Abstract
Background
Change in glomerular filtration rate (GFR) is important for clinical decision making. GFR estimates from serum creatinine provide an unbiased but imprecise estimate of GFR at single time points. However, the accuracy of estimated GFR over time is not well known.
Study Design
Longitudinal study of diagnostic test accuracy
Settings and participants
Four clinical trials with longitudinal measures of GFR and serum creatinine on the same day, including subjects with and without kidney disease, with a wide range of kidney function, diverse racial backgrounds and varied clinical characteristics.
Index test
GFR estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Reference test
GFR measured using urinary clearance of 125I-iothalamate
Results
Data included 19,735 GFR measurements in 3531 subjects over mean follow up of 2.6 years. Mean at baseline for measured and estimated GFR and error (measured GFR – estimated GFR) were 73.1 (95% CI, 71.6 to 74.5), 72.7 (95% CI, 71.5 to 74.0) and 0.14 (95% CI, −0.35 to 0.63) ml/min/1.73 m2, respectively. The mean rate of change in measured and estimated GFR and error was −2.3 (95% CI, −2.4 to −2.1), −2.2 (95% CI, −2.4 to −2.1) and −0.09 (95% CI, −0.24 to 0.05) ml/min/1.73 m2 per year (p <.001, p <.001, and p = 0.2 respectively). The variability (ie, SD) among subjects in rate of change in measured GFR, estimated GFR and error was 4.3, 3.4 and 3.3 ml/min/1.73 m2 per year, respectively. Only 15% of subjects had a rate of change in error of more than 3 ml/min/1.73 m2 per year, and only 2% had a rate of change in error larger than 5% per year.
Limitations
Subject characteristics were not available over time.
Conclusion
Accuracy of GFR estimates did not change over time. Clinicians should interpret changes in estimated GFR over time as reflecting changes in measured GFR rather than changes in errors in the GFR estimates in most individuals.
Evaluation and management of chronic kidney disease (CKD) requires monitoring of kidney function over time. The best overall measure of kidney function is the glomerular filtration rate (GFR). The gold standard for the measurement of GFR is the urinary clearance of an exogenous filtration marker, which is expensive, inconvenient, and may vary during the day. In clinical practice, levels of endogenous filtration markers in the serum, such as creatinine, are used to estimate the GFR, but they are also affected by physiological processes other than GFR. For creatinine, the non-GFR determinants include generation from dietary intake or muscle catabolism, tubular secretion, and extra-renal elimination1. Estimating equations use readily measured clinical variables as surrogates for these unmeasured non-GFR determinants, and render more accurate estimates than the serum level alone 2–3. These equations are widely used in clinical practice. Indeed serum creatinine is measured more than 280 million times per year in the US and estimated GFR (eGFR) based on serum creatinine is reported by more than 80% of clinical laboratories when serum creatinine is ordered 4–5.
While GFR estimating equations improve assessment of kidney function, the non-GFR determinants of serum creatinine may vary among individuals and over time, leading to errors in the estimated GFR. GFR estimating equations have been developed and extensively validated at single time points, but their performance over time is not well known6–8. In this study, we evaluate the accuracy of eGFR over time in diverse study populations with and without chronic kidney disease and diabetes over a wide range of measured GFR. We estimate GFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation 7.
METHODS
Study population
We included all four studies from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) that included repeated GFR, measured using urinary clearance of 125I-iothalamate, and serum creatinine that was measured on the same day as the measured GFR. The details of studies included in the CKD-EPI collaboration have been previously described7. Studies included in the pooled data set were Modification of Diet in Renal Disease (MDRD) Study, African American Study of Kidney Disease and Hypertension (AASK), the Captopril Trial by the Collaborative Study Group (CSG) (herein referred to as CSG), and Diabetes Complications and Control Trial (DCCT). All studies have been previously described in detail 9–14. The MDRD Study tested protein intake and blood pressure control in patients with moderate non-diabetic kidney disease with GFR between 13 and 55 ml/min/1.73 m2. AASK tested the effectiveness of three antihypertensive regimens and two levels of BP control in African American individuals with hypertensive kidney disease and GFR between 20–65 ml/min per 1.73m2. CSG compared captopril with placebo in type 1 diabetics with proteinuria. DCCT studied the effects of intensive insulin therapy on the development and progression of microvascular complications in type 1 diabetics with negligible to minimal proteinuria. Thus, pooled, the dataset included people with and without kidney disease, who have a wide range of GFR, diverse racial backgrounds and varied clinical characteristics.
