Abstract
The regenerating islet-derived members (Reg), a group of small secretory proteins, which are involved in cell proliferation or differentiation in digestive organs, are upregulated in several gastrointestinal cancers, functioning as trophic or antiapoptotic factors. Regenerating islet-derived type IV (RegIV), a member of the Reg gene family, has been reported to be overexpressed in gastroenterological cancers. RegIV overexpression in tumor cells has been associated with carcinogenesis, cell growth, survival and resistance to apoptosis. Cancer tissue expressing RegIV is generally associated with more malignant characteristics than that without such expression, and RegIV is considered a novel prognostic factor as well as diagnostic marker in some gastroenterological cancers. We previously investigated the expression levels of RegIV mRNA of 202 surgical colorectal cancer specimens with quantitative real-time reverse-transcriptase polymerase chain reaction and reported that a higher level of RegIV gene expression was a significant independent predictor of colorectal cancer. The biologic functions of RegIV protein in cancer tissue, associated with carcinogenesis, anti-apoptosis and invasiveness, are being elucidated by molecular investigations using transfection techniques or neutralizing antibodies of RegIV, and the feasibility of antibody therapy targeting RegIV is being assessed. These studies may lead to novel therapeutic strategies for gastroenterological cancers expressing RegIV. This review article summarizes the current information related to biological functions as well as clinical importance of RegIV gene to clarify the significance of RegIV expression in gastroenterological cancers.
Keywords: Regenerating islet-derived type IV protein, Gastrointestinal neoplasms, Prognosis, Epidermal growth factor receptor/protein kinase B
INTRODUCTION
It is generally accepted that cancer develops as a result of multiple genetic alterations. A better understanding of the changes in gene expression that occur during carcinogenesis may lead to improvements in diagnosis, treatment and prevention. Identification of novel biomarkers for cancer diagnosis and novel targets for treatment is a major goal[1]. Genes encoding transmembrane/secretory proteins strongly expressed in cancer may be ideal biomarkers for cancer diagnosis[2]. In addition, if the function of the gene product is involved in the neoplastic process, such genes may constitute a therapeutic target.
The regenerating islet-derived members (Reg) are a family of genes belonging to the calcium-dependent lectin (C-type lectin) gene superfamily[3-6]. Reg represents a group of small secretory proteins that are essential for cell regeneration and proliferation and form an immune system[7,8]. Reg plays a wide range of roles in human physiology as well as in disease[9-15] (Table 1). Among the human Reg family, special attention has been paid to Regenerating islet-derived type IV (RegIV), the most recently discovered member. RegIV was originally isolated from a cDNA library of ulcerative colitis tissues by Hartupee et al[3]. It is expressed not only in various normal tissues such as stomach, colon, small intestine and pancreas[16,17], but also in various malignant diseases, including colorectal[18], gastric[19], pancreatic[20] and gallbladder cancer[21]. The biological functions of RegIV in cancers are not fully understood; however, several possible functions have been proposed.
Table 1.
Human Reg family
| Superfamily member | Length of amino acid | Chromosome localization | Main function |
| RegIA | 166 | 2p12 | Islet cell regeneration, diabetogenesis and pancreatic lithogenesis |
| RegIB | 166 | 2p12 | Islet cell regeneration, diabetogenesis and pancreatic lithogenesis |
| RegIIIA | 175 | 2p12 | Cell proliferation and differentiation in pancreatic inflammation and liver carcinogenesis |
| RegIIIG | 175 | 2p12 | Lectin-mediated innate immunity by binding intestinal bacteria, forming symbiotic host-microbial relationships |
| RegIV | 158 | 1p12 | Cell proliferation and cell regeneration. Carcinogenesis and anti-apoptosis in gastroenterological cancers |
Reg: Regenerating gene; RegIV: Regenerating islet-derived type IV.
MOLECULAR CHARACTERISTICS OF REGIV AND OTHER REG GENES
Using sequence analysis, Hartupee et al[3] mapped the RegIV gene to chromosome 1 and determined that the RegIV gene contains 6 exons, the structure of which is preserved among members of the Reg gene family. Zhang et al[22] also showed that most members of the Reg family have similar organization with respect to exon number and chromosome location.
Previous studies have revealed that the Reg family shares strong similarities with C-type lectin[3], which is distinguished from other lectins by sharing a calcium-dependent carbohydrate recognition domain (CRD). This domain of lectin might account for the complex events induced by RegIV and other Reg genes[23-25]. Unlike RegIV, however, other Reg genes are present at low or undetectable levels in most tumors[11]. Analysis of the unique structural similarities and differences between RegIV and other members of this gene family are expected to provide a basis for investigations of structure-function relations in this gene family.
As for binding of RegIV to other molecules or putative receptors, detailed interactions have not been elucidated. Ho et al[26] recently provided evidence that human RegIV binds to polysaccharides and mannan in the absence of calcium, unlike other C-type lectins. Utilizing nuclear magnetic resonance to elucidate the structural basis for carbohydrate recognition of RegIV, they found that RegIV has two calcium-independent mannan-binding sites serving as CRDs, suggesting a potential role in specific carbohydrate recognition[26]. These findings might provide clues to understanding the sugar-binding role of RegIV proteins, as well as molecular interactions with currently unknown receptors.
REGIV OVEREXPRESSION IN GASTROENTEROLOGICAL CANCER
Although various normal tissues express RegIV, expression levels are much lower in normal tissues than in cancerous tissues[27]. Violette et al[11] showed that the RegIV is more strongly expressed in colorectal tumors (particularly in mucinous carcinomas) and normal small intestine than in normal colorectal tissue. They also demonstrated that RegIV-positive tumor cells display different phenotypes: mucus-secreting, enterocyte-like, or undifferentiated.
Several studies have demonstrated overexpression of RegIV in gastric cancer. Oue et al[16] reported that RegIV expression was significantly higher in gastric carcinoma than in normal tissue on quantitative real-time polymerase chain reaction (PCR). Moreover, RegIV expression was associated with both intestinal mucin phenotype and neuroendocrine differentiation.
