Abstract
Large conductance, Ca2+/voltage-sensitive K+ channels (BK channels) are well characterized, but their physiological roles, often determined through pharmacological manipulation, are less clear. Iberiotoxin is considered the “gold standard” antagonist, but cost and membrane-impermeability limit its usefulness. Economical and membrane-permeable alternatives could facilitate the study of BK channels. Thus, we characterized the effect of penitrem A, a tremorigenic mycotoxin, on BK channels and demonstrate its utility for studying vascular function in vitro and in vivo. Whole-cell currents from human embryonic kidney 293 cells transfected with hSlo α or α + β1 were blocked >95% by penitrem A (IC50 6.4 versus 64.4 nM; p < 0.05). Furthermore, penitrem A inhibited BK channels in inside-out and cell-attached patches, whereas iberiotoxin could not. Inhibitory effects of penitrem A on whole-cell K+ currents were equivalent to iberiotoxin in canine coronary smooth muscle cells. As for specificity, penitrem A had no effect on native delayed rectifier K+ currents, cloned voltage-dependent Kv1.5 channels, or native ATP-dependent KATP current. Penitrem A enhanced the sensitivity to K+-induced contraction in canine coronary arteries by 23 ± 5% (p < 0.05) and increased the blood pressure response to phenylephrine in anesthetized mice by 36 ± 11% (p < 0.05). Our data indicate that penitrem A is a useful tool for studying the role of BK channels in vascular function and is practical for cell and tissue (in vitro) studies as well as anesthetized animal (in vivo) experiments.
Introduction
Large conductance, Ca2+/voltage-sensitive channels (BK channels), composed of pore-forming α and auxiliary β1 subunits, may be key regulators of arterial tone via negative feedback (Nelson et al., 1995; Jaggar et al., 2000). Indeed, altered α or β1 expression is associated with vascular dysfunction in hypertension, diabetes, and aging (Liu et al., 1998; Brenner et al., 2000; Amberg et al., 2003; Borbouse et al., 2009). Genetic deletion of α or β1 subunits produces hypertension; however, in addition to presumed changes in total peripheral resistance, the role of adrenal BK channels to regulate aldosterone production must be considered (Sausbier et al., 2005; Grimm et al., 2009). In contrast to the original report (Brenner et al., 2000), a recent study indicated that β1 knockout mice are normotensive (Xu et al., 2011). Thus, much remains to be determined about the physiological roles of BK channels and/or compensations in response to BK channel gene deletion.
Adding complexity to our understanding of BK channel function is heterogeneity between vascular beds (Yang et al., 2009). Although BK channels seem to be extremely important in regulating cerebral vascular tone (Brayden and Nelson, 1992; Knot et al., 1998), these channels are arguably less important in skeletal muscle arteries (Kotecha and Hill, 2005; Yang et al., 2009) or coronary arteries (Rogers et al., 2006; Borbouse et al., 2010a,b). Clearly, the regulation of BK channels is extremely complex and governed by multiple endogenous signaling molecules (Hou et al., 2009). Thus, understanding the physiological roles and mechanisms of BK channel activation will require additional research using a combination of genetic models and pharmacological tools.
Since the identification and characterization of peptide toxins in scorpion venom, iberiotoxin has become the “gold standard” for pharmacological study of BK channels because of its selectivity and potency (IC50 0.25 nM) (Galvez et al., 1990). However, another study determined that the IC50 of iberiotoxin is much higher (33–371 nM) than the concentrations commonly used (Lippiat et al., 2003). In addition, iberiotoxin is prohibitively expensive for many isolated organ and whole-animal studies, and its membrane impermeability creates difficulty for cell-attached or inside-out patch-clamp experiments. It would be beneficial to identify an economical and membrane-permeable alternative to facilitate physiological and pharmacological studies of BK channels. Penitrem A is one such candidate molecule (Knaus et al., 1994) and has been used previously as an inhibitor of BK channels in smooth muscle (Cotton et al., 1997; Borbouse et al., 2009; Asano et al., 2010).
Little, however, is known about the basic pharmacological properties of penitrem A. For example, the IC50 for a block of BK channels has not been published, nor are there reports of its specificity in regard to other K+ channels. Our study was designed to characterize some pharmacological properties of penitrem A as a BK channel antagonist and demonstrate its utility in determining the physiological role of BK channels in vascular smooth muscle. Experiments were performed at the cell, tissue, and animal levels. We used patch-clamp techniques to assess the inhibitory effect, determine whether the β1 subunit influenced block, and illustrate the advantages of a membrane-permeable inhibitor. We measured the isometric tension of coronary arteries and the blood pressure of anesthetized mice to determine whether penitrem A altered vascular reactivity in vitro or in vivo. We conclude that penitrem A, compared with iberiotoxin, is an antagonist of BK channels that is comparable in potency and efficacy, but is more useful and less expensive.
Materials and Methods
Animal Models.
