Abstract
Slc26 anion transporters play crucial roles in transepithelial Cl− absorption and HCO3− secretion; Slc26 protein mutations lead to several diseases. Slc26a9 functions as a Cl− channel and electrogenic Cl−-HCO3− exchanger, and can interact with CFTR. Slc26a9(−/−) mice have reduced gastric acid secretion, yet no human disease is currently associated with SLC26A9 coding mutations. Therefore, we tested the function of non-synonymous, coding, single nucleotide polymorphisms (cSNPs) of SLC26A9. Presently, eight cSNPs are NCBI-documented: Y70N, T127N, I384T, R575W, P606L, V622L, V744M and H748R. Using two-electrode voltage-clamp and anion selective electrodes, we measured the biophysical consequences of these cSNPs. Y70N (cytoplasmic N-terminus) displays higher channel activity and enhanced Cl−-HCO3− exchange. T127N (transmembrane) results in smaller halide currents but not for SCN−. V622L (STAS domain) and V744M (STAS adjacent) decreased plasma membrane expression which partially accounts for decreased whole cell currents. Nevertheless, V622L transport is reduced to ~50%. SLC26A9 polymorphisms lead to several function modifications (increased activity, decreased activity, altered protein expression) which could lead to a spectrum of pathophysiologies. Thus, knowing an individual’s SLC26A9 genetics becomes important for understanding disease potentially caused by SLC26A9 mutations or modifying diseases, e.g., cystic fibrosis. Our results also provide a framework to understand SLC26A9 transport modalities and structure-function relationships.
Keywords: SLC26A9, single nucleotide polymorphisms, voltage clamp, Cl− channel, intracellular pH, Xenopus oocytes
Introduction
Transepithelial chloride absorption and bicarbonate secretion are coupled to fluid secretion and thus important for the normal function of most epithelia. Such coupling is well illustrated in cystic fibrosis tissues in which mutations of cystic fibrosis transmembrane conductance regulator (CFTR, a Cl− channel) reduce Cl− and HCO3− secretion leading to extracellular dehydration especially in the lung (Riordan, et al., 1989). Similarly, other diseases such as congenital chloride diarrhea, Pendred syndrome and deafness due to mutations in anion transport of SLC26 family members (Moseley, et al., 1999; Taylor, et al., 2002; Wall, 2006; Wangemann, et al., 2007) also perturb fluid transport.
The human SLC26 transporter family encodes 10 different proteins, and is a part of the “sulfate permease” (i.e. SulP) protein family with homologues in bacteria, plants (Hawkesford, 2003; Takahashi, et al., 1997), fungi (Cherest, et al., 1997; van de Kamp, et al., 2000) and other animals (Dorwart, et al., 2008; Saier, et al., 1999). Slc26 proteins play multiple physiological roles in the transport of several anions, including SO42−, Cl−, I−, HCO3−, SCN−, OH−, NO3−, formate− and oxalate2− (Bissig, et al., 1994; Chang, et al., 2009a; Chang, et al., 2009b; Jiang, et al., 2002; Karniski, et al., 1998; Kim, et al., 2005; Moseley, et al., 1999; Mount and Romero, 2004; Satoh, et al., 1998; Scott and Karniski, 2000; Shcheynikov, et al., 2006b; Soleimani, et al., 2001; Xie, et al., 2002). Some Slc26 proteins have tissue-specific expression-patterns while others are widespread (Mount and Romero, 2004). Structurally, these Slc26 proteins assemble as dimers (Detro-Dassen, et al., 2008) with 12 predicted transmembrane spans (Shelden, et al., 2010), and a cytoplasmic COOH-terminus containing a STAS (Sulfate Transporter Anti-Sigma) domain (Aravind and Koonin, 2000). Slc26 STAS domains can participate in protein-protein interactions with the R-region of CFTR (Avella, et al., 2011b; Chang, et al., 2009a; Homma, et al., 2010; Ko, et al., 2002; Ko, et al., 2004; Rode, et al., 2012) and also control Slc26 transport function (Everett, et al., 1997; Hastbacka, et al., 1996; Makela, et al., 2002; Rossi and Superti-Furga, 2001). Moreover, disease-causing mutations identified SLC26A2, -A3 and -A4, are mutations within the STAS domains (Everett, et al., 1997; Hastbacka, et al., 1996; Makela, et al., 2002; Rossi and Superti-Furga, 2001). While these Slc26 proteins transport a variety of anions, play a variety of physiological roles, and are associated with several genetic disorders, the molecular components accounting for this diversity are only just being elucidated.
Slc26a9 functions as a Cl−-HCO3− exchanger, a Cl− channel and a Na+ coupled transporter (Chang, et al., 2009b; Dorwart, et al., 2007; Xu, et al., 2005). The Slc26a9 protein is localized to epithelia of the stomach (Xu, et al., 2005) and lung (Chang, et al., 2009b; Lohi, et al., 2002; Xu, et al., 2005; Xu, et al., 2008), although mRNA is also detectable in brain, heart, kidney, small intestine, thymus, and ovary (Chang, et al., 2009b). Slc26a9 and CFTR have overlapping expression in gut and lung epithelia, making protein-protein interactions important. Slc26a9 channel and transporter activity is inhibited by interaction of its STAS domain with the R-region of CFTR in Xenopus oocytes (Chang, et al., 2009a). Interestingly, after cAMP-activation CFTR activity is enhanced with co-expression of SLC26A9 (NCBI accession number: NP_443166.1; MIM# 608481) in mammalian cells (Avella, et al., 2011b; Bertrand, et al., 2009). One group found that cellular HCO3− secretion leading to gastric surface pH increase is mediated by Slc26a9 Cl−-HCO3− exchange (Demitrack, et al., 2010). Slc26a9 deletion in mice causes decreased gastric acid secretion, loss of tubulovesicles (young mice) and reduction in parietal cells (adult mice) (Xu, et al., 2008). Human SLC26A9 has become a novel candidate gene apparently associated with inner ear development (Urness, et al., 2010) as well as in antipsychotic responses (McClay, et al., 2011). Moreover, a recent GWAS analysis of Cystic Fibrosis patients indicates that expression of human SLC26A9 is associated with some CF phenotypes (Li, et al., 2011). Combined these studies have led to the hypothesis that SLC26A9 mutations may impart large (monogenic) or small (polygenic) phenotypic effects in cystic fibrosis, stomach-related diseases and/or other diseases in humans. Thus, functional analyses of SLC26A9 genetic polymorphisms are clinically important.
Recently, eight non-synonymous coding SNPs of SLC26A9 were reported in the public SNP database (Table 1; http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=115019): Y70N (rs75021701); T127N (rs77497889); I384T (rs112659452); R575W1 (rs139697920); P606L (rs74146719); V622L (rs34992672); V744M (rs3811428) and H748R (rs16856462). This database reporting of human genetic variations, however, has not been complemented by functional or cell biological characterization. In this study, we examined the functional properties and the surface protein expression of SLC26A9 SNPs using the Xenopus oocyte expression system. Our results reveal critical roles of residues at positions 70 and 127 in determining the currents mediated by SLC26A9. Additionally our results indicate that the STAS domain is more than a structural motif and protein-interaction domain; STAS seems to also control SLC26A9 surface protein expression.
Table 1.