In pancreatic ductal adenocarcinoma[20] as well as in gallbladder carcinoma[21] amplification of RegIV in normal tissues was not apparent on quantitative real-time PCR. In contrast, high levels of RegIV were found in cancer tissues. These data suggest that overexpression of RegIV is associated with generating and maintaining cancer tissue.
IMPORTANT ROLES OF REGIV IN CANCER TISSUES
Overexpression of RegIV is an early event in carcinogenesis
Carcinogenesis is a multistep process involving somatic mutations or epigenetic changes affecting tumor suppressor genes and oncogenes. Several studies have indicated that RegIV may participate in early carcinogenesis in certain cancers.
Most colorectal carcinomas are thought to develop through the “adenoma-to carcinoma sequence” model[28], in which adenomas are recognized as precursor lesions of the vast majority of colorectal cancers. Elucidation of the adenoma-carcinoma sequence along with its corresponding molecular genetic alterations will significantly enhance our understanding of the pathogenesis of colorectal carcinoma. However, since research on genetic mutations of adenomas is scant, the mechanism of the adenoma-adenocarcinoma sequence remains elusive.
Zhang et al[9,14] generated a large collection of candidate, differentially expressed genes in primary colorectal adenomas and found that RegIV was one of the differentially expressed genes in colorectal adenoma and adenocarcinoma. They proposed that overexpression of RegIV may be an early event in colorectal carcinogenesis.
In gastric cancer, one of the important precancerous changes is intestinal metaplasia caused by chronic inflammation[29]. Oue et al[27] performed serial analysis of gene expression of gastric carcinoma and identified several genes potentially involved in invasion, metastasis and carcinogenesis, and reported that RegIV is a candidate gene for cancer-specific expression. They also performed immunohistochemical analysis of RegIV in gastric tissues and showed that RegIV protein was immunohistochemically expressed in the goblet cells of intestinal metaplasia of the stomach and gastric carcinoma, suggesting an association of RegIV protein with intestinal differentiation of the stomach and the pathogenesis of intestinal-type gastric carcinoma[16].
Gallbladder carcinoma is also thought to arise from epithelial dysplasia, and dysplasia appears to arise from metaplasia[30]. Tamura et al[21] reported that RegIV participates in gallbladder carcinogenesis via intestinal metaplasia because RegIV expression was found not only in the cancer cells but also in the intestinal metaplastic epithelium of patients with adenomyomatosis. In contrast, RegIV expression was never apparent in the normal epithelium of the gallbladder.
Intraductal papillary mucinous neoplasms (IPMN) of the pancreas show a wide spectrum of histological differentiation from hyperplasia and adenoma, and the existence of an adenoma-carcinoma sequence has been documented[31-33]. Adsay et al[34-36] suggested that the intestinal-type IPMN to colloid carcinoma sequence is a distinct pathway of carcinogenesis involving intestinal-related genes caudal-type homeobox transcription factor 2 (CDX2) and Mucin 2 intestinal (MUC2). They described this pathway as the “intestinal” pathway of carcinogenesis. CDX2 is a transcriptional factor that is important for the maintenance of intestinal identity[37], and MUC2 is a major mucin detected in intestinal epithelium[38]. Nakata et al[38] analyzed RegIV and CDX2 expressions in patients with IPMNs using immunohistochemical staining and microdissection-based quantitative real-time reverse transcription PCR. The positive rates of both RegIV protein and mRNA expression were significantly higher in intestinal-type IPMN than in the other types of IPMN. A significant correlation between RegIV and CDX2 mRNA levels was also demonstrated. They concluded that RegIV plays an important role in differentiation of the “intestinal”pathway of IPMN and may be regulated by CDX2.
Carcinogenesis is a complex, multistep process involving somatic mutations or epigenetic changes affecting tumor suppressor genes and oncogenes[39,40]. RegIV may contribute a part of these process, however further investigation is essential for comprehensive elucidation.
RegIV as an antiapoptotic factor
Advanced malignancies are often associated with poor responses to chemotherapy or radiation. Additional generic alterations in tumorigenesis create a permissive environment for clonal expansion of cells that are resistant to apoptosis[41]. So far, considerable attention has been given to the B-cell lymphoma 2 (Bcl-2) family of genes as possible regulators of intrinsic tumor resistance to therapy[42]. Repressors of programmed cell death, such as Bcl-2 and B-cell lymphoma-extra large (Bcl-xL), are known to decrease radiation- and chemotherapy-induced apoptotic cell death in cell cultures[43] via the Akt signaling pathway, which is an important determinant of the response to anticancer therapy[44]; overexpression of these genes suggests a poor prognosis in colon cancer[45]. However, key cellular factors that regulate expression of anti-apoptotic genes in tumors are not fully clarified. Defining dominant pathways responsible for regulating apoptosis could broaden current strategies for therapeutic intervention.
Bishunpuri et al[46] investigated possible roles of RegIV in colon cancer cells, using an in vitro radiation-survival colony assay. Colon cancer cells were cultured with or without recombinant human RegIV (rhR4) and exposed to 4 Gy of irradiation. After irradiation, colony counts increased significantly in rhR4-treated cell lines, but decreased in untreated cells. In the absence of irradiation, rhR4 treatment did not alter the numbers of colonies in treated cells. These data indicate that RegIV promotes tumor cell survival following a potent apoptotic stimulus. Furthermore, to establish a causative association between RegIV and the anti-apoptotic genes Bcl-2 and Bcl-xL, rhR4 was added to cultures of colon carcinoma cell lines, and Bcl-2 and Bcl-xL mRNA expression levels were analyzed. Both Bcl-2 and Bcl-xL expression levels increased significantly after rhR4-treatment in colon cancer cell lines, indicating that exogenous RegIV regulates expression of the Bcl-2 and Bcl-xL.