All animal procedures and protocols were approved by institutional committees and followed guidelines set forth in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011). Male C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) 10 to 12 weeks of age were used for in vivo blood pressure experiments. As described below, mice were anesthetized to a surgical plane of anesthesia before experiments and euthanized thereafter. Coronary arteries from male mongrel dogs were used for in vitro isometric tension experiments as described previously (Rogers et al., 2006). In brief, anesthetized dogs were used for unrelated experiments involving a thoracotomy; the heart was fibrillated and removed to isolate coronary arteries. For patch-clamp experiments on native vascular smooth muscle cells, arteries were obtained from a variety of different species (rat, mouse, dog, and pig). Arteries (cerebral, coronary, femoral, and aorta) were taken from animals euthanized after unrelated experiments.
Cell Culture and Transfection.
HEK 293 cells (Agilent Technologies, Santa Clara, CA) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, and culture flasks were incubated in a 5% CO2 incubator at 37°C. Plasmids encoding hSlo α and hSlo α + β1 were kindly provided by Dr. Jonathan Lippiat (University of Leeds, Leeds, UK) (Lippiat et al., 2003), and hKV1.5 plasmid was a generous gift from Dr. Jeffrey R. Martens (University of Michigan, Ann Arbor, MI) (McEwen et al., 2007). Cells were transiently transfected with pIRES-hSloα or pIRES-hSloαβ1 and pmaxGFP (AMAXA, Gaithersburg, MD) using Lipofectamine LTX with PLUS reagent (Invitrogen, Carlsbad, CA). Cells at 50 to 70% confluence in 35-mm dishes were transfected with 0.5 to 2.5 μg of DNA, and currents were recorded from green fluorescent protein-positive cells 1 to 3 days later. Transfected cells were selected in media supplemented with 0.5 mg/ml G418 (Invitrogen), 1% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Electrophysiology.
BK channel currents were recorded at room temperature from inside-out and whole-cell patches as described previously (Asano et al., 2010). The bath flowed ∼2 to 3 ml/min into a ∼0.2- to 0.3-ml chamber throughout the recordings. For whole-cell recordings, bath solution contained 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, and 5 mM Tris, pH 7.4. Pipette solution contained 140 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM HEPES, 1 mM Mg-ATP, 0.1 mM Na-GTP, and 5 mM Tris, pH 7.1. Inside-out recordings were made in symmetrical 140 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM HEPES, and 5 mM Tris, pH 7.1 (pCa 7; Maxchelator; http://www.stanford.edu/∼cpatton/maxc.html). Stock solutions of penitrem A (MP Biomedicals, Solon, OH) were prepared in dimethyl sulfoxide and diluted 1:10,000 for experiments. Iberiotoxin (AnaSpec, Inc., San Jose, CA) was dissolved in water and diluted 1:1000 for experiments.
Isometric Tension Studies.
Isometric tension was measured from segments of canine left circumflex coronary arteries as described previously (Rogers et al., 2006). Arteries were denuded of endothelium, and the lack of a relaxant response to acetylcholine (10 μM) in precontracted arterial tissue confirmed the functional loss of endothelium. For K+ concentration-response experiments, KCl was added cumulatively in the presence or absence of 1 μM penitrem A.
Blood Pressure Measurements.
Mean arterial pressure was measured as described previously (Ohanyan et al., 2011). Mice were anesthetized with sevoflurane gas (supplemented with O2) and placed on a heating table to maintain body temperature at 37°C. A polyethylene catheter was placed into the jugular vein for bolus administrations of heparin (50 U/ml) and hexamethonium (5 mg/kg), a ganglionic blocker. A micro-tip transducer (Millar Instruments Inc., Houston, TX) was placed in the femoral artery to measure mean arterial pressure and heart rate. Data were recorded with a Power Lab acquisition system (ADInstruments, Colorado Springs, CO). Phenylephrine (0.5 μg/kg/min) and penitrem A (100 μg/kg/min) were administered intravenously. Stock solutions of penitrem A were dissolved in ethanol and subsequently diluted in lactated Ringer's solution; phenylephrine was diluted in lactated Ringer's solution.
Statistics.
Data are presented as the mean and S.E. of the number of samples (e.g., number of patches, cells, dogs, or mice) as described in the text and figure legends. When two values were compared, paired or unpaired t tests were used as appropriate. When three values were compared, one-way analysis of variance (ANOVA) was used. Current-voltage relationships and concentration-response curves were analyzed by two-way ANOVA. Bonferroni post hoc tests followed ANOVA to determine differences. In all tests, p < 0.05 was considered significant.
Results
Penitrem A Inhibits BK Channels in a Concentration-Dependent Manner: Influence of the β1 Subunit.
