Abstract
Since the initial discovery of replication protein A (RPA) as a DNA replication factor, much progress has been made on elucidating critical roles for RPA in other DNA metabolic pathways. RPA has been shown to be required for DNA replication, DNA repair, DNA recombination, and the DNA damage response pathway with roles in checkpoint activation. This review summarizes the current understanding of RPA structure, phosphorylation and protein-protein interactions in mediating these DNA metabolic processes.
Keywords: RPA, phosphorylation, DNA replication, DNA repair, DNA Damage Response, Checkpoint Activation, Review
2. INTRODUCTION
Few if any proteins have as much impact on mammalian DNA metabolic processes as the single-stranded DNA (ssDNA) binding protein, RPA. These processes include DNA replication, DNA repair, DNA recombination, telomere maintenance and the DNA damage response and checkpoint activation. The abundance of RPA and the ability to interact with numerous DNA metabolic proteins are key elements for the involvement in these processes. In recent years, studies have demonstrated that RPA is not only required for these DNA metabolic pathways, but also that it plays a regulatory role in determining what pathways become activated. In this regulatory role, the structure of RPA and changes in the structure as a consequence of phosphorylation influence the DNA binding mode and more importantly which proteins can interact with RPA. These recent studies suggest that RPA acts to control the flow of traffic along the genomic highway which helps to govern what DNA metabolic pathways are utilized in the cell. This becomes extremely critical when the cell needs to halt DNA replication, activate the DNA damage response and initiate DNA repair in response to genomic stress and DNA damaging events. RPA is an excellent candidate to help control this ‘traffic’ with its involvement in all DNA metabolic pathways. In this review, we will highlight the newer advances in our understanding of RPA and focus on the mechanism of how RPA can act to direct traffic at the busy intersections of the multiple DNA metabolic pathways.
3. RPA STRUCTURE AND FUNCTION
3.1. Protein Structure and DNA binding domains
RPA is a heterotrimeric protein that consists of 70 kDa, 32 kDa, and 14 kDa subunits. These subunits have been termed RPA1, RPA2 and RPA3, respectively. Despite the inability to determine the full length protein structure, many studies have elucidated structural features within each subunit. This work has led to a better understanding of how the full length protein functions. One of the major structural features of RPA is the presence of the oligonucleotide/oligosaccharide binding (OB) folds within the subunits (1, 2). This OB fold structure consists of beta sheets that form beta-barrel structures that can wrap around ssDNA. This is a common structural feature in ssDNA binding proteins (3, 4). The first RPA partial structure revealed two OB folds within the central region of RPA1 (amino acids 181-422) (1). These OB folds have been termed the DNA binding domain (DBD) A, composed of amino acids 181-290 and B, composed of amino acids 301-422. Further work revealed an OB fold structure in the C-terminus of RPA1, which was termed DBD C (4, 5). This OB fold comprises amino acids 436-616. Structural work with RPA2 and RPA3 revealed OB folds within each of these subunits, termed DBD D and E, respectively (2, 6). The DBD D is formed between amino acids 43-171 on RPA2, while DBD E is generated from the entire amino acids 1-121 of RPA3 (7). Although structural results with DBD E imply it is capable of ssDNA binding, this has not been observed (4, 7). The C-terminus of RPA3 is important for structural stability of the heterotrimer but other functions of RPA3 are not known. More recently, studies looking at the N-terminus of RPA1 have revealed a sixth OB fold (DBD F) residing in amino acids 1-110 (8). A recent study using computational methods and reactivity information for the intact heterotrimer is consistent with the structures formed by truncated RPA (9).
Other structural features include a linker region in RPA1 between DBD F and DBD A which likely enables a great deal of flexibility in the protein (8). Also, DBD F contains a basic cleft region that appears to be a critical feature for protein-protein interactions and DNA metabolic regulation (8). This domain will be discussed in greater detail in the sections pertaining to this regulation. DBD C contains a conserved zinc finger motif (Cys4-type) that has been shown to influence DNA binding (2, 10). RPA2 has a significant number of phosphorylatable sites within the unstructured N-terminus region (amino acids 1-40) that play an important role in DNA metabolic pathway regulation. As with the basic cleft region of RPA1, we will discuss this further within other sections of this review. RPA2 also contains a protein-protein interaction domain that resides in the C-terminus of the subunit. The trimerization of RPA1, RPA2 and RPA3 is mediated by DBD C, DBD D and DBD E (6, 10). The RPA complex is extremely stable and has been shown to be resistant to 6 M urea (11, 12).
3.2. DNA binding and polarity
Much work has been conducted with purified RPA and truncation mutants to elucidate its DNA binding characteristics. The culmination of this work has identified four distinct RPA ssDNA binding modes, an 8-10 nucleotides (nt), 12-23 nt, 23-27 nt and 30 nt binding modes. Each mode represents varying degrees of DBD contact and ssDNA occlusion. The affinity of RPA for ssDNA with an occluded binding size of 30 nt is extremely high with dissociation constants (KDs) in the low nM to high pM range (13). The affinity for each individual DBD, however, is relatively weak when compared with the heterotrimer, with KDs in the low micromolar range for DBD A (14). The 8-10 nt mode most likely represents contact of DBD A and B (1, 15). For example, biochemical studies have shown that DBD A and B have the highest affinity for ssDNA and are the primary contacts with ssDNA in the initial multistep binding process. The crystal structure of DBD AB bound to a dC8 ssDNA substrate reveals specific aromatic amino acid stacking with bases as well as hydrogen bonds between amino acids and bases (1). This structure demonstrates that 3 nt on the 5′ side of the ssDNA (dC1-3) reside within DBD A. There are 2 nt (dC4-5) that participate in hydrogen bonding between DBD A and B, then the 3 nt at the 3′ end of the ssDNA (dC6-8) reside within DBD B (1). RPA has been shown to have about a 10-50 fold preference for pyrimidine sequences compared with purines (16, 17). This likely relates to the base stacking and other interactions within the OB folds.
The 12-23 nt mode represents DBD A, B, C binding to ssDNA (18). This DNA binding mode was also demonstrated by Cai and colleagues (19). The 23-27 nt mode involves DBD A-D (18). The 30 nt binding mode represents a multistep binding and stretching out of DBDs A-D (20). There is also evidence that DBD F may contact ssDNA and play a role in the 30 nt binding mode (8). The exact role of these binding modes is not clear, but they likely play a role in mediating RPA ssDNA binding as well as specific protein-protein interactions. A recent review article highlights these binding modes and possible models for affecting DNA metabolic processes (21).
The DBD A and B domains bind in a specific polarity with respect to ssDNA binding (22-24). In this process, DBD A binds to the 5′ end of ssDNA whereas DBD B binds toward the 3′ end. In the multistep binding process to achieve the 30 nt binding mode, the other DBDs change conformation and align along the ssDNA. In vitro experiments with purified RPA demonstrate that with high concentrations of protein, multiple bound species can form on short 30-31 nt DNA substrates (20, 25). These species represent multiple RPAs bound to the DNA and likely represent the 8-10 nt binding mode (20). Kinetic analysis of RPA binding ssDNA suggest a near diffusion limited association rate, kon, ~ 2 nM−1 s−1 (16). These data combined suggest that the association rate of RPA for ssDNA is greater than the rate at which RPA elongates to form the 30 nt binding mode. This is significant as it may have impact during DNA metabolic processes when there is a high abundance of RPA compared with ssDNA. For example, this may occur at a site of replication fork collapse with RPA recruitment to ssDNA as a consequence of polymerase and helicase uncoupling (26, 27). In this scenario, RPA would likely bind in the 8-10 nt binding mode. The RPA DNA binding modes may help regulate protein-protein interactions, assist in enabling kinases to phosphorylate RPA2 (28) and influence the specific DNA metabolic pathways that occur.
RPA has a weak affinity for double-stranded DNA (dsDNA) with nearly a thousand fold difference in affinity compared with ssDNA (17, 29, 30). In fact, the dsDNA binding that is observed in vitro, is likely the result of RPA denaturation of duplex DNA followed by binding to the ssDNA (25, 29, 31). In this binding, the DBDs are believed to disrupt the hydrogen bonds between complementary DNA molecules. Biochemical studies using purified protein and synthetic DNA substrates demonstrate a preference for RPA binding to UV and cisplatin damaged duplex DNA when compared with undamaged duplex DNA (32-34). This enhanced affinity is believed to be the consequence of DNA unwinding and disruption of the hydrogen bonds which is induced by the localized DNA damage (35, 36). Studies looking at RPA binding to damaged ssDNA demonstrate that RPA has a decreased affinity when compared to undamaged ssDNA (25, 37). These results and damaged DNA binding are significant with respect to bulky DNA damage processing and will be discussed in more detail in the nucleotide excision repair section.
3.3. Phosphorylation of RPA2
RPA2 undergoes cell cycle-regulated phosphorylation, with phosphorylation by the cyclin-dependent kinase (CDK) family of kinases occurring during DNA replication and mitosis, and dephosphorylation occurring as cells progress into G1 (38, 39). Ser23 and Ser29 are CDK consensus sites, as defined by the S/T-P motif, and are phosphorylated by cyclin A-Cdk2 and cyclin B-Cdk1 during DNA replication and mitosis, respectively (40-44) (Figure 1). Similar to other kinase substrates, initial phosphorylation by Cdk1 and Cdk2 can prime RPA2 for phosphorylation at additional sites in response to DNA damage (45). There are many protein kinases that have an acidic or phosphorylated residue in their consensus phosphorylation motif, and some of these sites are “primed” by other protein kinases. That is, the first kinase provides a negative charge stimulating phosphorylation by the second kinase. Anantha et al. used the CDK inhibitor roscovitine to demonstrate this concept, decreasing phosphorylation of RPA sites Ser4, Ser8, Thr21 and Ser33, in addition to the CDK consensus site Ser29, following treatment with the topoisomerase I inhibitor, camptothecin (45). To provide additional evidence, these investigators replaced endogenous RPA2 with RPA2 containing a S23A CDK consensus site mutation. This drastically reduced the phosphorylation of Ser4, Ser8, Thr21, Ser29 and Ser33 in response to camptothecin. This is consistent with a previous report demonstrating decreased IR-induced phosphorylation of RPA2 when Ser23 and Ser29 are mutated to alanines (40). Overall, phosphorylation of RPA2 is not substantially stimulated in response to ionizing radiation and radiomimetics such as bleomycin as compared to agents that generate large amounts of ssDNA (45, 46). High doses of IR such as 50 Gy induce low amounts of phosphorylated RPA2 in asynchronous cells (40). Lower doses of IR or bleomycin generate weak Ser23 and Ser29 phosphorylation and only faint Ser4 and Ser8 phosphorylation is detected with phospho-specific antibodies (45, 46). These data are consistent with the requirement of the phosphorylation of Ser23 and to some extent the phosphorylation of Ser29 to stimulate additional phosphorylation of other serine and threonine residues in N-RPA2.
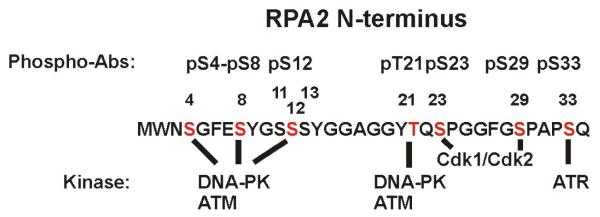
Figure 1.
RPA2 phosphorylation sites. N-terminal residues 1-34 are shown. Indicated above the sequence are sites for which phospho-specific antibodies are available. The putative kinases responsible for phosphorylation of specific residues are shown below the sequence.