Kidney Function Measures
In all studies, GFR was measured using urinary clearance of 125I-iothalamate and expressed adjusted for body surface area. For all studies, the first GFR measurement was considered as the baseline value and generally corresponded to the trial baseline period. For the DCCT, GFR measurements began to be performed midway through the trial, so baseline values do not correspond with the trial baseline. Serum creatinine was assayed in the individual study laboratories. Samples from the baseline visit were calibrated to standardized serum creatinine values ascertained by the Roche enzymatic method (Roche–Hitachi P-Module instrument with Roche Creatininase Plus assay, www.roche.com) at the Cleveland Clinic Research Laboratory as described previously 15. The same calibration was used for all subsequent visits.
GFR was estimated using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation as eGFR= 141 × min (Scr/κ, 1)α × max (Scr/κ, 1) −1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1; max indicates the maximum of Scr/ κ or 17. As sex and race do not change over time, changes over time in estimated GFR reflect changes in age and serum creatinine.
Analytic plan
The primary outcome was the difference between measured and estimated GFR at multiple time points, referred to herein as error. The secondary outcome was the difference between the slopes of measured and estimated GFR. We summarized the descriptive data for measured GFR, estimated GFR and error using means and standard deviations for continuous data or median and interquartile range as appropriate. In addition to mean error (bias), we also described the median error, the mean of the absolute values of the error, interquartile range (IQR) of error and probability that the estimate was within 30% (P30) of measured GFR 16.
We explored the change over time of measured GFR, estimated GFR and error using mixed models that modeled each of these variables as a linear function of time for each study individually and for all studies pooled together (see detailed methods in Item S1, available as online supplementary material). The mixed models allowed different random intercepts and slopes for each individual. These random intercepts and slopes were assumed to be normally distributed about a common mean intercept and slope with a common variance-covariance matrix, We analyzed GFR and serum creatinine on both the natural and logarithmic scales. We used the logarithmic scale in addition to the natural scale as it is consistent with previous work developing the CKD-EPI equation and when modelling decline in measured and estimated GFR, use of the logarithmic scale reduced heterogeneity, strengthened linearity, and improved correlation. Results from analyses conducted using the log transformed scale are presented as percentages. We also explored nonlinear patterns for change in error by testing quadratic terms in the pooled and individual studies. We tested for heterogeneity among studies by incorporating an interaction between study and time and comparing models with and without the interaction using the likelihood ratio test.
There is no widely accepted threshold for “acceptable errors” in GFR estimation over time, especially over a wide range of GFR. Based on discussion with clinicians about what they would consider clinically significant, we considered rates of change in error exceeding an absolute value of 3 ml/min/1.73 m2 per year or 5% per year as representative of potentially large errors. We considered differences in slope between estimated and measured GFR of similar magnitude as potentially large. These measures are equivalent for people with measured GFR of 60 ml/min/1.73 m2, but differ over a wide range of GFR, hence use of separate cutoff values on the natural and percent scale is informative. Statistical analyses were performed in SAS version 9.2 and R software.
RESULTS
Clinical Characteristics
The pooled data from the four studies included a total of 3531 subjects with measured GFR at baseline. Table 1 compares the clinical characteristics across the studies. The mean age was lower and the mean measured GFR was higher in CSG and DCCT compared to MDRD Study and AASK. The mean follow up time was 2.7 years in MDRD Study, 3.8 years in AASK, 1.9 years in CSG and 1.7 years in DCCT. Altogether there were 19,735 GFR measurements across all subjects during a mean follow up period of 2.6 years, including 8049 for MDRD Study, 8649 for AASK, 998 for CSG and 2039 for DCCT.
Table 1.