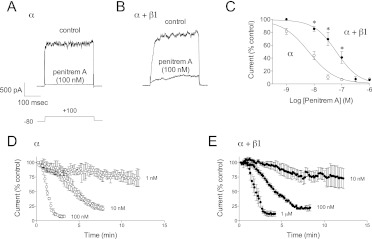
Whole-cell patch-clamp techniques were used to determine the effect of penitrem A on cloned BK channels (α or α + β1) expressed in HEK 293 cells. Currents at +100 mV were recorded under control conditions (in the presence of vehicle; 0.01% dimethyl sulfoxide) and after addition of penitrem A (Fig. 1, A and B). In cells expressing BK channels composed solely of α subunits, inhibition of current by 100 nM penitrem A was 93 ± 1% (n = 13 cells; Fig. 1, A and C). In contrast, when BK channels contained the regulatory β1 subunit 100 nM penitrem A blocked current 59 ± 9% (n = 9 cells; Fig. 1, B and C). Over several log orders of penitrem A concentrations, BK channels composed of α subunits alone were more sensitive to block than BK channels containing the β1 subunit (Fig. 1C). The half-maximal inhibitory concentration (IC50) of penitrem A for BK α was 6.4 nM (log IC50 = −8.20 ± 0.08; n = 7–16 cells). The IC50 of penitrem A on BK α + β1 subunits was 64.4 nM (log IC50 = −7.19 ± 0.07; n = 4–15 cells; p < 0.05). Hill slopes were equivalent (−0.91 ± 0.12 versus −1.07 ± 0.15). Penitrem A-induced block of whole-cell current seemed to be slower for channels containing the β1 subunit (Fig. 1, D and E; note concentration differences).
Fig. 1.
Presence of the β1 subunit decreases the sensitivity of BK channels to penitrem. A and B, representative traces of BK current at +100 mV in HEK 293 cells transfected with hSlo α (A) or hSlo α + β1 (B). Whole-cell current was measured before (control) and after the addition of penitrem A (100 nM). C, group data (n = 4–16 cells at each concentration) show the effect of BK channel subunit composition on sensitivity to block by penitrem A. *, p < 0.05 between the two groups as determined by two-way ANOVA. D and E, group data (n = 3–9) show time- and concentration-dependent block of BK α (D) and α + β1 (E) by penitrem A. Note that compared with α a 10× higher concentration of penitrem A is required to achieve a similar time-dependent block in α + β1.
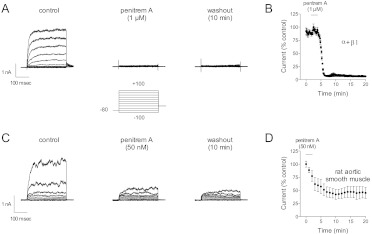
The inhibitory effect of penitrem A on BK channel current was, for all practical purposes, irreversible (Fig. 2). When current in cells expressing BK channels composed of α + β1 subunits was inhibited by 1 μM penitrem A 10 or more min of washing was an insufficient time for appreciable reversal (Fig. 2A). After 2 min of penitrem A (1 μM) exposure current was inhibited 91 ± 2% (n = 5; Fig. 2B). Cells were studied in a ∼0.2- to 0.3-ml chamber that was perfused at ∼2 to 3 ml/min; however, washing cells for 14 min with penitrem A-free bath solution did not allow BK current to recover (Fig. 2B; current at the 20-min mark was only 6 ± 2% of the control level). Similar irreversibility was observed in native smooth muscle cells (e.g., rat aorta) with lower concentrations of penitrem A (e.g., 50 nM; Fig. 2, C and D).
Fig. 2.
Irreversible block of BK channels by penitrem A. A, current traces are shown from a representative HEK 293 cell expressing BK channels composed of α + β1 subunits. The voltage template is shown below the current. Penitrem A (1 μM) abolished current within 1 to 2 min; however, 10 min of washing in penitrem A-free bath solution was not sufficient time for the current to recover appreciably. B, group data (n = 5 cells) illustrate the practical irreversibility of a 2-min exposure to penitrem A; BK current at +100 mV is plotted versus time. C, current traces are shown from a representative rat aortic smooth muscle cell. The voltage template was the same as in A and B. D, penitrem A (50 nM) reduced native BK current and was practically irreversible (n = 5).
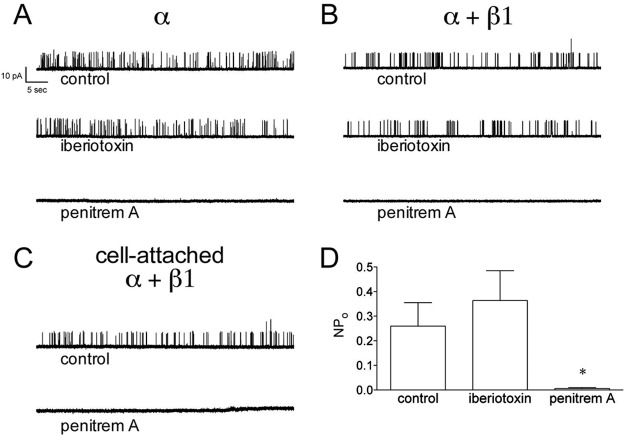
Penitrem A, but Not Iberiotoxin, Inhibits BK Channels in Inside-Out Patches.