A specific form of RPA2 phosphorylated on Ser23 and Ser29 is the predominant form of RPA2 observed in mitosis (39, 44, 47). In unstressed cells, Ser23 phosphorylation occurs during S-phase; however, Ser29 phosphorylation is only observed during mitosis (45, 46, 48). This unique phospho-isoform of RPA2 suggests a functional role for RPA in mitosis. RPA is excluded from the chromosomes during all phases of mitosis (46). Phosphorylation of RPA2 decreases RPA binding to dsDNA (47) which may facilitate the removal of RPA from chromosomal DNA during mitosis. RPA2 is rapidly dephosphorylated following cytokinesis and relocates to the nucleus in the G1 phase of the cell cycle (46). This is in agreement with the rapid dephosphosphorylation of RPA observed in a Xenopus in vitro system during mitotic exit (49). DNA damage occurring during mitosis causes a delay in mitotic progression and activates a spindle assembly checkpoint (50, 51). Interestingly, RPA distribution within the cell changes when cells are exposed to DNA damage during mitosis. RPA relocates back to the nucleus and binds to DNA (46). RPA2, previously phosphorylated at Ser23 and Ser29, is further phosphorylated on residues Ser4, Ser8, and Thr21. These phosphorylation events are coincident with an extended duration of BubR1 phosphorylation and a delay in progression into G1 after DNA damage (46, 48). Cells expressing an RPA2 mutant with alanine substitutions at Ser23 and Ser29 were further delayed in exiting mitosis following mitotic DNA damage, implicating RPA2 phosphorylation in mitotic checkpoint recovery (48). It is not clear how RPA2 phosphorylation promotes mitotic exit after damage. Continued effort to identify the molecular consequences of specific phosphorylation sites is needed to identify the precise role of RPA in mitosis.
In response to DNA damage, RPA2 is phosphorylated at multiple sites by the phosphoinositide 3-kinase-related kinase family of kinases, including ATM (ataxia-telangiectasia mutated), ATR (ATM and Rad3-related) and DNA-PK (DNA-dependent protein kinase) (52-56). ATM and DNA-PK have been shown to phosphorylate S/T-Q consensus sites Thr21 and Ser33 in vitro as well as other non-consensus sites using mass spectrometry, phospho-peptide mapping and phospho-specific antibodies (54, 55, 57, 58) (Figure 1). Other sites known to be phosphorylated on the N-terminus of RPA2 in response to DNA damage include the non-consensus S/T-Q residues Ser4, Ser8 (59-61) and Ser12 (unpublished data). ATM and DNA-PK are capable of phosphorylating serines and threonines that are not S/T-Q consensus sites in DNA repair proteins, BRCA1, Artemis, Xrcc4 and Ku (62-65). Enrichment of negative, hydrophobic residues or another serine surrounding the phosphorylation site enhances substrate recognition by DNA-PK and ATM (66, 67). The sequence surrounding Ser4, Ser8 and Ser12, includes hydrophobic as well as negatively charged residues and other serines, MWN (S)GFE (S)YGS (S)SYG, making Ser4, Ser8 and Ser12 adequate substrates for both DNA-PK and ATM. There is evidence from in vitro experiments for the direct phosphorylation of RPA2 by ATR where FLAG-tagged ATR was immunoprecipitated from lysates and combined with purified recombinant RPA in an immune complex kinase assay (68). In addition, immunoprecipitated ATR phosphorylated a GST-RPA2 fusion protein (69). Mutations in the fusion protein at the S/T-Q consensus sites, Thr21 and Ser33, decreased phosphorylation by ATR, suggesting either one or both of these sites are substrates for ATR (69). We have observed only Ser33 phosphorylation by ATR in vitro (unpublished data). Consistent with in vitro observations, in mitotically arrested HeLa cells Ser33 is not phosphorylated and only ATM, DNA-PK and CDKs have been shown to be involved in phosphorylation of RPA2 in response to DNA damage (45, 46). Nocodazole-arrested HeLa cells treated with ATM and DNA-PK inhibitors before IR decreased Ser4, Ser8 and Thr21 phosphorylation while Seckel cells deficient in ATR activity showed little difference in Ser4, Ser8 and Thr21 phosphorylation with or without transgenic expression of ATR (46). In agreement with these findings, Anantha et al. did not observe Ser33 phosphorylation in nocodazole arrested cells treated with bleomycin (48). In asynchronous cells, ATR activity is required for IR-, UV-hydroxyurea-, cisplatin- and etoposide-induced phosphorylation of RPA2 (54, 56, 70, 71). Interestingly, ATM and DNA-PK have also been shown to be required for RPA2 phosphorylation when cells are treated with UV (55, 60) while cisplatin-induced phosphorylation requires ATR and DNA-PK but not ATM activity (72). Both purified ATM and DNA-PK phosphorylate recombinant RPA at similar residues, including Ser4, Ser8, Thr21, and to a lesser degree Ser33 (55, 57, 58). Taken together, these observations implicate Ser33 primarily as an ATR substrate and Ser4, Ser8, and Thr21 as ATM and DNA-PK substrates.
While ATM and ATR are known to be involved in checkpoint signaling, DNA-PK is involved in and most often associated with initiating non-homologous end joining (NHEJ). DNA-PK phosphorylates several proteins in the NHEJ pathway but primarily DNA-PK autophosphorylation and Artemis phosphorylation regulate NHEJ-catalyzed repair (73, 74). Although DNA-PK helps initiate NHEJ, previous studies have also suggested DNA-PK plays a role in cell cycle checkpoint functions but the mechanism remains unclear (45, 53, 72). M059J is a human glioma cell line deficient in DNA-PK. In M059J cells, the camptothecin-induced phosphorylation of RPA2 is deficient and these cells are hypersensitive to cell killing by camptothecin. Moreover, these cells fail to suppress replication in response to camptothecin. Recently, cooperation between DNA-PK and ATR in activating checkpoint signaling has been shown in sapacitabine treated cells (75). It is of interest that ATR phosphorylates DNA-PK in response to UV irradiation in replicating cells. Combined, these observations implicate a role for DNA-PK phosphorylation of RPA2 and suggest that ATR and DNA-PK cooperate in the response to replication stress. Currently, little is known about the role of DNA-PK in checkpoint activation and its cooperation with ATR and it will be interesting to determine how these proteins coordinate together in the activation of the checkpoint response.
In addition to the PIKK family of kinases, other proteins involved in DNA repair facilitate phosphorylation of RPA2. The MRN complex along with CtIP work together in the processing of DSBs and form regions of ssDNA during DNA damage-induced homologous recombination repair (76, 77). The MRN/CtIP generated ssDNA promotes RPA binding and phosphorylation by ATR, ATM and DNA-PK. Two recent studies revealed that the FHA and BRCT domains of Nbs1 bind phosphorylated motifs in CtIP and recruit CtIP to DSBs (78, 79). Interestingly, Nbs1 is phosphorylated at Ser278 which maps to a site located within the second BRCT domain that potentially could regulate CtIP protein interaction (78). This may explain the loss of RPA phosphorylation in cells expressing an Nbs1 Ser278Ala/Ser343Ala phosphomutant (61). The FHA and BRCT domains of Nbs1 also function together to interact with the mediator/scaffold protein MDC1 (79). MDC1 directly interacts with ATM through its FHA domain and stimulates phosphorylation of ATM substrates including RPA2 (80). This has been shown by depletion of MDC1 expression with RNAi decreasing replication stress-induced phosphorylation of RPA2 (81). Another mediator/scaffold protein, 53BP1 interacts with RPA2 and the protein-protein interaction is disrupted following camptothecin treatment (82). Down regulation of 53BP1 expression by RNAi decreased camptothecin-induced RPA2 phosphorylation possibly through an interaction between DNA-PK and 53BP1 (82). While the precise function of MCPH1 has not been determined, MCPH1 is a mediator of the response to DNA damage (83). MCPH1 contains one N-terminal and two C-terminal BRCT domains and may also act as a scaffold protein, recruiting phosphorylated checkpoint and DNA repair proteins to sites of DNA damage. Down regulation of MCPH1 eliminated UV-induced phosphorylation of RPA2 (84). This could be due to a decrease in RPA recruitment to chromatin which would reduce ATR binding to chromatin and stimulation of kinase activity.
3.4. Protein-protein interactions
In order for RPA to direct and coordinate protein traffic at sites of DNA damage it is important to possess a high binding affinity for ssDNA. However, more importantly it is essential to interact with multiple proteins and exchange protein binding partners in an organized and controlled process. RPA interacts with multiple proteins through interactions with the N-terminus of RPA1. One mechanism for the regulation of protein interactions with RPA involves the phosphorylated N-terminal domain of RPA2. Evidence for this hypothesis is demonstrated by the interaction between an aspartic acid substituted 35 residue peptide of RPA2 corresponding to the N-terminal domain of RPA2 and the basic cleft present in the N-terminus of RPA1 (85, 86). Further support is demonstrated with recombinant RPA partially phosphorylated by DNA-PK that undergoes a structural change involving the B-DNA binding domain of RPA1 (87). This study used partially proteolyzed RPA that did not contain the N-terminal domain of RPA1 (87). While interaction of the N-terminus of RPA2 with RPA1 has not been demonstrated with the complete heterotrimeric protein, notably Liu and colleagues do demonstrate that the phosphorylated RPA2 subunit interacts with a proteolyzed portion of the RPA1 subunit of the intact purified heterotrimeric protein. The N-terminus of RPA1 is attached to the rest of RPA1 through a flexible linker (88). This flexibility may be important to allow the N-terminus to function as a protein interaction domain and to be flexible enough to interact with the phosphorylated N-terminus of RPA2.
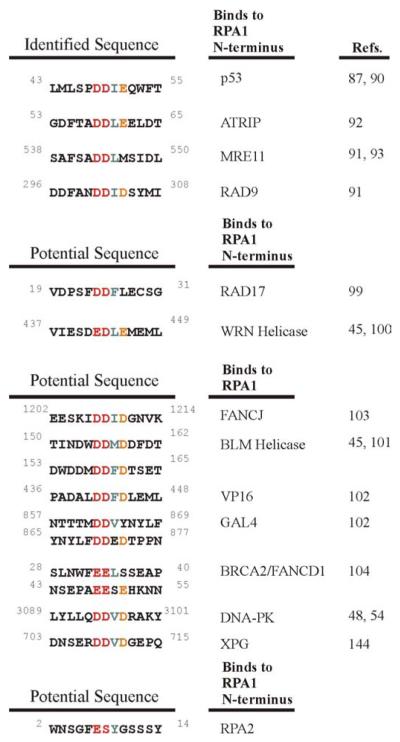
Proteins that have been identified to bind to the N-terminus of RPA1 include p53, ATRIP, Mre11 and Rad9 (Figure 2) (86, 89-92). All of these proteins contain an acidic alpha helical domain that binds to the basic cleft of the N-terminus of RPA1 through electrostatic contributions. Mutation of two aspartic acid residues Asp58 and Asp59 in a 107 residue peptide of ATRIP decreased binding to the N-terminus of RPA1 (91). Comparable decreases in interaction with RPA1 were also observed with mutations of Asp543 and Asp544 in Mre11 and Asp301 and Asp302 in Rad9 (90, 92). Alternatively, a single rfa1-t11 mutation in S. cerevisiae, Lys-45-Glu, within the N-terminal basic cleft binding domain of RPA1, equivalent to a mutation at human Arg 41 RPA1, leads to chromosomal instability, sensitivity to UV radiation and a loss of recruitment of checkpoint proteins (93-96). The rfa-t11 mutant binds weakly to Ddc2 (Atrip) (93). Rad17 is part of the Rad17-Rfc2-5 clamp loading complex that loads the checkpoint clamp Rad9-Rad1-Hus1. RPA recruits the Rad17-Rfc2-5 complex to damaged DNA through an interaction with Rad17 (97). This interaction is completely abolished with the mutant rfa-t11 protein indicating that RPA17 also binds to the N-terminus of RPA1 (98). There are many other checkpoint response and DNA repair proteins that interact with RPA1 possibly via this binding motif (Figure 2) (99-102). Recently, an acidic domain in Rad52 from budding yeast was identified that is required for interaction with RPA1 (103). A recognized N-terminal acidic domain of BRCA2 and the FANCJ helicase interact with RPA1, possibly to the N-terminus of RPA1 (104, 105). In order for RPA2 to function in a regulatory role, the best fit binding motif for RPA2 would include Glu7 and a negatively charged phosphorylated Ser8 mimicking another Glu or Asp. Phosphorylation of Ser4 and Ser12 would provide additional negative charges similar to the Rad9 sequence identified to bind to RPA1. Combined with the ability of RPA’s DNA binding polarity directing initial protein complexes, checkpoint response and DNA repair proteins are potentially assembled and disassembled from protein complexes by DNA damage-induced phosphorylation of RPA2 (47, 106, 107), implicating both the N-terminus of RPA1 and RPA2 in directing traffic and coordinating changes in protein complexes at sites of DNA damage.
