Abstract
The interaction of dopamine (DA) and α-synuclein (α-S) can lead to protein misfolding and neuronal death triggered by oxidative stress relevant to the progression of Parkinson’s disease (PD). In this study, interfacial properties associated with DA-induced α-S aggregation under various solution conditions (i.e., pH, ionic strength) were investigated in vitro. The electrochemical oxidation of tyrosine (Tyr) residues in α-S was detected in the presence of DA. DA concentration dependence was analyzed and found to significantly affect α-S aggregation pathways. At low pH, DA was shown to be stable and produced no observable difference in interfacial properties. Between pH 7 and 11, DA promoted α-S aggregation. Significant differences in oxidation current signals in response to high pH and ionic strength suggested the importance of initial interactions in the stabilization of toxic oligomeric structures and subsequent off-pathways of α-S. Our results demonstrate the importance of solution interactions with α-S and the unique information that electrochemical techniques can provide for the investigation of α-S aggregation at early stages, an important step toward the development of future PD therapeutics.
Keywords: Dopamine, electroanalysis, α-synuclein, aggregation, tyrosine, Parkinson’s disease
The neuropathology associated with α-synuclein (α-S) is found in several neurodegenerative diseases including Parkinson’s disease (PD), dementia with Lewy bodies, Lewy body variants of Alzheimer’s disease, and multiple system atrophy.1,2 α-S exists as a soluble protein in healthy individuals; however, in PD patients, it forms insoluble aggregates found as fibrillar plaques that affect the dopaminergic neurons in the substantia nigra pars compacta.3,4 Moreover, mutations of α-S (A53T, A30P, and E46K) that accelerate the rate of α-S aggregation are responsible for familial PD, suggesting a link between α-S and the neuronal toxicity of neurodegenerative pathogenesis.5 As the major component of Lewy bodies, these pathological inclusions may constitute the toxicity associated with neurodegeneration.
The mechanism of α-S aggregation is still uncertain; however, several studies have suggested that it may be the result of α-S accumulation by gene triplication, and several point mutations indicate that the protein preferentially adopts a β-sheeted conformation.6,7 This conformation ultimately leads to the accumulation of fibrils, which constitutes the formation of Lewy bodies.8
Although the biological role of α-S has yet to be proven, it has been shown to exist in cytosolic and membrane- or vesicle-bound states.9 Several in vivo studies indicated that overexpression of α-S induced noticeable changes in membrane fluidity and vesicular trafficking.10−13 Dopamine (DA) storage in synaptic vesicles plays a crucial role in DA oxidative stress because it easily undergoes oxidative pathways in cytosolic conditions and, in due course, leads to the production of reactive oxygen species (ROS).14 This suggests that the influence of α-S on DA storage is important because it preserves vesicle formation and the stability of DA within these vesicles. The reduced availability of vesicles for DA storage can therefore lead to the production of ROS and subsequent oxidative stress, which are detrimental to biological components. Along with oxidative stress, DA and its oxidative derivatives have been described to noncovalently inhibit α-S aggregation by forming nonspecific hydrophobic interactions.15 Numerous in vitro studies using atomic force microscopy and electron microscopy have shown that α-S also exists in oligomeric morphologies.16,17 The effect of DA on α-S inhibits the formation of α-S fibrils, and promotes the formation of toxic oligomeric forms.18 Mutations in α-S that are implicated in familial PD have also been shown to form oligomeric structures. This finding suggests a strong link between α-S protofibrils and the early onset of PD. The α-S-DA construct inhibits the formation of elongated β-pleated sheet fibrils and remains in a protofibril morphology and amorphous aggregates. The oxidative intermediates of DA have been recently identified as inducers of off-pathways to form SDS-resistant α-S oligomers, which were thioflavin T negative.19−22 In this report, we present the electrochemical behavior of α-S in the presence of DA under oxidative stress conditions.