Eight non-synonymous, cSNPs of hSLC26A9 in NCBI database a
| Protein residue | DbSNP rs# Cluster id | Hetero-zygosity | Validationb | Minor allele frequency | Primers for mutagenesis |
|---|---|---|---|---|---|
|
| |||||
| Y70N | rs75021701 | 0.038 | 1, 2, 3 | 0.0312 | F: 5′-ccaagtacaagattaaagacaacatcattcctgacctgctc-3′ |
| R: 5′-gagcaggtcaggaatgatgttgtctttaatcttgtacttgg-3′ | |||||
|
| |||||
| T127N | rs77497889 | 0.001 | 1, 3 | N.Dc | F: 5′-cagatggtgccaggtaactttgccgttatcagc-3′ |
| R: 5′-gctgataacggcaaagttacctggcaccatctg-3′ | |||||
|
| |||||
| I384T | rs112659452 | 0.500 | N.D. | N.D. | F: 5′-cagcaacttctttggctccttctttaaaactcatgtcatttgctg-3′ |
| R: 5′-cagcaaatgacatgagttttaaagaaggagccaaagaagttgctg-3′ | |||||
|
| |||||
| R575Wd | rs139697920 | 0.001 | N.D. | N.D. | F: 5′-caagaagcaggagaagtggagaatgaggcccac-3′ |
| R: 5′-gtgggcctcattctccacttctcctgcttcttg-3′ | |||||
|
| |||||
| P606L | rs74146719 | 0.044 | 1, 2, 3 | 0.0402 | F: 5′-tttgagaatgcgctccccaccgacccc-3′ |
| R: 5′-ggggtcggtggggagcgcattctcaaa-3′ | |||||
|
| |||||
| V622L | rs34992672 | 0.005 | 1, 2, 3 | 0.0035 | F: 5′-gctaacggcaccagcctgtcctatatcacct-3′ |
| R: 5′-aggtgatataggacaggctggtgccgttagc-3′ | |||||
|
| |||||
| V744M | rs3811428 | 0.008 | 1, 2, 3 | 0.0022 | F: 5′-ggcaaatgctagagacatgaccccaggacacaa-3′ |
| R: 5′-ttgtgtcctggggtcatgtctctagcatttgcc-3′ | |||||
|
| |||||
| H748R | rs16856462 | 0.196 | 1, 2, 3 | 0.0731 | F: 5′-cgtgaccccaggacgcaacttccaagggg-3′ |
| R: 5′-ccccttggaagttgcgtcctggggtcacg-3′ | |||||
The SLC26A9 cSNPs were collected from NCBI database on 12/7/2010 and were updated on 02/21/2012.
Validation: 1) Validated by multiple, independent submissions to the refSNP cluster. 2) Validated by frenquency or genotype data: minor alleles observed in at least two chromosomes. 3): SNP has been sequenced in 1000Genome project.
N.D. = not determined.
R575W is replaced by R575Q in SLC26A9 cSNPs database ( 02/21/2012).
Methods and Materials
Animal health and welfare
X. laevis were housed and cared for in accordance and approval of the Institutional Care and Use Committees of Mayo Clinic College of Medicine.
SLC26A9 and its eight SNPs constructs
Subcloing triple FLAG-tagged SLC26A9
Cloning of SLC26A9 (NM_052934.3) and Slc26a9 (NM_177243.4) was recently reported by our group (Chang, et al., 2009b). The human SLC26A9’s opening reading frame was amplified by PCR reaction, sequence verified and subcloned into the Xenopus expression vector pGEMHE. The SLC26A9-pGEMHE was used as template for generating triple FLAG-tagged SLC26A9 (3FLAG-SLC26A9) by blunt ligation PCR. Two primers were used (forward primer: 5′ CATGATATCGACTACAAGGATGACGATGACAAGCAGCCCAGGCCCCGCT 3′ and reverse primer: 5′ ATCTTTATAATCACCGTCATGGTCTTTGTAGTCGCTCATATCTGGGGCATTTACAAGCA 3′, nucleotides coding for the tags are underlined) to insert a 66-bp sequence encoding a triple FLAG tag (“DYKDHDGDYKDHDIDYKDDDDK”) after the first two residues (“MS-”) of human SLC26A9. 3xFLAG did not alter function or expression of SLC26A9 or cSNPs.
Site-directed mutagenesis of human SLC26A9
Eight non-synonymous variants (Y70N, T127N, I384T, R575W, P606L, V622L, V744M and H748R; found in Table 1) were constructed by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). For surface expression assay, the pGEMHE construct containing coding region for triple FLAG-tagged SLC26A9 was used as template for generating eight non-synonymous variants (Y70N, T127N, I384T, R575W, P606L, V622L, V744M and H748R). The positive clones were verified by sequencing prior to experiments. SLC26A9 cSNPs (including constructs encoding the triple FLAG-tagged fusion proteins) were subcloned into pGEMHE (Chang, et al., 2009a; Chang, et al., 2009b). In the following section, the SLC26A9 indicate the “SLC26A9 - wild type” for functional analysis and “triple FLAG-tagged SLC26A9 - wild type” for surface protein expression detection. The SLC26A9-cSNPs (Y70N, T127N, I384T, R575W, P606L, V622L, V744M and H748R) indicate “triple FLAG-tagged cSNPs” for the surface protein expression detection.
Position predictions for SLC26A9-cSNPs
To predict SLC26A9 cSNP locations, an alignment of sequences of the cyanobacterial bicarbonate transporter – BicA (a member of SulP/SLC26 family), SHST1 (Stylosanthes hamata high-affinity sulfate transporter), human SLC26A2 and human SLC26A9 was performed by ClustalW2 (Supp. Figure S1) (Larkin, et al., 2007). The border of SLC26A9 transmembrane regions are predicted by the topology of BicA (Shelden, et al., 2010). The border of STAS domain and its variable loop are based on the SpoIIAA and other Slc26 proteins (Aravind and Koonin, 2000; Pasqualetto, et al., 2010).
Oocyte experiments
Female X. laevis were purchased from Nasco (Fort Atkinson, WI). Oocytes were removed and collagenase dissociated (Romero, et al., 1998a; Romero, et al., 1998b). Capped cRNA was synthesized with the T7 mMessage mMachine kit (Ambion, Austin, TX). 50 nl of water or RNA solution (3ng cRNA coding for human SLC26A9 or cSNPs) were injected into stage V/VI Xenopus oocytes (Chang, et al., 2009a; Chang, et al., 2009b). Equal injected cRNA was confirmed by UV absorbance (A260) and ethidium bromide staining (Supp. Figure S2). Oocytes were maintained at 16°C (OR3 media) and studied 3–7 days after injection.
Electrophysiology
Solutions
All solutions were either ND96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.5) or iso-osmotic ion replacements (Table 2) (Chang, et al., 2009a; Chang, et al., 2009b; Sciortino and Romero, 1999).
Table 2.
Experimental solution composition
| Standard ND96 | 0 Cl−/gluconate ND96 | 0 Cl−/Br− ND96 | 0 Cl−/I− ND96 | 0 Cl−/NO3− ND96 | 0 Cl−/SCN− ND96 | Final Conc. (mM) | ND96 with 33mM HCO3− | Final conc. (mM) |
|---|---|---|---|---|---|---|---|---|
| NaCl | Na.gluconate | NaBr | NaI | NaNO3 | NaSCN | 96 | NaCl | 63 |
| KCl | K.gluconate | KBr | KI | KNO3 | KSCN | 2 | KCl | 2 |
| MgCl2 | Mg.(gluconate)2 | MgBr2 | MgI2 | Mg(NO3)2.6H2O | Mg.(gluconate)2 | 1 | MgCl2 | 1 |
| CaCl2 | Ca.(gluconate)2 | CaBr2 | CaI2 | Ca(NO3)2.4H2O | Ca(SCN)2 | 1.8 | CaCl2 | 1.8 |
| HEPES | HEPES | HEPES | HEPES | HEPES | HEPES | 5.0 | HEPES | 5.0 |
| NaHCO3a | 33 | |||||||
| pH | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | ||
| Osm | 200±2 | 200±2 | 200±2 | 200±2 | 200±2 | 200±2 |
Before adding NaHCO3, bring solution to 90% volume, and regulate pH to 7.500 by NaOH. Then add 33mM NaHCO3, stir, and bring to 100% volume. Solution was bubbled with 5% CO2 for 30–40 minutes before experiments.
Anion substitution
Bathing solutions were gravity perfused from the 140 ml syringes at ~5ml/min. With a chamber volume of 0.5ml, i.e., the bathing solution is completely changed in 15s. For anion substitution experiments, the bath solution was changed in the following order: 0 Cl−/gluconate, Br−, I−, NO3−, SCN−. The oocyte was maintained three minutes in each Cl− replacement solution to make sure the current response was complete.
Two-Electrode Voltage Clamp
Electrodes were filled with 3 M KCl and had resistances of 0.2–0.5 megaohms. Oocytes were clamped at −60 mV, and current was constantly monitored and recorded at 10 Hz (Warner Inst. Co., Oocyte Clamp OC-725C). Voltage steps pulses (75 ms) were executed from −160 to +60 mV in 20 mV steps; the resulting I–V traces were filtered at 2 kHz (8 pole Bessel filter) and sampled at 10 kHz. Data were acquired and analyzed using Pulse and PulseFit (HEKA Instruments, Germany) (Chang, et al., 2009a; Chang, et al., 2009b; Sciortino and Romero, 1999). A KCl salt bridge was used to prevent artificial liquid junction when Cl− was replaced by various anions.
