Abstract
System xc− represents an intriguing target in attempts to understand the pathological states of the central nervous system. Also called a cystine-glutamate antiporter, system xc− typically functions by exchanging one molecule of extracellular cystine for one molecule of intracellular glutamate. Nonvesicular glutamate released during cystine-glutamate exchange activates extrasynaptic glutamate receptors in a manner that shapes synaptic activity and plasticity. These findings contribute to the intriguing possibility that extracellular glutamate is regulated by a complex network of release and reuptake mechanisms, many of which are unique to glutamate and rarely depicted in models of excitatory signaling. Because system xc− is often expressed on non-neuronal cells, the study of cystine-glutamate exchange may advance the emerging viewpoint that glia are active contributors to information processing in the brain. It is noteworthy that system xc− is at the interface between excitatory signaling and oxidative stress, because the uptake of cystine that results from cystine-glutamate exchange is critical in maintaining the levels of glutathione, a critical antioxidant. As a result of these dual functions, system xc− has been implicated in a wide array of central nervous system diseases ranging from addiction to neurodegenerative disorders to schizophrenia. In the current review, we briefly discuss the major cellular components that regulate glutamate homeostasis, including glutamate release by system xc−. This is followed by an in-depth discussion of system xc− as it relates to glutamate release, cystine transport, and glutathione synthesis. Finally, the role of system xc− is surveyed across a number of psychiatric and neurodegenerative disorders.
I. Introduction
Our limited understanding of neuroscience has contributed to the lack of well tolerated treatments for most if not all diseases of the brain and is exemplified by the large gaps in our understanding of glutamate signaling. Glutamate is often described as the primary excitatory neurotransmitter in the brain and may be present in up to 80% of all synapses (Greenamyre et al., 1988; Coyle and Puttfarcken, 1993; Moghaddam and Adams, 1998b; Tapia et al., 1999; Marino et al., 2001; Franks et al., 2002; Javitt et al., 2011), yet we are only now unmasking how the many components of this complex network of receptors, membrane-expressed enzymes, transporters, and release mechanisms function in an integrated manner to regulate the neurotransmitter actions of this amino acid.
System xc− is an example of a poorly understood source of glutamate release that may exert profound control over multiple aspects of synaptic function. Originally identified more than 30 years ago as a sodium-independent glutamate transporter (Bannai and Kitamura, 1980), system xc− seems to function as a cystine-glutamate antiporter or exchanger that couples the uptake of one molecule of cystine to the release of one molecule of glutamate (Bannai, 1986; Piani and Fontana, 1994; Lo et al., 2008; Bridges et al., 2012). As depicted in Fig. 1, nonvesicular glutamate release by system xc−, which is highly expressed by astrocytes, regulates synaptic activity by stimulating extrasynaptic receptors (Pow, 2001; Baker et al., 2002; Moran et al., 2005; Kupchik et al., 2012). The potential for system xc− to contribute to multiple aspects of neuronal function and plasticity is evident from the finding that increased cystine-glutamate exchange influences the capacity of some cells to undergo long-term potentiation and long-term depression (Moussawi et al., 2009, 2011). Not surprisingly, the rate of cystine-glutamate exchange is currently being targeted for numerous disorders of the central nervous system (CNS1), demonstrating the tremendous therapeutic potential stemming from a more complete understanding of the components of the complex, unique network regulating glutamate signaling.
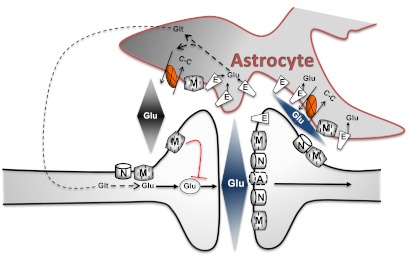
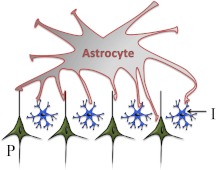
Fig. 1.
This schematic depicts an excitatory synapse that is ensheathed by astrocytic processes in an asymmetrical manner, resulting in a greater distance between glial processes and neurons on the presynaptic aspect of the synapse. Glu release in response to synaptic activation quickly reaches a concentration in the synaptic cleft capable of stimulating low-affinity AMPA receptors (A) that are located proximal to the release sites, as well as laterally distributed high-affinity receptors including NMDA (N) and mGlu receptors (M). Diffusion and clearance by excitatory amino acid transporters (E) are thought to rapidly lower the levels of extracellular glutamate in the synaptic cleft. Glutamine (Glt) is released by astrocytes and is considered to be a critical substrate needed to replenish neuronal glutamate stores. In addition to glutamine, astrocytes are also capable of releasing glutamate. The illustration depicts system xc− (X), which exchanges cystine (C-C) and glutamate on a 1:1 stoichiometry, the direction of the exchange being determined by relative substrate concentration gradients. Glutamate release from system xc−, which is also described as a cystine-glutamate exchanger or antiporter, has been shown to regulate synaptic neurotransmitter release by stimulating extrasynaptic glutamate receptors.
Although the contribution of system xc− activity to CNS diseases is the primary topic of this review, multiple nonvesicular release mechanisms may contribute to extracellular levels of glutamate (Kimelberg et al., 1990; Parpura et al., 1994; Strange et al., 1996; Bezzi et al., 1998, 2004; Basarsky et al., 1999; Araque et al., 2000; Haydon, 2001; Pasti et al., 2001; Ye et al., 2003; Kreft et al., 2004; Zhang et al., 2004). Nonvesicular release mechanisms are not typically incorporated into models of glutamate signaling (Franks et al., 2002; Rusakov et al., 2011), in part because very little is known regarding the importance of most of these mechanisms. In addition, the delayed awareness or acceptance of the potential for nonvesicular release to shape excitatory signaling can be attributed, in part, to the degree to which this network is unique to glutamate, often failing to conform to principles of neurotransmission that were largely established from the study of other neurotransmitters such as monoamines. The need to better understand these novel release mechanisms is evident from studies demonstrating that nonvesicular glutamate release has been implicated in diverse CNS functions ranging from activation of NMDA receptors to neurodevelopment (Kihara et al., 1989; Hirai et al., 1999; Jabaudon et al., 1999; Simonian and Herbison, 2001; Rothman et al., 2003; Pirttimaki et al., 2011) as well as the therapeutic potential of system xc− in the treatment of multiple CNS disorders, as reviewed below.
Beyond advancing our understanding of excitatory signaling, the study of the cellular basis of glutamate homeostasis has the potential to profoundly change how we conceptualize the brain. Through the study of glutamate release and clearance (Storck et al., 1992; Levy et al., 1993; Lehre et al., 1995), it has become evident that excitatory signaling is uniquely dependent on coordinated activity between astrocytes and neurons. This has contributed to the intriguing hypothesis that astrocytes, historically viewed as metabolic or structural support cells, are directly involved in glutamate signal transduction. Astrocytes seem to contribute to virtually every aspect of synaptic transmission, from synaptogenesis and synaptic plasticity to synaptic pruning (Ullian et al., 2001, 2004; Stellwagen and Malenka, 2006; Stevens et al., 2007; Kucukdereli et al., 2011; Paolicelli et al., 2011; Min and Nevian, 2012) for review see (Haydon, 2001; Stevens, 2008; Eroglu and Barres, 2010; Schafer and Stevens, 2010). The regulation of synaptic activity by astrocytes is accomplished through the release of a variety of neuroactive molecules, including ATP, d-serine, glutamate, hevin, kynurenic acid, secreted protein acidic and rich in cysteine, and thrombospondins (Beattie et al., 2002; Newman, 2003; Shoptaw et al., 2003; Christopherson et al., 2005; Stellwagen and Malenka, 2006; Guidetti et al., 2007; Kucukdereli et al., 2011). In turn, astrocytes monitor neuronal activity through the expression of receptors activated by virtually every major neurotransmitter including glutamate, GABA, monoamines, acetylcholine, and endocannabinoids (Bormann and Kettenmann, 1988; Sontheimer et al., 1988; Glaum et al., 1990; Pruss et al., 1991; von Blankenfeld and Kettenmann, 1991; Wyllie et al., 1991; Zanassi et al., 1999; Grybko et al., 2010; Navarrete and Araque, 2010; Shen and Yakel, 2012). Because astrocytes have primarily nonoverlapping domains and may contact more than a million synapses (in the human cortex) (Bushong et al., 2002, 2004; Ogata and Kosaka, 2002; Oberheim et al., 2006), these cells are in a position to coordinate activity on a network level. In support, activation of a single astrocyte is sufficient to produce a widespread, synchronized transition to an up-state in membrane potential for a large population of cortical neurons (Poskanzer and Yuste, 2011). As a result, the study of astrocytes, including attempts to understand the cellular regulation of glutamate signaling, has the potential to revolutionize our understanding of the CNS, a development that may expedite our efforts to discover treatments for CNS disorders (McNally et al., 2008; Bernstein et al., 2009; Haydon et al., 2009; Miguel-Hidalgo, 2009; L'Episcopo et al., 2010; Rappold and Tieu, 2010; Zhao and Rempe, 2010; Halliday and Stevens, 2011; Li et al., 2011).
The regulation of excitatory signaling by system xc− is the primary focus of this article. To do this, we begin with a broad overview of glutamate homeostasis, including descriptions of the molecular and cellular basis of glutamate uptake and release mechanisms. Subsequently, we review the importance of cystine-glutamate exchange to excitatory signaling as well as cellular antioxidant capacity. Finally, we discuss the potential for system xc− to contribute to a wide array of CNS disorders.
II. Glutamate Homeostasis
The mammalian central nervous system contains abundant glutamate, with intracellular concentrations in the millimolar range and extracellular levels reported to be between 0.2 to 7 μM outside of the synapse (Benveniste et al., 1984; Ronne-Engström et al., 1995; Baker et al., 2003). As depicted in Fig. 1, excitatory signaling requires coordinated activity between astrocytes and neurons. Glutamate released from a presynaptic terminal is ultimate cleared from the cleft through diffusion followed by active transport via sodium-dependent glutamate transporters that are primarily expressed on astrocytes (Franks et al., 2002; Rusakov et al., 2011). Replenishing neuronal stores of glutamate is thought to be dependent on the conversion of glutamate to glutamine by astrocytes, enabling the subsequent release by astrocytes and uptake of glutamine by neurons (Tamarappoo et al., 1997; Bröer and Brookes, 2001; Kanamori and Ross, 2004).
Disruptions in glutamate homeostasis have been linked to numerous diseased states of the brain and can lead to widespread changes in synaptic activity. An example of this is cocaine addiction, which is associated with persistent changes in both the clearance and release of glutamate in the nucleus accumbens. It is noteworthy that efforts to restore glutamate homeostasis in the nucleus accumbens not only reduce compulsive drug seeking or craving in rodent and man but also have led to the normalization of a diverse set of indicators of neuronal function, ranging from long-term potentiation to dendritic spine morphology (Baker et al., 2003; LaRowe et al., 2006; Moussawi et al., 2009; Knackstedt et al., 2010). These studies complement the work by others indicating that basal, extrasynaptic glutamate or nonvesicular glutamate release contributes to receptor activation, receptor clustering, receptor trafficking, receptor desensitization, and neurodevelopment (Jabaudon et al., 1999; Featherstone et al., 2002; Xi et al., 2002b; Manent et al., 2005; Moran et al., 2005; Augustin et al., 2007; Pendyam et al., 2009; Moussawi et al., 2011). As a result, a key to understanding the pathogenesis of disorders of the brain will rely on a more complete characterization of the individual cellular process of release and uptake, as well as how their integrated function influences glutamate homeostasis.
A. Glutamate Synapse
The synaptic cleft, estimated at only 1 to 2% of the extracellular space (Rusakov et al., 1998), has long been the near-singular focus of our attempts to understand excitatory signaling. In the past decade, there has been a tremendous increase in our understanding of the glutamate synapse, the complexity of which is perhaps best reflected by a number of excellent models of glutamate signaling (Rusakov and Kullmann, 1998; Rusakov, 2001, 2011; Franks et al., 2002; Lehre and Rusakov, 2002; Rusakov and Lehre, 2002; Franks et al., 2003; Raghavachari and Lisman, 2004; Zheng et al., 2008). The initial step involving the fusion of the presynaptic membrane with one or more synaptic vesicles results in the release of 3000 to 10,000 molecules of glutamate per action potential (Clements, 1996; Barbour and Häusser, 1997; Pendyam et al., 2009), resulting in a concentration of glutamate in the synaptic cleft of approximately 0.5 to 1.0 mM (Rusakov, 2001). At an estimated distance of 20 nm (Franks et al., 2003), the postsynaptic membrane expresses metabotropic and ionotropic glutamate receptors, the latter estimated to number fewer than 100 in a typical synapse (Nusser et al., 1998; Takumi et al., 1999). The distribution of glutamate receptors is highly organized, reflecting, in part, relative affinities for glutamate. Low-affinity α-amino-3-hydroxy-5-methyl-4-isoazole-proprionic acid (AMPA) receptors are expressed on the closest aspect of the postsynaptic membrane relative to the release sites, high-affinity N-methyl-d-aspartic acid (NMDA) and metabotropic glutamate receptors being expressed more laterally (Fig. 1). This organization of glutamate receptors is required, in part because an estimated 50 to 90% of the 3000 to 10,000 glutamate molecules exit the cleft within 10 to 70 ms (Savtchenko and Rusakov, 2004); thus, the concentration of glutamate falls rapidly across temporal and spatial dimensions. Of course, activation of low-affinity AMPA receptors is more sensitive to spatiotemporal regulation than that of high-affinity glutamate receptors, such that lateral trafficking of AMPA receptors represents a key form of synaptic plasticity influencing synaptic strength (for review, see Rusakov et al., 2011). Moreover, the two types of ionotropic receptors are differentially regulated by glutamate clearance mechanisms.
B. Glutamate Clearance
The uptake of glutamate is primarily achieved by a family of sodium-dependent excitatory amino acid transporters (EAAT), of which five (EAAT1–5) have been cloned. The translocation of one glutamate molecule by EAATs is coupled to the influx of three molecules of Na2+ and one molecule of H+ into the cell and the efflux of one molecule of K+ (Zerangue and Kavanaugh, 1996b; Levy et al., 1998; Owe et al., 2006). The cotransport of cations permits the generation of the steep glutamate concentration gradients that exist in neurons and astrocytes and may serve as a signaling pathway in astrocytes (Langer and Rose, 2009). For example, synaptic activity leading to increased glutamate uptake produces sodium transients in astrocytes that have been linked to glycolysis (Pellerin and Magistretti, 1994; Takahashi et al., 1995) and changes in the surface expression of EAAT2 (Nakagawa et al., 2008).
EAATs exhibit a high capacity for clearing glutamate, thereby effectively buffering extracellular concentrations of glutamate. This is possible because glutamate transporters exhibit a surface density that is comparable with the number of glutamate molecules released after an action potential. Specifically, the surface density of glutamate transporters is approximately 10,000 transporters/μm2 (Lehre and Danbolt, 1998), and the average central synapse is capable of releasing two vesicles per second, each vesicle containing an estimated 4000 to 5000 molecules of glutamate (Clements, 1996; Barbour and Häusser, 1997; Pendyam et al., 2009). With an uptake capacity of 10 μM · ms−1, there is abundant EAAT clearance capacity to prevent glutamate transporters from being overwhelmed by even high rates of excitatory activit,y which can serve to limit glutamate diffusion to distal synapses (Asztely et al., 1997; Diamond and Jahr, 2000; Zheng et al., 2008).