Mitani et al[19] also transfected gastric cancer cells with vector expressing RegIV to investigate the biologic significance of RegIV. To evaluate the effects of RegIV on the response to 5-fluorouracil (5-FU) treatment, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays were performed. Overexpression of RegIV in gastric cancer cells was confirmed to significantly inhibit 5-FU-induced apoptosis compared with cells transfected with empty vector on both MTT assay and measurement of DNA fragments. They also examined the expression of dihydropyrimidine dehydrogenase (DPD), because DPD has important roles in the pharmacokinetics and toxicity of 5-FU[47,48]. Induction of DPD expression was demonstrated in cells overexpressing RegIV compared with a negative control on Western blotting. They next examined the relevance of RegIV expression to the response of gastric cancer to low-dose 5-FU and cisplatin in patients with recurrent gastric cancer. Among 36 patients who received this regimen, the response was no change or progressive disease in all 14 patients with RegIV expression, whereas partial responses were obtained in 8 (36.4%) of the 22 patients without RegIV expression (P = 0.013).
To investigate the relevance of RegIV to resistance to chemotherapy or radiotherapy, Eguchi et al[44] established stable RegIV-expressing cells by transfection of plasmid into RegIV-negative pancreatic cancer cells. Fluorescence-activated cell scanning analysis of radiated cells showed that the apoptotic population was 28.7% among radiated control cells, compared with only 10.7% among RegIV-expressing cells (P < 0.001). Similarly, the 50% inhibitory concentration (IC50) of gemcitabine was 100 nmol/L against RegIV-expressing cells, but only 30 nmol/L against control cells. These in vitro findings suggest that RegIV has anti-apoptotic properties in pancreatic cancer cells exposed to radiation and chemotherapy.
These studies revealed that RegIV not only promotes the expression of certain factors known as anti-apoptotic proteins, but also contributes to increasing resistance to apoptotic death during treatment in vivo as well as in vitro (Table 2).
Table 2.
Articles reporting regenerating islet-derived type IV as anti-apoptic factor
| Author | Year | Type of cancer | Result |
| Bishnupuri et al[46] | 2006 | Colorectal | RegIV induces cell survival against radiation, and regulates Bcl-2 and Bcl-xL |
| Mitani et al[19] | 2007 | Gastric | RegIV inhibited 5-fluorouracil-induced apoptosis, and induced DPD expression |
| Eguchi et al[44] | 2009 | Pancreatic | RegIV decreased the sensitivity to radiation and gemcitabine |
RegIV: Reporting regenerating islet-derived type IV; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma-extra large; DPD: Dihydropyrimidine dehydrogenase.
RegIV as a proinvasive factor
Rafa et al[49] investigated the potential function of RegIV as a proinvasive factor in colorectal cancer cells. Colon cancer cells secreting RegIV or not were used to analyze the autocrine and paracrine effects of RegIV. They evaluated the invasive properties of cancer cells by performing collagen type I invasion assays and calculating invasion index, which is useful for judging the invasion ability[50,51]. They demonstrated that cell lines which secreted RegIV were spontaneously invasive, whereas cells which did not secrete RegIV were non-invasive compared with positive control cells. They also added the RegIV protein to non-RegIV-secreting cell lines, and confirmed a dose-dependent increase in the invasive index. Addition of an anti-RegIV antibody to assays with the invasive cell lines significantly limited their invasive properties. These results suggest that RegIV promotes in vitro invasion of colon cancer cell lines in both an autocrine and a paracrine manner.
REGIV EXPRESSION AND CLINICOPATHOLOGICAL FEATURES
Several studies have contrasted clinicopathological features with expression levels of RegIV. Among 36 patients with colorectal cancer, Oue et al[16] demonstrated that lymph node metastasis was positive in 69.2% of the RegIV-positive group, but only 30.4% of RegIV-negative group, suggesting that RegIV contributes to lymph node metastasis in colorectal cancer. A relation between overexpression of RegIV and liver metastasis has also been demonstrated in patients with colorectal cancer[18,52]. One study of 30 patients with colorectal cancer showed that metastatic recurrence in the liver during follow-up was more frequently associated with the presence than the absence of RegIV staining (63% vs 9%, P = 0.010)[18]. Bishnupuri et al[53] suggested that liver metastasis might result from RegIV inducing matrix metalloproteinase-7,which promotes liver metastasis[54].
We studied surgical specimens of cancer tissue and adjacent normal mucosa obtained from 202 patients with colorectal cancer. We examined the relations between the expression levels of RegIV and clinicopathological features. High expression levels of RegIV were significantly related to well-differentiated histological type, deeper invasion, lymphatic invasion, liver metastasis, and advanced stage (stage IV). In contrast, RegIV expression levels were unrelated to age, gender, tumor size, tumor location, lymph node metastasis, and venous invasion[55].
In gastric cancer, positive RegIV expression has been associated with poorly differentiated tumors, although there was no clear correlation between RegIV expression and tumor depth or lymph node metastasis[16]. The results of an in vitro study by Kuniyasu et al[56] suggest that RegIV might accelerate peritoneal metastasis in gastric cancer.
These studies generally discussed the unfavorable impact of RegIV expression on clinicopathological features. Cell proliferation and anti-apoptotic effect induced by RegIV may accelerate progression of cancer.
RegIV as a prognostic factor
RegIV has recently been recognized to be associated with poor prognoses in specific gastroenterological cancers. Macadam et al[13] reported a significant increase in colorectal cancer-related deaths among patients with non-metastatic early stage disease whose tumors expressed Reg genes. This subgroup of patients was also at high risk for recurrent colorectal cancer after curative surgery, and such patients may benefit from adjuvant therapy[13].
We also demonstrated that RegIV is significantly associated with outcomes in colorectal cancer. In our study, overall survival rates were significantly higher in RegIV low patients (83.5%) than in RegIV high patients (55.1%, P = 0.002). On multivariate Cox regression analysis, tumor size, liver metastasis, and a higher level of RegIV gene expression (P = 0.029) were significant independent predictors of overall survival in colorectal cancer[55].