Figure 2.
Sequence alignment of identified and putative peptide sequences that bind to the N-terminal domain of RPA1.
If the N-terminus of RPA1 binds ssDNA and also functions as a protein interaction domain, what role would RPA2 phosphorylation have in modulating these functions? One very intriguing possibility is that the phosphorylation of RPA2 would promote protein interactions by competing with ssDNA for binding to the N-terminal domain of RPA1. The binding of phosphorylated RPA2 to RPA1 would release the RPA1 N-terminal domain from ssDNA and allow DNA damage response proteins to bind to RPA1. Indeed, Mre11 and Rad9 interactions with RPA increase when RPA2 is phosphorylated (106, 107). Furthermore, this scenario is consistent with titration experiments where the N-terminal basic cleft of RPA1 had the highest binding affinity for a p53 peptide, intermediate binding affinity for an RPA2 aspartic phospho-mimetic peptide and the lowest binding affinity for ssDNA (86). The conformational change induced by phosphorylation of RPA2 could also have an effect on the overall ssDNA binding affinity of RPA as well as promote the 8-10 nt binding mode. Previous results have shown that the N-terminus of RPA1 does not alter the binding affinity of RPA for longer, pyrimidine-rich ssDNA substrates (85). In addition, both RPA containing a deletion of the RPA1 N-terminal domain and hyperphosphorylated RPA bind longer, pyrimidine-rich ssDNA with affinity equal to wt-RPA (85, 108). However, when RPA binding to shorter or purine-rich ssDNA was observed phosphorylated RPA had a decreased DNA binding affinity (108, 109). It is reasonable to suggest that in this mechanism, RPA would bind ssDNA and subsequent recruitment of kinases would phosphorylate RPA2. Phosphorylated RPA2 would then interact with DBD F of RPA1, which would result in damage-specific interaction with proteins as well as shift ssDNA binding to the 8-10 nt mode by decreasing the DBD contacts with ssDNA.
An alternative possibility that also has experimental support is that phosphorylated RPA2 competes with proteins bound to RPA1 thus displacing them from RPA1. For example, ATRIP binds the N-terminus of RPA1. If ATR then initiates phosphorylation of RPA2, phosphorylated RPA2 could displace ATRIP and ATR from RPA. This would allow ATR/ATRIP to move to another unphosphorylated RPA molecule or another available substrate and also allow possible further phosphorylation of the initial RPA molecule by another kinase such as DNA-PK. Consistent with this idea, we and others have shown that interaction of DNA-PK with RPA1 decreased once RPA2 was phosphorylated (47, 53). Also, using purified proteins, we have recently shown that phosphorylation of RPA results in a decreased interaction with MRN (110).
Another protein binding motif resides in the C-terminal domain of RPA2. The C-terminal residues 200-270 of RPA2 interact with DNA repair proteins XPA, Rad52, checkpoint response protein Tipin and the recently identified annealing helicase HepA-related protein (HARP) also known as SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin (SMARCAL1) (111-115). These proteins bind to the winged helix-turn-helix domain within the C-terminus. Unlike proteins binding primarily to the basic cleft of the N-terminus of RPA1, DNA damage does not stimulate or alter the interaction between these proteins and RPA (108, 112, 113, 116). This suggests phosphorylation of the N-terminus of RPA2 does not have a regulatory role in protein interactions with these proteins. This could be significant for DNA repair pathway proteins so that protein-protein interactions are maintained following DNA damage and subsequent RPA phosphorylation. Two different peptide sequences have been identified in Rad52 that bind to both RPA1 and RPA2, respectively (103, 117), leaving open the possibility for phosphorylation-dependent regulation of protein binding in certain DNA repair pathways.
4. RPA IN DNA REPLICATION
RPA was first identified and purified as a protein factor required for both initiation and elongation phases of SV40 DNA replication (11, 12, 118). Much of our understanding of RPA in DNA replication comes from the SV40 viral replication system. In this system, the viral genome is replicated using the large T antigen helicase along with proteins supplied by the host cell. RPA is recruited to the origin of replication by T antigen and assists in the origin unwinding (11, 119, 120). The high ssDNA affinity and the ability to denature duplex DNA are the properties of RPA that seem important in the initial replication phases. Despite the difficulty in identifying the eukaryotic replication initiating helicase (now believed to be the MCM complex), it is generally believed that RPA functions similarly in eukaryotic DNA replication. Following DNA unwinding, RPA helps recruit polymerase alpha to the replication origins where RNA priming is required for initiation (121, 122). RPA stimulates polymerase alpha activity as well as processivity, and RPA reduces misincorporation by polymerase alpha. The interaction between RPA and polymerase alpha is mediated by the primase domain of polymerase alpha and the N-terminus of RPA1 (124, 125). RPA has been shown to bind in a specific polarity on ssDNA, which is believed to help stimulate the DNA polymerase alpha synthesis (22, 24).
In the elongation phase of DNA replication, RPA is believed to play a role in stimulating DNA polymerases delta and epsilon (126, 127). This stimulation could be the result of RPA and PCNA interaction (128, 129). Interaction of RPA with Dna2 and subsequent stimulation of cleavage also suggests a role in Okazaki fragment processing (130, 131). RPA is able to bind the 5′ primer ends as they are displaced by polymerase delta (130). RPA recruits the Dna2 endonuclease which cleaves the RPA bound RNA primer (130). Release of RPA allows FEN1 to cleave the remaining flap (131, 132). The interaction between Dna2 and RPA is mediated through the C-terminus of Dna2 and RPA1 as well as a secondary interaction between the N-terminal domains of Dna2 and RPA1 (131). The N-terminus of RPA1 is required to achieve maximum activity of Dna2 (131).
Phosphorylation of RPA2 has been shown to inhibit DNA replication (108, 133). Using purified proteins, we have shown that phosphorylated RPA has minimal interaction with polymerase alpha, an important feature for replication initiation (47, 108). These data suggest that following DNA damage, RPA phosphorylation may modulate the DNA metabolic pathways via RPA protein-protein interactions such that DNA replication is halted and DNA repair can be initiated. The observation that the N-terminus of RPA1 (N-RPA1) interacts with Dna2 for maximum activity, suggests that RPA2 phosphorylation and subsequent interaction with N-RPA1 could influence Okazaki fragment processing through Dna2 activity. Also, it is unclear how RPA phosphorylation influences the interaction with the MCM complex and RPA loading at replication origins.
5. RPA IN DNA REPAIR
5.1. Nucleotide excision repair
RPA has been shown to be required for nucleotide excision repair (NER) (134-136). Initially, RPA was believed to play some role in DNA damage recognition when studies demonstrated a binding preference to duplex damaged DNA compared with undamaged DNA (32, 34). When incubated with XPA, the RPA-XPA complex had a higher affinity for damaged DNA than either protein alone, and the denaturation ability of RPA is suppressed (36, 137). However, studies looking at the kinetics and protein assembly during NER suggest that RPA is recruited at a later stage in the pathway (138, 139). The identification of XPC-hHR23B as the global initiator of NER further supported a role for RPA in the later stages of NER (140). The DNA binding polarity of RPA, the decreased affinity of RPA for damaged ssDNA and the ability to interact with the endonucleases XPF-ERCC1 and XPG support a role in protein positioning at the damaged DNA site (23, 25, 141). RPA was shown to block endonuclease cleavage on the DNA strand it was bound (23). The binding polarity of RPA and decreased affinity for damaged ssDNA would position the RPA1 subunit to the 5′ side of the undamaged DNA strand opposite the lesion and protect the undamaged DNA strand from nuclease cleavage (25, 142). RPA1 interacts with XPG and would help position this endonuclease to the 3′ side of the lesion on the damaged DNA strand (23, 143). RPA2 interacts with XPA and XPF-ERCC1 which would help position XPF-ERCC1 to the 5′ side of the lesion (144, 145). Following cleavage by the endonuclease, RPA would be in position to stimulate the gap filling reaction performed by polymerase delta or epsilon (136, 139, 146). As mentioned above, RPA2 is phosphorylated in response to DNA damage. Following phosphorylation, RPA has a decreased ability to denature DNA strands, which would assist in maintaining a defined DNA structure around the lesion. This change in RPA conformation to likely the 8-10 nt binding mode could also assist in polymerase displacement of RPA from the ssDNA as it synthesizes the new DNA.
5.2. Base excision repair
Studies in yeast containing a mutant RFA1 gene (homolog to RPA1) demonstrate sensitivity to methyl methane sulfonate (MMS), which is an agent that produces DNA damage that is repaired by the base excision repair (BER) pathway (96). This data strongly suggests that RPA is required for BER. RPA has been shown to interact with DNA glycosylases in the BER pathway, including UNG2 and hMYH (111, 147, 148). Despite these interactions, it is unclear what role RPA has in the early steps of BER and glycosylase function. One possibility is that RPA directs DNA glycosylases to sites of DNA replication as a safeguard to replication-induced DNA damage (149). The role of RPA in long-patch BER where the damaged base and subsequent small oligonucleotide 2-8 bases are removed has been more extensively studied (129, 150, 151). RPA stimulates long patch BER by enhancing primer extension and unwinding the 5′ end of the downstream strand (152). In addition, RPA has been shown to stimulate DNA ligase I in the final step of BER (153).
5.3. Mismatch repair
The first evidence that RPA was required for mismatch repair (MMR) was from in vitro experiments neutralizing RPA activity with specific antibodies (154). Interestingly, in those studies, mutant RPA with a point mutation in the RPA1 zinc finger domain did not support MMR or DNA replication, but fully supported NER (154). This suggests that different protein-protein interactions occur throughout the RPA structure that can influence and regulate various DNA metabolic pathways. Further biochemical studies identified specific functions of RPA in the MMR pathway. One function of RPA is to bind ssDNA and protect the template strand from nuclease degradation (155). RPA also functions to stimulate the excision process of EXOI when a mismatched base is present (156, 157). Following removal of the mismatched base, RPA helps regulate and terminate EXOI excision. This was initially believed to be dependent on MutLalpha (158), but more recently, experiments with extracts devoid of MutLalpha have shown that RPA functions independently to terminate MutSalpha-activated EXOI excision (157). Phosphorylation of RPA has also been shown to help regulate the various roles of RPA in MMR. Guo and colleagues have shown that once RPA is bound to ssDNA and extensive excision has occurred, RPA becomes phosphorylated (109). The significance of this, is that following phosphorylation of RPA2, RPA changes structural conformation such that phosphorylated RPA2 interacts with DBD F of RPA1 (85). In this process, RPA DBDs C and D likely have decreased contact with ssDNA which weakens the overall affinity of RPA for ssDNA (21). This RPA phosphorylation event is prior to DNA re-synthesis and possibly allows polymerase delta to dissociate RPA from ssDNA during synthesis as a consequence of less DBD contact and weaker ssDNA affinity or possibly an effect on protein interactions.