An unresolved question is the degree to which EAATs provide temporal resolution, especially for the estimated 50 to 100 ionotropic receptors in the synapse (Nusser et al., 1998; Takumi et al., 1999). Studies examining the impact of EAAT blockade on ionotropic receptor activation have generated mixed results, at least some studies reporting an impact on NMDA or AMPA-mediated currents (for review, see Tzingounis and Wadiche, 2007; Tong and Jahr, 1994; Carter and Regehr, 2000; Diamond and Jahr, 2000, although see Hestrin et al., 1990; Isaacson and Nicoll, 1993; Sarantis et al., 1993). Although glutamate binding to EAATs occurs very rapidly (Diamond and Jahr, 1997), glutamate translocation may take 70 ms (Wadiche et al., 1995), which is equivalent to the estimated time needed for 90% of glutamate to diffuse out of some synaptic clefts even in the absence of EAAT activity (Savtchenko and Rusakov, 2004). These data indicate that diffusion may be the primary glutamate clearance mechanism in the synaptic cleft. Striking similarities exist for GABA, at least some studies demonstrating that diffusion is the primary synaptic clearance mechanism for this key amino acid transporter and that GABA transporters function to limit spillover into distinct signaling compartments (e.g., neighboring synapses) (Jensen et al., 2003; Overstreet and Westbrook, 2003; Gonzalez-Burgos et al., 2009).
The geometry of the synapse may be an important factor determining the extent of EAAT regulation of receptor activation patterns in the synapse (Bergles and Jahr, 1997), especially as it relates to the spatial relationship between astrocytes and the synaptic cleft (Sarantis and Mobbs, 1992). Although synapses are often described as being ensheathed by glial processes, the degree to which this occurs varies greatly. In the hippocampus, only 57% of synapses are apposed to astrocytic processes; even then, astrocytes contacted on average only 43% of the synapse (Ventura and Harris, 1999). It is noteworthy that glial contact seems to favor the postsynaptic aspects of a synapse, thereby differentially regulating the activation of extrasynaptic receptors expressed on post- versus presynaptic cells (Lehre and Rusakov, 2002). However, the spatial relationships between astrocytes and neurons are dynamic (Langle et al., 2002; Panatier et al., 2006; Lushnikova et al., 2009). Thus, the conditions dictating physical contact between astrocytes and neurons will have profound implications for multiple aspects of synaptic activity, including excitatory signaling in the normal and diseased state.
In some circumstances, EAATs may transiently increase extracellular glutamate concentrations in extracellular space neighboring the transporters because the translocation probability is approximately 50%. In other words, EAATs may limit diffusion by rapidly binding and releasing glutamate at a probability equivalent to the actual transport of the molecule into the cell. The degree to which this may contribute to the activation of receptors proximal to the transporters would depend on a number of factors, including the distance between receptors and transporters, the probability of transport after molecule unbinding—which may be very high (Leary et al., 2011)—and the affinity of receptors neighboring glutamate transporters. Unlike the potential modest effects on AMPA receptors, transporter inhibition produces a profound effect of the activation of metabotropic glutamate receptors (Scanziani et al., 1997; Semyanov and Kullmann, 2000; Brasnjo and Otis, 2001), which are often located extrasynaptically. A similar phenomenon may also contribute to activation of NMDA receptors in that inhibition of EAATS has been shown to increase activation of extrasynaptic NMDA receptors (Mulholland and Chandler, 2010).
A critical function of EAATs is to spatially isolate the glutamate signal, which is typically thought of in terms of limiting diffusion across multiple synapses. Although not fully incorporated in most models of glutamate signaling, astrocytes and neurons express multiple cellular mechanisms capable of releasing glutamate (Blakely et al., 1988; Kimelberg et al., 1990; Levi and Raiteri, 1993; Parpura et al., 1994; Strange et al., 1996; Basarsky et al., 1999; Parpura and Haydon, 2000; Baker et al., 2002; Ye et al., 2003; Zhou et al., 2005; Malarkey and Parpura, 2008; Vyleta and Smith, 2011). Furthermore, studies demonstrating 1) regulation of synaptic activity by nonvesicular glutamate after inhibition of glutamate transporters or 2) selective activation of extrasynaptic receptors indicate an extrasynaptic location for at least some of the release mechanisms (Jabaudon et al., 1999; Moran et al., 2005; Mulholland and Chandler, 2010). Thus, spatial resolution provided by EAATs may also involve regulating the diffusion of glutamate into the synapse, not only from distal synapses but also from extrasynaptic release sources as depicted in Fig. 2. In support of such an idea, constitutive activation of NMDA receptors by nonvesicular release has been detected after inhibition of EAAT function (Jabaudon et al., 1999; Angulo et al., 2004; Cavelier and Attwell, 2005), posing the scenario that nonvesicular release of glutamate into the extrasynaptic compartment is incapable of stimulating high-affinity glutamate receptors in the synapse unless EAAT function is compromised. One such mechanism of nonvesicular release involves the exchange of an extracellular molecule of cystine for an intracellular molecule of glutamate by system xc−, which has been shown to be a key determinant of the activation of extrasynaptic group II metabotropic glutamate receptors (mGluRsII). EAATs have been shown to clear glutamate released by system xc−, supporting the idea that these transporters function to compartmentalize extracellular glutamate into multiple domains (Baker et al., 2002; Melendez et al., 2005).
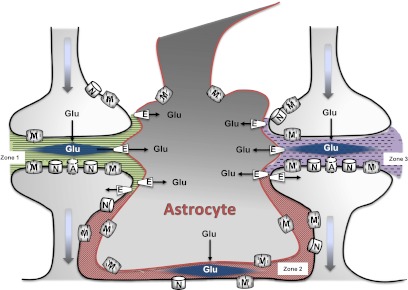
Fig. 2.
This illustration depicts extracellular Glu that is compartmentalized into distinct microdomains. In synapses ensheathed by astrocytes, excitatory amino acid transporters (E) are thought to limit overflow of glutamate and thereby inhibit cross-talk between neighboring synapses (depicted as zone 1 and zone 3) even under periods of high neuronal activity. This provides spatial resolution by restricting the source of glutamate capable of stimulating NMDA (N), AMPA (A), and metabotropic (M) receptors. In addition to the cleft, the extrasynaptic space separating synapses (zone 2) may be a critical site for glutamatergic signaling. Within this space, glutamate transporters, astrocytic glutamate release mechanisms, and functional extrasynaptic receptors are expressed. This supports the intriguing scenario that multiple signaling microdomains may separate neighboring synapses.
C. Glutamate Release
Extracellular glutamate is present in vivo in micromolar concentrations outside the synapse. Although vesicular glutamate release into the synapse has been shown to contribute to basal extrasynaptic glutamate levels in the striatum (Newcomb and Palma, 1994; You et al., 1994), alternative release mechanisms independent of voltage-gated calcium or sodium channels seem to be the primary sources of extrasynaptic glutamate (Bradford et al., 1987; Miele et al., 1996; Timmerman and Westerink, 1997; Baker et al., 2002). Such mechanisms include vesicular release from astrocytes (Parpura et al., 1994; Bezzi et al., 1998, 2004; Araque et al., 2000; Haydon, 2001; Pasti et al., 2001; Kreft et al., 2004; Zhang et al., 2004) as well as nonvesicular release from cystine-glutamate exchange (Bannai et al., 1986; Murphy et al., 1990; Warr et al., 1999; Moran et al., 2005), astrocytic hemichannels (Ye et al., 2003), and volume-sensitive organic anion channels (Kimelberg et al., 1990; Strange et al., 1996; Basarsky et al., 1999). In addition to release, extracellular glutamate levels may also accumulate after hydrolysis of N-acetylaspartylglutamate (Blakely et al., 1988; Zhou et al., 2005) or glutamine (Mena et al., 2005).
The study of glutamate release mechanisms has been instrumental in recent efforts to characterize astrocytes as cells critical to information processing in the brain rather than cells simply involved in metabolic or structural support. An initial step in this realization was the discovery that astrocytes are capable of glutamate release involving exocytosis (for review, see Haydon, 2001; Newman, 2003). Vesicles capable of uptake, storage, and release of glutamate have been identified in hippocampal astrocytes (Bezzi et al., 2004; Kreft et al., 2004; Zhang et al., 2004). The loading of these vesicles involves the activity of vesicular glutamate transporters; the fusion of these vesicles to the plasma membrane has been shown to be calcium-dependent. Unlike neurons, the process is dependent not on the opening of voltage-gated calcium channels but instead on inositol trisphosphate-mediated calcium release from endoplasmic reticulum after activation of a variety of G-coupled receptors (Volterra and Meldolesi, 2005). Receptors that may be capable of elevating intracellular levels of calcium expressed by astrocytes include metabotropic glutamate, muscarinic, histamine, substance P, and noradrenergic receptors (for review, see Verkhratsky et al., 1998).
The importance of astrocytes as signaling cells was also aided by studies linking nonvesicular release by system xc− with CNS activity in the normal and diseased states. As described below, system xc− typically mediates the stoichiometric exchange of extracellular cystine for intracellular glutamate. This process significantly contributes to the concentration of extracellular glutamate, at least in the striatum, where it has been shown to contribute up to 60% of the 1 to 3 μM glutamate existing outside of the synapse (Baker et al., 2002).
Given the extrasynaptic location of high-affinity glutamate receptors, including NMDA and metabotropic glutamate receptors (Alagarsamy et al., 2001; Tamaru et al., 2001), extrasynaptic glutamate can activate mechanisms capable of regulating synaptic activity (Figs. 1 and 2). One route would involve the stimulation of high-affinity group II/III metabotropic glutamate receptors, which are negatively coupled to synaptic release of glutamate or other neurotransmitters such as dopamine (Baskys and Malenka, 1991; Cochilla and Alford, 1998; Hu et al., 1999b; Baker et al., 2002; Xi et al., 2002a; Moran et al., 2005). Alternatively, activation of group I metabotropic glutamate or NMDA receptors also occurs after glutamate release from astrocytes (Parpura et al., 1994; Araque et al., 1998; Parpura and Haydon, 2000; D'Ascenzo et al., 2007). As a result, glutamate release from astrocytes may potentially contribute to CNS disease states linked to altered glutamate signaling. System xc− is the primary subject of this review, in part because there are a number of excellent reviews of glutamate release from astrocytes involving other mechanisms (Haydon, 2001; Zhou et al., 2005; Montana et al., 2006; Bergersen and Gundersen, 2009; Hamilton and Attwell, 2010).
III. Cystine Transport
A. System xc−
Although the action of system xc− as an antiporter/exchanger is well recognized today, it was first identified and functionally characterized in mammalian cells as a cystine transporter (Bannai and Kitamura, 1980). Given the very low levels of cysteine found in plasma and cerebrospinal fluid, its sensitivity to oxidation, and a limited capacity to synthesize l-cysteine from methionine via the transsulfuration pathway (McBean, 2012), the transport of l-cystine represents the primary delivery mechanism for this vital sulfur-containing amino acid. Early studies, most often carried out in culture with non-CNS cells, established the defining properties of system xc− (Bannai and Kitamura, 1980; Bannai and Ishii, 1982; Bannai, 1986). Most important was the observation that l-glutamate was not only a competitive inhibitor of cystine uptake by system xc− but that the dicarboxylic amino acid also served equally well as a substrate. The Km values for the uptake of either cystine or glutamate were typically in the 50 to 100 μM range, with each capable of acting as a competitive inhibitor of the other. This was central to establishing that system xc− was indeed an antiporter that mediated a 1:1 exchange of an intracellular and extracellular amino acid, with the directionality of the exchange dictated by the relative concentration gradients of the substrates across the cell membrane. Given that the intracellular concentration of glutamate is well in excess of cystine in almost all mammalian cells, system xc− essentially serves to couple the import of one molecule of cystine with the export of one molecule of glutamate.
These initial studies also demonstrated that the system xc− activity was chloride-dependent, sodium-independent, and electroneutral, properties that clearly differentiate it from the sodium-dependent, electrogenic EAATs (Waniewski and Martin, 1984; Bannai et al., 1986). An examination of pH dependence of uptake led to the conclusion that both cystine and glutamate were transported in an anionic form (Bannai and Kitamura, 1981). The ability of glutamate to interact with system xc− also prompted pharmacological comparisons with other components of the EAA system (Bridges et al., 2001; Patel et al., 2004; Balazs et al., 2006; Beart and O'Shea, 2007; Bridges and Patel, 2009). The EAA receptor agonists NMDA, kainate, AMPA, and 1-aminocyclopentane-trans-1,3-dicarboxylic acid were all shown to be inactive as system xc− inhibitors. Likewise, the well characterized EAAT substrate and inhibitors l- and d-aspartate, l-trans-2,4-pyrrolidine dicarboxylate, and dihydrokainate exhibited no cross-reactivity with system xc−. Collectively, these studies demonstrate that system xc− exhibits a distinct pharmacological profile within the EAA system and lay a foundation for the development of selective substrates and inhibitors (Patel et al., 2010).
B. Alternative Routes of Cystine Transport
Overlapping substrate specificity is a hallmark of amino acid transporters in mammalian cells (Hediger et al., 2004), probably reflecting the critical need for these small molecules in cellular function and survival. Thus, given the importance of maintaining sufficient intracellular levels of cysteine, it comes as no surprise that in addition to system xc−, there are a number of alternate routes of entry for cystine and cysteine, both outside and within the CNS (Wade and Brady, 1981; Bannai, 1984; Knickelbein et al., 1997; McBean, 2002). It is noteworthy that the EAATs have been implicated in the transport of both cystine and cysteine (Zerangue and Kavanaugh, 1996c; McBean, 2002; Chen and Swanson, 2003). The strongest case can be made for the uptake of cysteine by the neuronal transporter EAAT3, because the Imax (maximal flux rate determined electrophysiologically) for cysteine is comparable with that of glutamate (Zerangue and Kavanaugh, 1996c). It is also noteworthy in this regard that EAAT3 knockout mice exhibit decreased neuronal levels of glutathione (Aoyama et al., 2006). Cysteine is also a substrate for the sodium-dependent transporters known as alanine-serine-cysteine transporter 1 (SLC1A4) and 2 (SLC1A5), both of which are members of the functionally defined acid-sensing ion channel system (Zerangue and Kavanaugh, 1996a; Kanai and Hediger, 2004). One of the most active of the cystine transporters is bo,+AT (SLC7A9) (Rajan et al., 1999), which is present in the brush-border membranes of the kidney. Like system xc−, it is a member of the SLC7 family, although it is found as a heterodimer, rBAT acting as the “heavy chain” rather than 4F2hc (Verrey et al., 2004).
