine in place of one of the two single stranded uridines in the R17 replicase translational operator results in a much tighter binding to R17 coat protein. The complex containing the variant RNA is stable to gel electrophoresis and has a binding constant about 50 times greater than the one with wild type RNA. The nearly thirty percent increase in the free energy of binding for the variant RNA is primarily due to a more favorable enthalpy of interaction. A possible explanation for this surprising result is that the U to C change leads to a greater extent of formation of a transient covalent complex between the protein and the RNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carey J., Cameron V., de Haseth P. L., Uhlenbeck O. C. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983 May 24;22(11):2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- Carey J., Lowary P. T., Uhlenbeck O. C. Interaction of R17 coat protein with synthetic variants of its ribonucleic acid binding site. Biochemistry. 1983 Sep 27;22(20):4723–4730. doi: 10.1021/bi00289a017. [DOI] [PubMed] [Google Scholar]
- Carey J., Uhlenbeck O. C. Kinetic and thermodynamic characterization of the R17 coat protein-ribonucleic acid interaction. Biochemistry. 1983 May 24;22(11):2610–2615. doi: 10.1021/bi00280a003. [DOI] [PubMed] [Google Scholar]
- Krug M., de Haseth P. L., Uhlenbeck O. C. Enzymatic synthesis of a 21-nucleotide coat protein binding fragment of R17 ribonucleic acid. Biochemistry. 1982 Sep 14;21(19):4713–4720. doi: 10.1021/bi00262a030. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Uhlenbeck O. C. Nucleoside and nucleotide inactivation of R17 coat protein: evidence for a transient covalent RNA-protein bond. Biochemistry. 1985 Jul 16;24(15):4239–4244. doi: 10.1021/bi00336a064. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Sasmor H., Betz J. L. A perfectly symmetric lac operator binds the lac repressor very tightly. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6785–6789. doi: 10.1073/pnas.80.22.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafranski P., Zagorski V., Khrobochek Ia, zagorska P. Otnoshenie belka obolochki k RNK bakteriofaga f2 v transliatsionnom repressornom komplekse. Mol Biol (Mosk) 1975 Jan-Feb;9(1):78–85. [PubMed] [Google Scholar]
- Simons A., Tils D., von Wilcken-Bergmann B., Müller-Hill B. Possible ideal lac operator: Escherichia coli lac operator-like sequences from eukaryotic genomes lack the central G X C pair. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1624–1628. doi: 10.1073/pnas.81.6.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzyk R. M., Koontz S. W., Schimmel P. A covalent adduct between the uracil ring and the active site of an aminoacyl tRNA synthetase. Nature. 1982 Jul 8;298(5870):136–140. doi: 10.1038/298136a0. [DOI] [PubMed] [Google Scholar]
- Thurlow D. L., Ehresmann C., Ehresmann B. Nucleotides in 16S rRNA that are required in unmodified form for features recognized by ribosomal protein S8. Nucleic Acids Res. 1983 Oct 11;11(19):6787–6802. doi: 10.1093/nar/11.19.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Carey J., Romaniuk P. J., Lowary P. T., Beckett D. Interaction of R17 coat protein with its RNA binding site for translational repression. J Biomol Struct Dyn. 1983 Oct;1(2):539–552. doi: 10.1080/07391102.1983.10507460. [DOI] [PubMed] [Google Scholar]