Subject Characteristics at baseline
| Pooled (N=3531) |
MDRD (N=840) |
AASK (N=1094) |
CSG (N=360) |
DCCT (N=1237) |
|
|---|---|---|---|---|---|
| Age, years | 43 ± 15 | 52 ± 12 | 55 ± 11 | 34 ± 8 | 30 ± 7 |
| Female | 1491 (42%) | 332 (40%) | 424 (39%) | 169 (47%) | 566 (46%) |
| Black | 1226 (35%) | 66 (8%) | 1094 (100%) | 24 (7%) | 42 (3%) |
| Diabetes | 1640 (46%) | 43 (5%) | 0 (0%) | 360 (100%) | 1237 (100%) |
| BMI, kg/m2 | 27 ± 5 | 27 ± 5 | 31 ± 7 | 25 ± 4 | 25 ± 3 |
| BSA m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 |
| Serum creatinine, mg/dL | 1.5 ± 0.8 | 2.2 ± 0.9 | 1.8 ± 0.6 | 1.2 ± 0.5 | 0.8 ± 0.1 |
| mGFR, mL/min/1.73 m2 | 73 ± 43 | 33 ± 12 | 46 ± 13 | 75 ± 33 | 124 ± 22 |
| eGFR, mL/min/1.73 m2 | 73 ± 38 | 36 ± 14 | 50 ± 16 | 77 ± 28 | 117 ± 13 |
Values are mean ± SD or n (%). eGFR calculated using the CKD-EPI equation.
Conversion factors for units: serum creatinine in mg/dL to micromol/L, ×88.4; GFR in ml/min/1.73 m2 to ml/s/1.73 m2, ×0.01667.
MDRD - Modification of Diet in Renal Disease; AASK - African American Study of Kidney Disease and Hypertension; CSG - Collaborative Study Group; DCCT - Diabetes Control and Complications Trial; eGFR, estimated glomerular filtration rate; mGFR, measured glomerular filtration rate; BSA, body surface area; BMI, body mass index.
Changes in Measured and Estimated GFR and Error over Time
Descriptive data for measured GFR, estimated GFR and CKD-EPI equation performance over time in each study show that both measured and estimated GFR decreased over time in MDRD, AASK and CSG (Table S1). Measured GFR was stable over time in DCCT, whereas estimated GFR decreased. In each study, the performance of the CKD-EPI equation was consistent over time.
Table 2 shows results from the mixed model in the pooled dataset and by study. The mean measured and estimated GFR at baseline were both 73 ml/min/1.73 m2, with mean slope in measured and estimated GFR of −2.3 (95% CI, −2.4 to −2.1) and −2.2 (95% CI, −2.4 to −2.1) ml/min/1.73 m2 per year, respectively, [percentage reduction of −7.1% (95% CI, −7.6% to −6.6%) for both)] Mean error at baseline was very small [0.14 (95% CI, −0.35 to 0.63) ml/min/1.73 m2] and did not change significantly over time [mean rate of change in error of −0.09 (95% CI, −0.24 to 0.05) ml/min/1.73 m2 per year [percentage reduction of −0.1% (95% CI, −0.4% to 0.1%) per year]. The variability (ie, SD) among subjects in changes in measured GFR, estimated GFR and error was 4.3, 3.4 and 3.3 ml/min/1.73 m2 per year, respectively (percentage change of 15.2%, 15.6%, and 3.2% per year, respectively).
Table 2.
mGFR, eGFR and Difference (Error) at Baseline and Change Over Time
| Pooled (N=3531) |
MDRD (N=840) |
AASK (N=1094) |
CSG (N=360) |
DCCT (N=1237) |
|
|---|---|---|---|---|---|
| No. of subject-time measurements | 19735 | 8049 | 8649 | 998 | 2039 |
| Max no. of measurements per person | 15 | 15 | 15 | 6 | 3 |