Miyagawa et al[57] estimated RegIV mRNA levels in the peritoneal washes of 95 patients with gastric cancer by real-time reverse transcription-PCR. The RegIV mRNA level was correlated with the extent of wall penetration and peritoneal metastases. They also found that the outcomes of RegIV-positive patients were significantly worse than those of RegIV-negative patients. Multivariate analysis suggested that RegIV is an independent prognostic factor[57].
Tao et al[58] measured RegIV mRNA levels by immunohistochemical staining of tissue, and enzyme-linked immunosorbent assay of serum. Their results confirmed that the mean survival time was significantly shorter in patients with RegIV-positive gastric cancer than in those with RegIV-negative gastric cancer (P = 0.013)[58].
In gallbladder cancer, Tamura et al[21] reported that high expression of RegIV correlated with a well-differentiated phenotype accompanied by better outcomes, where lower expression correlated with a poorly differentiated phenotype accompanied by worse survival, suggesting that loss of RegIV expression might be associated with more malignant characteristics.
According to these data, RegIV expression is generally associated with poor outcomes in colorectal cancer and gastric cancer. Further studies will hopefully clarify differences in clinical outcomes according to the type of cancer.
RegIV as a diagnostic biomarker
Serum biomarkers for the detection of cancer are needed in order to find a larger number of candidates for suspected cancer. Several studies have assessed the feasibility of using RegIV as a serum diagnostic marker (Table 3). Oue et al[18] measured serum RegIV levels in patients with colorectal cancer by enzyme-linked immunosorbent assay to investigate the diagnostic potential of RegIV. Increased preoperative levels of RegIV were found in a low numbers of serum samples from patients with stage 0-III colorectal cancer, indicating that serum RegIV is unsuitable for the detection of early colorectal cancer. In contrast, in patients with stage IV disease, the serum RegIV concentration was significantly higher in the presence than in the absence of liver metastasis, suggesting that RegIV is a good marker for metastatic recurrence in the liver after resection of colorectal cancer.
Table 3.
Articles reporting regenerating islet-derived type IV as biomarker
| Author | Year | Type of cancer | Result |
| Oue et al[18] | 2007 | Colorectal | Serum RegIV concentration was significantly elevated in patients with liver metastases than those without |
| Mitani et al[19] | 2007 | Gastric | Presurgical serum RegIV was significantly elevated compared with healthy individuals |
| Kobayashi et al[59] | 2010 | Gastric | Presurgical serum RegIV was significantly different in early and advanced cancer, compared with healthy individuals |
RegIV: Reporting regenerating islet-derived type IV.
Mitani et al[19] demonstrated that the serum RegIV concentration in presurgical patients with gastric cancer was significantly higher than that in healthy individuals (1.96 ± 0.17 μg/L vs 0.52 ± 0.05 μg/L, P < 0.001). The diagnostic sensitivity of serum RegIV (36.1%) was superior to that of serum carcinoembryonic antigen (CEA, 11.5%) or carbohydrate antigen 19-9 (CA19-9, 13.1%).
Kobayashi et al[59] also evaluated the usefulness of serum RegIV levels as a diagnostic marker for gastric cancer. They collected pretreatment serum samples from patients with gastric cancer and healthy control subjects without cancer. RegIV levels were significantly higher in patients with early gastric cancer (median 8.42 μg/L) than in the control subjects (median 5.01 μg/L) (P < 0.001). RegIV levels were also higher in patients with advanced gastric cancer (median 13.12 μg/L) than in those with early gastric cancer (P < 0.02). The sensitivity for gastric cancer was 73.0%, the specificity was 70.8%, and the accuracy was 71.8%, which is superior to the respective values for CEA and CA19-9. Serum RegIV levels were thus suggested to be potentially useful as a screening marker for gastric cancer, including early disease.
In pancreatic cancer, Takehara et al[20] detected significantly elevated serum RegIV levels, measured with the use of an enzyme-linked immunosorbent assay system, in patients with early-stage pancreatic ductal adenocarcinomas. Their findings suggested that RegIV may become a new serological marker of pancreatic ductal adenocarcinoma.
Serum RegIV level can be a useful indicator to distinguish between patients with cancer and healthy subjects. RegIV has the potential to be used as a screening serum marker for certain cancers, including cancers in the early stages.
SIGNALING PATHWAY OF REGIV, AND REGIV-TARGETED THERAPY
Monoclonal antibody therapy has become an important option for the management of gastroenterological cancer. Bevacizumab, a humanized monoclonal antibody to vascular endothelial growth factor, is currently approved in combination with intravenous 5-FU-based regimens for first-line treatment of metastatic colorectal cancer. Besides the anti-angiogenesis factor antibody, antibodies against circulating ligands, such as hepatocyte growth factor[60] and interleukin-6[61], are under review as anticancer drugs.
The signaling pathway activated by RegIV is poorly understood; however, Bishunpuri et al[53] recently demonstrated that RegIV is likely to function as an anti-apoptotic factor in colon cancers through the phosphorylation of Akt and epidermal growth factor receptor (EGFR). EGFR expression and activation are common in adenocarcinomas and are associated with poor prognoses[62-68]. The importance of EGFR in colorectal cancer has been underpinned by the clinical use of the cetuximab, a therapeutic monoclonal antibody that binds to the external domain of EGFR and blocks ligand-mediated dimerization and activation[68].
Akt is another important downstream target of EGFR because EGFR activates Akt via a signaling pathway involving phosphoinositide 3-kinase (PI3K) and phosphoinositide-dependent kinase-1[69,70]. Akt overexpression and activation by phosphorylation at Thr308 and Ser473 are well-established early events in sporadic colon carcinogenesis and are detectable in the neoplasmic epithelium, but are generally absent in normal colonic epithelium[71].