5.4. DNA double-strand breaks and recombination
RPA has been shown to have roles in the repair of DNA double-strand breaks (DSBs) and recombination (159). Depletion of RPA inhibits Rad51 DNA repair foci, which highlights RPA bound ssDNA as a nucleation point for recombination proteins (160). Following DNA strand resection and the generation of 3′ overhangs at DSBs, RPA ssDNA binding likely protects the DNA and prevents secondary structure formation. RPA subsequently recruits Rad52 to the ssDNA overhangs via a direct protein-protein interaction (117). The RPA-Rad52 interaction occurs in a positively charged alpha helical structure domain on Rad52 (amino acids 247-275) (111, 161). This domain contains the Arg-Gln-Lys sequence which is similar to SMARCAL1, Tipin, Ung2 and XPA sequences that interact with the C-terminus of RPA2 (111, 113). Budding yeast contain an additional acidic domain (amino acids 288-327) in Rad52 that has been shown to be crucial for RPA interaction as well as recruitment to DNA repair centers (103). The RPA-Rad52 interaction is mediated through the C-terminus of RPA2 and thought to be in DBD A and a portion of DBD B of RPA1 as well (111, 161). The interaction between Rad52 and RPA increases the ssDNA binding affinity of the complex and promotes stoichiometric interactions (161). It was suggested that similar to the p53 interaction with RPA (86), the acidic domain of Rad52 could interact with RPA and modulate ssDNA binding and/or structural conformation (103). RPA has to be released from ssDNA to enable Rad51 to bind the 3′ ssDNA overhangs and promote strand invasion (162). This process appears to be regulated by Rad52, and more recently, RPA phosphorylation (163). The rfa1-t11 mutant described in previous sections, results in recombination defects and likely stems from effects on RPA release from ssDNA, where Rad52 is unable to promote release of RPA from ssDNA and enable Rad51 exchange (164, 165). Recent work has suggested that RPA phosphorylation can regulate DSB repair and recombination (45, 160, 163, 166). Phosphorylated RPA has been shown to interact more efficiently with Rad51 and Rad52 (166). Similar to other DNA repair pathways and based on previous findings that phosphorylations weaken RPA-ssDNA binding affinity, phosphorylation of RPA promotes the transfer of ssDNA to Rad52 (163). This is likely facilitated by the conformational change in RPA when RPA2 becomes phosphorylated, promoting the 8-10 nt binding mode. In this mechanism, RPA phosphorylation would be important for Rad52 mediator functions and promote Rad51 exchange and RPA dissociation from ssDNA. However, the interaction between RPA and Rad52 may be more important for homologous recombination activity in S. cerevisiae than in mammalian systems. In human homologous recombination repair, BRCA2 plays a major role in Rad51 localization and function (167, 168). BRCA2 contains three OB folds similar in structure to the OB folds of RPA and binds ssDNA (169). A small 70 amino acid highly acidic protein DSS1 binds to one of the OB folds of BRCA2 and acts as a modifier of BRCA2 function (170). A fungal ortholog of BRCA2, Brh2 promotes the nucleation of Rad51 nucleoprotein filament formation (171). Nucleation of Rad51 onto ssDNA requires the removal of RPA from ssDNA. It seems reasonable to suggest that the acidic helical protein DSS1 binds to the basic cleft of the N-terminal domain of RPA1, facilitating the removal of RPA from ssDNA by BRCA2. This would allow Rad51 binding and accelerate nucleoprotein filament formation of Rad51. The role, if any, of DSS1, BRCA2 and the phosphorylation of RPA2 in the exchange of RPA for Rad51 are important questions that need to be answered.
5.5. Telomere maintenance
Studies in yeast were the first to identify a role for RPA in telomere maintenance (172). Telomere length was synergistically shortened in a double mutant where the human Ku and RPA homologs were mutated. Single mutants of the RPA homolog, however, do not display loss of telomere length (172). Similarly, it has been shown that yeast Taz1 (ortholog of TRF2 and TRF1) and RPA work synergistically to prevent telomere loss (173). It is believed that loss of both Taz1 and RPA disrupt the telomeric ssDNA binding protein Pot1 function which leads to telomere loss (173). One mechanism proposed for maintaining telomeric DNA was that RPA could protect the ssDNA from degradation (173). An alternative mechanism could be that RPA could play a role in telomere replication. Recent work has shown that RPA can unwind G-quadruplex structures formed from telomeric sequences (174). These G-quadruplex structures in the absence of RPA could ultimately inhibit telomerase activity. Rubtsova and colleagues were able to show that RPA stimulates telomerase activity in vitro (175). The stimulation of telomerase by RPA could be the result of disruption of DNA structures that inhibit telomerase or RPA could play a role in displacing other proteins from telomeric DNA that could inhibit telomerase. In alternative lengthening of telomere (ALT) cells, RPA also plays a role in maintaining telomere length (176). In this pathway, RPA appears to play a role in telomere capping to prevent accumulation of single-stranded telomeric DNA (176). Interestingly, a truncation of RFA2p (homolog of RPA2 N-terminal 1-40 amino acid phosphorylation domain) in yeast was shown to reduce telomere length (177). It was determined that the N-terminus of RFA2 was important for the interaction and recruitment of the telomeric ssDNA binding protein Est1p, which is required for telomerase activity (178). This suggests that RPA phosphorylation may assist in modulating telomere length.
6. ROLES OF RPA PHOSPHORYLATION IN THE DNA DAMAGE RESPONSE AND CHECKPOINT ACTIVATION
While the phosphorylation of RPA2 has been studied for many years, the precise role remains obscure. It has been suggested that RPA2 mutants that have aspartic acid substitutions to mimic phosphorylation are unable to associate with replication centers (59, 70). These data are based on RPA2 phospho-mimetic mutants competing with endogenous RPA2 to complex with endogenous RPA1 in unstressed cells. While the proposal is plausible, other possible conclusions are conceivable. Additional data strengthening this argument will be needed. For example, the comparison of co-localization of exogenous wild-type and phospho-mimetic RPA2 with incorporated BrdU and the simultaneous RNAi knockdown of endogenous RPA2 would provide additional support. However, indirectly Francon and colleagues provide support for this model in another study, where they demonstrated that only hypophosphorylated RPA2 associated with initiation and elongation stages of DNA replication (49). In UV-irradiated cells, hyperphosphorylation of RPA2 is associated with the cessation of cell cycle progression and a decrease in replication activity (70). These data are consistent with the decreased support of in vitro SV40 DNA replication activity from cell extracts containing hyperphosphorylated RPA2 (108, 133, 179, 180). How phosphorylation of RPA2 affects DNA replication remains to be identified. One possibility is that the interaction between DNA polymerase alpha and RPA that occurs during the synthesis and processing of Okazaki fragments (125, 181) is inhibited by the hyperphosphorylation of RPA2 (108, 182). A second possibility involves the minichromosome maintenance (MCM) 2-7 helicase complex, which functions in both the initiation and the elongation of replication forks (183, 184). In budding yeast RPA and Cdc45, a replication co-factor, are required to interact with MCM2 to initiate the activation of replication origins and maintain helicase activity during elongation (121, 122, 185, 186). The hyperphosphorylation of RPA2 may play a role in controlling these activities such that RPA phosphorylation disrupts the protein-protein interactions.
There is evidence that phosphorylation of RPA2 is required for resistance to camptothecin, cisplatin and etoposide. Cancer cell lines that have suppressed the DNA damage response and do not phosphorylate RPA2 failed to complete DNA replication following cisplatin treatment and were hypersensitive to cisplatin and etoposide (187) . These data are consistent with previous results that utilized U2OS cells expressing a phospho-mutant form of RPA2 containing alanines at Ser23 and Ser29. These cells accumulated in S-phase while cells expressing wild-type RPA2 continued to cycle into G2 phase after camptothecin exposure (45). A similar phenotype was observed with ATR mutant cells, which were impeded in progressing through S-phase with consequent survival defects in response to cisplatin treatment (188). DNA-PK deficient M059J human glioblastoma cells unable to phosphorylate RPA2 exhibited enhanced sensitivity to camptothecin as compared to M059K cells expressing DNA-PK (53). Taken together, these results are indicative of a strong correlation between the ability of cells to efficiently hyperphosphorylate RPA2 in response to DNA damage and cellular survival.
7. CONCLUDING REMARKS
Before RPA was identified as the eukaryotic single-stranded DNA binding protein (SSB), it was argued that a mammalian SSB was not necessary. The logic of the argument was that mammalian Okazaki fragments are much shorter than bacterial Okazaki fragments (189) and the shorter regions of exposed ssDNA could be protected by the replication protein complex itself (190, 191). With the discovery of RPA, it is now known that SSB proteins are conserved throughout all kingdoms of life and retain central roles in the maintenance of genomic integrity (13, 192). If the primary role of RPA is to bind to ssDNA with very high affinity, the equally important secondary role of RPA is to direct and coordinate the assembly and disassembly of proteins to DNA during replication, recombination and DNA repair. To do this efficiently RPA must associate and disassociate from numerous proteins and ssDNA. There is increasing evidence that RPA binding to ssDNA is highly dynamic consistent with the different DNA binding modes (28, 109, 193). This can be critical to RPA function. It is tempting to speculate that the dynamic nature of RPA binding allows RPA to migrate along ssDNA. This would be analogous to recent studies with E. coli SSB that diffused along ssDNA in response to an elongating RecA nucleoprotein filament (194). This would give RPA an additional dimension in directing and mobilizing traffic at the lesion site. Additionally, to prevent increased destabilization of the damaged site, hyperphosphorylated RPA loses the ability to denature dsDNA yet maintains binding to ssDNA while during DNA replication in unstressed cells, RPA does not become hyperphosphorylated and continues to facilitate helicase activity. To associate and disassociate from recruited proteins, we propose a competition-type mechanism where the phosphorylation of the N-terminus of RPA2 allows it to compete with other substrates for binding to the N-terminal domain of RPA1. Future work will define how the competing protein interactions are regulated with RPA and how RPA directs the vast traffic of proteins needed to accurately maintain the genome.
ACKNOWLEDGEMENTS
We are grateful to members of the Patrick and Oakley laboratories for helpful comments during the preparation of this manuscript. Financial support from the American Cancer Society ACS RSG-06163-01-GMC to S.M.P. and ACS RSG-10-031-01-CCG to G.G. O. is acknowledged.