Ion-Selective Microelectrodes
Ion-selective microelectrodes were manufactured and used to monitor pHi and intracellular Cl− ([Cl−]i) of oocytes as described previously (Chang, et al., 2009a; Chang, et al., 2009b; Romero, et al., 1998a; Romero, et al., 2000; Sciortino and Romero, 1999). Electrodes were connected to a high-impedance electrometer (WPI-FD223 for intracellular pH (pHi) and Vm experiments), and digitized output data (filtered at 10Hz) were acquired at 0.5 Hz as previously (Chang, et al., 2009a; Chang, et al., 2009b; Romero, et al., 1998a; Romero, et al., 2000; Sciortino and Romero, 1999). All ion-selective microelectrodes had slopes of −54 to −57 mV/decade ion concentration (or activity). pH electrodes were calibrated at pH 6.0 and 8.0; and Cl− electrodes were calibrated at 10mM NaCl and 100mM NaCl.
Calculations
Oocytes were perfused with ND96 for 5 min, at which time initial pHi or initial pCli was measured. The solution was switched to CO2/HCO3− for 8–10 min, and the final pHi was measured at the stable trough. ΔpHCO2 = Final pHi(CO2) – Initial pHi(CO2); ΔpH(0Cl-CO2) = Final pHi(0Cl-CO2) – Initial pHi(0Cl-CO2); (dpHi/dt)CO2 is the linear pHi slope for a 15–45s segment at the pHi trough. Likewise, (dpHi/dt)(0Cl-CO2) is the linear pHi slope during the response to 0Cl−. [HCO3−]i was calculated from the Henderson-Hasselbach equation (Roos and Boron, 1981). Δ[Cl−]i = Final pCli – Initial pCli ; Δd[Cl−]i/dt = Δ[Cl−]i/t (min); “n” indicates the total number of experiments, and oocytes were harvested from at least two donor animals.
Oocyte Surface Protein Expression
Oocyte surface protein expression of human SLC26A9 and its eight SNPs were measured using biotinylation as well as immunolocalization.
Biotinylation assay
Cell surface protein isolation kit (Pierce, Rockford, IL) was used according to the manufacturer instructions. 72 h after cRNA injection (3ng), groups of 20 oocytes were incubated for 1h at 4°C in PBS that contained 0.25mg/ml Sulfo-NHS-biotin. Then, the non-reactive biotin was quenched and cells were disrupted by lysis buffer with protease inhibitor cocktail (Sigma P8340). The homogenate was incubated at 4°C with rotation mixing for 1 h, and then incubated with neutravidin agarose in the mini-column (kit). The non-bound proteins were washed from the column. The bound, biotinlyated protein was eluted from the column by SDS sample buffer containing 50mM DTT, precipitated and size-fractionated by SDS-PAGE, then transferred onto PVDF membrane. The PVDF membrane was blocked in 5% non-fat milk/PBST and then probed with an anti-FLAG mouse monoclonal primary antibody overnight at 4°C. After washing and incubation with secondary antibodies - HRP-conjugated goat-anti-mouse polyclonal antibody, the immunoblotted proteins were visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Experiments were repeated at least 3 times and results from representative immunoblots are shown.
Immunolocalization
Xenopus oocytes were injected with H2O or 3ng cRNAs coding for triple-FLAG tagged human SLC26A9 or its eight non-synonymous SNPs (3xFLAG-SNP) respectively. Three days after injection, the oocytes were fixed in 4% PFA/PBS for 1 h, followed by 100 mM glycine in PBS for 30 min. Then the oocytes were stored in 30% sucrose at 4°C overnight and frozen in OCT compound (Tissue-tek) with liquid nitrogen. 10 μm cryostat sections were cut, the sections were blocked in 5% non-fat milk, 1% BSA in PBS for 1h. Oocytes stained with only the secondary antibody were controls for background staining. Mouse monoclonal anti-FLAG primary antibody was used at a dilution of 1:1000 overnight, followed by incubation with a secondary anti-mouse Cy3 antibody at a dilution of 1:1,000. Sections were observed using fluorescent microscopy (Zeiss Observer Z1), and images were collected using the AxioVision program (Zeiss).
Statistical analysis
Ion activities or currents are quantified as the mean ± s.e.m (6–12 oocytes in each experimental group from at least two donor Xenopus). Statistical analysis was performed with One-way ANOVA and pairwise comparison between groups was performed using Tukey test (p < 0.05; MINITAB software, USA).
Results
Coding non-synonymous single nucleotide polymorphisms (SNPs) of SLC26A9
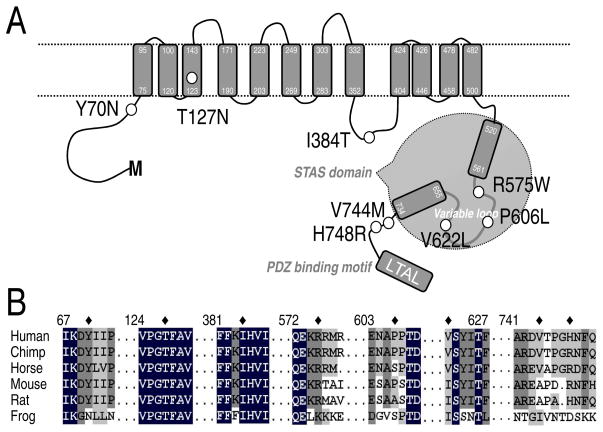
In the NCBI SNP database, eight coding, non-synonymous nucleotide polymorphisms of human SLC26A9 are publically available (Table 1). The mutations resulting from these missense SNPs of SLC26A9 are Y70N (rs75021701); T127N (rs77497889); I384T (rs112659452); R575W (rs139697920); P606L (rs74146719); V622L (rs34992672); V744M (rs3811428) and H748R (rs16856462). To postulate possible roles of these cSNP mutants, we made a topology prediction for SLC26A9 (Figure 1A). The SLC26A9 transmembrane topology is based on the cyanobacterial bicarbonate transporter (BicA), a member of SulP (SLC26) family (Shelden, et al., 2010). The border of STAS domain and its variable loop are based on the alignment with SpoIIAA protein and other Slc26 proteins (Aravind and Koonin, 2000; Pasqualetto, et al., 2010). Figure 1A illustrates the predicted locations of these cSNPs: Y70N (rs75021701in cytoplasmic N-terminus and close to the TM-1 (transmembrane domain 1); T127N (rs77497889) in TM-3; I384T (rs112659452) in an intracellular loop between TM-8 and -9; R575W (rs139697920), P606L (rs74146719) and V622L (rs34992672) in the variable loop of STAS domain; V744M (rs3811428) and H748R (rs16856462) in the C-terminus close to the STAS domain.
Figure 1. Location and coding residue’s conservation of SLC26A9 cSNPs.
A. Proposed SLC26A9topology indicating the positions of cSNPs. Transmembrane topology is based on the cyanobacterial bicarbonate transporter, BicA, a member of SulP (SLC26A) family (Shelden, et al., 2010). The border of STAS domain and its variable loop are based on the alignment with SpoIIAA protein and other Slc26 proteins (Aravind and Koonin, 2000; Pasqualetto, et al., 2010). PDZ-binding motif is proposed by(Chang, et al., 2009b). Nonsynonymous cSNPs are indicated by black circles. B. Selective alignment of SLC26A9 amino acid sequences 67-73, 124-130, 381-387, 572-578, 603-609, 622-627, 741-751 with corresponding regions of other Slc26a9 proteins. The NCBI/GenBank accession numbers for these sequences are human SLC26A9 (Homo sapiens isoform a; NP_443166), chimpanzee Slc26a9 (Pan troglodytes isoform 2; XP_514143), horse Slc26a9 (Equus caballus; XP_001490875), mouse Slc26a9 (Mus musculus; NP_796217), rat Slc26a9 (Rattus norvegicus; NP_001100642), frog Slc26a9 (Xenopus tropicalis; NP_001096210). The asterisk indicates the coding residue of SLC26A9 cSNPs. The intensity of the shading corresponds to the consensus level of the conserved residues in the gene family.
We postulated that conserved residues should play important roles in ion transport function or trafficking of the SLC26A9 proteins to the cell surface. Next, to determine the potential significance of these residues, we made an alignment for Slc26a9 proteins from ifferent species (Figure 1B). Among these eight mutated residues; Y70, T127, I384 and R575 are conserved in Slc26a9 proteins of mammalian species including chimpanzee, horse, mouse and rat. Notably, T127 belongs to a high conservative sequence (-VPGTFAV-) of Slc26a9 proteins. And the “GxF” motif is conserved among all Slc26 members except SLC26A11 (data not shown).