Bishunpuri et al[53] examined the effects of purified rhR4 on colon adenocarcinoma cells to determine the signaling pathways responsive to RegIV. They reported that rhR4 treatment resulted in rapid phosphorylation of EGFR at Tyr992 and Tyr1068 and Akt at Thr308 and Ser473. They concluded that RegIV is a potent transactivator of the EGFR/PI3K/Akt signaling pathway via an unknown receptor and proposed that disruption of PI3K signaling may have utility as a novel therapeutic intervention for colorectal cancer.
To examine the role of RegIV overexpression in pancreatic cancer cells, Takehara et al[20] constructed several expression vectors designed to express siRNA specific to RegIV and transfected them into a pancreatic cancer cell line that endogenously expressed RegIV at a high level. The cell line had a knockdown effect on endogenous RegIV transcription, resulting in a significant reduction in the number of viable cells as measured by MTT assay as well as by colony formation assay compared with negative controls. RegIV was thus suggested to have a critical role in pancreatic cancer cell survival and growth. They also generated recombinant human RegIV and incubated pancreatic cancer cells in its presence to examine whether RegIV activates the Akt signaling pathway. Recombinant human RegIV treatment was confirmed to significantly increase phosphorylated Akt, suggesting that RegIV stimulates cell growth via the Akt signaling pathway in pancreatic cancer cells[20]. Furthermore, by using monoclonal antibodies specific to RegIV, they succeeded in neutralizing secreted RegIV in the culture medium in vitro and found that treatment with these neutralizing antibodies significantly suppressed pancreatic cancer cell growth by blocking Akt phosphorylation.
Legoffic et al[72] similarly examined the effect of RegIV antibody by Western blotting analysis in mice with pancreatic cancer. They confirmed that the injection of RegIV antibody significantly reduced the intra-tumor level of proteins associated with apoptosis (Akt, Bcl-2 and Bcl-xL) after treatment compared with a control group, resulting in significant reduction of tumor.
The results of these studies suggest that RegIV may contribute to cell proliferation and anti-apoptosis by transactivating EGFR via an unknown receptor (Figure 1). Although a cell surface receptor for RegIV has not been identified, there are numerous examples where EGFR is transactivated by signaling molecules that do not bind directly to receptor. Bishnupuri et al[53] speculated that the RegIV receptor might be a G protein-coupled receptor, because ligands capable of transactivating EGFR usually act on such receptors. They are now focusing on identifying the RegIV binding receptor and further delineating primary intracellular signaling events[53].
Figure 1.
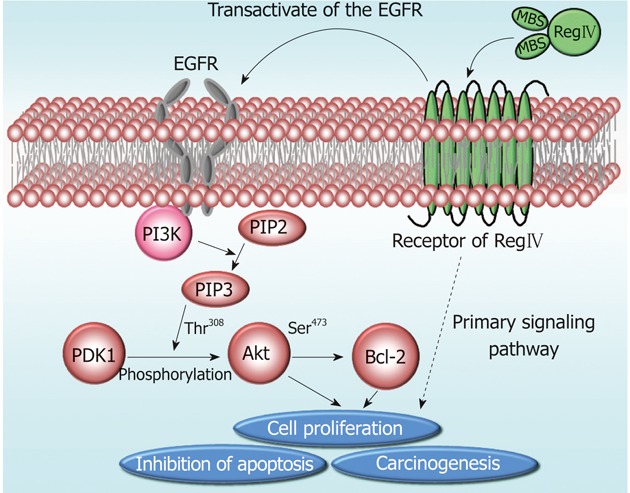
Schematic representation of the regenerating islet-derived type IV signaling pathway. Regenerating islet-derived type IV (RegIV) contributes to cell proliferation, inhibition of apoptosis, and carcinogenesis via protein kinase B (Akt) signaling pathway by transactivating the epidermal growth factor receptor (EGFR). The primary signaling pathway is now being investigated. MBS: Mannan-binding sites; PI3K: Phosphoinositide 3-kinase; PIP2: Phosphatidylinositol 4,5-bisphosphate; PIP3: Phosphatidylinositol 4,5-triphosphate; PDK1: Phosphoinositide-dependent kinase-1; Bcl-2: B-cell lymphoma 2.
The findings outlined above suggest the feasibility of antibody therapy targeting RegIV. Neutralizing antibody therapy targeting RegIV may lead to novel therapeutic strategies for gastroenterological cancers expressing RegIV (Table 4).
Table 4.
Articles reporting regenerating islet-derived type IV-targetted therapy
| Author | Year | Method/type of cancer | Result |
| Bishunpuri et al[53] | 2006 | Transfection of rhR4/colorectal | Increase in pEGFR and pAkt |
| Takehara et al[20] | 2006 | Transfection of rhR4 and siRNA/pancreatic | Significant reduction in viable cells andincrease in pAkt |
| Legoffic et al[72] | 2009 | RegIV antibody injection/pancreatic | Significant reductions in Akt, Bcl-2 and Bcl-xL |
rhR4: Recombinant human regenerating islet-derived type IV; RegIV: Human regenerating islet-derived type IV; siRNA: Small interfering RNA; pEGFR: Phosphorylated epidermal growth factor receptor; pAkt: Phosphorylated protein kinase B; Akt: Protein kinase B; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma-extra large.
CONCLUSION
This is the first systematic review of the RegIV gene as related to gastroenterological cancer, focusing on its role in cancer tissue and its impact on clinical outcomes. RegIV is generally upregulated in gastroenterological cancers, including those of the stomach, colorectum, and pancreas, as well as in benign diseases such as ulcerative colitis. Available evidence suggests that the basic biological effects of RegIV seem to be induction of cellular proliferation, invasion and inhibition of apoptosis, resulting in relatively worse clinicopathological features, or worse survival in patients with high-RegIV expression than those without.