Abbreviations
- Replication protein A
RPA
- single-stranded DNA
ssDNA
- oligonucleotide/oligosaccharide binding
OB
- DNA binding domain
DBD
- double-stranded DNA
dsDNA
- cyclin-dependent kinase
CDK
- phosphoinositide 3-kinase-related kinase
PIKK
- ataxia-telangiectasia mutated
ATM
- DNA-dependent protein kinase
DNA-PK
- non-homologous end joining
NHEJ
- nucleotide excision repair
NER
- methyl methane sulfonate
MMS
- base excision repair
BER
- mismatch repair
MMR
- double-strand breaks
DSBs
- alternative lengthening of telomere
ALT
9. REFERENCES
- 1.Bochkarev A, Pfuetzner RA, Edwards AM, Frappier L. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature. 1997;385:176–181. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]
- 2.Bochkarev A, Bochkareva E, Frappier L, Edwards AM. The crystal structure of the complex of replication protein A subunits RPA32 and RPA14 reveals a mechanism for single-stranded DNA binding. Embo J. 1999;18:4498–504. doi: 10.1093/emboj/18.16.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murzin AG. OB (oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. Embo J. 1993;12:861–7. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philipova D, Mullen JR, Maniar HS, Lu J, Gu C, Brill SJ. A hierarchy of SSB protomers in replication protein A. Genes Dev. 1996;10:2222–2233. doi: 10.1101/gad.10.17.2222. [DOI] [PubMed] [Google Scholar]
- 5.Brill SJ, Bastin-Shanower S. Identification and characterization of the fourth single-stranded-DNA binding domain of replication protein A. Mol.Cell Biol. 1998;18:7225–7234. doi: 10.1128/mcb.18.12.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochkareva E, Frappier L, Edwards AM, Bochkarev A. The RPA32 subunit of human replication protein A contains a single-stranded DNA-binding domain. J.Biol Chem. 1998;273:3932–3936. doi: 10.1074/jbc.273.7.3932. [DOI] [PubMed] [Google Scholar]
- 7.Bochkareva E, Korolev S, Lees-Miller SP, Bochkarev A. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. Embo J. 2002;21:1855–63. doi: 10.1093/emboj/21.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daughdrill GW, Ackerman J, Isern NG, Botuyan MV, Arrowsmith C, Wold MS, Lowry DF. The weak interdomain coupling observed in the 70 kDa subunit of human replication protein A is unaffected by ssDNA binding. Nucleic Acids Res. 2001;29:3270–6. doi: 10.1093/nar/29.15.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuss JE, Sweeney DJ, Alter GM. Prediction of and experimental support for the three-dimensional structure of replication protein A. Biochemistry. 2009;48:7892–905. doi: 10.1021/bi801896s. [DOI] [PubMed] [Google Scholar]
- 10.Bochkareva E, Korolev S, Bochkarev A. The role for zinc in replication protein A. J Biol Chem. 2000;275:27332–8. doi: 10.1074/jbc.M000620200. [DOI] [PubMed] [Google Scholar]
- 11.Wold MS, Kelly T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc.Natl.Acad.Sci.U.S.A. 1988;85:2523–2527. doi: 10.1073/pnas.85.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairman MP, Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988;7:1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu.Rev.Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 14.Arunkumar AI, Stauffer ME, Bochkareva E, Bochkarev A, Chazin WJ. Independent and coordinated functions of replication protein A tandem high affinity single-stranded DNA binding domains. J Biol Chem. 2003;278:41077–82. doi: 10.1074/jbc.M305871200. [DOI] [PubMed] [Google Scholar]
- 15.Blackwell LJ, Borowiec JA. Human replication protein A binds single-stranded DNA in two distinct complexes. Mol.Cell Biol. 1994;14:3993–4001. doi: 10.1128/mcb.14.6.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrick SM, Turchi JJ. Stopped-flow kinetic analysis of replication protein A-binding DNA: damage recognition and affinity for single-stranded DNA reveal differential contributions of k (on) and k (off) rate constants. J Biol Chem. 2001;276:22630–7. doi: 10.1074/jbc.M010314200. [DOI] [PubMed] [Google Scholar]
- 17.Kim C, Snyder RO, Wold MS. Binding properties of replication protein A from human and yeast cells. Mol.Cell Biol. 1992;12:3050–3059. doi: 10.1128/mcb.12.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastin-Shanower SA, Brill SJ. Functional analysis of the four DNA binding domains of replication protein A. The role of RPA2 in ssDNA binding. J Biol Chem. 2001;276:36446–53. doi: 10.1074/jbc.M104386200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai L, Roginskaya M, Qu Y, Yang Z, Xu Y, Zou Y. Structural characterization of human RPA sequential binding to single-stranded DNA using ssDNA as a molecular ruler. Biochemistry. 2007;46:8226–33. doi: 10.1021/bi7004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salas TR, Petruseva I, Lavrik O, Saintome C. Evidence for direct contact between the RPA3 subunit of the human replication protein A and single-stranded DNA. Nucleic Acids Res. 2009;37:38–46. doi: 10.1093/nar/gkn895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanning E, Klimovich V, Nager AR. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 2006;34:4126–37. doi: 10.1093/nar/gkl550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolpashchikov DM, Khodyreva SN, Khlimankov DY, Wold MS, Favre A, Lavrik OI. Polarity of human replication protein A binding to DNA. Nucleic Acids Res. 2001;29:373–9. doi: 10.1093/nar/29.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Laat WL, Appeldoorn E, Sugasawa K, Weterings E, Jaspers NG, Hoeijmakers JH. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev. 1998;12:2598–2609. doi: 10.1101/gad.12.16.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iftode C, Borowiec JA. 5′ --> 3′ molecular polarity of human replication protein A (hRPA) binding to pseudo-origin DNA substrates. Biochemistry. 2000;39:11970–81. doi: 10.1021/bi0005761. [DOI] [PubMed] [Google Scholar]
- 25.Patrick SM, Turchi JJ. Replication protein A (RPA) binding to duplex cisplatin-damaged DNA is mediated through the generation of single-stranded DNA. J Biol Chem. 1999;274:14972–8. doi: 10.1074/jbc.274.21.14972. [DOI] [PubMed] [Google Scholar]
- 26.Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19:1040–1052. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacek M, Walter JC. A requirement for MCM7 and Cdc45 in chromosome unwinding during eukaryotic DNA replication. Embo J. 2004;23:3667–76. doi: 10.1038/sj.emboj.7600369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackwell LJ, Borowiec JA, Masrangelo IA. Single-stranded-DNA binding alters human replication protein A structure and facilitates interaction with DNA-dependent protein kinase. Mol.Cell Biol. 1996;16:4798–4807. doi: 10.1128/mcb.16.9.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lao Y, Lee CG, Wold MS. Replication protein A interactions with DNA. Characterization of double-stranded DNA-binding/helix-destabilization activities and the role of the zinc-finger domain in DNA interactions. Biochemistry. 1999;38:3974–3984. doi: 10.1021/bi982371m. [DOI] [PubMed] [Google Scholar]
- 30.Wold MS, Weinberg DH, Virshup DM, Li JJ, Kelly TJ. Identification of cellular proteins required for simian virus 40 DNA replication. J.Biol.Chem. 1989;264:2801–2809. [PubMed] [Google Scholar]
- 31.Treuner K, Ramsperger U, Knippers R. Replication protein A induces the unwinding of long double-stranded DNA regions. J.Mol.Biol. 1996;259:104–112. doi: 10.1006/jmbi.1996.0305. [DOI] [PubMed] [Google Scholar]
- 32.Burns JL, Guzder SN, Sung P, Prakash S, Prakash L. An affinity of human replication protein A for ultraviolet-damaged DNA. J.Biol Chem. 1996;271:11607–11610. doi: 10.1074/jbc.271.20.11607. [DOI] [PubMed] [Google Scholar]
- 33.Clugston CK, McLaughlin K, Kenny MK, Brown R. Binding of human single-stranded DNA binding protein to DNA damaged by the anticancer drug cis-diamminedichloroplatinum (II) Cancer Res. 1992;52:6375–6379. [PubMed] [Google Scholar]
- 34.Patrick SM, Turchi JJ. Human replication protein A preferentially binds cisplatin-damaged duplex DNA in vitro. Biochemistry. 1998;37:8808–8815. doi: 10.1021/bi9730590. [DOI] [PubMed] [Google Scholar]
- 35.Buschta-Hedayat N, Buterin T, Hess MT, Missura M, Naegeli H. Recognition of nonhybridizing base pairs during nucleotide excision repair of DNA. Proc Natl Acad Sci U S A. 1999;96:6090–5. doi: 10.1073/pnas.96.11.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Missura M, Buterin T, Hindges R, Hubscher U, Kasparkova J, Brabec V, Naegeli H. Double-check probing of DNA bending and unwinding by XPA-RPA: an architectural function in DNA repair. Embo J. 2001;20:3554–64. doi: 10.1093/emboj/20.13.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Yang Z, Utzat CD, Liu Y, Geacintov NE, Basu AK, Zou Y. Interactions of human replication protein A with single-stranded DNA adducts. Biochem J. 2005;385:519–26. doi: 10.1042/BJ20041151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brill SJ, Stillman B. Yeast replication factor-A functions in the unwinding of the SV40 origin of DNA replication. Nature. 1989;342:92–95. doi: 10.1038/342092a0. [DOI] [PubMed] [Google Scholar]
- 39.Din S, Brill SJ, Fairman MP, Stillman B. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 1990;4:968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- 40.Liu VF, Weaver DT. The ionizing radiation-induced replication protein A phosphorylation response differs between ataxia telangiectasia and normal human cells. Mol.Cell Biol. 1993;13:7222–7231. doi: 10.1128/mcb.13.12.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henricksen LA, Wold MS. Replication protein A mutants lacking phosphorylation sites for p34cdc2 kinase support DNA replication. J.Biol Chem. 1994;269:24203–24208. [PubMed] [Google Scholar]
- 42.Dutta A, Stillman B. cdc2 family kinases phosphorylate a human cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 1992;11:2189–2199. doi: 10.1002/j.1460-2075.1992.tb05278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan ZQ, Amin AA, Gibbs E, Niu H, Hurwitz J. Phosphorylation of the p34 subunit of human single-stranded-DNA-binding protein in cyclin A-activated G1 extracts is catalyzed by cdk-cyclin A complex and DNA-dependent protein kinase. Proc.Natl.Acad.Sci.U.S.A. 1994;91:8343–8347. doi: 10.1073/pnas.91.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang F, Newport JW. Distinct roles of cdk2 and cdc2 in RP-A phosphorylation during the cell cycle. J.Cell Sci. 1993;106:983–994. doi: 10.1242/jcs.106.3.983. [DOI] [PubMed] [Google Scholar]
- 45.Anantha RW, Vassin VM, Borowiec JA. Sequential and synergistic modification of human RPA stimulates chromosomal DNA repair. J Biol Chem. 2007;282:35910–23. doi: 10.1074/jbc.M704645200. [DOI] [PubMed] [Google Scholar]
- 46.Stephan H, Concannon C, Kremmer E, Carty MP, Nasheuer HP. Ionizing radiation-dependent and independent phosphorylation of the 32-kDa subunit of replication protein A during mitosis. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oakley GG, Patrick SM, Yao J, Carty MP, Turchi JJ, Dixon K. RPA phosphorylation in mitosis alters DNA binding and protein-protein interactions. Biochemistry. 2003;42:3255–3264. doi: 10.1021/bi026377u. [DOI] [PubMed] [Google Scholar]
- 48.Anantha RW, Sokolova E, Borowiec JA. RPA phosphorylation facilitates mitotic exit in response to mitotic DNA damage. Proc Natl Acad Sci U S A. 2008;105:12903–8. doi: 10.1073/pnas.0803001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francon P, Lemaitre JM, Dreyer C, Maiorano D, Cuvier O, Mechali M. A hypophosphorylated form of RPA34 is a specific component of pre-replication centers. J Cell Sci. 2004;117:4909–20. doi: 10.1242/jcs.01361. [DOI] [PubMed] [Google Scholar]