Several cSNPs change SLC26A9 Cl− current magnitude
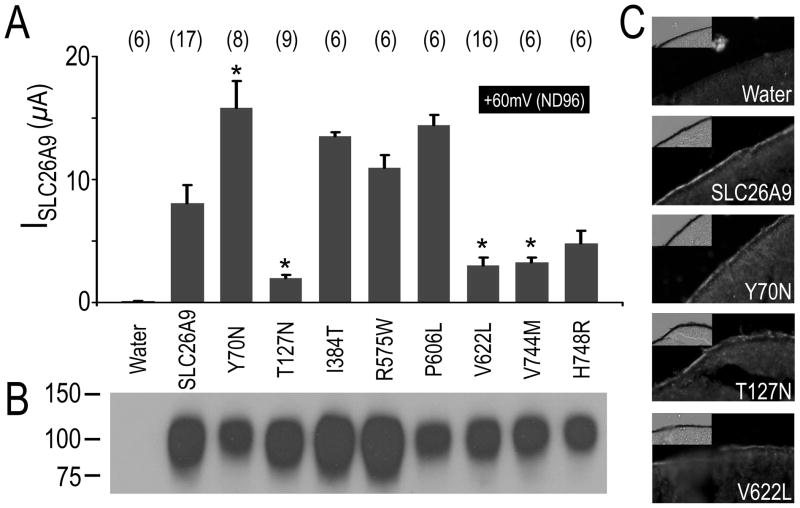
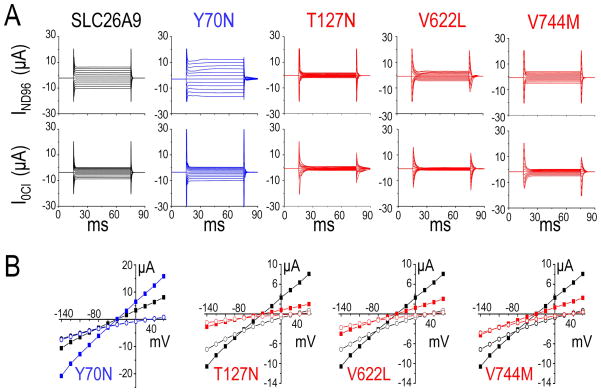
Large Cl− currents have been reported from two-electrode voltage clamp in the Xenopus oocytes expressing mouse Slc26a9 (Romero, et al., 2006) or human SLC26A9 (Dorwart, et al., 2007). To understand the possible SLC26A9 cSNPs pathophysiology, these point mutations were created in the template of SLC26A9 and expressed in Xenopus oocytes for functional analysis. The Cl− current (Figure 2A) of control oocytes injected with SLC26A9’s cRNA was compared with that of oocytes injected with same amount cRNA (Supp. Figure S2) of the eight SLC26A9 cSNPs. The oocytes were clamped at −60 mV. As previously reported for mouse Slc26a9 (Chang, et al., 2009b) and human SLC26A9 (Dorwart, et al., 2007), SLC26A9 (ISLC26A9; Figure 2A; Figure 3A, B- black) showed large voltage-dependent I (I–V)-responses which were reduced, changed reversal potential (Erev) and slightly rectified when bath Cl− was replaced by gluconate (0 Cl−/ND96 solution, pH7.5). Compared with ISLC26A9, four cSNPs changed current magnitudes with statistical significance (p<0.01) (Figure 2A) but had similar overall I–V shapes (Figure 3A, B). Y70N (rs75021701) (Figure 3A, B-blue) responded to similar maneuvers with larger than wild-type currents. Conversely, smaller ISLC26A9’s were elicited in T127N (rs77497889), V622L (rs34992672) and V744M (rs3811428) in the presence or absence of Cl− (Figure 2A; Figure 3A, B- red). The I–V relationships illustrate that these changes are maintained over the entire voltage range tested (Figure 3B). To highlight the differences among the SLC26A9 and its cSNPs, currents are plotted at +60mV in the ND96 solution (Figure 3A). Figure 3A shows that IY70N is ~two fold increased (15.81±2.2 μA, p<0.01) at +60 mV compared to ISLC26A9 (8.08 ± 1.47 μA). T127N (rs77497889), V622L (rs34992672) and V744M (rs3811428) both display decreased currents at +60 mV (ND96) (n≥6, p<0.005) (Figure 3A). None of these SLC26A9/cSNPs-currents were observed in the water-control oocytes (data not shown), as previous report (Chang, et al., 2009b).
Figure 2. Currents mediated by SLC26A9, cSNPs and surface protein expression.
A, Pooled histograms of mean currents at +60mV (ND96 solution) resulting from voltage clamp experiments of Xenopus oocytes injected with cRNAs of SLC26A9 and its eight cSNPs respectively. The number of replications in each group is displayed above each bar as well as (*) indicating statistical difference from SLC26A9 (P<0.01). B, Representative western blot of biotinylated proteins (~0.5 oocyte/lane) that were isolated from oocytes expressing triple FLAG-tagged SLC26A9 & cSNPs. And oocytes injecting with water is choose as negative control. Molecular weight markers are displayed to the left of the panel. C, Sections of oocytes injecting with cRNA coding for triple-FLAG tagged SLC26A9 and selective cSNPs (Y70N, T127N and V622L) were labeled with the antibody against the FLAG epitope. Cy3 fluorescence was detected on the membrane of oocytes expressing SLC26A9, Y70N, T127N and V622L, but not water-injected control.
Figure 3.
cSNPs alter Cl− channel function of SLC26A9.
A. In voltage clamp experiments, current sweeps resulting from the voltage step protocol in ND96 (upper) and a Cl− free ND96 (lower) for SLC26A9 (black), Y70N (blue), T127N (red), V622L (red) and V744M (red). B. Paired comparison of I–V relationships for Y70N (blue), T127N (red), V622L (red), V744M (red) and SLC26A9 (black) respectively. The recordings were made first in ND96 (square) and then in 0 Cl−/ND96 solution (circle) from Xenopus oocytes injected with corresponding cRNAs. The number of experiments for each group is shown in Figure 2A as “(#)”.
Cell surface detection of SLC26A9 and its non-synonymous cSNPs
To investigate the effect of SLC26A9 cSNPs on protein expression and trafficking, cell surface biontinylation assay and immunohistochemistry assays were performed on Xenopus oocytes expressing SLC26A9 and its cSNPs. To maximize the specificity of detection, we subcloned a triple FLAG epitope fused to the N-terminus of human SLC26A9 and its eight cSNPs respectively (see Materials and Methods). This 3xFLAG-tag allowed us to use a mouse anti-FLAG monoclonal for protein detection of all of the constructs. Voltage clamp experiments found that these triple FLAG tagged SLC26A9/cSNPs show the same function as its non-tagged counterparts respectively (data not shown), especially for Y70N (rs75021701), T127N (rs77497889) and V622L (rs34992672). Thus the constructs encoding the triple FLAG tagged SLC26A9/cSNPs were used in our surface expression detection experiments (Figure 2B,C).
As shown in Figure 2B, the cell surface expression was detected by biotinylation assay for SLC26A9 and its eight cSNPS. The band corresponding to SLC26A9/cSNPs is 100kD, which is close to the predicted monomer size of triple FLAG-tagged SLC26A9 protein (813 amino acid, 90kD). No bands were detected for the control water-injected oocytes suggesting the signals were responsible for SLC26A9/cSNPs surface expression. Although it was not quantified, the bands corresponding to Y70N (rs75021701), P606L (rs74146719), V622L (rs34992672), V744M (rs3811428) and H748R (rs16856462) were slightly less in density than the band for SLC26A9 while the bands for T127N (rs77497889), I384T (rs112659452) and R575W (rs139697920) were similar or slightly higher in density than the band for SLC26A9 in three independent experiments.
To further detect the surface protein expression level of Y70N (rs75021701), T127N (rs77497889) and V622L (rs34992672), immunohistochemistry of crysectioned oocytes was used, followed by protein detection with the anti-FLAG antibody (Figure 2C). The fluorescence signals on plasma membrane of oocytes expressing T127N (rs77497889) are similar to that of SLC26A9, while the fluorescence signals for Y70N (rs75021701) and V622L (rs34992672) are slightly less than that of SLC26A9. These results are consistent with those of the biotinylation experiments.