IV. System xc− and Glutathione Synthesis in the Central Nervous System
System xc− plays a particularly significant role in the CNS, where the demand for glutathione is high (Halliwell, 2006; Lin and Beal, 2006), the cysteine/cystine ratio in the cerebrospinal fluid greatly favors cystine, and the transporters discussed above are localized in a manner that preferentially transports cystine into glia and cysteine into neurons (Dringen et al., 1999; Dringen et al., 2000; Wang and Cynader, 2000; Conrad and Sato, 2012). Referred to as “cystine/cysteine cycle,” this pathway begins with the system xc−-mediated uptake of cystine into astrocytes, a process driven by the high intracellular concentration of glutamate present in these cells (see Fig. 3). In contrast, mature neurons seem to have a very limited capacity to directly transport cystine and express negligible levels of system xc−. Once in the astrocytes, the cystine is rapidly reduced to cysteine and serves as a rate-limiting precursor in the synthesis of GSH (Sagara et al., 1993). The efflux of GSH from astrocytes and its subsequent metabolism by γ-glutamyl-transpeptidase and aminopeptidase N provides an extracellular source of cysteine that is transported into neurons to support glutathione production in these cells (Yudkoff et al., 1990; Dringen et al., 1999, 2001; Wang and Cynader, 2000). Additional studies indicate that antioxidant support provided by these astrocytes is not limited to the efflux of glutathione, but should also be expanded to include the export of cysteine (Anderson et al., 2007; Banjac et al., 2008; Conrad and Sato, 2012). Thus, accumulating evidence suggests that extracellular pools of cysteine in the CNS may be able to be maintained, at least in part, in a manner that is not dependent upon the export of glutathione. Furthermore, the cysteine seems capable of also contributing to oxidative protection and may in some cases be able to compensate for oxidative stress caused by low levels of GSH (Banjac et al., 2008). It is noteworthy that whether the result of producing and releasing cysteine or glutathione, the first step in these pathways is the transport of cystine into astrocytes through system xc.
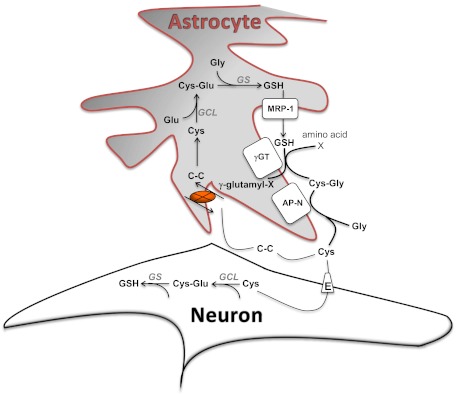
Fig. 3.
System xc− (X)-mediated cystine uptake is a critical determinant of cellular antioxidant capabilities. In astrocytes, cystine (C-C) transported into the cell is reduced into Cys, which can then be used for GSH synthesis by the sequential activity of glutamate cysteine ligase (GCL) and GSH synthetase (GS). GSH can be released by astrocytes via muldrug resistance-asociated protein 1 (MRP-1) transporters, where it is degraded by γ-glutamyl-transpeptidase (γGT) and aminopeptidase N (AP-N). This provides an extracellular source of cysteine that is transported into neurons by the neuronal glutamate transporter EAAT3 (E) to support glutathione production in these cells. In addition, cysteine may also oxidize in the oxygen-rich extracellular space, thereby supplying cystine to support system xc− activity.
Although mechanistic and pharmacological characterizations of system xc− have benefited greatly from the use of cultured cells, the translation and applicability of the results to physiological function in vivo is not necessarily straightforward. Given 1) the rapidity with which cysteine in media oxidizes to cystine under typical culture conditions, 2) the inability of many cells to synthesize cysteine, and 3) the dependence of many cell types on adequate intracellular glutathione levels for survival, it is not that surprising that many cell types express system xc− when cultured (Conrad and Sato, 2012). Indeed, cells that may not express system xc− in vivo may be dependent upon inducing the expression of the transporter to survive in vitro (Watanabe and Bannai, 1987). Likewise, the expression of system xc− could also change with developmental age. For example, even though it is accepted that mature neurons express little or no system xc−, the transporter is expressed on immature cortical cells and is critical to cell survival. This was shown to be the case on the basis of the vulnerability of these neurons to quisqualate-mediated damage that was, in turn, attributable to oxidative damage and GSH depletion resulting from system xc− inhibition rather than excitatory amino acid receptor-mediated excitotoxicity (Murphy et al., 1989; Shih et al., 2003, 2006). An additional factor to consider is that xCT mRNA is not constitutively expressed in most tissue, including the liver, heart, kidney, and lung; a notable exception to this is the brain (Taguchi et al., 2007).
A. System xc− and GSH Signaling
With the growing appreciation of the role that system xc− plays in the synthesis of GSH in astrocytes come questions as to whether changes in the activity of the antiporter (and precursor availability) could ultimately influence the functional roles of GSH within the CNS, particularly those linked with signal transduction (Janáky et al., 1999; Filomeni et al., 2002). This is a complex question, because there are multiple mechanisms through which GSH is known to influence signaling pathways, some quite general and others more specifically linked to individual transmitter systems. At a very basic level, glutathione serves as a primary regulator of the overall redox state of cells as well as a key intermediate in detoxification pathways. Consequently, variations in glutathione levels can serve as a general indicator of cell health or stress and can be linked to global cellular functions such as growth, cell-cycle regulation, survival, and programmed cell death. Evidence is also emerging that the level of glutathione may itself act as a signal to turn specific pathways on or off, although it is difficult to separate whether the signal is actually free glutathione or a secondary protein disulfide that serves as a trigger depending upon its redox state. A recent example of such signaling seems to couple glutathione levels to the regulation of system xc− in astrocytes. Thus, depletion of glutathione in primary astrocytes that have been differentiated with dibutyryl-cAMP induces an up-regulation of system xc− expression as measured by activity, protein, and mRNA (Seib et al., 2011). The response seems to be both cell- and phenotype-specific, because similar treatments of other cell types or nondifferentiated protoplasmic astrocytes failed to mimic the marked increase in system xc− activity. The time course of the changes in system xc− is temporally linked to GSH levels, and the induction could be prevented with coadministration of a cell-permeant glutathione-prodrug, such as GSH-ethylester or N-acetylcysteine. How secondary “redox sensors” may or may not be involved in this pathway, however, remains to be seen.
As the major cellular thiol, glutathione also serves to modulate the activity of proteins possessing critical cysteine-residues that are redox sensitive. Changes in the redox state of these “switches” have been shown to be capable of either increasing or decreasing protein activity (Janssen-Heininger et al., 2008). Because these proteins can include receptors, enzymes, and transporters linked with specific transmitter systems, it provides a more direct link to synaptic transmission. Examples include the excitatory amino acid transporters (Trotti et al., 1997) and NMDA receptors (Lipton et al., 2002) within the excitatory glutamate system; succinic semialdehyde dehydrogenase (Kim et al., 2009), GABA (Pan et al., 2000; Calero and Calvo, 2008), and glycine receptors (Pan et al., 1995) within inhibitory systems; as well as transient receptor potential channels (Song et al., 2011), ryanodine receptors (Bodhinathan et al., 2010), acid-sensing ion channels (Nelson et al., 2006), and potassium channels (DiChiara and Reinhart, 1997), among many others. Whether changes in glutathione levels and system xc− activity are linked in a manner that matches the dynamic regulation of these transmitter-linked proteins remains to be investigated.
Beyond the ability of glutathione to modulate protein activity through redox modulation, a number of studies suggest that the tripeptide can directly interact with receptors, potentially acting as an agonist, antagonist or modulator. In addition to its export from astrocytes, glutathione has been shown to be released upon K+-depolarization in a calcium-dependent manner, suggestive of a neuronal compartment (Zängerle et al., 1992). It has also been reported to evoke excitatory potentials in rat cortical slices and to regulate the release of other transmitters, although it remains to be determined whether these actions are mediated via a specific “glutathione receptor” or through other systems, such as excitatory amino acid receptors (Shaw et al., 1996; Janáky et al., 1999; Oja et al., 2000). Radioligand binding studies have lent added strength to the idea that glutathione can directly interact with sites on neurotransmitter receptors, although the results have, again, made little progress in addressing the issue of specificity of action. For example, glutathione has been reported to inhibit the binding of [3H]3-((±)-2-carboxypiperazin-4-yl)-[1,2]propyl-1-phosphonic acid to the glutamate site on NMDA receptors, as well as both enhance and reduce the binding of [3H]MK-801 to its site in the receptor channel (Ogita et al., 1995; Janáky et al., 1999), suggesting that glutathione actions may vary between agonist and antagonist on the basis of specific conditions. Glutathione was also shown to block the binding of radiolabeled AMPA and kainate to non-NMDA receptors (Varga et al., 1989). In a study employing computational modeling, the amino acid binding pockets common to family C G-protein-coupled receptors were probed in silico for the potential to accommodate glutathione (Wang et al., 2006). Using both functional and binding assays, glutathione was identified as an orthosteric agonist at the fish 5.24 receptor and a positive modulator of the rat calcium sensor receptor. Finally, numerous studies have directly examined the binding of [3H]glutathione to CNS membrane preparations (for review see Janáky et al., 1999). Collectively, these studies indicate that glutathione binding is specific, represents multiple sites with differing affinities, and can be distinguished on the basis of binding to glutathione-using enzymes as well as excitatory amino acid receptors. Although the presence of such glutathione sites is intriguing, a clear association between a glutathione binding site, a molecularly characterized receptor, and a well defined physiological response has yet to emerge. Given this state of development, the physiological consequences of changes in glutathione levels as a result of alterations in system xc− activity also remain speculative.
V. Role of System xc− in Disease
As described above and depicted in Fig. 3, system xc− is capable of contributing to the antioxidant capacity of a cell through the maintenance of glutathione levels and to glutamate homeostasis. The dual nature of system xc− may be advantageous because it permits increased antioxidant capacity via cystine uptake to buffer the potential toxic effects of glutamate release. Alternatively, this subjects glutamate release by system xc− activity to changes in oxidative stress, a seemingly undesirable outcome given the need for highly regulated excitatory signaling for purposes ranging from signal fidelity to cell survival. Control of glutamate release by free radicals has been shown by studies demonstrating that free-radical scavengers reduce system xc− mediated glutamate release (Barger et al., 2007). The efficiency of this system is also problematic because extracellular glutamate can outcompete cystine for transport by system xc− to the point of increased neurotoxicity (Murphy et al., 1990), an outcome that is exacerbated by impaired glutamate transport by EAATs (Lewerenz et al., 2006). As a result, it is important to stress that the dual nature of system xc− is heavily regulated by a variety of cellular mechanisms because it is not the sole determinant of either extracellular glutamate or intracellular glutathione. Thus, the degree to which system xc− contributes to CNS disorders, or its value as a therapeutic target, is determined, at least in part, by the status of a complex network of cellular mechanisms. Although this review focuses on system xc−, it is important to note that impaired glutamate transport by EAATs is common to many CNS disorders, which probably compounds the impact on glutamate homeostasis of any changes in system xc−. As described below, a great deal of work has implicated system xc− in a wide array of CNS disorders, which is impressive given that it is a relatively recent addition to the complex network that regulates glutamate, an amino acid that has been studied for more than 60 years (Stern et al., 1949; for review, see Watkins and Jane, 2006).
A. Psychiatric Disorders
1. Drug Addiction.
Drug addiction is a chronic relapsing disorder hypothesized to be produced by drug-induced neuroplasticity that renders users vulnerable to craving-inducing stimuli. Drug-induced plasticity that may result in the addiction phenotype includes long-term changes in the activity of corticostriatal pathways, which originate in the prefrontal cortex and project to the nucleus accumbens (Kalivas et al., 2005; Uys and LaLumiere, 2008; Kalivas, 2009; Wolf, 2010). Activation of this circuit or related structures correlates with craving in humans and is necessary for reinstatement in rodents (Volkow et al., 1991, 1999, 2005; Breiter et al., 1997; McFarland and Kalivas, 2001; Park et al., 2002).
Cocaine or other drugs of abuse produce a persistent reduction in cystine-glutamate exchange via system xc− in the nucleus accumbens that may contribute to the pathological glutamate signaling linked to addiction (Baker et al., 2003; Madayag et al., 2007; Kau et al., 2008; Knackstedt et al., 2009). In particular, repeated cocaine use significantly increases the Km of [35S]cystine uptake in nucleus accumbens tissue slices (Baker et al., 2003; Madayag et al., 2007; Kau et al., 2008). Second, blockade of system xc− produces a significant decrease in extracellular glutamate levels in the nucleus accumbens of drug-naïve rats but not cocaine-withdrawn rats (Baker et al., 2003). Both findings suggest that the activity of system xc− is reduced in cocaine-withdrawn rats.
The functional consequence of a reduction in cystine-glutamate exchange is a persistent decrease in basal, extrasynaptic glutamate levels in the nucleus accumbens core (Pierce et al., 1996; Reid and Berger, 1996; Breiter et al., 1997). Under normal conditions, high-affinity glutamate receptors, including group I mGluRs, group II mGluRs, and NMDA receptors, are activated by nonvesicular glutamate, glutamate release from astrocytes, and/or exhibit a pattern of tonic activation (Jabaudon et al., 1999; Angulo et al., 2004; D'Ascenzo et al., 2007; Moussawi et al., 2011; Kupchik et al., 2012), but see Cavelier et al., 2005). Although the impact of reduced basal glutamate levels on group I mGluRs and NMDA receptors has yet to be determined, group II mGluRs exhibit reduced tonic activation in rats withdrawn from repeated cocaine (Xi et al., 2002b). As depicted in Fig. 4, diminished group II mGluR inhibition of vesicular release may contribute to increased cocaine-evoked glutamate (and possibly dopamine) signaling in the nucleus accumbens observed in rats withdrawn from repeated cocaine (Madayag et al., 2010). It is noteworthy that infusion of cystine into the nucleus accumbens of cocaine-withdrawn rats elevates extracellular glutamate to near control levels, and this prevents cocaine-induced elevations in glutamate after a cocaine challenge (Baker et al., 2003). Furthermore, cystine-glutamate antiporter activity can be targeted to prevent cocaine-induced plasticity (Madayag et al., 2007). In particular, daily administration of the cysteine prodrug N-acetylcysteine during cocaine self-administration prevents the reduction in system xc− activity as well as the changes previously observed involving extracellular glutamate (Madayag et al., 2007).
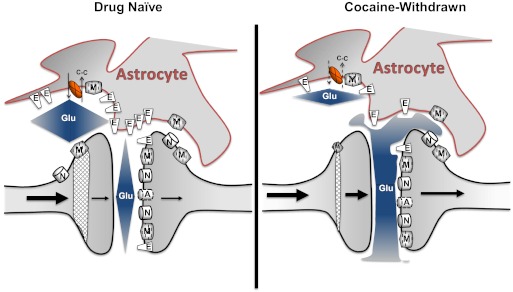
Fig. 4.
This model illustrates cocaine-induced changes in the nucleus accumbens core. Relative to drug-naive counterparts, cocaine-withdrawn rats exhibit reduced glutamate release by system xc− (X), reduced glutamate clearance by the excitatory amino acid transporter GLT-1 (E)—both of which may contribute to synaptic overflow of Glu after a cocaine injection. As depicted, reduced system xc− activity results in reduced basal extrasynaptic glutamate, which contributes to diminished mGluR2 (M)-mediated inhibition of synaptic glutamate. In addition, reduced glutamate clearance permits glutamate to spill over into compartments that are more distal. As discussed in the text, the nature of glutamate dysfunction may not be due solely to increased activity of postsynaptic metabotropic, AMPA (A), or NMDA (N) receptors but could be due to altered patterns of activation arising from a loss of signal fidelity that results from “cross talk” of glutamate from distinct microdomains. C-C, cystine.