- 50.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–10. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardwick KG, Murray AW. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol. 1995;131:709–20. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Guan J, Wang H, Perrault AR, Wang Y, Iliakis G. Replication protein A2 phosphorylation after DNA damage by the coordinated action of ataxia telangiectasia-mutated and DNA-dependent protein kinase. Cancer Res. 2001;61:8554–8563. [PubMed] [Google Scholar]
- 53.Shao RG, Cao CX, Zhang H, Kohn KW, Wold MS, Pommier Y. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J. 1999;18:1397–1406. doi: 10.1093/emboj/18.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Block WD, Yu Y, Lees-Miller SP. Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucleic Acids Res. 2004;32:997–1005. doi: 10.1093/nar/gkh265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oakley GG, Loberg LI, Yao J, Risinger MA, Yunker RL, Zernik-Kobak M, Khanna KK, Lavin MF, Carty MP, Dixon K. UV-induced hyperphosphorylation of replication protein a depends on DNA replication and expression of ATM protein. Mol.Biol.Cell. 2001;12:1199–1213. doi: 10.1091/mbc.12.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barr SM, Leung CG, Chang EE, Cimprich KA. ATR kinase activity regulates the intranuclear translocation of ATR and RPA following ionizing radiation. Curr.Biol. 2003;13:1047–1051. doi: 10.1016/s0960-9822(03)00376-2. [DOI] [PubMed] [Google Scholar]
- 57.Zernik-Kobak M, Vasunia K, Connelly M, Anderson CW, Dixon K. Sites of UV-induced phosphorylation of the p34 subunit of replication protein A from HeLa cells. J.Biol.Chem. 1997;272:23896–23904. doi: 10.1074/jbc.272.38.23896. [DOI] [PubMed] [Google Scholar]
- 58.Niu H, Erdjument-Bromage H, Pan ZQ, Lee SH, Tempst P, Hurwitz J. Mapping of amino acid residues in the p34 subunit of human single-stranded DNA-binding protein phosphorylated by DNA-dependent protein kinase and Cdc2 kinase in vitro. J.Biol.Chem. 1997;272:12634–12641. doi: 10.1074/jbc.272.19.12634. [DOI] [PubMed] [Google Scholar]
- 59.Vassin VM, Wold MS, Borowiec JA. Replication Protein A (RPA) Phosphorylation Prevents RPA Association with Replication Centers. Mol.Cell Biol. 2004;24:1930–1943. doi: 10.1128/MCB.24.5.1930-1943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cruet-Hennequart S, Coyne S, Glynn MT, Oakley GG, Carty MP. UV-induced RPA phosphorylation is increased in the absence of DNA polymerase eta and requires DNA-PK. DNA Repair (Amst) 2006;5:491–504. doi: 10.1016/j.dnarep.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Manthey KC, Opiyo S, Glanzer JG, Dimitrova D, Elliott J, Oakley GG. NBS1 mediates ATR-dependent RPA hyperphosphorylation following replication-fork stall and collapse. J Cell Sci. 2007;120:4221–9. doi: 10.1242/jcs.004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–6. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 63.Chan DW, Ye R, Veillette CJ, Lees-Miller SP. DNA-dependent protein kinase phosphorylation sites in Ku 70/80 heterodimer. Biochemistry. 1999;38:1819–28. doi: 10.1021/bi982584b. [DOI] [PubMed] [Google Scholar]
- 64.Yu Y, Wang W, Ding Q, Ye R, Chen D, Merkle D, Schriemer D, Meek K, Lees-Miller SP. DNA-PK phosphorylation sites in XRCC4 are not required for survival after radiation or for V (D)J recombination. DNA Repair (Amst) 2003;2:1239–52. doi: 10.1016/s1568-7864(03)00143-5. [DOI] [PubMed] [Google Scholar]
- 65.Ma Y, Pannicke U, Lu H, Niewolik D, Schwarz K, Lieber MR. The DNA-dependent protein kinase catalytic subunit phosphorylation sites in human Artemis. J Biol Chem. 2005;280:33839–46. doi: 10.1074/jbc.M507113200. [DOI] [PubMed] [Google Scholar]
- 66.Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J.Biol.Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 67.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–6. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 68.Unsal-Kacmaz K, Sancar A. Quaternary structure of ATR and effects of ATRIP and replication protein A on its DNA binding and kinase activities. Mol.Cell Biol. 2004;24:1292–1300. doi: 10.1128/MCB.24.3.1292-1300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olson E, Nievera CJ, Klimovich V, Fanning E, Wu X. RPA2 is a direct downstream target for ATR to regulate the S-phase checkpoint. J Biol Chem. 2006 doi: 10.1074/jbc.M605121200. [DOI] [PubMed] [Google Scholar]
- 70.Olson E, Nievera CJ, Klimovich V, Fanning E, Wu X. RPA2 is a direct downstream target for ATR to regulate the S-phase checkpoint. J Biol Chem. 2006;281:39517–33. doi: 10.1074/jbc.M605121200. [DOI] [PubMed] [Google Scholar]
- 71.Rossi R, Lidonnici MR, Soza S, Biamonti G, Montecucco A. The dispersal of replication proteins after Etoposide treatment requires the cooperation of Nbs1 with the ataxia telangiectasia Rad3-related/Chk1 pathway. Cancer Res. 2006;66:1675–83. doi: 10.1158/0008-5472.CAN-05-2741. [DOI] [PubMed] [Google Scholar]
- 72.Cruet-Hennequart S, Glynn MT, Murillo LS, Coyne S, Carty MP. Enhanced DNA-PK-mediated RPA2 hyperphosphorylation in DNA polymerase eta-deficient human cells treated with cisplatin and oxaliplatin. DNA Repair (Amst) 2008;7:582–96. doi: 10.1016/j.dnarep.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Goodarzi AA, Yu Y, Riballo E, Douglas P, Walker SA, Ye R, Harer C, Marchetti C, Morrice N, Jeggo PA, Lees-Miller SP. DNA-PK autophosphorylation facilitates Artemis endonuclease activity. Embo J. 2006;25:3880–9. doi: 10.1038/sj.emboj.7601255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui X, Yu Y, Gupta S, Cho YM, Lees-Miller SP, Meek K. Autophosphorylation of DNA-dependent protein kinase regulates DNA end processing and may also alter double-strand break repair pathway choice. Mol Cell Biol. 2005;25:10842–52. doi: 10.1128/MCB.25.24.10842-10852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Matsuda A, Plunkett W. Ataxia-telangiectasia and Rad3-related and DNA-dependent protein kinase cooperate in G2 checkpoint activation by the DNA strand-breaking nucleoside analogue 2′-C-cyano-2′-deoxy-1-beta-D-arabino-pentofuranosylcytosine. Mol Cancer Ther. 2008;7:133–42. doi: 10.1158/1535-7163.MCT-07-0416. [DOI] [PubMed] [Google Scholar]
- 76.Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP. Human CtIP promotes DNA end resection. Nature. 2007;450:509–14. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen L, Nievera CJ, Lee AY, Wu X. Cell cycle-dependent complex formation of BRCA1. CtIP.MRN is important for DNA double-strand break repair. J Biol Chem. 2008;283:7713–20. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- 78.Williams RS, Dodson GE, Limbo O, Yamada Y, Williams JS, Guenther G, Classen S, Glover JN, Iwasaki H, Russell P, Tainer JA. Nbs1 flexibly tethers Ctp1 and Mre11-Rad50 to coordinate DNA double-strand break processing and repair. Cell. 2009;139:87–99. doi: 10.1016/j.cell.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lloyd J, Chapman JR, Clapperton JA, Haire LF, Hartsuiker E, Li J, Carr AM, Jackson SP, Smerdon SJ. A supramodular FHA/BRCT-repeat architecture mediates Nbs1 adaptor function in response to DNA damage. Cell. 2009;139:100–11. doi: 10.1016/j.cell.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, Alt FW, Chen J. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 81.Peng A, Chen PL. NFBD1/Mdc1 mediates ATR-dependent DNA damage response. Cancer Res. 2005;65:1158–1163. doi: 10.1158/0008-5472.CAN-04-2508. [DOI] [PubMed] [Google Scholar]
- 82.Yoo E, Kim BU, Lee SY, Cho CH, Chung JH, Lee CH. BP1 is associated with replication protein A and is required for RPA2 hyperphosphorylation following DNA damage. Oncogene. 2005 doi: 10.1038/sj.onc.1208710. [DOI] [PubMed] [Google Scholar]
- 83.O’Driscoll M, Jackson AP, Jeggo PA. Microcephalin: a causal link between impaired damage response signalling and microcephaly. Cell Cycle. 2006;5:2339–44. doi: 10.4161/cc.5.20.3358. [DOI] [PubMed] [Google Scholar]
- 84.Rai R, Dai H, Multani AS, Li K, Chin K, Gray J, Lahad JP, Liang J, Mills GB, Meric-Bernstam F, Lin SY. BRIT1 regulates early DNA damage response, chromosomal integrity, and cancer. Cancer Cell. 2006;10:145–57. doi: 10.1016/j.ccr.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Binz SK, Lao Y, Lowry DF, Wold MS. The phosphorylation domain of the 32-kDa subunit of replication protein A (RPA) modulates RPA-DNA interactions. Evidence for an intersubunit interaction. J.Biol.Chem. 2003;278:35584–35591. doi: 10.1074/jbc.M305388200. [DOI] [PubMed] [Google Scholar]
- 86.Bochkareva E, Kaustov L, Ayed A, Yi GS, Lu Y, Pineda-Lucena A, Liao JC, Okorokov AL, Milner J, Arrowsmith CH, Bochkarev A. Single-stranded DNA mimicry in the p53 transactivation domain interaction with replication protein A. Proc Natl Acad Sci U S A. 2005;102:15412–7. doi: 10.1073/pnas.0504614102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Kvaratskhelia M, Hess S, Qu Y, Zou Y. Modulation of replication protein A function by its hyperphosphorylation-induced conformational change involving DNA binding domain B. J Biol Chem. 2005;280:32775–83. doi: 10.1074/jbc.M505705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jacobs DM, Lipton AS, Isern NG, Daughdrill GW, Lowry DF, Gomes X, Wold MS. Human replication protein A: global fold of the N-terminal RPA-70 domain reveals a basic cleft and flexible C-terminal linker. J Biomol NMR. 1999;14:321–31. doi: 10.1023/a:1008373009786. [DOI] [PubMed] [Google Scholar]
- 89.Lin YL, Chen C, Keshav KF, Winchester E, Dutta A. Dissection of functional domains of the human DNA replication protein complex replication protein A. J.Biol Chem. 1996;271:17190–17198. doi: 10.1074/jbc.271.29.17190. [DOI] [PubMed] [Google Scholar]
- 90.Xu X, Vaithiyalingam S, Glick GG, Mordes DA, Chazin WJ, Cortez D. The basic cleft of RPA70N binds multiple checkpoint proteins, including RAD9, to regulate ATR signaling. Mol Cell Biol. 2008;28:7345–53. doi: 10.1128/MCB.01079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ball HL, Ehrhardt MR, Mordes DA, Glick GG, Chazin WJ, Cortez D. Function of a conserved checkpoint recruitment domain in ATRIP proteins. Mol Cell Biol. 2007;27:3367–77. doi: 10.1128/MCB.02238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olson E, Nievera CJ, Liu E, Lee AY, Chen L, Wu X. The Mre11 complex mediates the S-phase checkpoint through an interaction with replication protein A. Mol Cell Biol. 2007;27:6053–67. doi: 10.1128/MCB.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 94.Kanoh Y, Tamai K, Shirahige K. Different requirements for the association of ATR-ATRIP and 9-1-1 to the stalled replication forks. Gene. 2006;377:88–95. doi: 10.1016/j.gene.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 95.Grandin N, Charbonneau M. Control of the yeast telomeric senescence survival pathways of recombination by the Mec1 and Mec3 DNA damage sensors and RPA. Nucleic Acids Res. 2007;35:822–38. doi: 10.1093/nar/gkl1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Umezu K, Sugawara N, Chen C, Haber JE, Kolodner RD. Genetic analysis of yeast RPA1 reveals its multiple functions in DNA metabolism. Genetics. 1998;148:989–1005. doi: 10.1093/genetics/148.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou L, Liu D, Elledge SJ. Replication protein A-mediated recruitment and activation of Rad17 complexes. Proc.Natl.Acad.Sci.U.S.A. 2003;100:13827–13832. doi: 10.1073/pnas.2336100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Majka J, Binz SK, Wold MS, Burgers PM. Replication protein A directs loading of the DNA damage checkpoint clamp to 5′-DNA junctions. J Biol Chem. 2006;281:27855–61. doi: 10.1074/jbc.M605176200. [DOI] [PubMed] [Google Scholar]