Results and Discussion
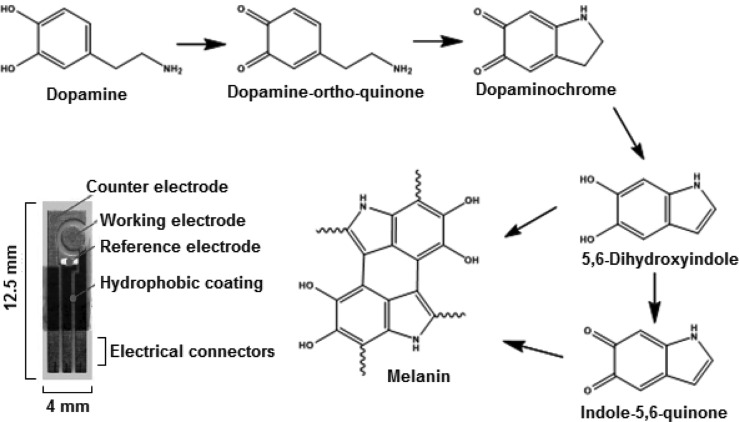
Electrochemical techniques such as cyclic voltammetry (CV) and square-wave voltammetry (SWV) have recently been successfully applied to monitoring early interfacial changes attributed to peptide aggregation.23−34 The electrochemical activity of DA has been well-documented in neurochemical studies performed in vitro and in vivo.35−39 As shown is Figure 1, DA can be easily oxidized on a carbon electrode to form quinonic molecules. DA oxidation results in three anodic peaks at 0.18, 0.77, and 0.92 V (vs Ag/AgCl, data not shown), which are representative of its oxidation into dopamine-o-quinone and other DA reactive quinones (e.g., dopaminochrome, indole-5,6-quinone, and 5,6-dihydroxyindole). In order to understand the effect of DA oxidation on α-S, SWV of Tyr was studied in the presence and absence of DA under various experimental conditions. The peak oxidation potentials of DA did not overlap with that of Tyr oxidation, which was observed at ∼0.55 V (vs Ag/AgCl). As shown in Figure 2A, the Tyr oxidation signal was enhanced in the presence of DA, which might have functioned as a mediator to facilitate the electron exchange with the electrode surface. After 24 h incubation, an enhanced Tyr current signal was observed in the presence of DA when compared with the Tyr signal in the presence of fresh DA solution (Figure 2B). It was found that Tyr oxidation increased in the presence of quinonic intermediates. However, in the presence of excess DA in solution (at higher concentrations), the effect of the quinones facilitating the electron flow from Tyr was disrupted due to the fouling of the electrode surface. Thus, DA-mediated electron transfer of Tyr oxidation was best observed in the presence of low DA concentrations.
Figure 1.
Oxidation states of dopamine (DA). Inset shows the screen-printed carbon strip electrode (SPCS) used for the electrochemical oxidation measurements.
Figure 2.
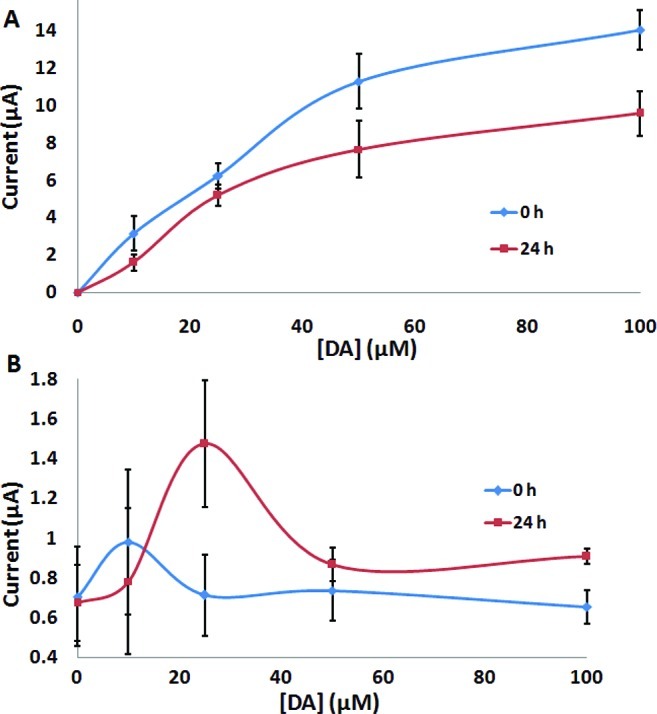
Anodic current signals were plotted against the dopamine (DA) concentration in the presence of 25 μM free Tyr using square-wave voltammetry at a carbon paste electrode following changes in electrochemical oxidation signal of (A) DA at ∼0.18 V and (B) Tyr at ∼0.55 V (vs Ag/AgCl).