Multiple preclinical studies indicate that system xc− represents a novel therapeutic target for the treatment of cocaine addiction. Acute or repeated administration of N-acetylcysteine has been shown to inhibit cocaine-induced behaviors used to model aspects of addiction. Acute administration of the cysteine prodrugs N-acetylcysteine or l-2-oxothiazolidine-4-carboxylate blocks cocaine-induced reinstatement (Baker et al., 2003). Subsequent studies indicate that the short-term effects of N-acetylcysteine were dependent on intact system xc− activity and mGluRII receptor activation (Moran et al., 2005; Kau et al., 2008). Later, studies demonstrated the capacity of repeated N-acetylcysteine to block multiple measures of cocaine seeking produced by the drug, drug-paired discrete cues, and the drug environment (Madayag et al., 2007; Reichel et al., 2011). Furthermore, N-acetylcysteine has been shown to reduce drug seeking for nicotine and heroin, suggesting potential efficacy against numerous drugs of abuse (Zhou and Kalivas, 2008; Knackstedt et al., 2009). These findings are critical for the potential therapeutic efficacy of any novel treatment to reduce compulsive drug seeking, which can be generated by a wide-array of external stimuli and often involves use of multiple drugs.
The nature of N-acetylcysteine-induced increases in system xc− activity may be more complex than simply increasing extracellular levels of cystine, a necessary substrate. N-Acetylcysteine does not seem to be transported into the cell by system xc− (Kupchik et al., 2012). Instead, the internalization of either N-acetylcysteine or its metabolic product cysteine is achieved by cysteine transporters (Kupchik et al., 2012). Once cysteine is elevated inside the cell, system xc− activity is augmented. This could occur either by intracellular cysteine, supporting the cystine/cysteine cycle described in section IV, or through a mechanism dependent on intracellular signaling. In either scenario, the actions of N-acetylcysteine at the cellular or behavioral level require increased system xc− activity (Baker et al., 2008; Kau et al., 2008; Kupchik et al., 2012).
The assessment of the therapeutic potential of any medication for addictive disorders will need to include endpoints in addition to altered drug craving. For instance, studies using N-acetylcysteine have shown a reduction in drug seeking without a change in the reinforcing properties of the drug (Madayag et al., 2007; Murray et al., 2012). Consistent with these findings, N-acetylcysteine does not alter cocaine-induced rush or euphoria when administered to drug-dependent humans (Amen et al., 2011). These data pose an intriguing question regarding the use of N-acetylcysteine as a treatment for addiction: is the value of such treatment significantly diminished if the short-term, euphoric properties of the previously abused drug persist in a manner that could maintain recreational drug use, provided there is a reduction in compulsive, uncontrollable drug use? Another important point regarding the potential therapeutic utility of cysteine prodrugs or other approaches that exert antioxidant properties and support glutathione synthesis is the benefit of diminished toxicity associated with certain drugs of abuse. This includes neurotoxicity produced by methamphetamine (Fukami et al., 2004), hepatotoxicity produced by long-term alcohol administration (Pivetta et al., 2006), and tumor formation after chronic smoking (Balansky et al., 2011). As a result, the impact of this approach on quality of life measures may be an important benefit of the approach that is difficult to capture in most clinical studies.
The effectiveness of repeated N-acetylcysteine administration in reducing drug seeking may be due to the reversal of at least a subset of drug-induced alterations in plasticity that contributes to drug seeking. This possibility was posed by studies demonstrating that repeated N-acetylcysteine administration resulted in enduring suppression of drug seeking even when testing was conducted at a time in which the drug would have been cleared from the body (Madayag et al., 2007; Kau et al., 2008; Zhou and Kalivas, 2008). Direct support for this compelling hypothesis has been generated by recent studies conducted by Moussawi et al. (2009, 2011). For instance, N-acetylcysteine reversed a deficit in the capacity of cocaine-withdrawn rats to exhibit long-term potentiation or long-term depression in the nucleus accumbens core after stimulation of the prefrontal cortex.
The therapeutic potential of N-acetylcysteine in treating addictive disorders established by the above preclinical studies have been largely confirmed by clinical studies. Specifically, N-acetylcysteine has been shown to reduce craving and or use of a variety of controlled substances including cocaine and tobacco (LaRowe et al., 2007; Mardikian et al., 2007; Knackstedt et al., 2009; Amen et al., 2011). In addition, N-acetylcysteine has been shown to reduce pathological gambling and lessen other types of compulsive behaviors, such as trichotillomania (Grant et al., 2007, 2009; Knackstedt et al., 2009).
It may seem paradoxical that targeting cystine-glutamate exchange, which increases nonvesicular glutamate, is effective in reducing drug seeking in rodents or drug-craving and use in humans because these behaviors require increased glutamate signaling (Cornish and Kalivas, 2000; Baker et al., 2003; Di Ciano and Everitt, 2003; McFarland et al., 2003; LaRowe et al., 2006). As discussed above, however, this may reflect the existence of multiple, functionally distinct pools of glutamate. Cocaine increases glutamate in the synaptic cleft after corticostriatal activation, thereby generating behaviors dependent upon postsynaptic receptor stimulation (Cornish and Kalivas, 2000; McFarland et al., 2003; Di Ciano and Everitt, 2004; Suto et al., 2004). Conversely, N-acetylcysteine promotes glutamate release into the extrasynaptic compartment, resulting in the stimulation of group II mGluRs (Baker et al., 2002, 2003; Moran et al., 2005). By stimulating extrasynaptic group II mGluRs without exerting postsynaptic effects, extrasynaptic glutamate seems to inhibit synaptic release (Baker et al., 2002; Moran et al., 2005). To the extent that NR2B receptors are located outside the synapse, this hypothesis is supported by the recent observation that glutamate release from astrocytes seems to stimulate extrasynaptic but not synaptic NMDA receptors (D'Ascenzo et al., 2007).
Disruptions in glutamate homeostasis after repeated cocaine administration are not restricted to reduced system xc− activity but also include reduced activity of excitatory amino acid transporters (Knackstedt and Kalivas, 2007; Knackstedt et al., 2010). Given the role of EAATs to compartmentalize glutamate into functionally distinct pools (e.g., synaptic, extrasynaptic), it is possible that receptors located in the synapse or in the extrasynaptic compartment are being stimulated by glutamate diffusing across microdomains. Thus, as depicted in Fig. 4, the nature of glutamate dysfunction in addiction may arise from a loss of signal fidelity instead of, or in addition to, abnormal levels of receptor activation.
2. Schizophrenia.
Abnormal excitatory signaling in the prefrontal cortex has been proposed to contribute to schizophrenia (Weinberger, 1987; Bunney and Bunney, 2000; Chavez-Noriega et al., 2002). Synchronized pyramidal cell activity in the prefrontal cortex is required for normal cognitive processing, and the level of synchronized oscillatory activity can be measured using electroencephalograms (Howard et al., 2003; Haenschel et al., 2009; Uhlhaas and Singer, 2010; Lewis et al., 2012). Measuring distinct frequencies, including slow (θ, β) and fast (γ) frequency bands, during rest or while performing cognitive tasks can be used to study aspects of network synchrony (Singer, 1999; Buzsáki and Draguhn, 2004; Fries, 2009; Gonzalez-Burgos et al., 2009). It is noteworthy that schizophrenia is associated with reduced synchrony of pyramidal cells in the prefrontal cortex as reflected by abnormalities in low- (θ/β) and high-frequency (γ) bands in a manner that has been linked to symptoms of the disease (Uhlhaas and Singer, 2010; Lisman, 2011). As a result, it is critical to understand the cellular mechanisms that regulate synchronized cellular activity within the cortex.
In addition to frequently studied mechanisms implicated in regulating cortical synchrony, such as GABAergic interneurons (Lewis et al., 2005; Lewis and Moghaddam, 2006), astrocytes are well positioned to regulate cortical activity at the network level (see Fig. 5). Specifically, astrocytes have been estimated to regulate up to 2 million synapses in the human cortex (Oberheim et al., 2006). Although astrocytes are capable of influencing synaptic activity through the release of a variety of molecules, glutamate seems to be particularly important. In support, neuronal synchrony in the hippocampus or cortex has been linked to glutamate release by astrocytes (Angulo et al., 2004; Poskanzer and Yuste, 2011), with the most recent study demonstrating network-wide synchrony after the stimulation of a single astrocyte. These studies clearly indicate a need to more closely examine the role of astrocytes, cells capable of regulating neuronal signaling, possibly through the regulation of glutamate homeostasis, in schizophrenia.
Fig. 5.
This schematic depicts aspects of cellular organization in the cortex that may be relevant for synchronization of network activity. GABAergic interneurons (I) are thought to contribute to network synchrony, in part because they synapse onto multiple pyramidal cells (P). The cellular architecture also supports a role for astrocytes in network synchronization. As described in section V.A.2, a single astrocyte in the human cortex has a domain that contains an estimated 2 million synapses and is capable of altering the membrane potential of large populations of neurons.
Aside from the direct study of astrocytes, glutamate dysfunction in schizophrenia has attracted intense interest; however, the precise role of this amino acid is equivocal because evidence indicates that either an increase or a decrease in glutamate signaling may occur. A hypoglutamatergic state is supported by functional imaging studies revealing diminished activation of the frontal cortex in schizophrenics (Barch et al., 2001; MacDonald et al., 2005). Furthermore, noncompetitive NMDA receptor antagonists, including phencyclidine, exacerbate symptoms in schizophrenic patients and produce a broad range of schizophrenia-like symptoms in humans and rats (Luby et al., 1959; Pearlson, 1981; Javitt and Zukin, 1991; Krystal et al., 1994; Malhotra et al., 1997); thus, hypoglutamatergic function through NMDA receptors is sufficient to produce a schizophrenia-like state. In contrast, a hyperglutamatergic hypothesis stems from evidence that 1) noncompetitive NMDA receptor antagonists increase extracellular glutamate levels in the prefrontal cortex, which generates an increase in the firing rate of cortical neurons (Moghaddam and Adams, 1998a; Lorrain et al., 2003; Jackson et al., 2004), and 2) these changes are necessary for the psychotomimetic effects of phencyclidine (Moghaddam and Adams, 1998a; Homayoun et al., 2005). The uncertainty regarding the nature of glutamate dysfunction in schizophrenia may arise, in part, from the existence of multiple, distinct pools of glutamate as discussed above.
A reduction in system xc− activity could account for the seemingly discrepant hypo- and hyperglutamatergic hypotheses of schizophrenia. Specifically, reduced system xc− activity could result in an apparent hypofunction, because glutamate released by cystine-glutamate exchange is cleared by GLT-1 transporters (Baker et al., 2002), the activity of which contributes to measures of brain activity using functional imaging techniques (Bonvento et al., 2002; Aubert et al., 2007). Thus, reduced system xc− activity could result in diminished glutamate clearance through sodium-dependent glutamate transporters, giving the appearance of reduced activity levels when using functional imaging techniques. In addition, reduced system xc− activity may lead to NMDA receptor hypofunction through at least two mechanisms. First, glutamate release from system xc− may result in activation of NMDA receptors. NR2B-containing NMDA receptors have been shown to be activated by glutamate released from astrocytes (D'Ascenzo et al., 2007). Furthermore, the cysteine prodrug N-acetylcysteine seems to increase NMDA receptor activation because it restores deficient mismatch negativity, which is an auditory evoked-potential component related to NMDA receptor function (Lavoie et al., 2008). In addition to direct activation of the receptor by glutamate, NMDA receptor hypoactivity could stem from reduced extracellular glutathione levels, which would result in diminished activation of the extracellular redox site of the NMDA receptor (Köhr et al., 1994) or lower levels of the coagonist glycine that is released after the metabolism of extracellular glutathione by the membrane-bound enzyme γ-glutamyl transpeptidase (Abbott and Meister, 1986). The importance of glutathione is evident from the observation that glutathione depletion is sufficient to produce NMDA receptor hypofunction (Steullet et al., 2006) as well as several behavioral abnormalities used to model schizophrenia (Cabungcal et al., 2006). Thus, reduced system xc− may give rise to an apparent hypoglutamatergic state largely involving either decreased glutamate release into the extrasynaptic compartment or diminished glutathione levels after reduced cystine transport.
In contrast to the extrasynaptic compartment, reduced system xc− activity leads to a hyperglutamatergic state within the synapse. This occurs as a result of decreased activation of group II mGluRs (Baker et al., 2002; Moran et al., 2005), which have been shown to inhibit synaptic release of glutamate and dopamine (Hu et al., 1999a; Cartmell and Schoepp, 2000; Baker et al., 2002; Chaki et al., 2006) and supports the heightened glutamate and dopamine release implicated in schizophrenia. Collectively, these studies indicate that reduced system xc− may lead to an apparent hypo- and hyperglutamatergic state in the extrasynaptic and synaptic compartments, respectively. This scenario illustrates the need to better understand the complex nature of glutamate signaling as opposing homeostatic changes in glutamate levels in separate extracellular compartments as potential mechanisms underlying pathological states.
Extant data indicate that system xc− activity may be altered in patients with schizophrenia. An increase in the expression of group II mGluR has been linked to schizophrenia (Gupta et al., 2005), and this could arise in response to chronic understimulation of the receptor. Likewise, a modest increase in the levels of xCT, the catalytic subunit for system xc−, has been observed in post mortem tissue obtained from patients with schizophrenia (Baker et al., 2008). Similar to mGluRII, this may reflect a decrease in net function. This conclusion is supported by the observation that those with schizophrenia exhibit reduced glutathione levels in the prefrontal cortex (Do et al., 2000), which may reflect blunted system xc− activity as a result of the link between glutathione levels and cystine transport as discussed above (Bannai, 1984; Meister, 1991; Sies, 1999). Alternatively, reduced glutathione could result in blunted system xc− activity, because extracellular glutathione metabolism serves as a reservoir for extracellular cystine, which is critical in maintaining cystine-glutamate exchange (Deneke and Fanburg, 1989; Sato et al., 1999; Sies, 1999; Kim et al., 2001; Shih et al., 2006).
The neurodevelopmental aspects of schizophrenia may involve reduced activity of system xc− or related mechanisms. Nonvesicular glutamate has been shown to be a key regulator of postsynaptic development in Drosophila melanogaster by regulating the expression of postsynaptic glutamate receptors during synaptogenesis. Specifically, decreased nonvesicular glutamate release resulted in increased expression of postsynaptic glutamate receptors (Featherstone et al., 2002), an effect more recently linked to cystine-glutamate exchange (Augustin et al., 2007). It is noteworthy that postsynaptic glutamate receptor expression influences synapse morphology and presynaptic neurotransmitter release in D. melanogaster (Petersen et al., 1997; Sigrist et al., 2000). Nonvesicular glutamate release has also been shown to regulate cortical neuron migration during neurodevelopment (Manent et al., 2005). Glutamate has been previously shown to regulate neuroblast migration and to act as either an acceleratory or stop signal. Synaptic glutamate is unlikely to contribute to this effect because normal cortical development occurs in munc13-1/2 double knock-out mice that are incapable of vesicular release of neurotransmitters (Verhage et al., 2000). Rather, nonvesicular release seems to be the source of glutamate involved in neuronal development (Manent et al., 2005). Collectively, these data indicate that a disruption of nonvesicular release could disrupt cortical migration during development, affect synapse formation, and lead to altered neurotransmitter release, all of which seem to occur in schizophrenia.
Preclinical and clinical studies have established the therapeutic potential of N-acetylcysteine in treating schizophrenia and related disorders. Short-term or nonprolonged administration of N-acetylcysteine has been shown to attenuate the psychotomimetic effects of phencyclidine (Baker et al., 2008; Chen et al., 2010). In patients, repeated N-acetylcysteine has been shown to significantly improve symptoms of schizophrenia and bipolar disorder, as well as potentially reduce side effects produced by current antipsychotic medications (Berk et al., 2008a,b; Bulut et al., 2009). Thus, although the degree to which astrocytes, and more specifically system xc−, contribute to the pathology of schizophrenia is unknown, accumulating data indicate that cystine-glutamate exchange represents a novel, effective target in the treatment of this disease.