| mGFR | |||||
| Baseline (Intercept) | 73.1 (71.6, 74.5); <0.001 | 33.0 (32.2, 33.8); <0.001 | 46.7 (45.8, 47.5); <0.001 | 74.6 (71.3, 78.0); <0.001 | 123.9 (122.7, 125.1); <0.001 |
| Change (Slope) | −2.25 (−2.43, −2.06); <0.001 | −3.34 (−3.59, −3.09); <0.001 | −1.89 (−2.10, −1.69); <0.001 | −5.25 (−6.27, −4.24); <0.001 | −0.94 (−1.40, −0.48); <0.001 |
| % Change (Slope) | −7.1% (−7.6, −6.6); <0.001 | −15.7% (−16.8, −14.5); <0.001 | −8.1% (−8.8, −7.3); <0.001 | −11.8% (−13.7, −10.0); <0.001 | −0.8% (−1.2, −0.4); <0.001 |
| eGFR | |||||
| Baseline (Intercept) | 72.7 (71.5, 74.0); <0.001 | 35.1 (34.2, 36.0); <0.001 | 50.8 (49.7, 51.8); <0.001 | 76.7 (73.8, 79.6); <0.001 | 117.3 (116.6, 118.0); <0.001 |
| Change (Slope) | −2.22 (−2.37, −2.07); <0.001 | −3.37 (−3.64, −3.09); <0.001 | −2.19 (−2.40, −1.99); <0.001 | −4.52 (−5.46, −3.57); <0.001 | −1.06 (−1.31, −0.80); <0.001 |
| % Change (Slope) | −7.1% (−7.6, −6.6); <0.001 | −15.5% (−16.7, −14.3); <0.001 | −8.4% (−9.2, −7.6); <0.001 | −0.10%(−11.7, −8.2); <0.001 | −1.0% (−1.2, −0.7); <0.001 |
| Difference (Error) | |||||
| Baseline (Intercept) | 0.14 (−0.35, 0.63); 0.6 | −2.10 (−2.47, −1.73); <0.001 | −4.04 (−4.56, −3.52); <0.001 | −2.05 (−3.82, −0.28); 0.02 | 6.58 (5.39, 7.77); <0.001 |
| Change (Slope) | −0.09 (−0.24, 0.05); 0.2 | 0.01 (−0.11, 0.14); 0.8 | 0.28 (0.15, 0.42); <0.001 | −0.71 (−1.49, 0.08); 0.08 | 0.11 (−0.38, 0.59); 0.7 |
| % Change (Slope) | −0.1% (−0.4, 0.1); 0.2 | −0.3% (−0.7, 0.1); 0.1 | 0.2% (−0.1, 0.5); 0.3 | −1.6% (−2.6, −0.5); 0.004 | 0.1% (−0.3, 0.6); 0.5 |
Except where indicated, values shown are given as estimate (95% CI); p. Unit for mGFR and eGFR and for the difference (error) is ml/min/1.73 m2. Unit for change is ml/min/1.73 m2 per year computed using non-log transformed values or % per year computed log transformed values. Intercepts for the model using log transformed values are not shown.
Max, maximum; MDRD - Modification of Diet in Renal Disease; AASK - African American Study of Kidney Disease and Hypertension; CSG Collaborative Study Group; DCCT - Diabetes Control and Complications Trial; eGFR, estimated glomerular filtration rate; mGFR, measured glomerular filtration rate
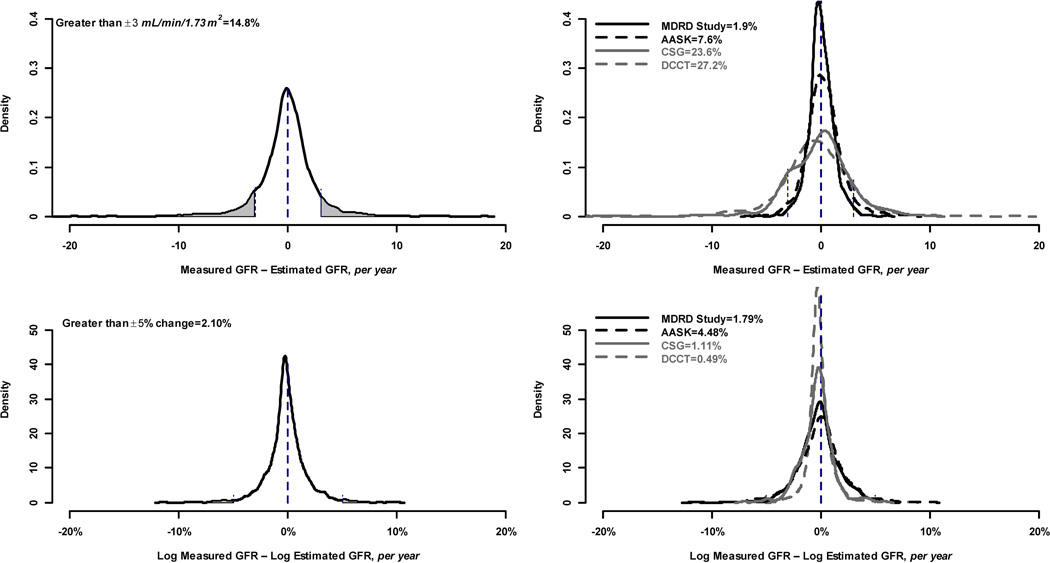
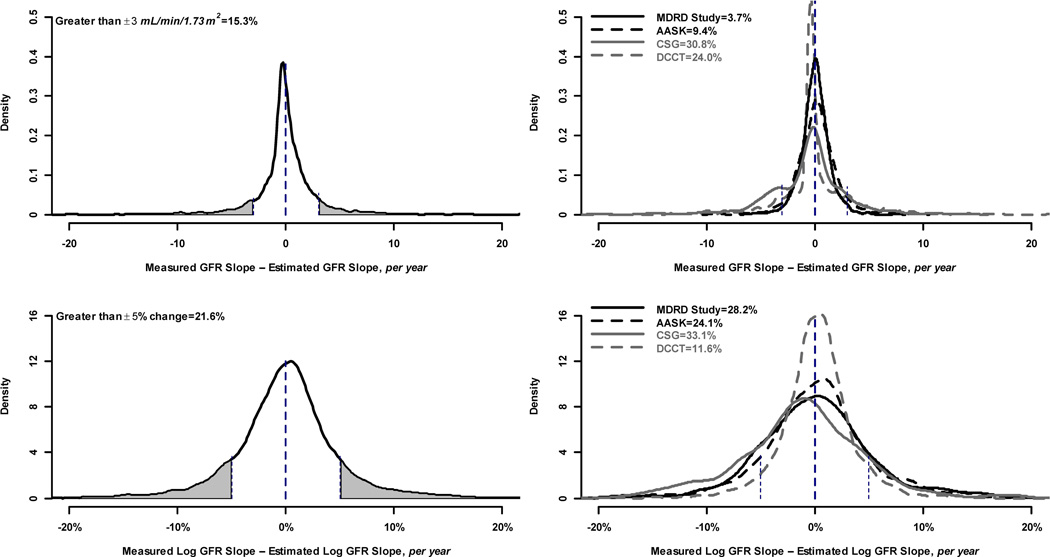
Only 14.8% of subjects had a change in error greater than ± 3 ml/min/1.73 m2 per year irrespective of baseline GFR (Figure 1, top left panel) and 2.1% of subjects had a change in error larger than 5% (Figure 1, bottom left). Similarly, 15.3% of subjects had a difference between the slopes of measured and estimated GFR that was greater than ± 3 ml/min/1.73 m2 per year (Figure 2, top left panel), whereas 21.6% of subjects had a difference between the slopes of measured and estimated GFR of larger than 5% (Figure 2, Figure 2, bottom left panel).
Figure 1.