Once DA is synthesized, it is immediately sequestered in the brain and exists in vesicles at low pH values. This environment retains DA stability and shields it from degradation of ROS and subsequent oxidative stress. At physiological pH, DA can auto-oxidize into ROS and interact with α-S.19Supporting Information Figure S2 demonstrates that DA degraded into its oxidative intermediates at pH 7 and 11 but remained stable at low pH values, retaining the same absorbance peak with a maximum absorbance value at 280 nm. At pH 4, the spectra showed no significant change over time and were indicative of DA’s stability at low pH. In an acidic environment, DA formed its oxidative intermediates, which were observed by the change in absorbance at pH 7. Over time, the peak at 280 nm became less defined and absorbance shifted to higher wavelengths due to increased light scattering. At pH 11, the absorbance spectrum showed a maximum at 300 nm, characteristic of the yellow chromophore dopamine-o-quinone, and changed over time. The absorbance values increased and shifted to a maximum absorbance at 280 nm as the solution turned brown. This result demonstrated that the degradation of DA over time resulted in the formation of reactive DA-intermediates at physiological pH.
We also investigated the interaction of DA intermediates with α-S at varying pH by monitoring their electrochemical oxidation (Supporting Information Figure S3). At pH 4, the kinetics curve showed no significant change over time. This was in agreement with UV–vis measurements (Supporting Information Figure S2) demonstrating the stability of DA at low pH. At pH 11, a significant fluctuation was observed. In the presence of DA, the oxidation process displayed an increase in the current intensity whereas in its absence, the current signal decreased. This indicated that DA was interacting with α-S in a conformation that facilitated Tyr oxidation on the electrode surface. This interaction increased aggregation and resulted in rapid structural variations. This provided electrochemical evidence that oxidative stress caused by reactive quinones was responsible for off-pathway α-S aggregation.
When DA was present in high concentrations, the current signals were similar to the control, but deviated at 5 h and reached a minimum after 24 h. Incubations of α-S in the presence of DA have been demonstrated to increase aggregation rates.40−42 The decrease in current signal was, therefore, attributed to the rapid progression of α-S aggregation, which ultimately led to no current signal, indicative of fully aggregated species. This occurred because aggregation obstructed the available Tyr residues from oxidizing at the electrode surface. Alternatively, melanin, a final product of DA oxidation, might have been formed when DA was found at such high concentrations. In its polymeric form, melanin could have consequently blocked the electrode surface and thus decreased the current signals.
In agreement with our electrochemical studies, DA-induced formation of SDS-insoluble multimeric complexes of human recombinant α-S protein was observed by denaturing SDS-PAGE and Western blot analysis (Figure 3). In comparison, the α-S-specific antibody detected only low molecular weight species in the sample of untreated recombinant α-S. In support of the present observations, our previous work with native PAGE had also demonstrated that our human recombinant α-S preparation predominantly contained monomeric and dimeric forms.25
Figure 3.