Similar to addiction, disruptions in glutamate homeostasis linked to schizophrenia are not restricted to changes in system xc− but also appear to involve changes in the activity of excitatory amino acid transporters (Bauer et al., 2008; Rao et al., 2012). Given the role of EAATs to compartmentalize glutamate into functionally distinct pools (e.g., synaptic, extrasynaptic), it is possible that receptors located in the synapse or in the extrasynaptic compartment are being stimulated by glutamate diffusing across microdomains. Thus, the nature of glutamate dysfunction in schizophrenia may arise from a loss of signal fidelity instead of, or in addition to, abnormal levels of receptor activation.
B. Neurodegenerative Disorders
Extracellular glutamate is highly regulated, in part, because of the excitotoxic effects of this amino acid. However, clearly establishing a role of system xc− in neuronal death is difficult, in part because system xc− is one of several mechanisms that serve as a point of interface between cellular antioxidant capacity and excitotoxicity. With the exception of gliomas (see section V.B.1), a neuroprotective effect is often attributed to the uptake of cystine and the subsequent increase in cellular antioxidant capacity linked to glutathione synthesis (see Fig. 3). In contrast, excitotoxicity, at least under certain circumstances, may arise as a result of system xc− glutamate release. In culture, glutamate release by system xc− has been shown to cause neuronal death—especially in the absence of sodium-dependent glutamate clearance and in experiments using high levels of cystine. This seems to be primarily a function of system xc− expressed by microglia, which have been shown to kill cerebellar granule cells (Piani and Fontana, 1994) and enhance amyloid-β induced neuronal death in cortical cultures (Qin et al., 2006). Glutamate release by activated microglia also causes oligodendrocyte death in culture and in the rat optic nerve, an effect at least partially attributed to impaired activity of sodium-dependent glutamate transporters (Domercq et al., 2007). In addition to microglia, system xc− on astrocytes also has the potential to contribute to neuronal death. Activation of astrocytes with IL-1β leads to increased expression of xCT and increased system xc− mediated glutamate release, which potentiates hypoxia-induced excitotoxicity of cortical neurons (Fogal et al., 2007; Jackman et al., 2010). Activation of NMDA receptors seems to be the main mediator of this system xc−-induced excitotoxicity (Qin et al., 2006; Fogal et al., 2007). Although system xc− has been shown to be capable of releasing enough glutamate to stimulate non-NMDA type glutamate receptors on Purkinje cells, this required a concentration of cystine that is far greater than what is observed in vivo (Warr et al., 1999).
The first evidence that system xc− could play a role in neuronal death was as a result not of excitotoxicity but instead of glutamate-induced oxidative stress (Murphy et al., 1990). Specifically, glutamate release, especially in the absence of glutamate uptake by EAATs, outcompeted cystine for transport into the cell. The subsequent toxicity occurred before glutamate receptors developed (Murphy and Baraban, 1990), an action subsequently shown to be due to depletion of glutathione and the accompanying oxidative stress (Ratan et al., 1994). Thus, in contrast to the enhancement of neurotoxicity caused by releasing glutamate, increased activity of system xc− activity can exert protective effects by generating and releasing glutathione. The protective effects occur across cell types such that overexpression of xCT in astrocytes has been shown to enhance glutathione release and protect neurons from oxidative stress (Shih et al., 2006). Not only does system xc− act to prevent oxidative stress but such stress also seems to be one of the main triggers for its up-regulation. Oxidative stress induced by exposure of Müller glial cells to xanthine/xanthine oxidase causes up-regulation of system xc− activity and xCT expression (Mysona et al., 2009) as does the depletion of glutathione in differentiated astrocytes (Seib et al., 2011). Likewise, oxidative stress and nitric oxide up-regulate system xc− activity and xCT expression in a retinal ganglion cell line (RGC-5; Dun et al., 2006). Thus, up-regulation of system xc− can be a protective mechanism against oxidative stress, and under certain circumstances, it can contribute to excitotoxicity as described below.
1. Gliomas.
Proliferation of gliomas, which are glia-derived tumors, is facilitated by their ability to generate toxic levels of glutamate, enabling the tumors to overcome physical growth restrictions through the destruction of neighboring tissue (for review, see de Groot and Sontheimer, 2011). Gliomas achieve excitotoxic levels of glutamate through high levels of system xc− activity coupled with the relative absence of sodium-dependent transport (Ye and Sontheimer, 1999; Ye et al., 1999; Kim et al., 2001; Takano et al., 2001; Rothstein, 2002; Chung et al., 2005). In addition, high system xc− activity displayed by gliomas also confers resistance to antineoplastic drugs or radiation therapy by maintaining high levels of glutathione (Chung et al., 2005). It is noteworthy that in addition to triggering excitotoxic pathology in the regions surrounding the tumor, the glutamate released by system xc− also seems to have an autocrine effect on the tumor cells that enhances migration and invasion (Lyons et al., 2007). Given the multiple actions of system xc− in gliomas, the potential use of inhibitors of system xc− as a treatment of brain tumors, both as a method of inhibiting their growth and as a method to reduce their associated seizure activity, is under active investigation (Chung and Sontheimer, 2009; Buckingham et al., 2011; de Groot and Sontheimer, 2011). In addition, restoring sodium-dependent transporter activity is sufficient to block the neurotoxic effects of gliomas, thereby clearly demonstrating the link between the excitotoxic effects of system xc− and diminished EAAT activity.
2. Amyotrophic Lateral Sclerosis.
Studies examining the role of system xc− in the pathology of amyotrophic lateral sclerosis (ALS) are lacking, but these efforts should be aided by the recent development of a double-knockout mouse of SOD1(−/−) and xCT(−/−). To date, however, these mice have been used only to examine changes in the function of red blood cells (Iuchi et al., 2008). Furthermore, the function of system xc− in other ALS models has not been has examined. However, ALS is clearly linked to changes in glutamate signaling (for review, see Rattray and Bendotti, 2006), is associated with altered levels and/or activity of glutathione (Cova et al., 2010; D'Alessandro et al., 2011), and has an astrocytic component (Boillée et al., 2006), all of which would be consistent with a change in system xc− activity.
Although the degree to which system xc− is involved in the pathology of the disease is unknown, it appears to be a novel therapeutic target for ALS. First, increased expression of Nrf2, a transcription factor that regulates the expression of xCT, produces a protective effect in mouse models of ALS (Vargas et al., 2008). Second, ceftriaxone prolongs survival in ALS models, and this may be due to increases in the expression of either GLT-1 or xCT (Rothstein et al., 2005; Lewerenz et al., 2009). N-Acetylcysteine administration reduces oxidative stress and mitochondrial dysfunction in human neuroblastoma cells (SH-SY5Y) expressing the G93A-SOD1 mutation (Beretta et al., 2003). N-Acetylcysteine also delayed the onset of motor impairments and prolonged survival in G93A-SOD1 mice (Andreassen et al., 2000, although see Jaarsma et al., 1998). In a randomized, double-blind, controlled trial, N-acetylcysteine produced only a modest, nonsignificant increase in survival, with no evidence of reduction in disease progression; however, the dose used was considerably lower than what has been used in clinical trials that have obtained a positive effect for other CNS disorders (i.e., 50 mg/day versus >1 g/day) (Louwerse et al., 1995; Grant et al., 2007; Berk et al., 2008a,b; Grant et al., 2009). Alternatively, the effect of N-acetylcysteine may depend on the type of ALS expressed. N-Acetylcysteine increased survival in a subgroups of patients with disease of the limbs onset, whereas it may have decreased survival in those with bulbar onset (Louwerse et al., 1995). This adds to existing data suggesting a different contribution of excitotoxicity in these types of ALS. For instance, riluzole, which reduces glutamate signaling, is more likely to promote survival in patients with ALS who have bulbar symptoms (Bensimon et al., 1994; Zoccolella et al., 2007); thus, N-acetylcysteine-induced glutamate release may be detrimental in this patient population.
3. Alzheimer's Disease.
Similar to ALS, studies examining the change in system xc− in Alzheimer's disease have not been conducted, although there is indirect evidence to suggest that system xc− may be up-regulated. Specifically, increased phosphorylation of α subunit of eukaryotic initiation factor 2 and activating transcriptional factor 4 expression, which increase xCT expression, have been detected in the brains of patients with Alzheimer's disease (Lewerenz and Maher, 2009). Increased xCT expression in reactive microglia have been found in transgenic mice expressing mutant human amyloid precursor protein or those injected with amyloid-β (Qin et al., 2006).
The above studies suggest that system xc− activity may be altered in Alzheimer's disease; however, the impact of this change is unclear. In a study on probable Alzheimer's disease patients, treatment with N-acetylcysteine to drive system xc− caused a beneficial trend in all measures tested and significant improvement in certain cognitive tasks (Adair et al., 2001). The release of glutamate from system xc−, especially the degree to which this causes activation of extrasynaptic NMDA receptors, may contribute to amyloid-β production and toxicity (Qin et al., 2006; Bordji et al., 2010). In contrast, cystine uptake by system xc− seems to exert powerful beneficial effects by lowering amyloid-β-induced oxidative stress (Olivieri et al., 2001), reversing abnormal protein expression associated with a mouse model of Alzheimer's (Robinson et al., 2011), preventing apoptosis triggered by oxidative stress or a toxin implicated in Alzheimer's (Onyango et al., 2005a; Tanel and Averill-Bates, 2007), and normalizing the activity of sodium-dependent glutamate transporters (Begni et al., 2004). The last of these indicates the complexity of isolating the dual actions of system xc− and the need for additional work.
4. Parkinson's Disease.
The pathogenesis of Parkinson's involves nigrostriatal toxicity that may be linked to mitochondrial dysfunction or oxidative stress (for review, see Yao and Wood, 2009; Surendran and Rajasankar, 2010). It is noteworthy that mitochondrial dysfunction, oxidative stress, and ultimate nigrostriatal toxicity may be due, in part, to diminished glutathione levels (Penugonda et al., 2005). Not surprisingly, the depletion in glutathione is linked to an increase in xCT protein, at least in rats receiving unilateral 6-hydroxydopamine lesions (Massie et al., 2008). In general, a neuroprotective role has been clearly established for glutathione and N-acetylcysteine. Under numerous experimental conditions, N-acetylcysteine has been shown to counter age-related mitochondrial damage (Banaclocha, 2001); prevent apoptosis (Molina-Jimenez et al., 2003) perhaps by decreasing p38 and c-Jun N-terminal kinase (Onyango et al., 2005b); and scavenge H2O2 and reactive quinones (Bagh et al., 2008). Collectively, these studies support the therapeutic potential of increasing system xc− activity in treating Parkinson's in a manner that may target the underlying pathological condition.
Preclinical studies support the therapeutic potential of N-acetylcysteine in the treatment of Parkinson's disease. In support, N-acetylcysteine produces beneficial effects in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Park et al., 2004; Aoyama et al., 2008), or in α-synuclein overexpressing and EAA carrier 1-null mice (Clark et al., 2010; Berman et al., 2011). In contrast to these studies, a recent report suggests that xCT deletion affords protection from 6-hydroxydopamine toxicity (Massie et al., 2011). The protective effect of lower levels of xCT was attributed to the decrease in extracellular glutamate found in the striatum of these mice—especially because these mice did not exhibit reduced levels of glutathione or increased markers of oxidative stress. As discussed above, a complex relationship exists between system xc− activity and cellular glutathione levels, such that impairing system xc− activity will not necessarily result in long-term reductions in glutathione, yet increased activity seems to successfully address deficits in this key antioxidant. Additional work is needed to understand the observed effect, especially in light of the positive data obtained in other models as well as the potential for system xc− to serve as an entry point for toxic substances as described in the next section.
The therapeutic efficacy of N-acetylcysteine in the treatment of Parkinson's disease has not been examined, although a clinical trial is currently ongoing. However, zonisamide has recently been shown to reduce disease severity in a clinical trial (Murata et al., 2001, 2007), and the mechanism of action underlying this therapeutic effect may involve increased system xc− activity. In support, repeated zonisamide administration to hemiparkinsonian mice resulted in increased glutathione synthesis as a result of zonisamide-induced up-regulation of system xc− (Asanuma et al., 2010).
C. Neurotoxins
It is noteworthy that system xc− may serve as an entry point for damaging substances including viruses and neurotoxins (Warren et al., 2004; Kaleeba and Berger, 2006; Liu et al., 2009). In terms of the latter, evidence suggests that it may mediate the actions of putative environmental neurotoxins including β-N-methyl-l-alanine (BMAA) and β-N-oxalyl-l-α,β-diaminopropionic acid. BMAA is a nonprotein amino acid that is produced by cyanobacteria and has been implicated in neurodegenerative diseases (Bradley and Mash, 2009) such as Alzheimer's disease and ALS; high levels of BMAA have been found in the brains of patients with Alzheimer's disease and ALS (Pablo et al., 2009). BMAA can act as a direct excitotoxin by acting on glutamate receptors (Weiss and Choi, 1988), but it can also act on system xc−. BMAA inhibits system xc−-mediated cystine uptake, thereby leading to decreased cellular glutathione levels and oxidative stress. In addition, BMAA seems to be transported by system xc− and thereby increases glutamate release in a manner sufficient to produce excitotoxicity (Liu et al., 2009). Therefore, BMAA creates the worst possible array of effects on system xc−: it prevents the beneficial effect of cystine uptake and causes the harmful effect of enhancing glutamate release.
The amino acid β-N-oxalyl-l-α,β-diaminopropionic acid is produced by seeds from plants of the genus Lathyrus and has been implicated in the death of motor neurons that occurs in neurolathyrism. It has also been shown to compete with cystine at system xc− and, as an alternate substrate, to cause increased glutamate release (Warren et al., 2004). The possibility that these environmental factors play a role in the development or progression of neurodegenerative diseases through actions on system xc− requires further study.
VI. Summary
Billions of dollars have been spent to fund over a million studies devoted to understanding the neuron, a cell held in such prominence that it provides the field its name. In comparison, there have been fewer than 40,000 publications examining the role of astrocytes, a cell type as numerous as neurons in the human brain. Unfortunately, our knowledge of the neuron has yet to result in the development of effective, well tolerated treatment for most, if not all, pathological conditions of the brain. The limitations in our understanding of the brain are reflected by the large gaps in our understanding of glutamate, the primary excitatory neurotransmitter in the brain (Greenamyre et al., 1988; Coyle and Puttfarcken, 1993; Moghaddam and Adams, 1998b; Tapia et al., 1999; Marino et al., 2001; Franks et al., 2002). It is clear that the network regulating glutamate signaling is vastly more complex than for most other neurotransmitters and involves highly regulated interactions between neurons and astrocytes as well as possibly other cells in the brain. System xc− represents an example of this unique, complex network. By releasing glutamate, this mechanism contributes to glutamate receptor activation. In so doing, system xc− and related release mechanisms support the view that there may be multiple, highly specialized signaling compartments between neurons and astrocytes that are distinct from the synapse. As the primary route for cystine uptake, system xc− also supports glutathione synthesis, especially in glia, and thereby supports cellular antioxidant defenses. This poses an intriguing but perhaps perplexing interaction between oxidative stress and excitatory signaling, a further indication of the potentially unappreciated level of complexity related to glutamate activity. Although much remains to be learned regarding the contribution of system xc− to a variety of CNS diseases, the dual role of this mechanism positions it as a potential therapeutic target in diseases linked to either oxidative stress or pathological excitatory signaling. To date, encouraging preclinical or clinical data have been obtained in a wide array of CNS diseases, ranging from Alzheimer's disease and addiction to Parkinson's disease and schizophrenia. Continued efforts toward understanding the contribution of system xc− and related mechanisms have the potential to reshape how we view even basic tenets of neuroscience and to identify novel therapeutic targets.