Proportion of study subjects with large rate of change in error. Top row shows proportion with large changes defined by ± 3 ml/min per 1.73 m2. Bottom row shows proportion with large changes defined by 5% (±0.05 change on the log scale). Left hand column shows proportion with large changes in the pooled studies. Right hand column shows proportion with large changes within each study. Vertical lines indicate the definition of large change within each panel, and the shaded portion of the curve indicates the proportion of study subjects with a large change.
Figure 2.
Proportion of study subjects with large differences between slopes in measured and estimated GFR. Top row shows proportion with large differences defined by ± 3 ml/min per 1.73 m2. Bottom row shows proportion with large differences defined by 5% (±0.05 change on the log scale). Left hand column shows proportion with large differences in the pooled studies. Right hand column shows proportion with differences within each study. Vertical lines indicate the definition of large difference within each panel, and the shaded portion of the curve indicates the proportion of study subjects with a large difference.
There was substantial heterogeneity among studies in changes in error (p = 0.002). At baseline, mean error ranged from 6.6 ml/min/1.73 m2 for DCCT to −4.0 ml/min/1.73 m2 for AASK. For each study, the mean rate of change in error was small [range of −0.71 (95% CI, −1.49 to 0.08) to 0.28 (95% CI, 0.15 to 0.42) ml/min/1.73 m2 per year [percentage change of −1.6% (95% CI, −2.6% to −0.5%) to 0.2% (95% CI, −0.1% to 0.5%) per year] (Table 2). Most changes in the error were small (SD of the between-subjects slopes for MDRD, AASK, CSG and DCCT were 1.13, 1.29, 2.97, and 2.49 ml/min/1.73 m2 per year respectively). There was also heterogeneity among the studies in the proportions of individuals with large changes in error: 1.9%, 7.6%, 23.6% and 27.2% of subjects in the four studies, respectively, had rates of change in error greater than ±3 ml/min/1.73 m2 per year (Figure 1, top right panel). On the percent scale, the studies with higher mean levels of GFR had lower proportions of large changes in error: 1.8%, 4.5%, 1.1% and 0.5% of subjects in the four studies, respectively, had rates of change in error greater than 5% (Figure 1, bottom right panel). Similar findings were observed for differences between slopes of measured and estimated GFR. Correlations between slopes for estimated and measured GFR for the four studies were 0.74. 0.58, 0.36 and 0.01, respectively, on the natural scale, and 0.87, 0.83, 0.70 and 0.07, respectively, on the percent scale. The right hand panel of Figure 2 shows the variability among studies in the proportion of individuals with large differences between the slopes of measured vs estimated GFR, with CSG and DCCT again having higher proportions of large difference between slopes on the natural than the percent scale.