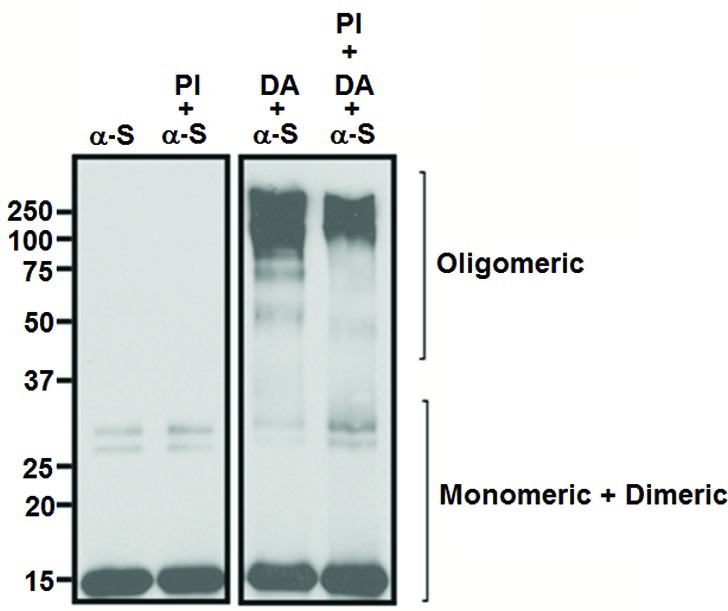
Western blotting analysis of α-S (50 μM) demonstrated that DA incubation led to the formation of SDS-insoluble multimeric complexes. Samples containing α-S oligomers after incubation with DA (2 mM) for 16 h were separated by SDS-PAGE. Protease inhibitors (PI) were used to avoid protein degradation during sample incubation.
Ionic strength has a significant role in inducing α-S aggregation.40−42 The interaction of increased electrostatic forces has been shown to aid in the stabilization of intermediate forms.40−42 Preaggregation of α-S has also been demonstrated to result in the initial destabilization of soluble monomers and the loss of an ordered structure.23,24 Increased ionic strength would accelerate the process of destabilization by binding to α-S and assisting in the formation of its nucleated form. This supports our electrochemical data, where kinetic curves were recorded at varying salt concentrations (Figure 4A and B). Electrostatic stability and the formation of intermediate forms were observed in the presence of 200 mM NaCl. Here, the conformation of α-S presented the most dynamic characteristic having a large range of current intensity in the first 24 h. In the presence of high ionic strength, α-S went through dynamic structural changes in its early aggregation stages, which were aided by the additional electrostatic forces in the presence of 50 mM and 100 mM NaCl. Electrochemical data distinguished this interaction in its preaggregated form suggesting the importance of the protofibril arrangement. Ionic strength was, therefore, found to be essential in the stability of early intermediates of α-S as it nucleated and subsequently formed oligomers. The interaction of DA with α-S obstructed the available Try residues from oxidizing at the electrode surface. With 200 mM NaCl, the complexing of α-S and DA might have been hindered by the effect of high ionic strength resulting in similar current intensities to α-S aggregation in the absence of DA (Figure 5).
Figure 4.
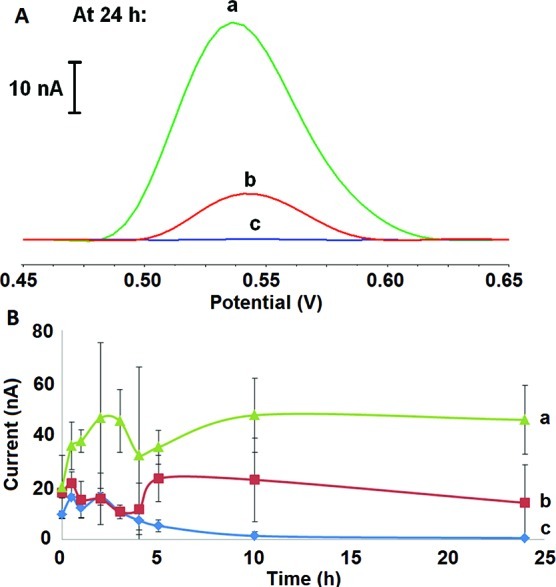
(A) Square-wave voltammograms of Tyr oxidation in α-S that was incubated with varying concentrations of DA for 24 h. (B) Plot for the dependence of Tyr oxidation current signals on time with (a) α-S in the presence of 0.5 mM DA, (b) α-S only, and (c) α-S in the presence of 5 mM DA in PBS buffer at pH 7.4 and 37 ± 1 °C with shaking for 24 h.
Figure 5.