Acknowledgments
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grant DA025617] (to D.A.B.) and the National Institutes of Health National Institute of Mental Health [Grant MH083417] (to D.A.B.).
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Bridges, Lutgen, Lobner, and Baker.
This article is available online at http://pharmrev.aspetjournals.org.
- ALS
- amyotrophic lateral sclerosis
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoazole-proprionic acid
- BMAA
- β-N-methyl-l-alanine
- CNS
- central nervous system
- EAA
- excitatory amino acid
- EAAT
- excitatory amino acid transporter
- mGluR
- metabotropic glutamate receptor
- MK-801
- dizocilpine maleate
- NMDA
- N-methyl-d-aspartic acid
- SOD
- superoxide dismutase.
References
- Abbott WA, Meister A. (1986) Intrahepatic transport and utilization of biliary glutathione and its metabolites. Proc Natl Acad Sci USA 83:1246–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair JC, Knoefel JE, Morgan N. (2001) Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology 57:1515–1517 [DOI] [PubMed] [Google Scholar]
- Alagarsamy S, Sorensen SD, Conn PJ. (2001) Coordinate regulation of metabotropic glutamate receptors. Curr Opin Neurobiol 11:357–362 [DOI] [PubMed] [Google Scholar]
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. (2011) Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 36:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CL, Iyer SS, Ziegler TR, Jones DP. (2007) Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol 293:R1069–R1075 [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Klivenyi P, Beal MF, Bush AI. (2000) N-acetyl-L-cysteine improves survival and preserves motor performance in an animal model of familial amyotrophic lateral sclerosis. Neuroreport 11:2491–2493 [DOI] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. (2004) Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci 24:6920–6927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Matsumura N, Watabe M, Nakaki T. (2008) Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. Eur J Neurosci 27:20–30 [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. (2006) Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci 9:119–126 [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. (2000) SNARE protein-dependent glutamate release from astrocytes. Neuroscience 20:666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. (1998) Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci 18:6822–6829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, Miyoshi K, Murata M. (2010) Neuroprotective effects of zonisamide target astrocyte. Ann Neurol 67:239–249 [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. (1997) Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron 18:281–293 [DOI] [PubMed] [Google Scholar]
- Aubert A, Pellerin L, Magistretti PJ, Costalat R. (2007) A coherent neurobiological framework for functional neuroimaging provided by a model integrating compartmentalized energy metabolism. Proc Natl Acad Sci USA 104:4188–4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. (2007) Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci 27:111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagh MB, Maiti AK, Jana S, Banerjee K, Roy A, Chakrabarti S. (2008) Quinone and oxyradical scavenging properties of N-acetylcysteine prevent dopamine mediated inhibition of Na+,K+-ATPase and mitochondrial electron transport chain activity in rat brain: implications in the neuroprotective therapy of Parkinson's disease. Free Radic Res 42:574–581 [DOI] [PubMed] [Google Scholar]
- Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I. (2008) Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology 33:1760–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. (2003) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–749 [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. (2002) The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22:9134–9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balansky R, Ganchev G, Iltcheva M, Steele VE, De Flora S. (2011) Prevention of cigarette smoke-induced lung tumors in mice by budesonide, phenethyl isothiocyanate, and N-acetylcysteine. Int J Cancer 126:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs R, Bridges RJ, Cotman CW. (2006) Excitatory Amino Acid Transmission in Health and Disease, Oxford University Press, New York [Google Scholar]
- Banaclocha MM. (2001) Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med Hypotheses 56:472–477 [DOI] [PubMed] [Google Scholar]
- Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kölle P, Tschoep K, Issels RD, Daniel PT, et al. (2008) The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene 27:1618–1628 [DOI] [PubMed] [Google Scholar]
- Bannai S. (1984) Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta 779:289–306 [DOI] [PubMed] [Google Scholar]
- Bannai S. (1986) Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem 261:2256–2263 [PubMed] [Google Scholar]
- Bannai S, Ishii T. (1982) Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol 112:265–272 [DOI] [PubMed] [Google Scholar]
- Bannai S, Kitamura E. (1980) Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem 255:2372–2376 [PubMed] [Google Scholar]
- Bannai S, Kitamura E. (1981) Role of proton dissociation in the transport of cystine and glutamate in human diploid fibroblasts in culture. J Biol Chem 256:5770–5772 [PubMed] [Google Scholar]
- Bannai S, Takada A, Kasuga H, Tateishi N. (1986) Induction of cystine transport activity in isolated rat hepatocytes by sulfobromophthalein and other electrophilic agents. Hepatology 6:1361–1368 [DOI] [PubMed] [Google Scholar]
- Barbour B, Häusser M. (1997) Intersynaptic diffusion of neurotransmitter. Trends Neurosci 20:377–384 [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. (2001) Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry 58:280–288 [DOI] [PubMed] [Google Scholar]
- Barger SW, Goodwin ME, Porter MM, Beggs ML. (2007) Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem 101:1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarsky TA, Feighan D, MacVicar BA. (1999) Glutamate release through volume-activated channels during spreading depression. J Neurosci 19:6439–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskys A, Malenka RC. (1991) Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol 444:687–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. (2008) Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res 104:108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beart PM, O'Shea RD. (2007) Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol 150:5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. (2002) Control of synaptic strength by glial TNFalpha. Science 295:2282–2285 [DOI] [PubMed] [Google Scholar]
- Begni B, Brighina L, Sirtori E, Fumagalli L, Andreoni S, Beretta S, Oster T, Malaplate-Armand C, Isella V, Appollonio I, et al. (2004) Oxidative stress impairs glutamate uptake in fibroblasts from patients with Alzheimer's disease. Free Radic Biol Med 37:892–901 [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 330:585–591 [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. (1984) Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 43:1369–1374 [DOI] [PubMed] [Google Scholar]
- Beretta S, Sala G, Mattavelli L, Ceresa C, Casciati A, Ferri A, Carrì MT, Ferrarese C. (2003) Mitochondrial dysfunction due to mutant copper/zinc superoxide dismutase associated with amyotrophic lateral sclerosis is reversed by N-acetylcysteine. Neurobiol Dis 13:213–221 [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Gundersen V. (2009) Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience 158:260–265 [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. (1997) Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19:1297–1308 [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, et al. (2008a) N-Acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 64:361–368 [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI. (2008b) N-Acetyl cysteine for depressive symptoms in bipolar disorder–a double-blind randomized placebo-controlled trial. Biol Psychiatry 64:468–475 [DOI] [PubMed] [Google Scholar]
- Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. (2011) N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann Neurol 69:509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HG, Steiner J, Bogerts B. (2009) Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert Rev Neurother 9:1059–1071 [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285 [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. (2004) Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7:613–620 [DOI] [PubMed] [Google Scholar]
- Blakely RD, Robinson MB, Thompson RC, Coyle JT. (1988) Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate: subcellular and regional distribution, ontogeny, and the effect of lesions on N-acetylated-alpha-linked acidic dipeptidase activity. J Neurochem 50:1200–1209 [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. (2010) Redox sensitive calcium stores underlie enhanced after hyperpolarization of aged neurons: role for ryanodine receptor mediated calcium signaling. J Neurophysiol 104:2586–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S, Vande Velde C, Cleveland DW. (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52:39–59 [DOI] [PubMed] [Google Scholar]
- Bonvento G, Sibson N, Pellerin L. (2002) Does glutamate image your thoughts? Trends Neurosci 25:359–364 [DOI] [PubMed] [Google Scholar]
- Bordji K, Becerril-Ortega J, Nicole O, Buisson A. (2010) Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ss production. J Neurosci 30:15927–15942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Kettenmann H. (1988) Patch-clamp study of γ-aminobutyric acid receptor Cl− channels in cultured astrocytes. Proc Natl Acad Sci USA 85:9336–9340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford HF, Young AM, Crowder JM. (1987) Continuous glutamate leakage from brain cells is balanced by compensatory high-affinity reuptake transport. Neurosci Lett 81:296–302 [DOI] [PubMed] [Google Scholar]
- Bradley WG, Mash DC. (2009) Beyond Guam: the cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph Lateral Scler 10 (Suppl 2):7–20 [DOI] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS. (2001) Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron 31:607–616 [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, et al. (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611 [DOI] [PubMed] [Google Scholar]
- Bridges CC, Kekuda R, Wang H, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V. (2001) Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42:47–54 [PubMed] [Google Scholar]
- Bridges RJ, Natale NR, Patel SA. (2012) System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 165:20–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Patel SA. (2009) Pharmacology of glutamate transport in the CNS: substrates and inhibitors of excitatory amino acid transporters (EAATs) and the glutamate/cystine exchanger system xc−, in Topics in Medicinal Chemistry: Transporters as Targets for Drugs (Napier S, Bingham M. eds) pp 187–222, Springer, New York [Google Scholar]
- Bröer S, Brookes N. (2001) Transfer of glutamine between astrocytes and neurons. J Neurochem 77:705–719 [DOI] [PubMed] [Google Scholar]
- Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H. (2011) Glutamate release by primary brain tumors induces epileptic activity. Nat Med 17:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut M, Savas HA, Altindag A, Virit O, Dalkilic A. (2009) Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. World J Biol Psychiatry 10:626–628 [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. (2000) Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev 31:138–146 [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. (2004) Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci 22:73–86 [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. (2004) Neuronal oscillations in cortical networks. Science 304:1926–1929 [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Nicolas D, Kraftsik R, Cuénod M, Do KQ, Hornung JP. (2006) Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: relevance to schizophrenia. Neurobiol Dis 22:624–637 [DOI] [PubMed] [Google Scholar]
- Calero CI, Calvo DJ. (2008) Redox modulation of homomeric rho1 GABA receptors. J Neurochem 105:2367–2374 [DOI] [PubMed] [Google Scholar]
- Carter AG, Regehr WG. (2000) Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse. J Neurosci 20:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75:889–907 [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. (2005) Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol 564:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D. (2005) Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol 87:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Okuyama S. (2006) Group II metabotropic glutamate receptor-mediated regulation of dopamine release from slices of rat nucleus accumbens. Neurosci Lett 404:182–186 [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Schaffhauser H, Campbell UC. (2002) Metabotropic glutamate receptors: potential drug targets for the treatment of schizophrenia. Curr Drug Target CNS Neurol Disord 1:261–281 [DOI] [PubMed] [Google Scholar]
- Chen HH, Stoker A, Markou A. (2010) The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology (Berl) 209:343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. (2003) The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem 84:1332–1339 [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120:421–433 [DOI] [PubMed] [Google Scholar]
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25:7101–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WJ, Sontheimer H. (2009) Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-kappaB. J Neurochem 110:182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. (2010) Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS One 5:e12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD. (1996) Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci 19:163–171 [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Alford S. (1998) Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron 20:1007–1016 [DOI] [PubMed] [Google Scholar]
- Conrad M, Sato H. (2012) The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (-): cystine supplier and beyond. Amino Acids 42:231–246 [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova E, Bongioanni P, Cereda C, Metelli MR, Salvaneschi L, Bernuzzi S, Guareschi S, Rossi B, Ceroni M. (2010) Time course of oxidant markers and antioxidant defenses in subgroups of amyotrophic lateral sclerosis patients. Neurochem Int 56:687–693 [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695 [DOI] [PubMed] [Google Scholar]
- D'Alessandro G, Calcagno E, Tartari S, Rizzardini M, Invernizzi RW, Cantoni L. (2011) Glutamate and glutathione interplay in a motor neuronal model of amyotrophic lateral sclerosis reveals altered energy metabolism. Neurobiol Dis 43:346–355 [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. (2007) mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA 104:1995–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J, Sontheimer H. (2011) Glutamate and the biology of gliomas. Glia 59:1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke SM, Fanburg BL. (1989) Regulation of cellular glutathione. Am J Physiol 257:L163–L173 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. (2003) Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci 117:952–960 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24:7167–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. (1997) Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci 17:4672–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. (2000) Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J Neurophysiol 83:2835–2843 [DOI] [PubMed] [Google Scholar]
- DiChiara TJ, Reinhart PH. (1997) Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci 17:4942–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuénod M. (2000) Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci 12:3721–3728 [DOI] [PubMed] [Google Scholar]
- Domercq M, Sánchez-Gómez MV, Sherwin C, Etxebarria E, Fern R, Matute C. (2007) System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol 178:6549–6556 [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Gros C, Hirrlinger J. (2001) Aminopeptidase N mediates the utilization of the GSH precursor CysGly by cultured neurons. J Neurosci Res 66:1003–1008 [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267:4912–4916 [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y, Mysona B, Van Ells T, Amarnath L, Ola MS, Ganapathy V, Smith SB. (2006) Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 324:189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. (2010) Regulation of synaptic connectivity by glia. Nature 468:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Broadie K. (2002) Developmental regulation of glutamate receptor field size by nonvesicular glutamate release. Nat Neurosci 5:141–146 [DOI] [PubMed] [Google Scholar]
- Filomeni G, Rotilio G, Ciriolo MR. (2002) Cell signalling and the glutathione redox system. Biochem Pharmacol 64:1057–1064 [DOI] [PubMed] [Google Scholar]
- Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. (2007) System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J Neurosci 27:10094–10105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Bartol TM, Jr, Sejnowski TJ. (2002) A Monte Carlo model reveals independent signaling at central glutamatergic synapses. Biophys J 83:2333–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. (2003) Independent sources of quantal variability at single glutamatergic synapses. J Neurosci 23:3186–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. (2009) Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32:209–224 [DOI] [PubMed] [Google Scholar]
- Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. (2004) Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res 1016:90–95 [DOI] [PubMed] [Google Scholar]
- Glaum SR, Holzwarth JA, Miller RJ. (1990) Glutamate receptors activate Ca2+ mobilization and Ca2+ influx into astrocytes. Proc Natl Acad Sci USA 87:3454–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Rotaru DC, Zaitsev AV, Povysheva NV, Lewis DA. (2009) GABA transporter GAT1 prevents spillover at proximal and distal GABA synapses onto primate prefrontal cortex neurons. J Neurophysiol 101:533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Odlaug BL. (2007) N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry 62:652–657 [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW. (2009) N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 66:756–763 [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, Maragos WF, Albin RL, Penney JB, Young AB. (1988) Glutamate transmission and toxicity in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry 12:421–430 [DOI] [PubMed] [Google Scholar]
- Grybko M, Sharma G, Vijayaraghavan S. (2010) Functional distribution of nicotinic receptors in CA3 region of the hippocampus. J Mol Neurosci 40:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. (2007) Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 55:78–92 [DOI] [PubMed] [Google Scholar]
- Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH. (2005) Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57:123–131 [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, Linden DE, Rodriguez E. (2009) Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci 29:9481–9489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. (2011) Glia: initiators and progressors of pathology in Parkinson's disease. Mov Disord 26:6–17 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658 [DOI] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. (2010) Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11:227–238 [DOI] [PubMed] [Google Scholar]