Recent studies revealed that the serum RegIV level can be a novel biomarker to detect patients with colorectal, gastric, and pancreatic cancer. These studies suggested RegIV has the potential to be used as a screening serum marker. In addition, our multivariate analysis suggested that overexpression of the RegIV gene is a useful prognostic biomarker in patients with colorectal cancer, which corresponds to other reports.
The signaling pathway activated by RegIV is not fully understood. However, recent studies demonstrated that RegIV is a potent activator of the EGFR/Akt signaling cascade, which is associated with cell survival and proliferation. Further investigation of RegIV, particularly its cell surface receptors and signaling pathways, will further our understanding of the basic mechanisms of this gene. Strategies designed to reduce endogenous RegIV expression or to block downstream signaling warrant additional investigations to delineate their potential roles in the prevention or treatment of established gastrointestinal adenocarcinomas.
Footnotes
Peer reviewer: Noriko Nakajima, MD, PhD, Associate Professor, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Nihon University School of Medicine, 1-8-13 Kandasurugadai Chiyoda-ku, Tokyo 101-8309, Japan
S- Editor Cheng JX L- Editor Cant MR E- Editor Xiong L
References
- 1.Yasui W, Oue N, Ito R, Kuraoka K, Nakayama H. Search for new biomarkers of gastric cancer through serial analysis of gene expression and its clinical implications. Cancer Sci. 2004;95:385–392. doi: 10.1111/j.1349-7006.2004.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckhaults P, Rago C, St Croix B, Romans KE, Saha S, Zhang L, Vogelstein B, Kinzler KW. Secreted and cell surface genes expressed in benign and malignant colorectal tumors. Cancer Res. 2001;61:6996–7001. [PubMed] [Google Scholar]
- 3.Hartupee JC, Zhang H, Bonaldo MF, Soares MB, Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim Biophys Acta. 2001;1518:287–293. doi: 10.1016/s0167-4781(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 4.Lasserre C, Simon MT, Ishikawa H, Diriong S, Nguyen VC, Christa L, Vernier P, Brechot C. Structural organization and chromosomal localization of a human gene (HIP/PAP) encoding a C-type lectin overexpressed in primary liver cancer. Eur J Biochem. 1994;224:29–38. doi: 10.1111/j.1432-1033.1994.tb19991.x. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty C, Katsumata N, Myal Y, Schroedter IC, Brazeau P, Murphy LJ, Shiu RP, Friesen HG. Age-related changes in peptide-23/pancreatitis-associated protein and pancreatic stone protein/reg gene expression in the rat and regulation by growth hormone-releasing hormone. Endocrinology. 1995;136:1843–1849. doi: 10.1210/endo.136.5.7720628. [DOI] [PubMed] [Google Scholar]
- 6.Katsumata N, Chakraborty C, Myal Y, Schroedter IC, Murphy LJ, Shiu RP, Friesen HG. Molecular cloning and expression of peptide 23, a growth hormone-releasing hormone-inducible pituitary protein. Endocrinology. 1995;136:1332–1339. doi: 10.1210/endo.136.4.7895644. [DOI] [PubMed] [Google Scholar]
- 7.Dusetti NJ, Frigerio JM, Fox MF, Swallow DM, Dagorn JC, Iovanna JL. Molecular cloning, genomic organization, and chromosomal localization of the human pancreatitis-associated protein (PAP) gene. Genomics. 1994;19:108–114. doi: 10.1006/geno.1994.1019. [DOI] [PubMed] [Google Scholar]
- 8.Broekaert D, Eyckerman S, Lavens D, Verhee A, Waelput W, Vandekerckhove J, Tavernier J. Comparison of leptin- and interleukin-6-regulated expression of the rPAP gene family: evidence for differential co-regulatory signals. Eur Cytokine Netw. 2002;13:78–85. [PubMed] [Google Scholar]
- 9.Zhang Y, Lai M, Gu X, Luo M, Shao L. Reg IV, a differentially expressed gene in colorectal adenoma. Chin Med J (Engl) 2003;116:918–922. [PubMed] [Google Scholar]
- 10.Zenilman ME, Kim S, Levine BA, Lee C, Steinberg JJ. Ectopic expression of reg protein: A marker of colorectal mucosa at risk for neoplasia. J Gastrointest Surg. 1997;1:194–201; discussion 201-202. doi: 10.1016/s1091-255x(97)80109-6. [DOI] [PubMed] [Google Scholar]
- 11.Violette S, Festor E, Pandrea-Vasile I, Mitchell V, Adida C, Dussaulx E, Lacorte JM, Chambaz J, Lacasa M, Lesuffleur T. Reg IV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer. 2003;103:185–193. doi: 10.1002/ijc.10788. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki Y, Ishihara S, Miyaoka Y, Rumi MA, Sato H, Kazumori H, Adachi K, Takasawa S, Okamoto H, Chiba T, et al. Reg protein is overexpressed in gastric cancer cells, where it activates a signal transduction pathway that converges on ERK1/2 to stimulate growth. FEBS Lett. 2002;530:59–64. doi: 10.1016/s0014-5793(02)03398-7. [DOI] [PubMed] [Google Scholar]
- 13.Macadam RC, Sarela AI, Farmery SM, Robinson PA, Markham AF, Guillou PJ. Death from early colorectal cancer is predicted by the presence of transcripts of the REG gene family. Br J Cancer. 2000;83:188–195. doi: 10.1054/bjoc.2000.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Lai M, Lv B, Gu X, Wang H, Zhu Y, Zhu Y, Shao L, Wang G. Overexpression of Reg IV in colorectal adenoma. Cancer Lett. 2003;200:69–76. doi: 10.1016/s0304-3835(03)00460-9. [DOI] [PubMed] [Google Scholar]