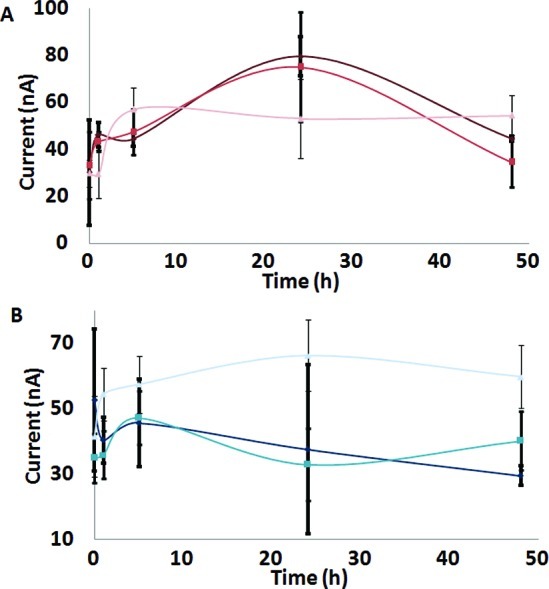
Plot for the dependence of Tyr oxidation in α-S under various ionic strength conditions. α-S (20 μM) was incubated in the absence (A) and presence (B) of 100 μM DA with 50 (black line), 100 (blue line), and 200 (light blue line) mM NaCl in PBS buffer at pH 7.4 and 37 ± 1 °C for 48 h.
The importance of analyzing early solvent interactions during α-S aggregation is essential for the development of future PD therapeutics. Our in vitro studies demonstrated the advantage of using CV and SWV since these techniques allow monitoring changes in interfacial properties of α-S at early stages. Our findings suggest that the oxidative stress effect of DA has a significant influence on α-S by altering its aggregation pathway when pH and ionic strength conditions are varied.
Methods
Materials
All chemicals, including dopamine HCl (DA, 98%) and l-tyrosine (Tyr, 98%), were purchased from Sigma-Aldrich (Oakville, ON). Human α-synuclein, (α-S, recombinant, Escherichia coli) was purchased from EMD Chemicals (Gibbstown, NJ). All solutions were prepared with ultrapure water using a Cascada LS (Pall Co., NY) water purification system.
Electrochemical Analysis
Cyclic voltammetry (CV) and square-wave voltammetry (SWV) were performed with a μAutolab-III electrochemical analyzer (Metrohm, Switzerland) operated in conjunction with its three general-purpose electrochemistry software (GPES). The planar screen-printed carbon strip (SPCS) electrodes were kindly donated by Professor Eiichi Tamiya (Osaka University, Japan) and Biodevice Technology Ltd. (Ishikawa, Japan). The three-electrode system was composed of a carbon electrode (geometric working area: 2.64 mm2) as the working electrode, a carbon counter-electrode, and the reference electrode (Ag/AgCl). Measurements were performed with aliquots of 20 μL of sample where the SPCS electrodes were used once and discarded. CV measurements were performed with DA and free l-Tyr with a three-electrode system that consisted of a carbon paste electrode (CPE) as the working electrode, a Ag/AgCl reference electrode, and a Pt wire auxiliary electrode. Carbon paste was prepared with a 70:30 (w/w) composition of graphite powder and mineral oil, respectively. The paste was packed into a Teflon tube with a Cu wire serving as the electrical contact with a geometric electrode surface area of 0.033 cm2. All measurements were taken at room temperature (24 ± 1 °C).
To induce aggregation, α-S was incubated with DA at 37 ± 1 °C with a thermal block-shaker (Fisher Scientific) at 300 rpm. Samples were prepared in 50 mM phosphate buffer at pH 7.4, and 5 mM sodium acetate/5 mM sodium phosphate, at pH 4, 7, and 11. Aliquots (20 μL) were removed and spotted on the three-electrode area of the SPCS surface. CV measurements were recorded from −0.05 to 1.20 V (vs Ag/AgCl) with scan rates at 10, 50 100, 250, and 500 mV/s. SWV was employed from −0.05 to 1 V (vs Ag/AgCl) with a step potential of 5 mV and amplitude of 25 mV at 50 Hz. Raw data were treated with Savitzky-Golay level 3 smoothing and baseline corrected to a peak width of 0.003 V. Each electrochemical measurement condition was performed for at least three replicates (n = 3).