- Haydon PG. (2001) GLIA: listening and talking to the synapse. Nat Rev Neurosci 2:185–193 [DOI] [PubMed] [Google Scholar]
- Haydon PG, Blendy J, Moss SJ, Rob Jackson F. (2009) Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology 56 (Suppl 1):83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. (2004) The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch 447:465–468 [DOI] [PubMed] [Google Scholar]
- Hestrin S, Sah P, Nicoll RA. (1990) Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron 5:247–253 [DOI] [PubMed] [Google Scholar]
- Hirai K, Yoshioka H, Kihara M, Hasegawa K, Sakamoto T, Sawada T, Fushiki S. (1999) Inhibiting neuronal migration by blocking NMDA receptors in the embryonic rat cerebral cortex: a tissue culture study. Brain Res Dev Brain Res 114:63–67 [DOI] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. (2005) Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol 93:1989–2001 [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. (2003) Gamma oscillations correlate with working memory load in humans. Cereb Cortex 13:1369–1374 [DOI] [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. (1999a) The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther 289:412–416 [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. (1999b) The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther 289:412–416 [PubMed] [Google Scholar]
- Isaacson JS, Nicoll RA. (1993) The uptake inhibitor L-trans-PDC enhances responses to glutamate but fails to alter the kinetics of excitatory synaptic currents in the hippocampus. J Neurophysiol 70:2187–2191 [DOI] [PubMed] [Google Scholar]
- Iuchi Y, Kibe N, Tsunoda S, Okada F, Bannai S, Sato H, Fujii J. (2008) Deficiency of the cystine-transporter gene, xCT, does not exacerbate the deleterious phenotypic consequences of SOD1 knockout in mice. Mol Cell Biochem 319:125–132 [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Guchelaar HJ, Haasdijk E, de Jong JM, Holstege JC. (1998) The antioxidant N-acetylcysteine does not delay disease onset and death in a transgenic mouse model of amyotrophic lateral sclerosis. Ann Neurol 44:293. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler BH, Gerber U. (1999) Inhibition of uptake unmasks rapid extracellular turnover of gluamate of nonvesicular origin. Proc Natl Acad Sci USA 96:8733–8738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. (2010) Regulation of system x(c)(-)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia 58:1806–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. (2004) NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA 101:8467–8472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janáky R, Ogita K, Pasqualotto BA, Bains JS, Oja SS, Yoneda Y, Shaw CA. (1999) Glutathione and signal transduction in the mammalian CNS. J Neurochem 73:889–902 [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, Bear MF, Umbricht D, Hajos M, Potter WZ, et al. (2011) Translating glutamate: from pathophysiology to treatment. Sci Transl Med 3:102mr2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308 [DOI] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. (2003) GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol 90:2690–2701 [DOI] [PubMed] [Google Scholar]
- Kaleeba JA, Berger EA. (2006) Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 311:1921–1924 [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650 [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. (2004) The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch 447:469–479 [DOI] [PubMed] [Google Scholar]
- Kanamori K, Ross BD. (2004) Quantitative determination of extracellular glutamine concentration in rat brain, and its elevation in vivo by system A transport inhibitor, alpha-(methylamino)isobutyrate. J Neurochem 90:203–210 [DOI] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. (2008) Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience 155:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M, Misu Y, Kubo T. (1989) Release by electrical stimulation of endogenous glutamate, Γ-aminobutyric acid, and other amino acids from slices of the rat medulla oblongata. J Neurochem 52:261–267 [DOI] [PubMed] [Google Scholar]
- Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK, Inatomi J, Sawa H, Ida Y, Endou H. (2001) Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta 1512:335–344 [DOI] [PubMed] [Google Scholar]
- Kim YG, Lee S, Kwon OS, Park SY, Lee SJ, Park BJ, Kim KJ. (2009) Redox-switch modulation of human SSADH by dynamic catalytic loop. EMBO J 28:959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. (1990) Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 10:1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. (2007) Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322:1103–1109 [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. (2010) Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein RG, Seres T, Lam G, Johnston RB, Jr, Warshaw JB. (1997) Characterization of multiple cysteine and cystine transporters in rat alveolar type II cells. Am J Physiol 273:L1147–L1155 [DOI] [PubMed] [Google Scholar]
- Köhr G, Eckardt S, Lüddens H, Monyer H, Seeburg PH. (1994) NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron 12:1031–1040 [DOI] [PubMed] [Google Scholar]
- Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. (2004) Properties of Ca(2+)-dependent exocytosis in cultured astrocytes. Glia 46:437–445 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214 [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. (2011) Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA 108:E440–E449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. (2012) The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry 71:978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. (2010) Glia as a turning point in the therapeutic strategy of Parkinson's disease. CNS Neurol Disord Drug Targets 9:349–372 [DOI] [PubMed] [Google Scholar]
- Langer J, Rose CR. (2009) Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol 587:5859–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langle SL, Poulain DA, Theodosis DT. (2002) Neuronal-glial remodeling: a structural basis for neuronal-glial interactions in the adult hypothalamus. J Physiol Paris 96:169–175 [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. (2006) Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict 15:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. (2007) Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry 164:1115–1117 [DOI] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, et al. (2008) Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 33:2187–2199 [DOI] [PubMed] [Google Scholar]
- Leary GP, Holley DC, Stone EF, Lyda BR, Kalachev LV, Kavanaugh MP. (2011) The central cavity in trimeric glutamate transporters restricts ligand diffusion. Proc Natl Acad Sci USA 108:14980–14985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. (1998) The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 18:8751–8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. (1995) Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 15:1835–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Rusakov DA. (2002) Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J 83:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G, Raiteri M. (1993) Carrier-mediated release of neurotransmitters. Trends Neurosci 16:415–419 [DOI] [PubMed] [Google Scholar]
- Levy LM, Lehre KP, Rolstad B, Danbolt NC. (1993) A monoclonal antibody raised against an [Na++K+]coupled L-glutamate transporter purified from rat brain confirms glial cell localization. FEBS Lett 317:79–84 [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. (1998) Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci 18:9620–9628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. (2009) Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem 111:332–343 [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Klein M, Methner A. (2006) Cooperative action of glutamate transporters and cystine/glutamate antiporter system xc− protects from oxidative glutamate toxicity. J Neurochem 98:916–925 [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Maher P. (2009) Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 284:1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. (2006) Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol 63:1372–1376 [DOI] [PubMed] [Google Scholar]
- Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT, Chui D, Hoi Yu AC. (2011) Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer's disease. Curr Alzheimer Res 8:67–80 [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795 [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA. (2002) Cysteine regulation of protein function–as exemplified by NMDA-receptor modulation. Trends Neurosci 25:474–480 [DOI] [PubMed] [Google Scholar]
- Lisman J. (2011) Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol http://dx.doi.org/10.1016/j.conb.2011.10.018 [DOI] [PMC free article] [PubMed]
- Liu X, Rush T, Zapata J, Lobner D. (2009) β-N-methylamino-l-alanine induces oxidative stress and glutamate release through action on system xc(-). Exp Neurol 217:429–433 [DOI] [PubMed] [Google Scholar]
- Lo M, Wang YZ, Gout PW. (2008) The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol 215:593–602 [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. (2003) Effects of ketamine and N-methyl-d-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706 [DOI] [PubMed] [Google Scholar]
- Louwerse ES, Weverling GJ, Bossuyt PM, Meyjes FE, de Jong JM. (1995) Randomized, double-blind, controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Arch Neurol 52:559–564 [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. (1959) Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 81:363–369 [DOI] [PubMed] [Google Scholar]
- Lushnikova I, Skibo G, Muller D, Nikonenko I. (2009) Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus 19:753–762 [DOI] [PubMed] [Google Scholar]
- Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. (2007) Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 67:9463–9471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. (2005) Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry 162:475–484 [DOI] [PubMed] [Google Scholar]
- Madayag A, Kau KS, Lobner D, Mantsch JR, Wisniewski S, Baker DA. (2010) Drug-induced plasticity contributing to heightened relapse susceptibility: neurochemical changes and augmented reinstatement in high-intake rats. J Neurosci 30:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. (2007) Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 27:13968–13976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. (2008) Mechanisms of glutamate release from astrocytes. Neurochem Int 52:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A. (1997) Clozapine blunts N-methyl-d-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry 42:664–668 [DOI] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. (2005) A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci 25:4755–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. (2007) An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry 31:389–394 [DOI] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. (2001) Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci 21:7001–7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie A, Schallier A, Kim SW, Fernando R, Kobayashi S, Beck H, De Bundel D, Vermoesen K, Bannai S, Smolders I, et al. (2011) Dopaminergic neurons of system x(c)−-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. FASEB J 25:1359–1369 [DOI] [PubMed] [Google Scholar]
- Massie A, Schallier A, Mertens B, Vermoesen K, Bannai S, Sato H, Smolders I, Michotte Y. (2008) Time-dependent changes in striatal xCT protein expression in hemi-Parkinson rats. Neuroreport 19:1589–1592 [DOI] [PubMed] [Google Scholar]
- McBean GJ. (2002) Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci 23:299–302 [DOI] [PubMed] [Google Scholar]
- McBean GJ. (2012) The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids 42:199–205 [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. (2008) Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr 13:501–510 [DOI] [PubMed] [Google Scholar]
- Meister A. (1991) Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther 51:155–194 [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. (2005) Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther 314:139–147 [DOI] [PubMed] [Google Scholar]
- Mena FV, Baab PJ, Zielke CL, Huang Y, Zielke HR. (2005) Formation of extracellular glutamate from glutamine: exclusion of pyroglutamate as an intermediate. Brain Res 1052:88–96 [DOI] [PubMed] [Google Scholar]
- Miele M, Boutelle MG, Fillenz M. (1996) The source of physiologically stimulated glutamate efflux from the striatum of conscious rats. J Physiol 497:745–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. (2009) The role of glial cells in drug abuse. Curr Drug Abuse Rev 2:72–82 [PubMed] [Google Scholar]
- Min R, Nevian T. (2012) Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 15:746–753 [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. (1998a) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352 [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. (1998b) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352 [DOI] [PubMed] [Google Scholar]
- Molina-Jimenez MF, Sanchez-Reus MI, Benedi J. (2003) Effect of fraxetin and myricetin on rotenone-induced cytotoxicity in SH-SY5Y cells: comparison with N-acetylcysteine. Eur J Pharmacol 472:81–87 [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. (2006) Vesicular transmitter release from astrocytes. Glia 54:700–715 [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. (2005) Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25:6389–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. (2011) Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci USA 108:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ. (2010) Inhibition of glutamate transporters couples to Kv4.2 dephosphorylation through activation of extrasynaptic NMDA receptors. Neuroscience 165:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Hasegawa K, Kanazawa I, and Japan Zonisamide on PD Study Group (2007) Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology 68:45–50 [DOI] [PubMed] [Google Scholar]
- Murata M, Horiuchi E, Kanazawa I. (2001) Zonisamide has beneficial effects on Parkinson's disease patients. Neurosci Res 41:397–399 [DOI] [PubMed] [Google Scholar]
- Murphy TH, Baraban JM. (1990) Glutamate toxicity in immature cortical neurons precedes development of glutamate receptor currents. Brain Res Dev Brain Res 57:146–150 [DOI] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2:1547–1558 [DOI] [PubMed] [Google Scholar]
- Murphy TH, Schnaar RL, Coyle JT. (1990) Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. Faseb J 4:1624–1633 [PubMed] [Google Scholar]
- Murray JE, Everitt BJ, Belin D. (2012) N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol 17:437–440 [DOI] [PubMed] [Google Scholar]
- Mysona B, Dun Y, Duplantier J, Ganapathy V, Smith SB. (2009) Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Müller glial cells. Cell Tissue Res 335:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Otsubo Y, Yatani Y, Shirakawa H, Kaneko S. (2008) Mechanisms of substrate transport-induced clustering of a glial glutamate transporter GLT-1 in astroglial-neuronal cultures. Eur J Neurosci 28:1719–1730 [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. (2010) Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68:113–126 [DOI] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. (2006) Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend 82:19–24 [DOI] [PubMed] [Google Scholar]
- Newcomb R, Palma A. (1994) Effects of diverse omega-conopeptides on the in vivo release of glutamic and gamma-aminobutyric acids. Brain Res 638:95–102 [DOI] [PubMed] [Google Scholar]
- Newman EA. (2003) New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci 26:536–542 [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. (1998) Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21:545–559 [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M. (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29:547–553 [DOI] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. (2002) Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113:221–233 [DOI] [PubMed] [Google Scholar]
- Ogita K, Enomoto R, Nakahara F, Ishitsubo N, Yoneda Y. (1995) A possible role of glutathione as an endogenous agonist at the N-methyl-d-aspartate recognition domain in rat brain. J Neurochem 64:1088–1096 [DOI] [PubMed] [Google Scholar]
- Oja SS, Janáky R, Varga V, Saransaari P. (2000) Modulation of glutamate receptor functions by glutathione. Neurochem Int 37:299–306 [DOI] [PubMed] [Google Scholar]
- Olivieri G, Baysang G, Meier F, Müller-Spahn F, Stähelin HB, Brockhaus M, Brack C. (2001) N-Acetyl-l-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: effects on beta-amyloid secretion and tau phosphorylation. J Neurochem 76:224–233 [DOI] [PubMed] [Google Scholar]
- Onyango IG, Bennett JP, Jr, Tuttle JB. (2005a) Endogenous oxidative stress in sporadic Alzheimer's disease neuronal cybrids reduces viability by increasing apoptosis through pro-death signaling pathways and is mimicked by oxidant exposure of control cybrids. Neurobiol Dis 19:312–322 [DOI] [PubMed] [Google Scholar]
- Onyango IG, Tuttle JB, Bennett JP., Jr(2005b) Activation of p38 and N-acetylcysteine-sensitive c-Jun NH2-terminal kinase signaling cascades is required for induction of apoptosis in Parkinson's disease cybrids. Mol Cell Neurosci 28:452–461 [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. (2003) Synapse density regulates independence at unitary inhibitory synapses. J Neurosci 23:2618–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owe SG, Marcaggi P, Attwell D. (2006) The ionic stoichiometry of the GLAST glutamate transporter in salamander retinal glia. J Physiol 577:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, Buck A, Mash DC. (2009) Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand 120:216–225 [DOI] [PubMed] [Google Scholar]
- Pan ZH, Bähring R, Grantyn R, Lipton SA. (1995) Differential modulation by sulfhydryl redox agents and glutathione of GABA- and glycine-evoked currents in rat retinal ganglion cells. J Neurosci 15:1384–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZH, Zhang X, Lipton SA. (2000) Redox modulation of recombinant human GABA(A) receptors. Neuroscience 98:333–338 [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. (2006) Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125:775–784 [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458 [DOI] [PubMed] [Google Scholar]
- Park SW, Kim SH, Park KH, Kim SD, Kim JY, Baek SY, Chung BS, Kang CD. (2004) Preventive effect of antioxidants in MPTP-induced mouse model of Parkinson's disease. Neurosci Lett 363:243–246 [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. (2002) Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci 22:2916–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747 [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. (2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA 97:8629–8634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. (2001) Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci 21:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Rajale T, O'Brien E, Burkhart DJ, Nelson JK, Twamley B, Blumenfeld A, Szabon-Watola MI, Gerdes JM, Bridges RJ, et al. (2010) Isoxazole analogues bind the system xc- transporter: structure-activity relationship and pharmacophore model. Bioorg Med Chem 18:202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Warren BA, Rhoderick JF, Bridges RJ. (2004) Differentiation of substrate and non-substrate inhibitors of transport system xc(-): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology 46:273–284 [DOI] [PubMed] [Google Scholar]