Findings in the overall dataset were consistent among subgroups of race and BMI (Table 3). Within one or two studies, there was a small but statistically significant differences in rate of change in error over time among subgroups defined by age and sex (Table 3).
Table 3.
Change in the Error Over Time by Subgroups
| Subgroup | MDRD (N=840) |
AASK (N=1094) |
CSG (N=360) |
DCCT (N=1237) |
|
|---|---|---|---|---|---|
| Sex | |||||
| Men | −0.07 (−0.22, 0.08); 0.4 | 0.10 (−0.06, 0.26); 0.2 | −0.55 (−1.66, 0.56); 0.3 | −0.17 (−0.77, 0.43); 0.6 | |
| Women | 0.14 (−0.07, 0.34); 0.2 | 0.59 (0.36, 0.82); <0.001 | −0.84 (−1.94, 0.26); 0.1 | 0.54 (−0.25, 1.33); 0.2 | |
| p for interaction | 0.1 | <0.001 | 0.7 | 0.1 | |
| Age (/5 y) | −0.08 (−0.13, −0.03); 0.002 | −0.04 (−0.10, 0.03); 0.3 | −0.15 (−0.67, 0.37); 0.6 | −0.34 (−0.70, 0.02); 0.06 | |
| Race | |||||
| White | 0.04 (−0.08, 0.17); 0.5 | NA | −0.84 (−1.62, −0.06); 0.04 | 0.11 (−0.38, 0.60); 0.7 | |
| Black | −0.41 (−0.95, 0.13); 0.1 | 0.28 (0.15, 0.42); <0.001 | 2.54 (−2.19, 7.28); 0.3 | 0.36 (−1.56, 2.28); 0.7 | |
| p for interaction | 0.07 | NA | 0.2 | 0.7 | |
| BMI (/2.5 kg/m2) | −0.05 (−0.11, 0.02); 0.2 | 0.03 (−0.03, 0.08); 0.3 | 0.39 (−0.16, 0.95); 0.2 | −0.14 (−0.55, 0.27); 0.5 | |
Except where indicated, values shown are given as estimate (95% CI); p.
\+Unit for change is ml/min/1.73 m2 per year;
BMI, body mass index; MDRD - Modification of Diet in Renal Disease; AASK - African American Study of Kidney is ease and Hypertension; CSG Collaborative Study Group; DCCT - Diabetes Control and Complications Trial
DISCUSSION
In this longitudinal evaluation of 19,735 GFR measurements in 3531 subjects from 4 studies over a mean follow-up of 2.6 years, we found the mean error in GFR estimates was small and did not change substantially over time. Only 15% had a change that was greater than ± 3 ml/min/1.73 m2 irrespective of the baseline GFR, and only 2% of subjects had a change in error that was larger than 5%, values that clinicians might consider as potentially large and of clinical significance. Significant differences among studies, in part related to range of GFR and rates of GFR decline. Overall, the CKD-EPI equation provided unbiased but imprecise estimates of measured GFR over time, with reasonable correlation between percent changes in measured and estimated GFR in studies in which GFR was declining. These findings are consistent with the previous literature on the accuracy of the CKD-EPI equation at a single time point 7, and have important implications for interpretation of GFR estimates in clinical practice.
In principle, error in GFR estimates reflects the effect of non-GFR determinants of creatinine unaccounted for by variables in the GFR estimating equation, biological variation and measurement errors in measured GFR and serum creatinine. To avoid systematic errors in measured GFR or serum creatinine, we used a single exogenous filtration marker across all studies and at all time points and calibrated creatinine assays in each study to reference materials at baseline and applied that calibration to all future time points. It is therefore most likely that the imprecision we observed in estimates of measured GFR over time reflect random changes in non-GFR determinants or GFR measurement error.