Western Blotting Analysis
Samples containing 2 μg of protein per lane were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 4% stacking gel and 12% resolving gel and transferred to nitrocellulose membranes. After blocking for 1 h with 5% skim milk powder in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4), blots were incubated overnight at 4 °C with anti-α-S antibody (610786, BD Transduction Laboratories, Mississauga, ON). After washes, blots were incubated with horseradish peroxidase conjugated secondary antibodies (Sigma, St. Louis, MO). Incubations with primary and secondary antibodies were performed in 1% skim milk powder in PBS. After three washes with PBS (containing 0.1% Tween-20), immunoactivity was detected by enhanced chemiluminescence assay (Amersham, Piscataway, NJ) with exposure to BioFlex MSI Film (Clonex, Mississauga, ON). Protease inhibitor cocktail was purchased from Roche Diagnostics (Mississauga, ON)
Supporting Information Available
Additional figures and experimental conditions. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors would like to acknowledge financial support from NSERC Discovery Grants to K.K. and to I.R.B and an Alzheimer Society of Canada Grant to K.K..
The authors declare no competing financial interest.
Author Contributions
T.C., A.M.C., X.R.C., and D.W.F.T. designed and performed experiments, analysed data, and wrote the paper; I.R.B. and K.K. provided biological and analytical tools, designed experiments, analysed data, and wrote the paper.
Supplementary Material
References
- Erickson J.; Wszolek Z.; Petrucelli L. (2005) Molecular pathogoenesis of Parkinson disease. Arch. Neurol. 62, 353–357. [DOI] [PubMed] [Google Scholar]
- Kruger R.; Kuhn W.; Muller T.; Woitalla D.; Graeber M.; Kosel S.; Przuntek H.; Epplen J. T.; Schols L.; Riess O. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 18, 106–108. [DOI] [PubMed] [Google Scholar]
- Dauer W.; Przedborski S. (2003) Parkinson’s Disease: Mechanisms and models. Neurology 39, 889–909. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M.; Lavedan C.; Leroy E.; Ide S.; Dehejia A.; Dutra A.; Pike B.; Root H.; Rubenstein J.; Boyer R.; Stenroos E. S.; Chandrasekharappa S.; Athanassiadou A.; Papapetropoulos T.; Johnson W. G.; Lazzarini A. M.; Duvoisin R. C.; Di Iorio G.; Golbe L. I.; Nussbaum. R. L. (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Conway K.; Lee S.-J.; Rochet J.-C.; Ding T.; Williamson R.; Lansbury P. Jr. (2000) Acceleration of oligomerization, not fibrillization is a shared property of both α-synuclein mutations linked to early-onset Parkinson’s disease: Implications for pathogensis and therapy. Proc. Natl. Acad. Sci. U.S.A. 97, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman E.; Giasson B. (2009) Molecular mechanisms of α-synuclein neurodegeneration. Biochim. Biophys. Acta 1792, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata A.; Maruyama M.; Kanazawa I.; Nukina N. (2001) α-synuclein affects the MAPK pathway and accelerates cell death. J. Biol. Chem. 276, 45320–45329. [DOI] [PubMed] [Google Scholar]
- Jenco J.; Rawlingson A.; Daniels B.; Morris A. (1998) Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by α- and β- synucleins. Biochemistry 37, 4901–4909. [DOI] [PubMed] [Google Scholar]
- Lotharius J.; Brundin P. (2002) Pathogenesis of Parkinson’s disease: Dopamine, vesicles and α-synuclein. Nat. Rev. Neurosci. 3, 932–942. [DOI] [PubMed] [Google Scholar]