- 15.Rechreche H, Montalto G, Mallo GV, Vasseur S, Marasa L, Soubeyran P, Dagorn JC, Iovanna JL. pap, reg Ialpha and reg Ibeta mRNAs are concomitantly up-regulated during human colorectal carcinogenesis. Int J Cancer. 1999;81:688–694. doi: 10.1002/(sici)1097-0215(19990531)81:5<688::aid-ijc3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Oue N, Mitani Y, Aung PP, Sakakura C, Takeshima Y, Kaneko M, Noguchi T, Nakayama H, Yasui W. Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol. 2005;207:185–198. doi: 10.1002/path.1827. [DOI] [PubMed] [Google Scholar]
- 17.Gu Z, Rubin MA, Yang Y, Deprimo SE, Zhao H, Horvath S, Brooks JD, Loda M, Reiter RE. Reg IV: a promising marker of hormone refractory metastatic prostate cancer. Clin Cancer Res. 2005;11:2237–2243. doi: 10.1158/1078-0432.CCR-04-0356. [DOI] [PubMed] [Google Scholar]
- 18.Oue N, Kuniyasu H, Noguchi T, Sentani K, Ito M, Tanaka S, Setoyama T, Sakakura C, Natsugoe S, Yasui W. Serum concentration of Reg IV in patients with colorectal cancer: overexpression and high serum levels of Reg IV are associated with liver metastasis. Oncology. 2007;72:371–380. doi: 10.1159/000113147. [DOI] [PubMed] [Google Scholar]
- 19.Mitani Y, Oue N, Matsumura S, Yoshida K, Noguchi T, Ito M, Tanaka S, Kuniyasu H, Kamata N, Yasui W. Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene. 2007;26:4383–4393. doi: 10.1038/sj.onc.1210215. [DOI] [PubMed] [Google Scholar]
- 20.Takehara A, Eguchi H, Ohigashi H, Ishikawa O, Kasugai T, Hosokawa M, Katagiri T, Nakamura Y, Nakagawa H. Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4. Cancer Sci. 2006;97:1191–1197. doi: 10.1111/j.1349-7006.2006.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura H, Ohtsuka M, Washiro M, Kimura F, Shimizu H, Yoshidome H, Kato A, Seki N, Miyazaki M. Reg IV expression and clinicopathologic features of gallbladder carcinoma. Hum Pathol. 2009;40:1686–1692. doi: 10.1016/j.humpath.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YW, Ding LS, Lai MD. Reg gene family and human diseases. World J Gastroenterol. 2003;9:2635–2641. doi: 10.3748/wjg.v9.i12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Xu J, Li N, Gao F, Huang P. RegIV potentiates colorectal carcinoma cell migration and invasion via its CRD domain. Cancer Genet Cytogenet. 2010;199:38–44. doi: 10.1016/j.cancergencyto.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Weis WI, Kahn R, Fourme R, Drickamer K, Hendrickson WA. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991;254:1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- 25.Kishore U, Eggleton P, Reid KB. Modular organization of carbohydrate recognition domains in animal lectins. Matrix Biol. 1997;15:583–592. doi: 10.1016/s0945-053x(97)90035-4. [DOI] [PubMed] [Google Scholar]
- 26.Ho MR, Lou YC, Wei SY, Luo SC, Lin WC, Lyu PC, Chen C. Human RegIV protein adopts a typical C-type lectin fold but binds mannan with two calcium-independent sites. J Mol Biol. 2010;402:682–695. doi: 10.1016/j.jmb.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 27.Oue N, Hamai Y, Mitani Y, Matsumura S, Oshimo Y, Aung PP, Kuraoka K, Nakayama H, Yasui W. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res. 2004;64:2397–2405. doi: 10.1158/0008-5472.can-03-3514. [DOI] [PubMed] [Google Scholar]
- 28.Lin YM, Furukawa Y, Tsunoda T, Yue CT, Yang KC, Nakamura Y. Molecular diagnosis of colorectal tumors by expression profiles of 50 genes expressed differentially in adenomas and carcinomas. Oncogene. 2002;21:4120–4128. doi: 10.1038/sj.onc.1205518. [DOI] [PubMed] [Google Scholar]
- 29.Asaka M, Sepulveda AR, Sugiyama T, Graham DY. Gastric Cancer. In: Mobley HLT, Mendz GL, Hazell SL, editors. Source Helicobacter pylori: Physiology and Genetics. Washington (DC): ASM Press;; 2001. [PubMed] [Google Scholar]
- 30.Wistuba II, Sugio K, Hung J, Kishimoto Y, Virmani AK, Roa I, Albores-Saavedra J, Gazdar AF. Allele-specific mutations involved in the pathogenesis of endemic gallbladder carcinoma in Chile. Cancer Res. 1995;55:2511–2515. [PubMed] [Google Scholar]
- 31.Tanaka M, Kobayashi K, Mizumoto K, Yamaguchi K. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol. 2005;40:669–675. doi: 10.1007/s00535-005-1646-4. [DOI] [PubMed] [Google Scholar]
- 32.Nagai E, Ueki T, Chijiiwa K, Tanaka M, Tsuneyoshi M. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called “mucinous ductal ectasia”. Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 1995;19:576–589. doi: 10.1097/00000478-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Sessa F, Solcia E, Capella C, Bonato M, Scarpa A, Zamboni G, Pellegata NS, Ranzani GN, Rickaert F, Klöppel G. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 34.Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, Brennan MF, Klimstra DS. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 36.Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA. 1999;96:7318–7323. doi: 10.1073/pnas.96.13.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakata K, Nagai E, Ohuchida K, Aishima S, Hayashi A, Miyasaka Y, Yu J, Mizumoto K, Tanaka M, Tsuneyoshi M. REG4 is associated with carcinogenesis in the ‘intestinal’ pathway of intraductal papillary mucinous neoplasms. Mod Pathol. 2009;22:460–468. doi: 10.1038/modpathol.2008.205. [DOI] [PubMed] [Google Scholar]