There are limited data on variation in non-GFR determinants of serum creatinine over time. Studies of variability over time in urinary creatinine excretion in normal subjects have shown intra-individual coefficients of variation (standard deviation divided by the mean) range from 10.5%–14.4%, suggesting random variation in creatinine generation 17–20. There are fewer data on variation in creatinine secretion. Systematic variation in dietary intake can affect both creatinine generation and secretion 21. Thus, it is to be expected that patient characteristics associated with changes in non-GFR determinants of serum creatinine would be associated with changes in error over time. However, our analysis is limited, because we did not explore changes in subject characteristics and their association to error over time because we did not have uniform follow up data on these variables.
Previous studies suggest that there is a reasonable amount of variability in measured GFR, due either to biological variability or measurement error. In a classic study using urinary clearance of inulin, Homer Smith documented a coefficient of variation for repeated GFR measurements in a single individual over time to be approximately 7.5%22. Other reports also show substantial variability in measured GFR over time. One analysis of the MDRD Study found that between-day coefficient of variations of 125I-iothalamate clearance ranged from 11.6 to 16.6% 23. Another report of the MDRD Study found a coefficient of variation between 2 GFR measures 3 months apart of 6.3% 21. A more recent study using data from MDRD Study and AASK combined showed a coefficient of variation of 11.9% of measures 2 months apart 24. Although it is difficult to compare the magnitude of variation in different variables across different studies, it is likely that changes in non-GFR determinants of serum creatinine, biological variation in measured GFR, and GFR measurement error all contribute to the variation in change in error in GFR estimation over time that we observed
Previous studies have evaluated the performance of GFR estimating equations over time using other statistical methods. In a previous report from the MDRD Study, rates of decline in measured vs. estimated GFR were compared using marginal models 25. The authors showed a mean (SD) change in measured and estimated GFR of 3.9 (7.2) and 2.8 (7.1) ml/min/1.73 m2 per year, respectively, which is a larger difference than we report here. Consistent with our results, the authors also found that differences in slope estimates between measured and estimated GFR was not related to demographic factors. In another study in AASK, time-to-event outcomes were compared using rates of changes in measured and estimated GFR 26. The association of baseline factors were similar with events defined by estimated GFR and measured GFR. In a study of 65 patients with CKD from Groningen, the Netherlands, the CKD-EPI equation accurately estimated the change in measured GFR over the 11 year follow-up, but underestimated the slope in measured GFR in the subset of patients who had a substantial progression 27. Overall, these reports are consistent with our findings, but the mixed model that we used is more robust for assessing changes over time in subjects with varying numbers of measurement and duration of follow-up, as observed in our study population.
Changes in estimated GFR are central to clinical decision making in daily practice. For example, they are used to make decisions regarding detection of CKD or acute kidney injury, use of iodinated or gadolinium contrast agents; and dosing of medications. Less frequently but importantly, they are also used in conjunction with signs and symptoms of kidney failure to determine optimal timing of initiation of dialysis, listing for a deceased donor kidney transplant, or pre-emptive transplant. Our data demonstrate that the error in GFR estimates is generally stable over time, with only 2–15% of changes in error large enough to be clinically significant. For clinical decision making, it is important to consider the clinical context when interpreting changes in estimated GFR. As such, for routine clinical decision making, we would suggest that it would be reasonable for clinicians to interpret a change in estimated GFR as a reflection of a change in measured GFR and act accordingly. However, if clinical circumstances suggest a change in non-GFR determinants of serum creatinine (for example, recent hospitalizations, decreased oral intake or refeeding after an illness, loss of muscle mass as with illness or amputation, or use of medications which inhibit creatinine secretion, such as trimethoprim), then the change in estimated GFR could reflect the change in non-GFR determinants of serum creatinine rather than a change in measured GFR 28. In all patients, clinicians should consider measuring GFR as a confirmatory test if more accurate information would improve clinical decision making.
The strengths of our study include a large pooled data set which allowed analysis of a very large number of GFR measurements in Black and White participants with a wide range in kidney function, a wide range of kidney disease diagnoses, including diabetes (which contributes a major share to the CKD burden), and sequential data from longitudinal follow up. These studies had a similar protocol for measuring GFR and calibration of serum creatinine which allowed for uniformity of analysis and interpretation. Differences among the studies allow us to demonstrate the variability in the error among populations. The use of CKD-EPI equation in the same study cohorts in which it was developed is a strength of our study, as it allows for a good fit at baseline, facilitating identification of deviations in fit over time. Finally, the use of a mixed model is a robust technique to evaluate both within and between individual variations in the change in error, and to handle missing follow-up data at random and irregularly spaced repeated observations
We acknowledge the several limitations in our study. First, we could not assess changes in subject characteristics over time that might affect the non GFR determinants of serum creatinine. Changes in estimated GFR in patients with rapidly changing non GFR determinants of serum creatinine are likely to be inaccurate. Second, although we had a diverse individual representation in terms of racial backgrounds and underlying disease, we had limited information on Hispanics, Asians and transplant recipients. Third, we did not have repeated measurements over short intervals to evaluate biologic variability in measured GFR and random error in GFR measurement. Fourth, use of the mixed model with missing data in some studies, does assume that the data are missing at random.