- Sharon R.; Bar-Josef I.; Betenski R.; Shen J.; Selkoe D. (2001) α-Synuclein occurs in lipid-rich molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 98, 9110–9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W.; Jonas A.; Clayton D.; George J. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 98, 9443–9449. [DOI] [PubMed] [Google Scholar]
- Sharon R.; Bar-Josef I.; Frosch M.; Walsh D.; Hamilton J.; Selkoe D. (2003) The formation of highly soluble oligomers of α-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 37, 583–595. [DOI] [PubMed] [Google Scholar]
- McLean P.; Kawamata H.; Ribich S.; Hyman B. (2000) Membrane association and protein conformation of α-synuclein in intact neurons. J. Biol. Chem. 275, 8812–8816. [DOI] [PubMed] [Google Scholar]
- Halliwell B.; Gutteridge J. (1985) Oxygen radicals and the nervous system. Trends Neurosci. 8, 22–26. [Google Scholar]
- Latawiec D.; Herrera F.; Bek A.; Losasso V.; Candotti M.; Benetti F.; Carlino E.; Kranjc A.; Lazzarino M.; Gustincich S.; Carloni P.; Legname G. (2010) Modulation of alpha-synuclein aggregation by dopamine analogs. PLOS One 5, 9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Rajamani S.; Kaylor J.; Han S.; Zhou F.; Fink A. (2004) The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrls. J. Biol. Chem. 279, 26846–26857. [DOI] [PubMed] [Google Scholar]
- Conway K.; Harper J.; Lansbury P. (1998) Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson’s disease. Nature 4, 1318–1320. [DOI] [PubMed] [Google Scholar]
- Mazzulli J.; Mishizen A.; Giasson B.; Lynch D.; Thomas S.; Nakashima A.; Nagatsu T.; Ota A.; Ischiropoulos H. (2006) Cytosolic catechols inhibit α-synuclein aggregation and facilitates the formation of intracellular soluble oligomeric intermediates. J. Neurosci. 26, 10068–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham C.; Leong S.; Ali F.; Kenche V.; Hill A.; Gras S.; Barnham K. J.; Cappai R. (2009) Dopamine and the dopamine oxidation product 5,6-dihyroxylindole promote distinct on-pathway and off-pathway aggregation of α-synuclein in a pH-dependent manner. J. Mol. Biol. 387, 771–785. [DOI] [PubMed] [Google Scholar]
- Xu J.; Kao S.-Y.; Lee F.; Song W.; Jin L.-W.; Yanker B. (2002) Dopamine-dependent neurotoxicity of α-synuclein: A mechanism for selective neurodegeneration in Parkinson’s disease. Nat. Med. 8, 600–606. [DOI] [PubMed] [Google Scholar]
- Hattori N.; Wang M.; Taka H.; Fujimura T.; Yoritaka A.; Kubo S.; Mochizuki H. (2009) Toxic effects of dopamine metabolism in Parkinson’s disease. Parkinsonism Relat. Disord. 15, S35–S38. [DOI] [PubMed] [Google Scholar]
- Leong S.; Pham C.; Galatis D.; Fodero-Tavoletti M.; Perez K.; Hill A. F.; Masters C. L.; Ali F. E.; Barnham K. J.; Cappai R. (2009) Formation of dopamine-mediated α-synuclein-soluble oligomers requires methionine oxidation. Free Radical Biol. Med. 46, 1328–1337. [DOI] [PubMed] [Google Scholar]
- Masarik M.; Stobiecka A.; Kizek R.; Jelen F.; Pechan Z.; Hoyer W.; Jovin T. M.; Subramaniam V.; Palecek E. (2004) Sensitive electrochemical detection of native and aggregated α-synuclein protein involved in Parkinson’s disease. Electroanalysis 16, 1172–1181. [Google Scholar]
- Palecek E.; Ostana V.; Masarik M.; Bertoncini C.; Jovin T. M. (2008) Changes in interfacial properties of α-synuclein preceding its aggregation. Analyst 133, 76–84. [DOI] [PubMed] [Google Scholar]
- Chan T.; Chow A.; Tang D.; Li Q.; Wang S.; Brown I.; Kerman K. (2010) Interaction of baicalein and copper with alpha-synuclein: Electrochemical approach to the Parkinson’s disease. J. Electroanal. Chem. 648, 151–155. [Google Scholar]
- Brabec V.; Schindlerova I. (1981) Electrochemical behaviour of proteins at graphite electrodes Part III. The effect of protein adsorption. Bioelectrochem. Bioenerg. 8, 451–458. [Google Scholar]