Iberiotoxin blocks BK channels by “plugging” the pore from the extracellular side (Giangiacomo et al., 1992); therefore, one cannot use bath application of iberiotoxin to block BK channels in cell-attached or inside-out patches. A common strategy to overcome this limitation is to backfill patch pipettes with iberiotoxin, thus allowing some time for control measurements while iberiotoxin diffuses to the tip. A membrane-permeable BK channel antagonist could strengthen experimental design and allow more time for control measurements and/or interventions before BK channel block. We compared the effects of iberiotoxin (100 nM) and penitrem A (100 nM) on BK channels in cell-attached and inside-out patches (Fig. 3). As expected, bath application of iberiotoxin to inside-out patches had no inhibitory effect on BK channel activity. In contrast, bath application of penitrem A inhibited BK channel activity in inside-out patches. Mean NPo of BK α alone under control conditions, with iberiotoxin, and with penitrem A was 0.077 ± 0.040, 0.103 ± 0.076, and 0.002 ± 0.001, respectively (p < 0.05 for penitrem A; n = 7–10). Likewise, mean NPo of BK α + β1 under control conditions, with iberiotoxin, and with penitrem A was 0.401 ± 0.158, 0.564 ± 0.235, and 0.015 ± 0.014, respectively (p < 0.05 for penitrem A; n = 7–13). Like the whole-cell recordings, BK channel block by penitrem A was not readily reversible with extensive washing (data not shown). In contrast to the whole-cell recordings where 100 nM penitrem A inhibited BK α + β1 by approximately 60% (Fig. 1), 100 nM penitrem A inhibited BK α + β1 in inside-out patches by 97 ± 1%. This increased inhibitory effect at the cytoplasmic face was also noted in the original description of penitrem A (Knaus et al., 1994).
Fig. 3.
Penitrem A blocks BK channels in inside-out and cell-attached patches. A and B, recordings of channel activity from representative inside-out patches are shown for BK α (A) or α + β1 (B). Patch potential was +40 mV, and solutions were symmetrical 140 mM K+ with 100 nM free Ca2+. Channel activity was recorded under control conditions and with bath application of iberiotoxin (100 nM) or penitrem A (100 nM). C, penitrem A blocks BK channel (α + β1) activity in a cell-attached patch. Channel activity was recorded under control conditions and with bath application of penitrem A (100 nM). D, group data (n = 23 patches) show that the exposure of the cytosolic side of inside-out patches to penitrem A, but not iberiotoxin, inhibits BK channel activity. *, p < 0.05 versus control by one-way ANOVA.
Specificity of Penitrem A: Block of BK, but Not KV or KATP Channels.
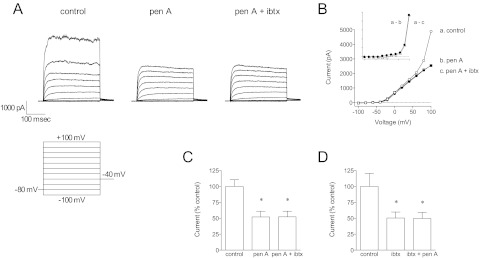
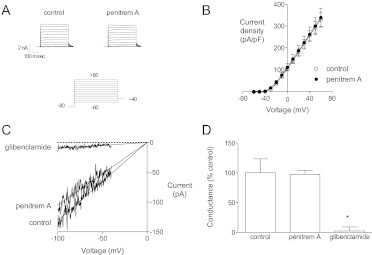
The effect of penitrem A to inhibit K+ current in coronary artery smooth muscle cells is equivalent to iberiotoxin (Fig. 4). In coronary myocytes, penitrem A (300 nM) inhibited BK current significantly, and the remaining current seemed to be mediated by KV channels (Fig. 4, A and B). After penitrem A treatment, iberiotoxin (100 nM) had no further inhibitory effect on current at +100 mV (Fig. 4C); when iberiotoxin was added first, penitrem A had no further inhibitory effect (Fig. 4D). It is unclear what KV channel types mediate the delayed rectifier K+ currents in smooth muscle, but KV1.5 has been suggested as a component (Chen et al., 2006; Dick et al., 2008). Thus, we determined whether penitrem A could inhibit cloned KV1.5 channels (Fig. 5, A and B). Whole-cell recordings of KV1.5 current were made from HEK 293 cells transfected with hKV1.5 plasmid (McEwen et al., 2007). Penitrem A (1 μM) had no effect on KV1.5 channels; current at +60 mV in the presence of penitrem A was 105 ± 6% of control (n = 5). We tested the effect of penitrem A on total outward K+ current in a variety of other native vascular smooth muscle cells (Supplemental Fig. 1). In each species and tissue penitrem A (1 μM) inhibited BK current (i.e., strongly outwardly rectifying current at positive potentials) and seemed to have little or no effect on native delayed rectifier channels.
Fig. 4.
Iberiotoxin and penitrem A block the same current in canine coronary smooth muscle cells. A, families of current traces from a representative dog coronary artery smooth muscle cell are shown under control conditions, with penitrem A (300 nM) and iberiotoxin (100 nM). These experiments were performed with a bath solution containing 0.01% bovine serum albumin to limit nonspecific binding of iberiotoxin to plastic. B, the current-voltage relationships from traces in A are shown. Penitrem A and iberiotoxin block the same current (inset, y scale is halved). C, group data (cells from n = 5 dogs) demonstrate that penitrem A blocks iberiotoxin-sensitive current; current at +100 mV is shown. D, iberiotoxin blocks penitrem A-sensitive current at +100 mV. *, p < 0.05 versus control by one-way ANOVA.
Fig. 5.