- 99.Shen JC, Lao Y, Kamath-Loeb A, Wold MS, Loeb LA. The N-terminal domain of the large subunit of human replication protein A binds to Werner syndrome protein and stimulates helicase activity. Mech Ageing Dev. 2003;124:921–30. doi: 10.1016/s0047-6374(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 100.Doherty KM, Sommers JA, Gray MD, Lee JW, von Kobbe C, Thoma NH, Kureekattil RP, Kenny MK, Brosh RM., Jr. Physical and functional mapping of the replication protein a interaction domain of the werner and bloom syndrome helicases. J Biol Chem. 2005;280:29494–505. doi: 10.1074/jbc.M500653200. [DOI] [PubMed] [Google Scholar]
- 101.Brosh RM, Jr., Li JL, Kenny MK, Karow JK, Cooper MP, Kureekattil RP, Hickson ID, Bohr VA. Replication protein A physically interacts with the Bloom’s syndrome protein and stimulates its helicase activity. J Biol Chem. 2000;275:23500–8. doi: 10.1074/jbc.M001557200. [DOI] [PubMed] [Google Scholar]
- 102.He Z, Brinton BT, Greenblatt J, Hassell JA, Ingles CJ. The transactivator proteins VP16 and GAL4 bind replication factor A. Cell. 1993;73:1223–1232. doi: 10.1016/0092-8674(93)90650-f. [DOI] [PubMed] [Google Scholar]
- 103.Plate I, Hallwyl SC, Shi I, Krejci L, Muller C, Albertsen L, Sung P, Mortensen UH. Interaction with RPA is necessary for Rad52 repair center formation and for its mediator activity. J Biol Chem. 2008;283:29077–85. doi: 10.1074/jbc.M804881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong JM, Ionescu D, Ingles CJ. Interaction between BRCA2 and replication protein A is compromised by a cancer-predisposing mutation in BRCA2. Oncogene. 2003;22:28–33. doi: 10.1038/sj.onc.1206071. [DOI] [PubMed] [Google Scholar]
- 105.Gupta R, Sharma S, Sommers JA, Kenny MK, Cantor SB, Brosh RM., Jr. FANCJ (BACH1) helicase forms DNA damage inducible foci with replication protein A and interacts physically and functionally with the single-stranded DNA-binding protein. Blood. 2007;110:2390–8. doi: 10.1182/blood-2006-11-057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robison JG, Elliott J, Dixon K, Oakley GG. Replication protein A and the Mre11.Rad50.Nbs1 complex co-localize and interact at sites of stalled replication forks. J.Biol.Chem. 2004;279:34802–34810. doi: 10.1074/jbc.M404750200. [DOI] [PubMed] [Google Scholar]
- 107.Wu X, Shell SM, Zou Y. Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene. 2005 doi: 10.1038/sj.onc.1208674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patrick SM, Oakley GG, Dixon K, Turchi JJ. DNA damage induced hyperphosphorylation of replication protein A. Characterization of DNA binding activity, protein interactions, and activity in DNA replication and repair. Biochemistry. 2005;44:8438–48. doi: 10.1021/bi048057b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo S, Zhang Y, Yuan F, Gao Y, Gu L, Wong I, Li GM. Regulation of replication protein A functions in DNA mismatch repair by phosphorylation. J Biol Chem. 2006;281:21607–16. doi: 10.1074/jbc.M603504200. [DOI] [PubMed] [Google Scholar]
- 110.Oakley GG, Tillison K, Opiyo SA, Glanzer JG, Horn JM, Patrick SM. Physical interaction between replication protein A (RPA) and MRN: involvement of RPA2 phosphorylation and the N-terminus of RPA1. Biochemistry. 2009;48:7473–81. doi: 10.1021/bi900694p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mer G, Bochkarev A, Gupta R, Bochkareva E, Frappier L, Ingles CJ, Edwards AM, Chazin WJ. Structural basis for the recognition of DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA. Cell. 2000;103:449–56. doi: 10.1016/s0092-8674(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 112.Unsal-Kacmaz K, Chastain PD, Qu PP, Minoo P, Cordeiro-Stone M, Sancar A, Kaufmann WK. The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol. 2007;27:3131–42. doi: 10.1128/MCB.02190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ciccia A, Bredemeyer AL, Sowa ME, Terret ME, Jallepalli PV, Harper JW, Elledge SJ. The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev. 2009;23:2415–25. doi: 10.1101/gad.1832309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bansbach CE, Betous R, Lovejoy CA, Glick GG, Cortez D. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 2009;23:2405–14. doi: 10.1101/gad.1839909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yusufzai T, Kong X, Yokomori K, Kadonaga JT. The annealing helicase HARP is recruited to DNA repair sites via an interaction with RPA. Genes Dev. 2009;23:2400–4. doi: 10.1101/gad.1831509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan J, Ghosal G, Chen J. The annealing helicase HARP protects stalled replication forks. Genes Dev. 2009;23:2394–9. doi: 10.1101/gad.1836409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park MS, Ludwig DL, Stigger E, Lee SH. Physical interaction between human RAD52 and RPA is required for homologous recombination in mammalian cells. J.Biol Chem. 1996;271:18996–19000. doi: 10.1074/jbc.271.31.18996. [DOI] [PubMed] [Google Scholar]
- 118.Wobbe CR, Weissbach L, Borowiec JA, Dean FB, Murakami Y, Bullock P, Hurwitz J. Replication of simian virus 40 origin-containing DNA in vitro with purified proteins. Proc.Natl.Acad.Sci.U.S.A. 1987;84:1834–1838. doi: 10.1073/pnas.84.7.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kenny MK, Lee SH, Hurwitz J. Multiple functions of human single-stranded-DNA binding protein in simian virus 40 DNA replication: single-strand stabilization and stimulation of DNA polymerases alpha and delta. Proc Natl Acad Sci U S A. 1989;86:9757–61. doi: 10.1073/pnas.86.24.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iftode C, Borowiec JA. Denaturation of the simian virus 40 origin of replication mediated by human replication protein A. Mol.Cell Biol. 1997;17:3876–3883. doi: 10.1128/mcb.17.7.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tanaka T, Nasmyth K. Association of RPA with chromosomal replication origins requires an Mcm protein, and is regulated by Rad53, and cyclin- and Dbf4-dependent kinases. EMBO J. 1998;17:5182–5191. doi: 10.1093/emboj/17.17.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zou L, Stillman B. Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol Cell Biol. 2000;20:3086–96. doi: 10.1128/mcb.20.9.3086-3096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maga G, Frouin I, Spadari S, Hubscher U. Replication protein A as a “fidelity clamp” for DNA polymerase alpha. J Biol Chem. 2001;276:18235–42. doi: 10.1074/jbc.M009599200. [DOI] [PubMed] [Google Scholar]
- 124.Braun KA, Lao Y, He Z, Ingles CJ, Wold MS. Role of protein-protein interactions in the function of replication protein A (RPA): RPA modulates the activity of DNA polymerase alpha by multiple mechanisms. Biochemistry. 1997;36:8443–8454. doi: 10.1021/bi970473r. [DOI] [PubMed] [Google Scholar]
- 125.Dornreiter I, Erdile LF, Gilbert IU, von Winkler D, Kelly TJ, Fanning E. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. EMBO J. 1992;11:769–776. doi: 10.1002/j.1460-2075.1992.tb05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waga S, Bauer G, Stillman B. Reconstitution of complete SV40 DNA replication with purified replication factors. J.Biol Chem. 1994;269:10923–10934. [PubMed] [Google Scholar]
- 127.Waga S, Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994;369:207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- 128.Loor G, Zhang SJ, Zhang P, Toomey NL, Lee MY. Identification of DNA replication and cell cycle proteins that interact with PCNA. Nucleic.Acids.Res. 1997;25:5041–5046. doi: 10.1093/nar/25.24.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dianov GL, Jensen BR, Kenny MK, Bohr VA. Replication protein A stimulates proliferating cell nuclear antigen-dependent repair of abasic sites in DNA by human cell extracts. Biochemistry. 1999;38:11021–5. doi: 10.1021/bi9908890. [DOI] [PubMed] [Google Scholar]
- 130.Bae SH, Bae KH, Kim JA, Seo YS. RPA governs endonuclease switching during processing of Okazaki fragments in eukaryotes. Nature. 2001;412:456–461. doi: 10.1038/35086609. [DOI] [PubMed] [Google Scholar]
- 131.Bae KH, Kim HS, Bae SH, Kang HY, Brill S, Seo YS. Bimodal interaction between replication-protein A and Dna2 is critical for Dna2 function both in vivo and in vitro. Nucleic Acids Res. 2003;31:3006–15. doi: 10.1093/nar/gkg422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kao HI, Campbell JL, Bambara RA. Dna2p helicase/nuclease is a tracking protein, like FEN1, for flap cleavage during Okazaki fragment maturation. J Biol Chem. 2004;279:50840–9. doi: 10.1074/jbc.M409231200. [DOI] [PubMed] [Google Scholar]
- 133.Carty MP, Zernik-Kobak M, McGrath S, Dixon K. UV light-induced DNA synthesis arrest in HeLa cells is associated with changes in phosphorylation of human single-stranded DNA-binding protein. EMBO J. 1994;13:2114–2123. doi: 10.1002/j.1460-2075.1994.tb06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coverley D, Kenny MK, Lane DP, Wood RD. A role for the human single-stranded DNA binding protein HSSB/RPA in an early stage of nucleotide excision repair. Nucleic Acids Res. 1992;20:3873–3880. doi: 10.1093/nar/20.15.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mu D, Park CH, Matsunaga T, Hsu DS, Reardon JT, Sancar A. Reconstitution of human DNA repair excision nuclease in a highly defined system. J Biol Chem. 1995;270:2415–8. doi: 10.1074/jbc.270.6.2415. [DOI] [PubMed] [Google Scholar]
- 136.Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hubscher U, Egly JM, Wood RD. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–68. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 137.Patrick SM, Turchi JJ. Xeroderma pigmentosum complementation group A protein (XPA) modulates RPA-DNA interactions via enhanced complex stability and inhibition of strand separation activity. J Biol Chem. 2002;277:16096–101. doi: 10.1074/jbc.M200816200. [DOI] [PubMed] [Google Scholar]
- 138.Volker M, Mone MJ, Karmakar P, van Hoffen A, Schul W, Vermeulen W, Hoeijmakers JH, van Driel R, van Zeeland AA, Mullenders LH. Sequential assembly of the nucleotide excision repair factors in vivo. Mol Cell. 2001;8:213–24. doi: 10.1016/s1097-2765(01)00281-7. [DOI] [PubMed] [Google Scholar]
- 139.Riedl T, Hanaoka F, Egly JM. The comings and goings of nucleotide excision repair factors on damaged DNA. Embo J. 2003;22:5293–303. doi: 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, Hanaoka F, Bootsma D, Hoeijmakers JH. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol.Cell. 1998;2:223–232. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 141.Matsunaga T, Park CH, Bessho T, Mu D, Sancar A. Replication protein A confers structure-specific endonuclease activities to the XPF-ERCC1 and XPG subunits of human DNA repair excision nuclease. J.Biol Chem. 1996;271:11047–11050. doi: 10.1074/jbc.271.19.11047. [DOI] [PubMed] [Google Scholar]
- 142.Evans E, Moggs JG, Hwang JR, Egly JM, Wood RD. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J. 1997;16:6559–6573. doi: 10.1093/emboj/16.21.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.He Z, Henricksen LA, Wold MS, Ingles CJ. RPA involvement in the damage-recognition and incision steps of nucleotide excision repair. Nature. 1995;374:566–569. doi: 10.1038/374566a0. [DOI] [PubMed] [Google Scholar]
- 144.Stigger E, Drissi R, Lee SH. Functional analysis of human replication protein A in nucleotide excision repair. J.Biol Chem. 1998;273:9337–9343. doi: 10.1074/jbc.273.15.9337. [DOI] [PubMed] [Google Scholar]