- Pearlson GD. (1981) Psychiatric and medical syndromes associated with phencyclidine (PCP) abuse. Johns Hopkins Med J 148:25–33 [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyam S, Mohan A, Kalivas PW, Nair SS. (2009) Computational model of extracellular glutamate in the nucleus accumbens incorporates neuroadaptations by chronic cocaine. Neuroscience 158:1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penugonda S, Mare S, Goldstein G, Banks WA, Ercal N. (2005) Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12. Brain Res 1056:132–138 [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. (1997) Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron 19:1237–1248 [DOI] [PubMed] [Google Scholar]
- Piani D, Fontana A. (1994) Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol 152:3578–3585 [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. (1996) Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci 16:1550–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttimaki TM, Hall SD, Parri HR. (2011) Sustained neuronal activity generated by glial plasticity. J Neurosci 31:7637–7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivetta LA, Pereira RP, Farinon M, de Bem AF, Perottoni J, Soares JC, Duarte MM, Zeni G, Rocha JB, Farina M. (2006) Ethanol inhibits delta-aminolevulinate dehydratase and glutathione peroxidase activities in mice liver: protective effects of ebselen and N-acetylcysteine. Environ Toxicol Pharmacol 21:338–343 [DOI] [PubMed] [Google Scholar]
- Poskanzer KE, Yuste R. (2011) Astrocytic regulation of cortical UP states. Proc Natl Acad Sci USA 108:18453–18458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV. (2001) Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia 34:27–38 [DOI] [PubMed] [Google Scholar]
- Pruss RM, Akeson RL, Racke MM, Wilburn JL. (1991) Agonist-activated cobalt uptake identifies divalent cation-permeable kainate receptors on neurons and glial cells. Neuron 7:509–518 [DOI] [PubMed] [Google Scholar]
- Qin S, Colin C, Hinners I, Gervais A, Cheret C, Mallat M. (2006) System xc- and apolipoprotein E expressed by microglia have opposite effects on the neurotoxicity of amyloid-beta peptide 1–40. J Neurosci 26:3345–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. (2004) Properties of quantal transmission at CA1 synapses. J Neurophysiol 92:2456–2467 [DOI] [PubMed] [Google Scholar]
- Rajan DP, Kekuda R, Huang W, Wang H, Devoe LD, Leibach FH, Prasad PD, Ganapathy V. (1999) Cloning and expression of a b(0,+)-like amino acid transporter functioning as a heterodimer with 4F2hc instead of rBAT. A new candidate gene for cystinuria. J Biol Chem 274:29005–29010 [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. (2012) Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J Affect Disord 136:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rappold PM, Tieu K. (2010) Astrocytes and therapeutics for Parkinson's disease. Neurotherapeutics 7:413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan RR, Murphy TH, Baraban JM. (1994) Macromolecular synthesis inhibitors prevent oxidative stress-induced apoptosis in embryonic cortical neurons by shunting cysteine from protein synthesis to glutathione. J Neurosci 14:4385–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray M, Bendotti C. (2006) Does excitotoxic cell death of motor neurons in ALS arise from glutamate transporter and glutamate receptor abnormalities? Exp Neurol 201:15–23 [DOI] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. (2011) Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther 337:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Berger SP. (1996) Evidence for sensitization of cocaine induced nucleus accumbens glutamate release. Neuroreport 7:1325–1329 [DOI] [PubMed] [Google Scholar]
- Robinson RA, Joshi G, Huang Q, Sultana R, Baker AS, Cai J, Pierce W, St Clair DK, Markesbery WR, Butterfield DA. (2011) Proteomic analysis of brain proteins in APP/PS-1 human double mutant knock-in mice with increasing amyloid beta-peptide deposition: insights into the effects of in vivo treatment with N-acetylcysteine as a potential therapeutic intervention in mild cognitive impairment and Alzheimer's disease. Proteomics 11:4243–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronne-Engström E, Carlson H, Liu Y, Ungerstedt U, Hillered L. (1995) Influence of perfusate glucose concentration on dialysate lactate, pyruvate, aspartate, and glutamate levels under basal and hypoxic conditions: a microdialysis study in rat brain. J Neurochem 65:257–262 [DOI] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hyder F, Shulman RG. (2003) In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu Rev Physiol 65:401–427 [DOI] [PubMed] [Google Scholar]
- Rothstein JD. (2002) Paving new pathways. Nat Med 8:938–940 [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, et al. (2005) Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433:73–77 [DOI] [PubMed] [Google Scholar]
- Rusakov DA. (2001) The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca(2+) depletion. Biophys J 81:1947–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Harrison E, Stewart MG. (1998) Synapses in hippocampus occupy only 1–2% of cell membranes and are spaced less than half-micron apart: a quantitative ultrastructural analysis with discussion of physiological implications. Neuropharmacology 37:513–521 [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. (1998) Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci 18:3158–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Lehre KP. (2002) Perisynaptic asymmetry of glia: new insights into glutamate signalling. Trends Neurosci 25:492–494 [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Savtchenko LP, Zheng K, Henley JM. (2011) Shaping the synaptic signal: molecular mobility inside and outside the cleft. Trends Neurosci 34:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara JI, Miura K, Bannai S. (1993) Maintenance of neuronal glutathione by glial cells. J Neurochem 61:1672–1676 [DOI] [PubMed] [Google Scholar]
- Sarantis M, Ballerini L, Miller B, Silver RA, Edwards M, Attwell D. (1993) Glutamate uptake from the synaptic cleft does not shape the decay of the non-NMDA component of the synaptic current. Neuron 11:541–549 [DOI] [PubMed] [Google Scholar]
- Sarantis M, Mobbs P. (1992) The spatial relationship between Muller cell processes and the photoreceptor output synapse. Brain Res 584:299–304 1992 [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274:11455–11458 [DOI] [PubMed] [Google Scholar]
- Savtchenko LP, Rusakov DA. (2004) Glutamate escape from a tortuous synaptic cleft of the hippocampal mossy fibre synapse. Neurochem Int 45:479–484 [DOI] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. (1997) Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature 385:630–634 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. (2010) Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans 38:476–481 [DOI] [PubMed] [Google Scholar]
- Seib TM, Patel SA, Bridges RJ. (2011) Regulation of the system x(C)- cystine/glutamate exchanger by intracellular glutathione levels in rat astrocyte primary cultures. Glia 59:1387–1401 [DOI] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. (2000) Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron 25:663–672 [DOI] [PubMed] [Google Scholar]
- Shaw CA, Pasqualotto BA, Curry K. (1996) Glutathione-induced sodium currents in neocortex. Neuroreport 7:1149–1152 [DOI] [PubMed] [Google Scholar]
- Shen JX, Yakel JL. (2012) Functional alpha7 nicotinic ACh receptors on astrocytes in rat hippocampal CA1 slices. J Mol Neurosci http://10.1007/s12031-012-9719-3 [DOI] [PMC free article] [PubMed]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. (2006) Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci 26:10514–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. (2003) Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 23:3394–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. (2003) Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry 64:1440–1448 [DOI] [PubMed] [Google Scholar]
- Sies H. (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921 [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Lachance PE, Lasko P, Schuster CM. (2000) Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature 405:1062–1065 [DOI] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE. (2001) Differing, spatially restricted roles of ionotropic glutamate receptors in regulating the migration of gnrh neurons during embryogenesis. J Neurosci 21:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. (1999) Neuronal synchrony: a versatile code for the definition of relations? Neuron 24:49-65, 111–125 [DOI] [PubMed] [Google Scholar]
- Song MY, Makino A, Yuan JX. (2011) Role of reactive oxygen species and redox in regulating the function of transient receptor potential channels. Antioxid Redox Signal 15:1549–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Kettenmann H, Backus KH, Schachner M. (1988) Glutamate opens Na+/K+ channels in cultured astrocytes. Glia 1:328–336 [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. (2006) Synaptic scaling mediated by glial TNF-alpha. Nature 440:1054–1059 [DOI] [PubMed] [Google Scholar]
- Stern JR, Eggleston LV, Hems R, Krebs HA. (1949) Accumulation of glutamic acid in isolated brain tissue. Biochem J 44:410–418 [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Neijt HC, Cuénod M, Do KQ. (2006) Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience 137:807–819 [DOI] [PubMed] [Google Scholar]
- Stevens B. (2008) Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals 16:278–288 [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178 [DOI] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. (1992) Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci USA 89:10955–10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol 270:C711–C730 [DOI] [PubMed] [Google Scholar]
- Surendran S, Rajasankar S. (2010) Parkinson's disease: oxidative stress and therapeutic approaches. Neurol Sci 31:531–540 [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. (2004) Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology 29:2149–2159 [DOI] [PubMed] [Google Scholar]
- Taguchi K, Tamba M, Bannai S, Sato H. (2007) Induction of cystine/glutamate transporter in bacterial lipopolysaccharide induced endotoxemia in mice. J Inflamm (Lond) 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. (1995) Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci USA 92:4616–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. (2001) Glutamate release promotes growth of malignant gliomas. Nat Med 7:1010–1015 [DOI] [PubMed] [Google Scholar]
- Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen OP. (1999) Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci 2:618–624 [DOI] [PubMed] [Google Scholar]
- Tamarappoo BK, Raizada MK, Kilberg MS. (1997) Identification of a system N-like Na(+)-dependent glutamine transport activity in rat brain neurons. J Neurochem 68:954–960 [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. (2001) Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience 106:481–503 [DOI] [PubMed] [Google Scholar]
- Tanel A, Averill-Bates DA. (2007) Inhibition of acrolein-induced apoptosis by the antioxidant N-acetylcysteine. J Pharmacol Exp Ther 321:73–83 [DOI] [PubMed] [Google Scholar]
- Tapia R, Medina-Ceja L, Peña F. (1999) On the relationship between extracellular glutamate, hyperexcitation and neurodegeneration, in vivo. Neurochem Int 34:23–31 [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. (1997) Brain microdialysis of GABA and glutamate: what does it signify? Synapse 27:242–261 [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. (1994) Block of glutamate transporters potentiates postsynaptic excitation. Neuron 13:1195–1203 [DOI] [PubMed] [Google Scholar]
- Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, Volterra A. (1997) Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci 9:1236–1243 [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. (2007) Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci 8:935–947 [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. (2010) Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11:100–113 [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. (2004) Role for glia in synaptogenesis. Glia 47:209–216 [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. (2001) Control of synapse number by glia. Science 291:657–661 [DOI] [PubMed] [Google Scholar]
- Uys JD, LaLumiere RT. (2008) Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol Disord Drug Targets 7:482–491 [DOI] [PubMed] [Google Scholar]
- Varga V, Janáky R, Marnela KM, Gulyás J, Kontro P, Oja SS. (1989) Displacement of excitatory amino acid receptor ligands by acidic oligopeptides. Neurochem Res 14:1223–1227 [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. (2008) Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci 28:13574–13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19:6897–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287:864–869 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. (1998) Glial calcium: homeostasis and signaling function. Physiol Rev 78:99–141 [DOI] [PubMed] [Google Scholar]
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 447:532–542 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. (1991) Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 148:621–626 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. (1999) Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry 156:19–26 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. (2005) Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci 25:3932–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6:626–640 [DOI] [PubMed] [Google Scholar]
- von Blankenfeld G, Kettenmann H. (1991) Glutamate and GABA receptors in vertebrate glial cells. Mol Neurobiol 5:31–43 [DOI] [PubMed] [Google Scholar]
- Vyleta NP, Smith SM. (2011) Spontaneous glutamate release is independent of calcium influx and tonically activated by the calcium-sensing receptor. J Neurosci 31:4593–4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade LA, Brady HM. (1981) Cysteine and cystine transport at the blood-brain barrier. J Neurochem 37:730–734 [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. (1995) Kinetics of a human glutamate transporter. Neuron 14:1019–1027 [DOI] [PubMed] [Google Scholar]
- Wang M, Yao Y, Kuang D, Hampson DR. (2006) Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J Biol Chem 281:8864–8870 [DOI] [PubMed] [Google Scholar]
- Wang XF, Cynader MS. (2000) Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem 74:1434–1442 [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. (1984) Characterization of L-glutamic acid transport by glioma cells in culture: evidence for sodium-independent, chloride-dependent high affinity influx. J Neurosci 4:2237–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr O, Takahashi M, Attwell D. (1999) Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol 514:783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BA, Patel SA, Nunn PB, Bridges RJ. (2004) The Lathyrus excitotoxin β-N-oxalyl-L-α,β-diaminopropionic acid is a substrate of the L-cystine/L-glutamate exchanger system xc. Toxicol Appl Pharmacol 200:83–92 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Bannai S. (1987) Induction of cystine transport activity in mouse peritoneal macrophages. J Exp Med 165:628–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JC, Jane DE. (2006) The glutamate story. Br J Pharmacol 147 (Suppl 1):S100–S108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. (1987) Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44:660–669 [DOI] [PubMed] [Google Scholar]
- Weiss JH, Choi DW. (1988) Beta-N-methylamino-L-alanine neurotoxicity: requirement for bicarbonate as a cofactor. Science 241:973–975 [DOI] [PubMed] [Google Scholar]
- Wolf ME. (2010) The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Mathie A, Symonds CJ, Cull-Candy SG. (1991) Activation of glutamate receptors and glutamate uptake in identified macroglial cells in rat cerebellar cultures. J Physiol 432:235–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. (2002a) Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300:162–171 [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. (2002b) Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther 303:608–615 [DOI] [PubMed] [Google Scholar]
- Yao Z, Wood NW. (2009) Cell death pathways in Parkinson's disease: role of mitochondria. Antioxid Redox Signal 11:2135–2149 [DOI] [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. (1999) Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci 19:10767–10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. (1999) Glioma cells release excitotoxic concentrations of glutamate. Cancer Res 59:4383–4391 [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23:3588–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Nylander I, Goiny M, O'Connor WT, Ungerstedt U, Terenius L. (1994) The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis–II. Effects of dopamine D1 and D2 receptor agonists. Neuroscience 63:427–434 [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Pleasure D, Cregar L, Lin ZP, Nissim I, Stern J, Nissim I. (1990) Glutathione turnover in cultured astrocytes: studies with [15N]glutamate. J Neurochem 55:137–145 [DOI] [PubMed] [Google Scholar]
- Zanassi P, Paolillo M, Montecucco A, Avvedimento EV, Schinelli S. (1999) Pharmacological and molecular evidence for dopamine D(1) receptor expression by striatal astrocytes in culture. J Neurosci Res 58:544–552 [DOI] [PubMed] [Google Scholar]
- Zängerle L, Cuénod M, Winterhalter KH, Do KQ. (1992) Screening of thiol compounds: depolarization-induced release of glutathione and cysteine from rat brain slices. J Neurochem 59:181–189 [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. (1996a) ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J Biol Chem 271:27991–27994 [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. (1996b) Flux coupling in a neuronal glutamate transporter. Nature 383:634–637 [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. (1996c) Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol 493:419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. (2004) Fusion-related release of glutamate from astrocytes. J Biol Chem 279:12724–12733 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Rempe DA. (2010) Targeting astrocytes for stroke therapy. Neurotherapeutics 7:439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Scimemi A, Rusakov DA. (2008) Receptor actions of synaptically released glutamate: the role of transporters on the scale from nanometers to microns. Biophys J 95:4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Neale JH, Pomper MG, Kozikowski AP. (2005) NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov 4:1015–1026 [DOI] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. (2008) N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry 63:338–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolella S, Beghi E, Palagano G, Fraddosio A, Guerra V, Samarelli V, Lepore V, Simone IL, Lamberti P, Serlenga L, et al. (2007) Riluzole and amyotrophic lateral sclerosis survival: a population-based study in southern Italy. Eur J Neurol 14:262–268 [DOI] [PubMed] [Google Scholar]