Penitrem A has no effect on KV1.5 or KATP channels. A, whole-cell recordings were made from HEK 293 cells transfected with hKV1.5. Representative KV1.5 currents in the absence or presence of 1 μM penitrem A are shown. B, group data (n = 5 cells) show that penitrem A (1 μM) has no effect on KV1.5 current. C, whole-cell recordings were made from mouse aortic smooth muscle cells. Cells were dialyzed with an ATP-free pipette solution to activate KATP current in symmetrical 140 mM K+. Current was recorded in response to voltage ramps from −100 to +100 mV under control conditions, with the addition of 1 μM penitrem A and 10 μM glibenclamide. Linear inward KATP current between −100 and −40 mV is shown. D, group data (n = 7 cells) show that glibenclamide, but not penitrem A, inhibits KATP channels in smooth muscle. *, p < 0.05 versus control by one-way ANOVA.
KATP channels are another major type of K+ channel in smooth muscle (Dart and Standen, 1995); therefore, we determined whether penitrem A could inhibit KATP channels. To activate KATP channels, we dialyzed murine aortic smooth muscle cells with ATP-free pipette solution. Membrane potential was ramped from −100 to +100 mV in symmetrical 140 mM K+. Current between −100 and −40 mV was linear, K+-selective, ATP-dependent, and glibenclamide-sensitive (Supplemental Fig. 2). We did not use any pharmacological KATP channel opener, because some of these drugs have been reported to activate BK channels (Gelband and McCullough, 1993). Glibenclamide (10 μM), but not penitrem A (1 μM), inhibited KATP channels in mouse aortic smooth muscle cells (Fig. 5C). In the presence of penitrem A KATP conductance was 98 ± 7% of control (n = 7; Fig. 5D).
Penitrem A Augments Smooth Muscle Contractility In Vitro and In Vivo.
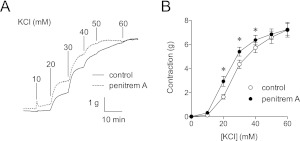
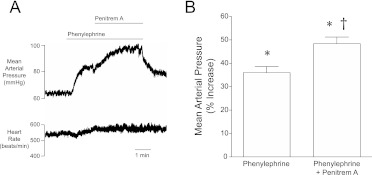
To test penitrem A as a functional inhibitor of smooth muscle BK channels in vitro, K+ concentration-response curves were constructed for canine coronary arteries in the presence or absence of 1 μM penitrem A. Inhibition of BK channels produced a leftward shift in the K+ concentration-response curve (Fig. 6). Contractions with 20, 30, and 40 mM K+ were increased 79 ± 12, 26 ± 5, and 12 ± 4%, respectively, by blocking BK channels with 1 μM penitrem A (p < 0.05; arteries from eight dogs). To test penitrem A as a functional inhibitor of smooth muscle BK channels in vivo blood pressure responses to phenylephrine, an α1 adrenergic agonist, were measured in anesthetized mice. Hexamethonium (5 mg/kg i.v.), a nicotinic receptor antagonist, was used to eliminate reflex responses to blood pressure changes. Phenylephrine (0.5 μg/kg/min i.v.) increased blood pressure to a steady-state level, and then addition of penitrem A (100 μg/kg/min i.v.) increased blood pressure further (Fig. 7A). In five hexamethonium-treated mice (Fig. 7B) phenylephrine increased mean blood pressure from 57 ± 2 to 77 ± 1 mm Hg (p < 0.05), whereas the addition of penitrem A further increased blood pressure to 84 ± 3 mm Hg (p < 0.05 versus control and phenylephrine).
Fig. 6.
Block of BK channels by penitrem A enhances K+-induced contraction. A, representative isometric contractions in canine coronary arteries are shown. K+-induced contractions were measured in the presence and absence of penitrem A (1 μM). BK channel block produced a leftward shift in the K+ concentration-response curve. B, group data (rings from n = 8 dogs) show that penitrem A enhanced sensitivity to K+-induced contraction. *, p < 0.05 versus control by two-way ANOVA.
Fig. 7.
Block of BK channels by penitrem A augments phenylephrine-induced blood pressure responses in mice. A, mean arterial pressure and heart rate are shown for a representative mouse. The mouse received phenylephrine (0.5 μg/kg/min i.v.), which increased pressure. There was no reflex change in heart rate, because the ganglionic blocker hexamethonium (5 mg/kg i.v.) was injected before the experiment. Penitrem A (100 μg/kg/min i.v.) was added and increased pressure further. B, group data (n = 5 mice) illustrate the effect of penitrem A to augment phenylephrine-induced pressor responses. * and † indicate significant difference (p < 0.05) from control and phenylephrine, respectively, by one-way ANOVA.