Y70N activates Cl−-HCO3− exchange by SLC26A9
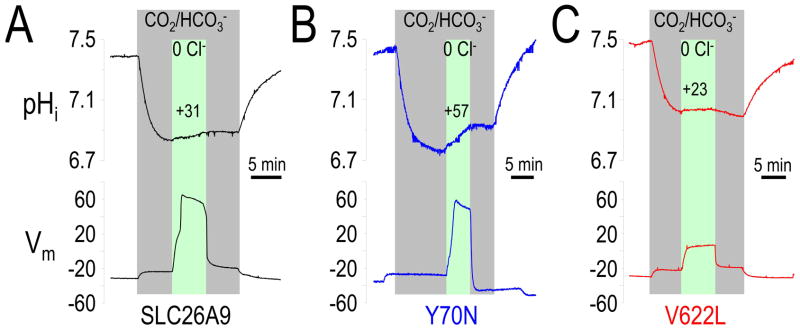
Several groups’ studies suggest that SLC26A9 (and mouse Slc26a9) function not only as a Cl− channel (Bertrand, et al., 2009; Chang, et al., 2009b; Dorwart, et al., 2007; Xu, et al., 2005; Xu, et al., 2008) but also a Cl−-HCO3− exchanger (Bertrand, et al., 2009; Chang, et al., 2009b; Dorwart, et al., 2007; Xu, et al., 2005; Xu, et al., 2008). Since large Cl− currents (Cl− channel activity) were observed for Y70N (rs75021701) (Figure 2A; Figure 3A), we sought to determine if the Cl−-HCO3− exchanger activity was simultaneously enhanced. To more directly evaluate Cl−-HCO3− exchange activity, we used pH microelectrodes to measure intracellular pH (pHi) changes after Cl− removal and readdition from a CO2/HCO3− bath solution for SLC26A9 and Y70N (Figure 4A, B). In water controls, Cl− replacement (with gluconate; 0Cl−) did not change pHi or Vm (data not shown), as previously described (Chang, et al., 2009b). However, Figure 4B illustrates that Y70N increased Cl−-HCO3− exchange activity, i.e., Y70N had an obvious depolarization and increased pHi change (initial rate = +57×10−5 pH unit/sec) after switching to the 0 Cl−/5%CO2 buffer. SLC26A9 (Figure 4A) alkalized at +31×10−5 pH unit/sec. These results illustrate that Y70N exhibits increased SLC26A9 function, both as a Cl− channel and as a Cl−-HCO3− exchanger (Table 3).
Figure 4. Cl−-HCO3− exchanger function of SLC26A9 changed by Y70N and V622L.
Representative pHi experiments for oocytes: SLC26A9 (black, left), Y70N (blue, middle) and V622L (red, right) in 5% CO2, 33mM HCO3− (pH7.5) solution (gray region). Membrane potential (Vm) was measured simultaneously. Extracellular Cl− removal (0 Cl−) (green region) depolarized the oocytes and increased pHi. The average dpHi/dt(0Cl-CO2) value (x10−5 pH unit/s) reflecting Cl−-HCO3− exchange function was displayed above the pH curve during 0 Cl−/HCO3− phrase. Average responses are given in Table 3.
Table 3.
HCO3− transport properties of SLC26A9, Y70N and V622L
| unit | SLC26A9 (n=6) | Y70N (n=6) | V622L (n=5) | |
|---|---|---|---|---|
| (dpHi/dt)CO2 | ×10−5 (pH unit/s) | −313 ± 25.4 | −289 ± 43.5 | −307 ±36.5 |
| Δ [HCO3−]CO2 | mM | 5.98 ± 0.78 | 6.30 ± 0.75 | 7.19 ± 1.23 |
| (dpHi/dt)(0Cl-CO2) | ×10−5 (pH unit/s) | +31.3 ± 3.1 | +57.0 ± 12.1 | +23.1 ±4.0 |
| Δ [HCO3−](0Cl-CO2) | mM | 0.79 ± 0.16 | 1.94 ± 0.45 | 0.40 ± 0.29 |
Calculations are as indicated under “Methods and Materials”. These data were collected using the two-electrode experiments (pH electrode plus voltage electrode) to Xenopus oocytes. The water-injected oocytes show no response to 0Cl−/HCO3−/5%CO2 (Cl−/HCO3− exchange activity) and its data is as same as previous report (data not shown) (Chang, et.al., 2008). The dpHi/dt(0Cl-CO2) values (bold) of Y70N and V622L, the Δ [HCO3−](0Cl-CO2) value (bold) of Y70N and V622L are significantly different from that of SLC26A9 wild type (P < 0.05).
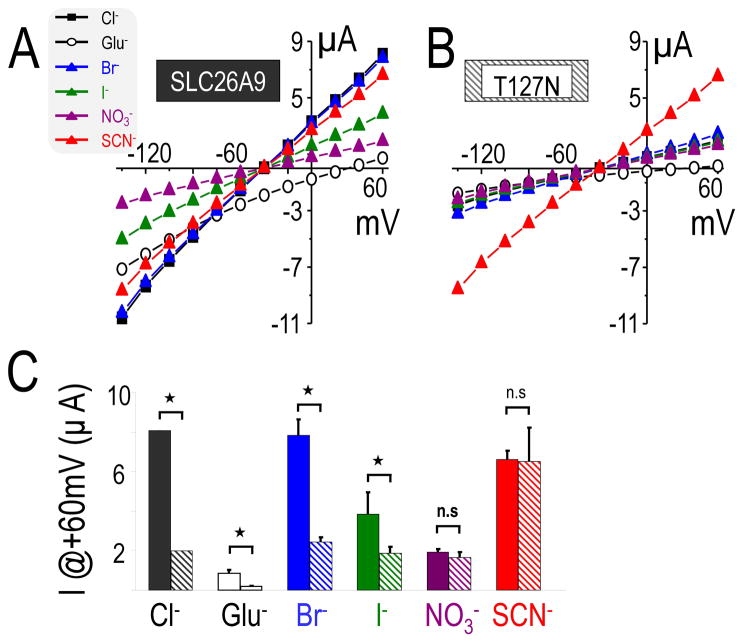
T127N decreases conductance of Cl−, Br−, I−, but neither NO3− nor SCN−
Based on the very low currents of T127N (rs77497889), we reasoned that the Cl−/HCO3− exchange of T127N (rs77497889) is only slightly different from the water–injected control oocytes. T127N (rs77497889) is the only SLC26A9 cSNP located in the transmembrane domain (Figure 1A), and transmembrane domains often play important roles in solute permeation of channels, and thus control ionic conductance. We focused on the anion conductance of T127N, rather than Cl−/HCO3− exchange. To further characterize the T127N-mediated current, we measured its conductance of different monovalent anions (Cl−, Br−, I−, NO3− and SCN−) compared to those of SLC26A9. We found an anion selectivity of SLC26A9 was similar to that previously reported (Dorwart, et al., 2007). That is, SLC26A9 conducted Br− similar to Cl−, but better than I− and NO3− (Figure 5A, C). We also found SLC26A9 conducted isothiocyanate (SCN−), and that the conductance of SCN− greater than that of I− and NO3− but slightly less than that of Br− and Cl−. Thus, SLC26A9 anion conductance has the rank order: Cl− ≈ Br− > SCN− > I− > NO3− ≫> Glu−. Figure 5B, C illustrates that IT127N had decreased conductance of not only for Cl− but also of Brand I− compared to ISLC26A9. However, the current carried by NO3− and SCN− were not decreased by T127N (rs77497889) compared to that of SLC26A9. Thus, the anion conductance of IT127N differed from SLC26A9: SCN− > Cl− ≈ Br− > I− > NO3− ≫ Glu−. With similar ionic replacements (Cl−, Br−, I−, NO3− and SCN−), water-injected oocytes display only minor currents (<100nA) (data not shown).
Figure 5. T127N changes the anion conductance order of SLC26A9.
A, I–V curve measured from voltage clamp experiments of Xenopus oocytes expressing SLC26A9(left) or T127N(right) in ND96 solution containing either 103.6mM Cl− or 103.6mM of the following anions: gluconate (Glu−), Br−, I−, NO3− and SCN−. B, Pooled histograms of mean currents at +60mV with each of anions (Cl−, Glu−, Br−, I−, NO3− and SCN−) measured in the oocytes expressing SLC26A9 (left) or T127N (right). “*” indicates significance (P<0.01) and “n.s” non-significance between specific pairs of anion substitution experiments.