- 145.Bessho T, Sancar A, Thompson LH, Thelen MP. Reconstitution of human excision nuclease with recombinant XPF-ERCC1 complex. J.Biol Chem. 1997;272:3833–3837. doi: 10.1074/jbc.272.6.3833. [DOI] [PubMed] [Google Scholar]
- 146.Shivji MK, Podust VN, Hubscher U, Wood RD. Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry. 1995;34:5011–5017. doi: 10.1021/bi00015a012. [DOI] [PubMed] [Google Scholar]
- 147.Nagelhus TA, Haug T, Singh KK, Keshav KF, Skorpen F, Otterlei M, Bharati S, Lindmo T, Benichou S, Benarous R, Krokan HE. A sequence in the N-terminal region of human uracil-DNA glycosylase with homology to XPA interacts with the C-terminal part of the 34-kDa subunit of replication protein A. J.Biol Chem. 1997;272:6561–6566. doi: 10.1074/jbc.272.10.6561. [DOI] [PubMed] [Google Scholar]
- 148.Parker A, Gu Y, Mahoney W, Lee SH, Singh KK, Lu AL. Human homolog of the MutY repair protein (hMYH) physically interacts with proteins involved in long patch DNA base excision repair. J Biol Chem. 2001;276:5547–55. doi: 10.1074/jbc.M008463200. [DOI] [PubMed] [Google Scholar]
- 149.Hagen L, Kavli B, Sousa MM, Torseth K, Liabakk NB, Sundheim O, Pena-Diaz J, Otterlei M, Horning O, Jensen ON, Krokan HE, Slupphaug G. Cell cycle-specific UNG2 phosphorylations regulate protein turnover, activity and association with RPA. Embo J. 2008;27:51–61. doi: 10.1038/sj.emboj.7601958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Matsumoto Y, Kim K, Bogenhagen DF. Proliferating cell nuclear antigen-dependent abasic site repair in Xenopus laevis oocytes: an alternative pathway of base excision DNA repair. Mol Cell Biol. 1994;14:6187–97. doi: 10.1128/mcb.14.9.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Krokan HE, Nilsen H, Skorpen F, Otterlei M, Slupphaug G. Base excision repair of DNA in mammalian cells. FEBS Lett. 2000;476:73–7. doi: 10.1016/s0014-5793(00)01674-4. [DOI] [PubMed] [Google Scholar]
- 152.DeMott MS, Zigman S, Bambara RA. Replication protein A stimulates long patch DNA base excision repair. J.Biol Chem. 1998;273:27492–27498. doi: 10.1074/jbc.273.42.27492. [DOI] [PubMed] [Google Scholar]
- 153.Ranalli TA, DeMott MS, Bambara RA. Mechanism underlying replication protein a stimulation of DNA ligase I. J Biol Chem. 2002;277:1719–27. doi: 10.1074/jbc.M109053200. [DOI] [PubMed] [Google Scholar]
- 154.Lin YL, Shivji MK, Chen C, Kolodner R, Wood RD, Dutta A. The evolutionarily conserved zinc finger motif in the largest subunit of human replication protein A is required for DNA replication and mismatch repair but not for nucleotide excision repair. J.Biol Chem. 1998;273:1453–1461. doi: 10.1074/jbc.273.3.1453. [DOI] [PubMed] [Google Scholar]
- 155.Ramilo C, Gu L, Guo S, Zhang X, Patrick SM, Turchi JJ, Li GM. Partial reconstitution of human DNA mismatch repair in vitro: characterization of the role of human replication protein A. Mol Cell Biol. 2002;22:2037–46. doi: 10.1128/MCB.22.7.2037-2046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Genschel J, Modrich P. Mechanism of 5′-directed excision in human mismatch repair. Mol Cell. 2003;12:1077–86. doi: 10.1016/s1097-2765(03)00428-3. [DOI] [PubMed] [Google Scholar]
- 157.Genschel J, Modrich P. Functions of MutLalpha, replication protein A (RPA), and HMGB1 in 5′-directed mismatch repair. J Biol Chem. 2009;284:21536–44. doi: 10.1074/jbc.M109.021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Y, Yuan F, Presnell SR, Tian K, Gao Y, Tomkinson AE, Gu L, Li GM. Reconstitution of 5′-directed human mismatch repair in a purified system. Cell. 2005;122:693–705. doi: 10.1016/j.cell.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 159.Heyer WD, Rao MR, Erdile LF, Kelly TJ, Kolodner RD. An essential Saccharomyces cerevisiae single-stranded DNA binding protein is homologous to the large subunit of human RP-A. EMBO J. 1990;9:2321–2329. doi: 10.1002/j.1460-2075.1990.tb07404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sleeth KM, Sorensen CS, Issaeva N, Dziegielewski J, Bartek J, Helleday T. RPA mediates recombination repair during replication stress and is displaced from DNA by checkpoint signalling in human cells. J Mol Biol. 2007;373:38–47. doi: 10.1016/j.jmb.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 161.Jackson D, Dhar K, Wahl JK, Wold MS, Borgstahl GE. Analysis of the human replication protein A:Rad52 complex: evidence for crosstalk between RPA32, RPA70, Rad52 and DNA. J Mol Biol. 2002;321:133–48. doi: 10.1016/s0022-2836(02)00541-7. [DOI] [PubMed] [Google Scholar]
- 162.New JH, Sugiyama T, Zaitseva E, Kowalczykowski SC. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A (see comments) Nature. 1998;391:407–410. doi: 10.1038/34950. [DOI] [PubMed] [Google Scholar]
- 163.Deng X, Prakash A, Dhar K, Baia GS, Kolar C, Oakley GG, Borgstahl GE. Human replication protein A-Rad52-single-stranded DNA complex: stoichiometry and evidence for strand transfer regulation by phosphorylation. Biochemistry. 2009;48:6633–43. doi: 10.1021/bi900564k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kantake N, Sugiyama T, Kolodner RD, Kowalczykowski SC. The recombination-deficient mutant RPA (rfa1-t11) is displaced slowly from single-stranded DNA by Rad51 protein. J Biol Chem. 2003;278:23410–7. doi: 10.1074/jbc.M302995200. [DOI] [PubMed] [Google Scholar]
- 165.Sugiyama T, Kantake N. Dynamic regulatory interactions of rad51, rad52, and replication protein-a in recombination intermediates. J Mol Biol. 2009;390:45–55. doi: 10.1016/j.jmb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 166.Wu X, Yang Z, Liu Y, Zou Y. Preferential localization of hyperphosphorylated replication protein A to double-strand break repair and checkpoint complexes upon DNA damage. Biochem J. 2005;391:473–80. doi: 10.1042/BJ20050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–57. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 168.West SC. Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol. 2003;4:435–45. doi: 10.1038/nrm1127. [DOI] [PubMed] [Google Scholar]
- 169.Yang H, Jeffrey PD, Miller J, Kinnucan E, Sun Y, Thoma NH, Zheng N, Chen PL, Lee WH, Pavletich NP. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science. 2002;297:1837–48. doi: 10.1126/science.297.5588.1837. [DOI] [PubMed] [Google Scholar]
- 170.Zhou Q, Kojic M, Cao Z, Lisby M, Mazloum NA, Holloman WK. Dss1 interaction with Brh2 as a regulatory mechanism for recombinational repair. Mol Cell Biol. 2007;27:2512–26. doi: 10.1128/MCB.01907-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang H, Li Q, Fan J, Holloman WK, Pavletich NP. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA-ssDNA junction. Nature. 2005;433:653–7. doi: 10.1038/nature03234. [DOI] [PubMed] [Google Scholar]
- 172.Smith J, Zou H, Rothstein R. Characterization of genetic interactions with RFA1: the role of RPA in DNA replication and telomere maintenance. Biochimie. 2000;82:71–8. doi: 10.1016/s0300-9084(00)00183-8. [DOI] [PubMed] [Google Scholar]
- 173.Kibe T, Ono Y, Sato K, Ueno M. Fission yeast Taz1 and RPA are synergistically required to prevent rapid telomere loss. Mol Biol Cell. 2007;18:2378–87. doi: 10.1091/mbc.E06-12-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Salas TR, Petruseva I, Lavrik O, Bourdoncle A, Mergny JL, Favre A, Saintome C. Human replication protein A unfolds telomeric G-quadruplexes. Nucleic Acids Res. 2006;34:4857–65. doi: 10.1093/nar/gkl564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rubtsova MP, Skvortsov DA, Petruseva IO, Lavrik OI, Spirin PV, Prasolov VS, Kisseljov FL, Dontsova OA. Replication protein A modulates the activity of human telomerase in vitro. Biochemistry (Mosc) 2009;74:92–6. doi: 10.1134/s0006297909010143. [DOI] [PubMed] [Google Scholar]
- 176.Grudic A, Jul-Larsen A, Haring SJ, Wold MS, Lonning PE, Bjerkvig R, Boe SO. Replication protein A prevents accumulation of single-stranded telomeric DNA in cells that use alternative lengthening of telomeres. Nucleic Acids Res. 2007;35:7267–78. doi: 10.1093/nar/gkm738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schramke V, Luciano P, Brevet V, Guillot S, Corda Y, Longhese MP, Gilson E, Geli V. RPA regulates telomerase action by providing Est1p access to chromosome ends. Nat Genet. 2004;36:46–54. doi: 10.1038/ng1284. [DOI] [PubMed] [Google Scholar]
- 178.Evans SK, Lundblad V. Est1 and Cdc13 as comediators of telomerase access. Science. 1999;286:117–20. doi: 10.1126/science.286.5437.117. [DOI] [PubMed] [Google Scholar]
- 179.Liu JS, Kuo SR, McHugh MM, Beerman TA, Melendy T. Adozelesin triggers DNA damage response pathways and arrests SV40 DNA replication through replication protein A inactivation. J.Biol.Chem. 2000;275:1391–1397. doi: 10.1074/jbc.275.2.1391. [DOI] [PubMed] [Google Scholar]
- 180.Wang Y, Zhou XY, Wang H, Huq MS, Iliakis G. Roles of replication protein A and DNA-dependent protein kinase in the regulation of DNA replication following DNA damage. J Biol Chem. 1999;274:22060–4. doi: 10.1074/jbc.274.31.22060. [DOI] [PubMed] [Google Scholar]
- 181.MacNeill SA. DNA replication: partners in the Okazaki two-step. Curr Biol. 2001;11:R842–4. doi: 10.1016/s0960-9822(01)00500-0. [DOI] [PubMed] [Google Scholar]
- 182.Binz SK, Sheehan AM, Wold MS. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair (Amst) 2004;3:1015–1024. doi: 10.1016/j.dnarep.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 183.Labib K, Tercero JA, Diffley JF. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science. 2000;288:1643–7. doi: 10.1126/science.288.5471.1643. [DOI] [PubMed] [Google Scholar]
- 184.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–74. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 185.Mimura S, Takisawa H. Xenopus Cdc45-dependent loading of DNA polymerase alpha onto chromatin under the control of S-phase Cdk. Embo J. 1998;17:5699–707. doi: 10.1093/emboj/17.19.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zou L, Stillman B. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science. 1998;280:593–6. doi: 10.1126/science.280.5363.593. [DOI] [PubMed] [Google Scholar]
- 187.Manthey KC, Glanzer JG, Dimitrova DD, Oakley GG. Hyperphosphorylation of replication protein A in cisplatin-resistant and -sensitive head and neck squamous cell carcinoma cell lines. Head Neck. 2009 doi: 10.1002/hed.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wilsker D, Bunz F. Loss of ataxia telangiectasia mutated- and Rad3-related function potentiates the effects of chemotherapeutic drugs on cancer cell survival. Mol Cancer Ther. 2007;6:1406–13. doi: 10.1158/1535-7163.MCT-06-0679. [DOI] [PubMed] [Google Scholar]
- 189.Kornberg A. DNA replication. W. H. Freeman; San Francisco: 1980. [Google Scholar]
- 190.Richter A, Sapp M, Knippers R. Are single-stranded DNA proteins needed for mammalian DNA replication? Trends Biochem Sci. 1986;11:283. [Google Scholar]
- 191.Chase JW, Williams KR. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–36. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- 192.Shereda RD, Kozlov AG, Lohman TM, Cox MM, Keck JL. SSB as an organizer/mobilizer of genome maintenance complexes. Crit Rev Biochem Mol Biol. 2008;43:289–318. doi: 10.1080/10409230802341296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Binz SK, Wold MS. Regulatory functions of the N-terminal domain of the 70-kDa subunit of replication protein A (RPA) J Biol Chem. 2008;283:21559–70. doi: 10.1074/jbc.M802450200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roy R, Kozlov AG, Lohman TM, Ha T. SSB protein diffusion on single-stranded DNA stimulates RecA filament formation. Nature. 2009;461:1092–7. doi: 10.1038/nature08442. [DOI] [PMC free article] [PubMed] [Google Scholar]