Discussion
The objective of this study was to better characterize penitrem A as an inhibitor of BK channels (Knaus et al., 1994). Penitrem A has been used previously as an inhibitor of BK channels in smooth muscle (Cotton et al., 1997; Borbouse et al., 2009; Asano et al., 2010). It is noteworthy, however, that the use of penitrem A as a BK channel antagonist has been without a complete understanding of its pharmacological properties. For example, the IC50 was not known, and it was unclear whether BK channel subunit composition might affect the block. We show that penitrem A inhibits BK α and α + β1 with IC50 values of 6.4 and 64.4 nM, respectively (Fig. 1). In addition, the use of penitrem A to study BK channels has been, until recently (Borbouse et al., 2009, 2010a,b), limited to in vitro studies, particularly patch-clamp experiments. Thus, we further investigated penitrem A as a BK channel inhibitor to assess vascular function in vitro and in vivo. We show that penitrem A enhances smooth muscle contraction in vitro (Fig. 6) and increases total peripheral resistance in vivo (Fig. 7). Information regarding the specificity of penitrem A against other ion channels was limited to Ca2+-activated Cl− channels (Sones et al., 2009), where it had very little effect. Here, we demonstrate no effect of penitrem A on native delayed rectifier, cloned KV1.5, or KATP channels (Figs. 4 and 5; Supplemental Figs. 1 and 2). Iberiotoxin and penitrem A are approximately equivalent in potency and efficacy (Fig. 4; Table 1), but penitrem A is less than 0.1% of the cost on a per-mole basis. We conclude that penitrem A is comparable with iberiotoxin in potency, efficacy, and selectivity and, further, penitrem A can be a more useful and economical alternative.
TABLE 1.
Characteristics of BK channel blockers
Numbers for Knaus et al. (1994) are estimations based on one dose (10 nM) that inhibited more than 50% of the current.
| Reference | Extracellular Block | Intracellular Block | IC50 α (or Undefined β*) | α + β1 | α + β2 | α + β3 | α + β4 | |
|---|---|---|---|---|---|---|---|---|
| nM | ||||||||
| Iberiotoxin | Galvez et al., 1990 | Yes | No | 0.25* | ||||
| Lippiat et al., 2003 | Yes | 33 | 371 | 39 | No block | |||
| Paxilline | Knaus et al., 1994 | Yes | Yes | <10* | ||||
| Sanchez and McManus, 1996 | Yes | 2 | ||||||
| Imlach et al., 2008 | Yes | 22 | 23 | 18 | ||||
| Lolitrem B | Dalziel et al., 2005 | Yes | 4 | |||||
| Imlach et al., 2008 | Yes | 4 | 5 | 2 | ||||
| Penitrem A | Knaus et al., 1994 | Yes | Yes | <10* | ||||
| This study | Yes | Yes | 6 | 64 |
BK channel subunit knockout mice have become a popular experimental model for studying smooth muscle reactivity, and a great deal of important information has been gleaned; however, this approach comes with certain limitations. For example, deletion of K+ channel genes elicits phenotypic compensations that may compromise interpretation (Nerbonne et al., 2008). Such changes may explain variable observations regarding blood pressure in BK channel subunit knockout mice. For instance, BK β1 subunit knockout mice were originally reported as hypertensive (Brenner et al., 2000; Plüger et al., 2000), but a recent study contradicted that (Xu et al., 2011). The earlier studies included acute measurements of blood pressure in conscious mice with arterial catheters, whereas the later study used radiotelemetry to monitor blood pressure continuously for 1 week. Both approaches are valid, and it remains unknown whether differences in the method of blood pressure measurement or the response to stress could explain the discrepancies; however, compensatory changes in other K+ channels or physiological mechanisms might be responsible for the observations of normal blood pressure in BK β1 subunit knockout mice. An alternative explanation is that BK channels may play a minimal role in regulating total peripheral resistance. Thus, the power of traditional pharmacological approaches to inhibit BK channels to elucidate a mechanism of action should not be underestimated, because we (Borbouse et al., 2009, 2010a,b) and others (Node et al., 1997) have demonstrated previously little, if any role, for BK channels in regulating coronary vascular tone by using penitrem A, charybdotoxin, or iberiotoxin. No comparable studies of organ blood flow are available for BK channel subunit knockout mice, probably because of the difficulty of instrumenting small animals. Studies of BK channels in regional and systemic hemodynamics might be made more readily in larger animals by using suitable pharmacological tools such as paxilline, lolitrem B, and penitrem A (Table 1). It must be kept in mind, however, that penitrem A was first described in the literature because of its tremorgenic effects (Cysewski et al., 1975; Hocking et al., 1988); therefore, its use in vivo may best be limited to anesthetized animals because of potential neurological side effects and convulsant properties.
Some characteristics of BK channel block by penitrem A are compared with those of other BK channel antagonists in Table 1. It is important to note that although experimental details such as patch potential and free Ca2+ are not identical, a fairly consistent picture emerges. Moreover, our data were generated by using the plasmids of Lippiat et al. (2003), allowing a relatively direct comparison. Penitrem A seems to be one-half to one log order more potent than iberiotoxin, whereas the presence of β1 subunits reduces sensitivity to either agent by a factor of 10. All of the pharmacological tools characterized in Table 1 block BK channels with similar efficacy (>95%). Penitrem A can inhibit BK channels whether it is applied from the intracellular or extracellular side of the membrane; this property is shared by paxilline and lolitrem B, but not iberiotoxin. We found that β1 subunits decrease BK channel sensitivity to penitrem A. This is different from paxilline or lolitrem B, which block BK α, α + β1, and α + β4 with similar potency (Imlach et al., 2008). Specificity for BK channels is another issue surrounding the available antagonists. Paxilline has been reported as an inhibitor of Ca2+-activated Cl− channels, whereas penitrem A and iberiotoxin have much less effect (Sones et al., 2009). In the original report (Knaus et al., 1994), it was mentioned that paxilline weakly blocks delayed rectifier K+ channels in mouse pancreatic β cells, but we show here that penitrem A has no effect on native delayed rectifier, cloned KV1.5, or KATP channels (Figs. 4 and 5; Supplemental Figs. 1 and 2). More studies will be necessary to determine possible off-target interactions of penitrem A; these, if any, may be expected to arise from introducing penitrem A into complex systems such as isolated organ or whole-animal experiments.