- Reynaud J.; Malfoy B.; Bere A. (1980) The electrochemical oxidation of three proteins: RNAase A, bovine serum albumin and concanavalin A at solid electrodes. J. Electroanal. Chem. 116, 595–606. [Google Scholar]
- Cai X.; Rivas G.; Farias P.; Shiraishi H.; Wang J.; Palecek E. (1996) Potentiometric stripping analysis of bioactive peptides at carbon electrodes down to subnanomolar concentrations. Anal. Chim. Acta 332, 49–57. [Google Scholar]
- MacDonald S.; Roscoe S. (1997) Electrochemical oxidation reactions of tyrosine, tryptophan and related dipeptides. Electrochim. Acta 42, 1189–1200. [Google Scholar]
- Brabec V.; Mornstein V. (1980) Electrochemical behaviour of proteins at graphite electrodes II. Electrooxidation of amino acids. Bioelectrochem. Bioenerg. 12, 159–165. [DOI] [PubMed] [Google Scholar]
- Vestergaard M.; Kerman K.; Saito M.; Nagatani N.; Takamura Y.; Tamiya E. (2005) A rapid label-free electrochemical detection and kinetic study of Alzheimer’s amyloid beta aggregation. J. Am. Chem. Soc. 127, 11892–11893. [DOI] [PubMed] [Google Scholar]
- Chikae M.; Fukuda T.; Kerman K.; Idegami K.; Miura Y.; Tamiya E. (2008) Amyloid-beta detection with saccharide immobilized gold nanoparticle on carbon electrode. Bioelectrochemistry 74, 118–123. [DOI] [PubMed] [Google Scholar]
- Veloso A. J.; Hung V. W. S.; Sindhu G.; Constantinof A.; Kerman K. (2009) Electrochemical oxidation of benzothiazole dyes for monitoring amyloid formation related to the Alzheimer’s disease. Anal. Chem. 81, 9410–9415. [DOI] [PubMed] [Google Scholar]
- Veloso A. J.; Yoshikawa H.; Cheng X. R.; Tamiya E.; Kerman K. (2011) Optical trapping for the characterization of amyloid-beta aggregation kinetics. Analyst 136, 4164–4167. [DOI] [PubMed] [Google Scholar]
- Keithley R. B.; Wightman R. M. (2011) Assessing principal component regression prediction of neurochemicals detected with fast-scan cyclic voltammetry. ACS Chem. Neurosci. 2, 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley R. B.; Takmakov P.; Bucher E. S.; Belle A. M.; Owesson-White C. A.; Park J.; Wightman R. M. (2011) Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal. Chem. 83, 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans A.; Keithley R. B.; Kita J. M.; Sombers L. A.; Wightman R. M. (2008) Dopamine detection with fast-scan cyclic voltammetry used with analog background subtraction. Anal. Chem. 80, 4040–4048. [DOI] [PubMed] [Google Scholar]
- Venton B. J.; Wightman R. M. (2003) Psychoanalytical electrochemistry: Dopamine and behaviour. Anal. Chem. 75, 414A–421A. [Google Scholar]
- DuVall S.; McCreery R. (2000) Self-catalysis by chatechols and quinones during heterogeneous electron transfer at carbon electrodes. J. Am. Chem. Soc. 122, 6759–6764. [Google Scholar]
- Cappai R.; Leck S.-L.; Tew D.; Williamson N.; Smith D.; Galatis D.; Sharples R. A.; Curtain C. C.; Ali F. E.; Cherny R. A.; Culvenor J. G.; Bottomley S. P.; Masters C. L.; Barnham K. J.; Hill A. F. (2005) Dopamine promotes α-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 19, 1377–1379. [DOI] [PubMed] [Google Scholar]
- Rekas A.; Knott R.; Sokolova A.; Barnham K.; Perez K.; Masters C.; Drew S. C.; Cappai R.; Curtain C. C.; Pham C. L. (2010) The structure of dopamine induced α-synuclein oligomers. Eur. Biophys. J. 39, 1407–1419. [DOI] [PubMed] [Google Scholar]
- Hoyer W.; Antony T.; Cherny D.; Heim G.; Jovin T.; Subramaniam V. (2002) Dependence of α-synuclein aggregate morphology on solution conditions. J. Mol. Biol. 322, 383–393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.