In summary, the CKD-EPI equation performs well over time. The finding of unbiased GFR estimates over time on average suggests that changes in estimated GFR reflect changes in measured GFR rather than changes in non-GFR determinants of serum creatinine.
Supplementary Material
Acknowledgements
Support: National Kidney Foundation Fellowship Award, CTSI, National Institutes of Health UO1 DK 053869, UO1 DK 067651, and UO1 DK 35073; K23 DK 081017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Supplementary Material
Table S1: Sample characteristics and performance of CKD-EPI equation over time by study.
Item S1: Detailed statistical methods.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
References
- 1.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 2.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Greene T, Sarnak MJ, et al. Effect of dietary protein restriction on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2006;48:879–888. doi: 10.1053/j.ajkd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 4.College of American Pathologists. Current Status Of Reporting Estimated Glomerular Filtration Rate (eGFR) 2011;Vol. 2011 [Google Scholar]
- 5.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clinical Chemistry. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens LA, Padala S, Levey AS. Advances in glomerular filtration rate-estimating equations. Curr Opin Nephrol Hypertens. 2010;19:298–307. doi: 10.1097/MNH.0b013e32833893e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. New England Journal Medicine. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 10.Levey A, Beck G, Bosch J, et al. Short-term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol. 1996;7:2097–2109. doi: 10.1681/ASN.V7102097. [DOI] [PubMed] [Google Scholar]
- 11.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and Statistical Aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14:S154–S165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 13.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting- enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. New England Journal Medicine. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Trial Research Group (DCCT) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 15.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21:797–807. [PMC free article] [PubMed] [Google Scholar]
- 17.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010 doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott PJ, Hurley PJ. Demonstration of individual variation in constancy of 24-hour urinary creatinine excretion. Clin Chim Acta. 1968;21:411–414. doi: 10.1016/0009-8981(68)90069-7. [DOI] [PubMed] [Google Scholar]
- 19.Greenblatt DJ, Ransil BJ, Harmati JS, Smith TW, Dohme DW, Koch-Weser J. Variability of 24-hour urinary creatinine excretion by normal subjects. J. Clin. Pharmacol. 1976;16:231. doi: 10.1002/j.1552-4604.1976.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 20.Newman DJ, Pugia MJ, Lott JA, Wallace JF, Hiar AM. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta. 2000;294:139–155. doi: 10.1016/s0009-8981(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Greene T, Schluchter MD, et al. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol. 1993;4:1159–1171. doi: 10.1681/asn.v451159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith HW. Comparative physiology of the kidney. In: Smith HW, editor. The Kidney: Structure and Function in Health and Disease. New York: Oxford Univ Press; 1951. pp. 520–574. [Google Scholar]
- 23.Perrone RD, Steinman TI, Beck GJ, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1990;16:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 24.Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56:39–49. doi: 10.1053/j.ajkd.2010.02.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie D, Joffe M, Brunelli S, et al. A comparison of change in measured and estimated glomerular filtration rate in patients with nondiabetic kidney disease. Clin J Am Soc Nephrol. 2008;3:1332–1338. doi: 10.2215/CJN.05631207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Lewis J, Appel L, et al. Validation of creatinine-based estimates of GFR when evaluating risk factors in longitudinal studies of kidney disease. J Am Soc Nephrol. 2006;17:2900–2909. doi: 10.1681/ASN.2005101106. [DOI] [PubMed] [Google Scholar]
- 27.Tent H, Waanders F, Krikken JA, et al. Performance of MDRD study and CKD-EPI equations for long-term follow-up of nondiabetic patients with chronic kidney disease. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr235. [DOI] [PubMed] [Google Scholar]
- 28.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 29.Singer JD, Willett JB. Applied Longitudinal Data Analysis:Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.