It is noteworthy, however, that the effects of penitrem A we observed in isolated arteries in vitro and on blood pressure in vivo were relatively straightforward and can be interpreted as specific effects on BK channels (Figs. 6 and 7). For example, in Fig. 6, the effect of penitrem A to increase smooth muscle contraction is consistent with what has been reported previously for iberiotoxin (Brayden and Nelson, 1992; Bratz et al., 2005). We assessed the effect of penitrem A on smooth muscle tone in vivo by measuring blood pressure in anesthetized mice (Fig. 7). C57BL/6 mice are a common background for engineered mice; therefore, these data may be a reference for those interested in using penitrem A in knockout or transgenic mice. A recent study demonstrated that lolitrem B had no effect on blood pressure, but decreased heart rate (Imlach et al., 2010). Our first impression was that these results could be explained by a reflex adjustment of heart rate, and thus cardiac output, and ultimately blood pressure, in response to an increase in total peripheral resistance. However, Imlach et al. went on to demonstrate an inhibitory effect of lolitrem B on heart rate in Langendorff-perfused hearts, where neural input is removed. The mechanisms by which lolitrem B reduces heart rate are unknown; however, it is important to note that they are not shared by penitrem A. When we used hexamethonium to block autonomic responses heart rate was unchanged during penitrem A-induced increases in blood pressure (Fig. 7).
We have demonstrated the efficacy and selectivity of penitrem A on BK channel function in vitro and in vivo; however, our study has several limitations that need to be addressed. First, penitrem A is well known to cause muscle tremor (Cysewski et al., 1975; Hocking et al., 1988), thus, the use of penitrem A to study the role of vascular BK channels might best be limited to anesthetized animals for in vivo experiments. Second, in addition to vascular smooth muscle BK channels, systemic penitrem A administration will inhibit BK channels in other cells and tissues that might affect the regulation of vascular tone and blood pressure. For instance, a recent study indicated that BK channels in astrocytes are key regulators of neurovascular coupling (Girouard et al., 2010). In addition, BK channels in endothelium may regulate membrane potential, Ca2+ influx, and the production of relaxing factors (Sandow and Grayson, 2009). Because BK channel expression is ubiquitous, in vivo experiments with penitrem A will require careful design and interpretation.
In conclusion, we have further characterized penitrem A as an inhibitor of BK channels, particularly BK α β1 channels that are generally found in smooth muscle. This information could be put to important use in determining the roles of BK channels in vascular function and serve as a complement to studies performed in BK channel subunit knockout mice. We demonstrate that penitrem A is a useful and economical alternative for studying the role of BK channels in vitro and in vivo. That is, we show that that the use of penitrem A is not limited to patch-clamp experiments, rather this agent is practical for tissue, organ, and whole-animal studies.
Supplementary Material
Acknowledgments
We thank Dr. Jonathan D. Lippiat (University of Leeds, Leeds, UK) for providing the two plasmids encoding human BK channels and Dr. Jeffrey R. Martens (University of Michigan, Ann Arbor, MI) for providing the KV1.5 plasmid.
This work was supported by a Research Funds Development Grant from West Virginia University [Grant RDG 1] and the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL092245, T32-HL090610].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- BK channel
- large conductance Ca2+/voltage-sensitive K+ channel
- KV channel
- voltage-dependent K+ channel
- KATP channel
- ATP-dependent K+ channel
- ANOVA
- analysis of variance
- HEK
- human embryonic kidney
- NPo
- total open probability.
Authorship Contributions
Participated in research design: Asano, Bratz, Tune, and Dick.
Conducted experiments: Asano, Bratz, Berwick, Fancher, and Tune.
Performed data analysis: Asano and Dick.
Wrote or contributed to the writing of the manuscript: Asano and Dick.