V622L reduces SLC26A9 function
Recently, STAS domains have emerged as a major form of regulation for SLC26 family transporters/channels (Chang, et al., 2009a; Ko, et al., 2004; Shcheynikov, et al., 2006a). V622L (rs34992672) is one of the cSNPs located in the STAS domain (Figure 1A) which changed SLC26A9 function. Although the surface protein expression of V622L is slightly reduced (Figure 2B,C), function may also be impaired. Again, we used pHi to quantify Cl−-HCO3− exchanger activity of V622L. As shown in Figure 4C shows that V622L reduced Cl−-HCO3− exchanger activity, i.e., V622L has a smaller depolarization and pHi change (initial rate (dpHi/dt) = +23×10−5 pH unit/sec after Cl− removal in the CO2/HCO3− buffer; Table 3). These results indicate that V622L decreased SLC26A9 Cl−-HCO3− exchange function, yet they are complicated due to simultaneous decrease protein expression.
Discussion
SLC26A9 protein functions as both a chloride channel and a Cl−-HCO3− exchanger involved in Cl− and HCO3− absorption as well as secretion in various epithelia (Chang, et al., 2009b; Dorwart, et al., 2007; Lohi, et al., 2002; Loriol, et al., 2008; Xu, et al., 2005). Numerous mutations in SLC26 genes have been shown to lead to human disorders (Dawson and Markovich, 2005; Everett and Green, 1999; Liu, et al., 2003; Markovich, 2001; Sindic, et al., 2007) and these disorders underscore the need to further our understanding of the SLC26 genes in human disease. Recently, a genome-wide association studies (GWAS) of neurocognition, as an indicator of antipsychotic treatment response in Schizophrenia, identified that one intronic SNP (rs11240594) of SLC26A9 was associated with olanzapine effects on neuro-processing speed, p =1.4×10−7) (McClay, et al., 2011). These investigators also noted that multiple neighboring SNPs were in linkage disequilibrium with this SLC26A9 SNP (rs11240594), apparently tagging the same signal (McClay, et al., 2011). These linkage data indicate that SLC26A9 gene is tightly linked with multiple neurocognitive domains in the brain and the patients with SLC26A9 allelic variations (i.e., cSNPs) may have increased risk of schizophrenia or brain dysfunction.
Deletion of Slc26a9 in mice resulted in the loss of gastric acid secretion and a moderate reduction in the number of parietal cells (Xu, et al., 2008). Microarray analysis and in situ hybridization showns that Slc26a9 is down-regulated in Fgf3−/−; Fgf10−/− (Fibroblast growth factor) mouse embryos (Urness, et al., 2010). Together these data and the human association of McClay and coworkers suggest that SLC26A9/Slc26a9 play multiple physiological roles being associated with human antipsychotic response, gastric acid secretion and otic development. These varied findings prompted us to investigate the potential effect of eight missense cSNPs of SLC26A9.
Y70N: a cSNP in cytoplasmic N-terminus
Little is known about the regulation and the structural elements of the N-terminus of Slc26 proteins as there are no crystal structures of Slc26 protein TMs or N-termini. There is expanding data that the N-termini of certain anion channels control function. For example, CFTR studies suggest that the cytoplasmic N-terminus is critical for the biosynthesis of this cAMP-activated chloride channel (Prince, et al., 1999). The CFTR N-terminus also controls PKA-dependent channel gating by interaction of the R-region via its highly conserved acidic stretch (Chappe, et al., 2005; Naren, et al., 1999). Moreover, the CFTR N-terminus physically links CFTR to proteins involved into membrane trafficking machinery (Bilan, et al., 2004; Naren, et al., 1998; Peters, et al., 2001). Several AP (adaptor protein complex) and GGA (Golgi-localized, γ-ear containing, Arf-binding adaptor protein) binding sites have been identified in the cytoplasmic, ClC chloride channel’s N-termini (Stauber and Jentsch, 2010). With these findings, we hypothesize that the SLC26A9 N-terminus may also play important functional roles. Our experiments reveal that Y70N displays enhanced SLC26A9 function. Y70 is located in between the SLC26A9 N-terminus and the first TM. These data, with our topology model, suggest that Y70 may be in a region of inter-domain structural flexibility which in turn amplifies activity.
T127N: a cSNP in the transmembrane domain
Multiple structure-function studies with CFTR have defined the molecular-level importance of critical functional domains and have correlated CFTR mutations with their clinical outcome (Hanrahan and Wioland, 2004; Sheppard and Welsh, 1999). However, the structure-function studies for the SLC26 family are just beginning. Recently Ohana et al. found by modeling the Slc26a6 TM onto the ClC chloride channel’s structure, two conserved negative residues E357 (Slc26a6) and E367 (Slc26a3) which play key roles in their unique transport modes (Ohana, et al., 2011). On the other hand, a significant number of conserved polar residues in transmembrane helices 1 and 2 of SulP family (plant and bacteria Slc26 family) were found by sequence and bioinformatics analysis (Leves, et al., 2008). Functional analysis results from SHST1-mutations (sulfate transporter) suggest that removal of even a single OH- group from these polar residues significantly affects transport. Figure 1A illustrates that T127N is predicted in TM-3 of SLC26A9, and IT127N anion conductance is decreased for Cl−, Br− and I−, yet unchanged for NO3− and SCN− (Figure 3, Figure 5). Interestingly, IT127N(SCN−) is the same magnitude as ISLC26A9(SCN−) and is dramatically increased from the basal current for T127N. Our sequence analysis revealed that the polar residue T127 is embedded in an undefined motif (GXF) which is highly conserved from SLC26A1 to SLC26A9 (not shown). We hypothesize that the polar T127 residue is located in a critical position adjacent to the SLC26A9 channel pore. By extension, T127N would then subtly change the pore conformation for some compact anions (Cl−, Br−and I−) but not for a resonant anion (NO3− and SCN−). Clearly, additional investigations will be needed to define the structural role of T127 in SLC26A9 function.
V622L: a cSNP in the STAS domain variable loop
Functional interactions of Slc26a3 and Slc26a6 STAS domains (C-terminus) with the R-region of CFTR have been previously reported to cause mutual stimulation of Slc26-activity and CFTR-channel activity (Ko, et al., 2004; Rakonczay, et al., 2008; Shcheynikov, et al., 2004).The situation appears more complex for the relationship of Slc26a9/SLC26A9 and R-CFTR We recently demonstrated that Slc26a9 function (Cl− channel and Cl−-HCO3− exchange) is inhibited by interaction of the CFTR R-region and the Slc26a9-STAS domain (Chang, et al., 2009a). This (R-region)-STAS interaction in Xenopus oocytes or of proteins in solution, did not require activation of PKA (Chang, et al., 2009a). Using HBE (human bronchial epithelial) cells, Bertrand et al. found SLC26A9 functions as an anion conductance which requires a functional interaction with PKA-activated CFTR (Bertrand, et al., 2009). Another group found SLC26A9 favors the biogenesis and/or stabilization of CFTR, leading to stimulated currents even with a non-SNP-mutation (L638P) which kills SLC26A9 function (Avella, et al., 2011a
Our knowledge of the STAS domain of Slc26 proteins stems from STAS’ weak but significant similarity with the Bacillus antisigma protein SpoIIAA (Aravind and Koonin, 2000). By mapping the conserved motifs onto Prestin STAS structure (Babu, et al., 2010; Pasqualetto, et al., 2010), the STAS domain seems to consist of six β-strands, surrounded by five α-helices. Babu and coworkers solved the STAS structure of E.coli YchM, finding the same basic structure, but that YchM is tightly associated with an acyl carrier protein and tightly associated with fatty acid synthesis (Babu, et al., 2010).The significance of this finding for vertebrate Slc26 proteins and their STAS-domains is currently unknown.