References
- Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. (2003) Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J Clin Invest 112:717–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano S, Tune JD, Dick GM. (2010) Bisphenol A activates Maxi-K (KCa1.1) channels in coronary smooth muscle. Br J Pharmacol 160:160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. (2009) Impaired function of coronary BKCa channels in metabolic syndrome. Am J Physiol Heart Circ Physiol 297:H1629–H1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbouse L, Dick GM, Payne GA, Berwick ZC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. (2010a) Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol 298:H1182–H1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. (2010b) Contribution of BKCa channels to local metabolic coronary vasodilation: effects of metabolic syndrome. Am J Physiol Heart Circ Physiol 298:H966–H973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratz IN, Dick GM, Partridge LD, Kanagy NL. (2005) Reduced molecular expression of K+ channel proteins in vascular smooth muscle from rats made hypertensive with Nω-nitro-l-arginine. Am J Physiol Heart Circ Physiol 289:H1277–H1283 [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. (1992) Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256:532–535 [DOI] [PubMed] [Google Scholar]
- Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. (2000) Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407:870–876 [DOI] [PubMed] [Google Scholar]
- Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. (2006) Key role of Kv1 channels in vasoregulation. Circ Res 99:53–60 [DOI] [PubMed] [Google Scholar]
- Cotton KD, Hollywood MA, McHale NG, Thornbury KD. (1997) Outward currents in smooth muscle cells isolated from sheep mesenteric lymphatics. J Physiol 503:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysewski SJ, Baetz AL, Pier AC. (1975) Penitrem A intoxication of calves: blood chemical and pathologic changes. Am J Vet Res 36:53–58 [PubMed] [Google Scholar]
- Dart C, Standen NB. (1995) Activation of ATP-dependent K+ channels by hypoxia in smooth muscle cells isolated from the pig coronary artery. J Physiol 483:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. (2008) Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol 294:H2371–H2381 [DOI] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. (1990) Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265:11083–11090 [PubMed] [Google Scholar]
- Gelband GH, McCullough JR. (1993) Modulation of rabbit aortic Ca2+-activated K+ channels by pinacidil, cromakalim, and glibenclamide. Am J Physiol Cell Physiol 264:C1119–C1127 [DOI] [PubMed] [Google Scholar]
- Giangiacomo KM, Garcia ML, McManus OB. (1992) Mechanism of iberiotoxin block of the large-conductance calcium-activated potassium channel from bovine aortic smooth muscle. Biochemistry 31:6719–6727 [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. (2010) Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A 107:3811–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. (2009) Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci U S A 106:11800–11805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking AD, Holds K, Tobin NF. (1988) Intoxication by tremorgenic mycotoxin (penitrem A) in a dog. Aust Vet J 65:82–85 [DOI] [PubMed] [Google Scholar]
- Hou S, Heinemann SH, Hoshi T. (2009) Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 24:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW, Dalziel JE. (2008) The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. J Pharmacol Exp Ther 327:657–664 [DOI] [PubMed] [Google Scholar]
- Imlach WL, Finch SC, Miller JH, Meredith AL, Dalziel JE. (2010) A role for BK channels in heart rate regulation in rodents. PLoS One 5:e8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (2011) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. (2000) Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278:C235–C256 [DOI] [PubMed] [Google Scholar]
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB., 3rd (1994) Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33:5819–5828 [DOI] [PubMed] [Google Scholar]
- Knot HJ, Standen NB, Nelson MT. (1998) Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol 508:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha N, Hill MA. (2005) Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca2+ signaling. Am J Physiol Heart Circ Physiol 289:H1326–H1334 [DOI] [PubMed] [Google Scholar]
- Lippiat JD, Standen NB, Harrow ID, Phillips SC, Davies NW. (2003) Properties of BKCa channels formed by bicistronic expression of hSloα and β1–4 subunits in HEK293 cells. J Membr Biol 192:141–148 [DOI] [PubMed] [Google Scholar]
- Liu Y, Hudetz AG, Knaus HG, Rusch NJ. (1998) Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circ Res 82:729–737 [DOI] [PubMed] [Google Scholar]
- McEwen DP, Schumacher SM, Li Q, Benson MD, Iñiguez-Lluhí JA, Van Genderen KM, Martens JR. (2007) Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem 282:29612–29620 [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. (1995) Relaxation of arterial smooth muscle by calcium sparks. Science 270:633–637 [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Gerber BR, Norris A, Burkhalter A. (2008) Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J Physiol 586:1565–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Kitakaze M, Kosaka H, Minamino T, Hori M. (1997) Bradykinin mediation of Ca2+-activated K+ channels regulates coronary blood flow in ischemic myocardium. Circulation 95:1560–1567 [DOI] [PubMed] [Google Scholar]
- Ohanyan VA, Guarini G, Thodeti CK, Talasila PK, Raman P, Haney RM, Meszaros JG, Damron DS, Bratz IN. (2011) Endothelin-mediated in vivo pressor responses following TRPV1 activation. Am J Physiol Heart Circ Physiol 301:H1135–H1142 [DOI] [PubMed] [Google Scholar]
- Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. (2000) Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res 87:E53–E60 [DOI] [PubMed] [Google Scholar]
- Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. (2006) H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol 291:H2473–H2482 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Grayson TH. (2009) Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 297:H1–H7 [DOI] [PubMed] [Google Scholar]
- Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, et al. (2005) Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation 112:60–68 [DOI] [PubMed] [Google Scholar]
- Sones WR, Leblanc N, Greenwood IA. (2009) Inhibition of vascular calcium-gated chloride currents by blockers of KCa1.1, but not by modulators of KCa2.1 or KCa2.3 channels. Br J Pharmacol 158:521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Garver H, Galligan JJ, Fink GD. (2011) Large-conductance Ca2+-activated K+ channel β1-subunit knockout mice are not hypertensive. Am J Physiol Heart Circ Physiol 300:H476–H485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, et al. (2009) Heterogeneity in function of small artery smooth muscle BKCa: involvement of the β1-subunit. J Physiol 587:3025–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.