Our analysis using a multiple sequence alignment indicates that there is sequence of variable length (variable loop) between the first α helix and the third β strand of Slc26 STAS domains. This variable loop also separates the STAS domain into two sequence-conserved regions. Interestingly the structure solved for prestin deleted this variable loop (Pasqualetto, et al., 2010); and YchM does not contain such an intervening sequence (Babu, et al., 2010). Without structure information for this STAS variable loop, we cannot anticipate or predict STAS structural elements or their potential regulatory roles. V622L is located in the SLC26A9 STAS domain within the variable loop (residues 562-654). In M. musculus, R. norvegicus and X. tropicalis, V622 is replaced by I622, a similar hydrophobic amino acid. V622L reduces the SLC26A9 Cl− current and Cl−-HCO3− exchange activity, but also slightly reduces protein expression. Despite only minor protein reduction, function of V622L-SLC26A9 is ~50% of wild-type SLC26A9. Thus, this cSNP in the STAS domain has an effect on both protein expression and activity of the resultant transporter-channel. Other experiments indicate that V622I increases ISLC26A9 (Chen and Romero, 2010). We postulate that replacement of I622 by V622 or L622 might cause progressive yet subtle alterations in conformation which result in changes of STAS domain tertiary structure. This change would then interfere with required interactions with other proteins or interdomain-regions. Together these findings indicate that the V622 is a controller of transport activity magnitude and proteins expression, and that side chain length of 622 has a role in controlling activity level.
Summary
We have functionally assessed eight missense cSNPs of SLC26A9. Four SLC26A9 cSNPs show significantly altered transport functions from wild-type: Y70N increased Cl− currents, while T127N, V622L and V744M decreased Cl− currents. Additionally, Y70N increased Cl−-HCO3− exchange activity, but T127N and V622L displayed decreased Cl−-HCO3− exchanger activity. The effects of T127N are more subtle: (a) ISLC26A9 was decreased for Cl−, Br− and I−, (b) ISLC26A9 was unchanged for NO3− and SCN−. That is, the anion conductance order of SLC26A9 was changed by T127N. Thus, we predicted that allelic variations in human SLC26A9 should change epithelial Cl− transport: N70 polymorphism would increase Cl− transport across epithelial cells while the others with N127, L622, and M744 polymorphisms would reduce Cl− transport. Thus, any disorder resulting from SLC26A9 interaction or functional presence (e.g., cystic fibrosis), would also be predicted to have altered pathophysiology due to allelic variations of SLC26A9.
Supplementary Material
Acknowledgments
We thank Heather L. Holmes and Elyse M. Scileppi for technical assistance.
We gratefully acknowledge the Support of NIH (R01-EY017732, P30-DK090728) and the Mayo Clinic Foundation.
Footnotes
R575W is replaced by R575Q in SLC26A9 cSNPs database ( 02/21/2012).
Supporting Information for this preprint is available from the Human Mutation editorial office upon request (humu@wiley.com)
References
- Aravind L, Koonin EV. The STAS domain - a link between anion transporters and antisigma-factor antagonists. Curr Biol. 2000;10(2):R53–5. doi: 10.1016/s0960-9822(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Avella M, Borgese F, Ehrenfeld J. Characterization of the L683P mutation of SLC26A9 in Xenopus oocytes. Biochim Biophys Acta. 2011a;1810(6):577–83. doi: 10.1016/j.bbagen.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Avella M, Loriol C, Boulukos K, Borgese F, Ehrenfeld J. SLC26A9 stimulates CFTR expression and function in human bronchial cell lines. J Cell Physiol. 2011b;226(1):212–23. doi: 10.1002/jcp.22328. [DOI] [PubMed] [Google Scholar]
- Babu M, Greenblatt JF, Emili A, Strynadka NC, Reithmeier RA, Moraes TF. Structure of a SLC26 anion transporter STAS domain in complex with acyl carrier protein: implications for E. coli YchM in fatty acid metabolism. Structure. 2010;18(11):1450–62. doi: 10.1016/j.str.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133(4):421–38. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilan F, Thoreau V, Nacfer M, Derand R, Norez C, Cantereau A, Garcia M, Becq F, Kitzis A. Syntaxin 8 impairs trafficking of cystic fibrosis transmembrane conductance regulator (CFTR) and inhibits its channel activity. J Cell Sci. 2004;117(Pt 10):1923–35. doi: 10.1242/jcs.01070. [DOI] [PubMed] [Google Scholar]
- Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem. 1994;269(4):3017–21. [PubMed] [Google Scholar]
- Chang M-H, Plata C, Sinđić A, Ranatunga WK, Chen AP, Zandi-Nejad K, Chan KW, Thompson J, Mount DB, Romero MF. Slc26a9 is inhibited by the R-region of CFTR via the STAS domain. J Biol Chem. 2009a;284(28306-18):28306–18. doi: 10.1074/jbc.M109.001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M-H, Plata C, Zandi-Nejad K, Sindic A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26A9 - anion exchanger, channel and Na+ transporter. J Membr Biol. 2009b;128(3):125–40. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappe V, Irvine T, Liao J, Evagelidis A, Hanrahan JW. Phosphorylation of CFTR by PKA promotes binding of the regulatory domain. Embo J. 2005;24(15):2730–40. doi: 10.1038/sj.emboj.7600747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A-P, Romero MF. Channel inhibitory region within the STAS domain of human SLC26A9. Faseb J. 2010;24:1002.7. [Google Scholar]
- Cherest H, Davidian JC, Thomas D, Benes V, Ansorge W, Surdin-Kerjan Y. Molecular characterization of two high affinity sulfate transporters in Saccharomyces cerevisiae. Genetics. 1997;145(3):627–35. doi: 10.1093/genetics/145.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Markovich D. Pathogenetics of the human SLC26 transporters. Curr Med Chem. 2005;12(4):385–96. doi: 10.2174/0929867053363144. [DOI] [PubMed] [Google Scholar]
- Demitrack ES, Soleimani M, Montrose MH. Damage to the gastric epithelium activates cellular bicarbonate secretion via SLC26A9 Cl(−)/HCO(3)(−) Am J Physiol Gastrointest Liver Physiol. 2010;299(1):G255–64. doi: 10.1152/ajpgi.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detro-Dassen S, Schanzler M, Lauks H, Martin I, Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C. Conserved Dimeric Subunit Stoichiometry of SLC26 Multifunctional Anion Exchangers. J Biol Chem. 2008;283(7):4177–88. doi: 10.1074/jbc.M704924200. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl channel regulated by the WNK kinases. J Physiol. 2007;584(Pt 1):333–45. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23:104–14. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17(4):411–22. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Everett LA, Green ED. A family of mammalian anion transporters and their involvement in human genetic diseases. Hum Mol Genet. 1999;8(10):1883–91. doi: 10.1093/hmg/8.10.1883. [DOI] [PubMed] [Google Scholar]
- Hanrahan JW, Wioland MA. Revisiting cystic fibrosis transmembrane conductance regulator structure and function. Proc Am Thorac Soc. 2004;1(1):17–21. doi: 10.1513/pats.2306009. [DOI] [PubMed] [Google Scholar]
- Hastbacka J, Superti-Furga A, Wilcox WR, Rimoin DL, Cohn DH, Lander ES. Atelosteogenesis type II is caused by mutations in the diastrophic dysplasia sulfate-transporter gene (DTDST): evidence for a phenotypic series involving three chondrodysplasias. Am J Hum Genet. 1996;58(2):255–62. [PMC free article] [PubMed] [Google Scholar]
- Hawkesford MJ. Transporter gene families in plants: the sulfate transporter gene family— redundancy or specialization? Physiol Plant. 2003;117:155–163. [Google Scholar]
- Homma K, Miller KK, Anderson CT, Sengupta S, Du GG, Aguinaga S, Cheatham M, Dallos P, Zheng J. Interaction between CFTR and prestin (SLC26A5) Biochim Biophys Acta. 2010;1798(6):1029–40. doi: 10.1016/j.bbamem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Grichtchenko, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277(37):33963–7. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber J, Murer H. Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol. 1998;275(1 Pt 2):F79–87. doi: 10.1152/ajprenal.1998.275.1.F79. [DOI] [PubMed] [Google Scholar]
- Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 Is a Cl− Channel Regulated by Intracellular pH. J Biol Chem. 2005;280(8):6463–70. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, et al. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. Embo J. 2002;21(21):5662–72. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6(4):343–50. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Leves FP, Tierney ML, Howitt SM. Polar residues in a conserved motif spanning helices 1 and 2 are functionally important in the SulP transporter family. Int J Biochem Cell Biol. 2008;40(11):2596–605. doi: 10.1016/j.biocel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Li X, Corvol H, Li W, Chiang T, Lin F, Boelle P-Y, Drumm M, Cutting G, Knowles M, Durie P, et al. Replication evidence that constituents of the apical plasma membrane contribute to Meconium ileus in Cystic Fibrosis. 25th N Am CF Conference.2011. [Google Scholar]
- Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F, Du LL, Welch KO, Petit C, Smith RJ, et al. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Mol Genet. 2003;12(10):1155–1162. doi: 10.1093/hmg/ddg127. [DOI] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem. 2002;277(16):14246–54. doi: 10.1074/jbc.M111802200. [DOI] [PubMed] [Google Scholar]
- Loriol C, Dulong S, Avella M, Gabillat N, Boulukos K, Borgese F, Ehrenfeld J. Characterization of SLC26A9, facilitation of Cl− transport by bicarbonate. Cell Physiol Biochem. 2008;22(1–4):15–30. doi: 10.1159/000149780. [DOI] [PubMed] [Google Scholar]
- Makela S, Kere J, Holmberg C, Hoglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2002;20(6):425–38. doi: 10.1002/humu.10139. [DOI] [PubMed] [Google Scholar]
- Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev. 2001;81(4):1499–533. doi: 10.1152/physrev.2001.81.4.1499. [DOI] [PubMed] [Google Scholar]
- McClay JL, Adkins DE, Aberg K, Bukszar J, Khachane AN, Keefe RS, Perkins DO, McEvoy JP, Stroup TS, Vann RE, et al. Genome-wide pharmacogenomic study of neurocognition as an indicator of antipsychotic treatment response in schizophrenia. Neuropsychopharmacology. 2011;36(3):616–26. doi: 10.1038/npp.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol. 1999;276(1 Pt 1):G185–92. doi: 10.1152/ajpgi.1999.276.1.G185. [DOI] [PubMed] [Google Scholar]
- Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch. 2004;447(5):710–21. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- Naren AP, Cormet-Boyaka E, Fu J, Villain M, Blalock JE, Quick MW, Kirk KL. CFTR chloride channel regulation by an interdomain interaction. Science. 1999;286(5439):544–8. doi: 10.1126/science.286.5439.544. [DOI] [PubMed] [Google Scholar]
- Naren AP, Quick MW, Collawn JF, Nelson DJ, Kirk KL. Syntaxin 1A inhibits CFTR chloride channels by means of domain-specific protein-protein interactions. Proc Natl Acad Sci U S A. 1998;95(18):10972–7. doi: 10.1073/pnas.95.18.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol. 2011;137(2):239–51. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetto E, Aiello R, Gesiot L, Bonetto G, Bellanda M, Battistutta R. Structure of the cytosolic portion of motor protein prestin and functional role of the STAS domain in SLC26/SulP anion transporters. J Mol Biol. 2010;400:448–462. doi: 10.1016/j.jmb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Peters KW, Qi J, Johnson JP, Watkins SC, Frizzell RA. Role of snare proteins in CFTR and ENaC trafficking. Pflugers Arch. 2001;443(Suppl 1):S65–9. doi: 10.1007/s004240100647. [DOI] [PubMed] [Google Scholar]
- Prince LS, Peter K, Hatton SR, Zaliauskiene L, Cotlin LF, Clancy JP, Marchase RB, Collawn JF. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tyrosine-based signal. J Biol Chem. 1999;274(6):3602–9. doi: 10.1074/jbc.274.6.3602. [DOI] [PubMed] [Google Scholar]
- Rakonczay Z, Jr, Hegyi P, Hasegawa M, Inoue M, You J, Iida A, Ignath I, Alton EW, Griesenbach U, Ovari G, et al. CFTR gene transfer to human cystic fibrosis pancreatic duct cells using a Sendai virus vector. J Cell Physiol. 2008;214(2):442–55. doi: 10.1002/jcp.21220. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rode B, Dirami T, Bakouh N, Rizk-Rabin M, Norez C, Lhuillier P, Lores P, Jollivet M, Melin P, Zvetkova I, et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum Mol Genet. 2012;21(6):1287–98. doi: 10.1093/hmg/ddr558. [DOI] [PubMed] [Google Scholar]
- Romero MF, Chang M-H, Plata C, Zandi-Nejad K, Broumand V, Sussman CR, Mount DB. “Physiology of electrogenic SLC26 paralogs” In - Epithelial Anion Transport in Health and Disease: the role of the SLC26 transporters family. Novartis Foundation Symposium. 2006;273:126–147. [PubMed] [Google Scholar]
- Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol. 1998a;274(2 Pt 2):F425–32. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+ driven anion exchanger (NDAE1): a new bicarbonate transporter. J Biol Chem. 2000;275:24552–24559. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- Romero MF, Kanai Y, Gunshin H, Hediger MA. Expression cloning using Xenopus laevis oocytes. Methods Enzymol. 1998b;296:17–52. doi: 10.1016/s0076-6879(98)96004-9. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Rossi A, Superti-Furga A. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene (SLC26A2): 22 novel mutations, mutation review, associated skeletal phenotypes, and diagnostic relevance. Hum Mutat. 2001;17(3):159–71. doi: 10.1002/humu.1. [DOI] [PubMed] [Google Scholar]
- Saier MH, Jr, Eng BH, Fard S, Garg J, Haggerty DA, Hutchinson WJ, Jack DL, Lai EC, Liu HJ, Nusinew DP, et al. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim Biophys Acta. 1999;1422(1):1–56. doi: 10.1016/s0304-4157(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Satoh H, Susaki M, Shukunami C, Iyama K, Negoro T, Hiraki Y. Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J Biol Chem. 1998;273(20):12307–15. doi: 10.1074/jbc.273.20.12307. [DOI] [PubMed] [Google Scholar]
- Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney, electrogenic Na+/HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol. 1999;277(4 Pt 2):F611–623. doi: 10.1152/ajprenal.1999.277.4.F611. [DOI] [PubMed] [Google Scholar]
- Scott DA, Karniski LP. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol. 2000;278(1):C207–C211. doi: 10.1152/ajpcell.2000.278.1.C207. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Kim KH, Kim KM, Dorwart MR, Ko SB, Goto H, Naruse S, Thomas PJ, Muallem S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl(−)/HCO3(−) selectivity by external Cl(−) J Biol Chem. 2004;279(21):21857–65. doi: 10.1074/jbc.M313323200. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006a;273:177–86. discussion 186–92, 261–4. [PubMed] [Google Scholar]
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by Slc26a3 and Slc26a6. J Gen Physiol. 2006b;127(5):511–24. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelden MC, Howitt SM, Price GD. Membrane topology of the cyanobacterial bicarbonate transporter, BicA, a member of the SulP (SLC26A) family. Mol Membr Biol. 2010;27(1):12–23. doi: 10.3109/09687680903400120. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(1 Suppl):S23–45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Sindic A, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Curr Opin Nephrol Hypertens. 2007;16(5):484–90. doi: 10.1097/MNH.0b013e3282e7d7d0. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280(2):F356–64. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- Stauber T, Jentsch TJ. Sorting motifs of the endosomal/lysosomal CLC chloride transporters. J Biol Chem. 2010;285(45):34537–48. doi: 10.1074/jbc.M110.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, Engler JA, Engler G, Van Montagu M, Saito K. Regulation of sulfur assimilation in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1997;94(20):11102–7. doi: 10.1073/pnas.94.20.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Metcalfe RA, Watson PF, Weetman AP, Trembath RC. Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab. 2002;87(4):1778–84. doi: 10.1210/jcem.87.4.8435. [DOI] [PubMed] [Google Scholar]
- Urness LD, Paxton CN, Wang X, Schoenwolf GC, Mansour SL. FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev Biol. 2010;340(2):595–604. doi: 10.1016/j.ydbio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kamp M, Schuurs TA, Vos A, van der Lende TR, Konings WN, Driessen AJ. Sulfur regulation of the sulfate transporter genes sutA and sutB in Penicillium chrysogenum. Appl Environ Microbiol. 2000;66(10):4536–8. doi: 10.1128/aem.66.10.4536-4538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SM. The renal physiology of pendrin (SLC26A4) and its role in hypertension. Novartis Found Symp. 2006;273:231–9. discussion 239–43, 261–4. [PubMed] [Google Scholar]
- Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3− secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292(5):F1345–53. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular and functional characterization of the Slc26A6 anion exchanger, functional comparison to Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- Xu J, Henriksnas J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl−/HCO3− exchange and is inhibited by NH4+ Am J Physiol Cell Physiol. 2005;289(2):C493–505. doi: 10.1152/ajpcell.00030.2005. [DOI] [PubMed] [Google Scholar]
- Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, et al. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci U S A. 2008;105(46):17955–17960. doi: 10.1073/pnas.0800616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.