- 39.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 40.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 41.Wils J, O’Dwyer P, Labianca R. Adjuvant treatment of colorectal cancer at the turn of the century: European and US perspectives. Ann Oncol. 2001;12:13–22. doi: 10.1023/a:1008357725209. [DOI] [PubMed] [Google Scholar]
- 42.Bonnotte B, Favre N, Moutet M, Fromentin A, Solary E, Martin M, Martin F. Bcl-2-mediated inhibition of apoptosis prevents immunogenicity and restores tumorigenicity of spontaneously regressive tumors. J Immunol. 1998;161:1433–1438. [PubMed] [Google Scholar]
- 43.Violette S, Poulain L, Dussaulx E, Pepin D, Faussat AM, Chambaz J, Lacorte JM, Staedel C, Lesuffleur T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498–504. doi: 10.1002/ijc.10146. [DOI] [PubMed] [Google Scholar]
- 44.Eguchi H, Ishikawa O, Ohigashi H, Takahashi H, Yano M, Nishiyama K, Tomita Y, Uehara R, Takehara A, Nakamura Y, et al. Serum REG4 level is a predictive biomarker for the response to preoperative chemoradiotherapy in patients with pancreatic cancer. Pancreas. 2009;38:791–798. doi: 10.1097/MPA.0b013e3181ac5337. [DOI] [PubMed] [Google Scholar]
- 45.Ogura E, Senzaki H, Yamamoto D, Yoshida R, Takada H, Hioki K, Tsubura A. Prognostic significance of Bcl-2, Bcl-xL/S, Bax and Bak expressions in colorectal carcinomas. Oncol Rep. 1999;6:365–369. doi: 10.3892/or.6.2.365. [DOI] [PubMed] [Google Scholar]
- 46.Bishnupuri KS, Luo Q, Korzenik JR, Henderson JO, Houchen CW, Anant S, Dieckgraefe BK. Dysregulation of Reg gene expression occurs early in gastrointestinal tumorigenesis and regulates anti-apoptotic genes. Cancer Biol Ther. 2006;5:1714–1720. doi: 10.4161/cbt.5.12.3469. [DOI] [PubMed] [Google Scholar]
- 47.Ukon K, Tanimoto K, Shimokuni T, Noguchi T, Hiyama K, Tsujimoto H, Fukushima M, Toge T, Nishiyama M. Activator protein accelerates dihydropyrimidine dehydrogenase gene transcription in cancer cells. Cancer Res. 2005;65:1055–1062. [PubMed] [Google Scholar]
- 48.Harris BE, Song R, Soong SJ, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990;50:197–201. [PubMed] [Google Scholar]
- 49.Rafa L, Dessein AF, Devisme L, Buob D, Truant S, Porchet N, Huet G, Buisine MP, Lesuffleur T. REG4 acts as a mitogenic, motility and pro-invasive factor for colon cancer cells. Int J Oncol. 2010;36:689–698. doi: 10.3892/ijo_00000544. [DOI] [PubMed] [Google Scholar]
- 50.Truant S, Bruyneel E, Gouyer V, De Wever O, Pruvot FR, Mareel M, Huet G. Requirement of both mucins and proteoglycans in cell-cell dissociation and invasiveness of colon carcinoma HT-29 cells. Int J Cancer. 2003;104:683–694. doi: 10.1002/ijc.11011. [DOI] [PubMed] [Google Scholar]
- 51.Bracke ME, Boterberg T, Bruyneel EA, Mareel MM. Collagen Invasion Assay. Methods Mol Med. 2001;58:81–89. doi: 10.1385/1-59259-137-X:081. [DOI] [PubMed] [Google Scholar]
- 52.Oliver L, Cordel S, Barbieux I, LeCabellec MT, Meflah K, Grégoire M, Vallette FM. Resistance to apoptosis is increased during metastatic dissemination of colon cancer. Clin Exp Metastasis. 2002;19:175–180. doi: 10.1023/a:1014510508664. [DOI] [PubMed] [Google Scholar]
- 53.Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130:137–149. doi: 10.1053/j.gastro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Kioi M, Yamamoto K, Higashi S, Koshikawa N, Fujita K, Miyazaki K. Matrilysin (MMP-7) induces homotypic adhesion of human colon cancer cells and enhances their metastatic potential in nude mouse model. Oncogene. 2003;22:8662–8670. doi: 10.1038/sj.onc.1207181. [DOI] [PubMed] [Google Scholar]
- 55.Numata M, Oshima T, Yoshihara K, Watanabe T, Tsuchida K, Tamagawa H, Yamamoto N, Shiozawa M, Morinaga S, Akaike M, et al. Relationship between RegIV gene expression to outcomes in colorectal cancer. J Surg Oncol. 2011;104:205–209. doi: 10.1002/jso.21906. [DOI] [PubMed] [Google Scholar]
- 56.Kuniyasu H, Oue N, Sasahira T, Yi L, Moriwaka Y, Shimomoto T, Fujii K, Ohmori H, Yasui W. Reg IV enhances peritoneal metastasis in gastric carcinomas. Cell Prolif. 2009;42:110–121. doi: 10.1111/j.1365-2184.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyagawa K, Sakakura C, Nakashima S, Yoshikawa T, Fukuda K, Kin S, Nakase Y, Shimomura K, Oue N, Yasui W, et al. Overexpression of RegIV in peritoneal dissemination of gastric cancer and its potential as A novel marker for the detection of peritoneal micrometastasis. Anticancer Res. 2008;28:1169–1179. [PubMed] [Google Scholar]
- 58.Tao HQ, He XJ, Ma YY, Wang HJ, Xia YJ, Ye ZY, Zhao ZS. Evaluation of REG4 for early diagnosis and prognosis of gastric cancer. Hum Pathol. 2011;42:1401–1409. doi: 10.1016/j.humpath.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi Y, Niwa Y, Tajika M, Kawai H, Kondo S, Hara K, Mizuno N, Hijioka S, Sawaki A, Matsuo K, et al. Serum tumor antigen REG4 as a useful diagnostic biomarker in gastric cancer. Hepatogastroenterology. 2010;57:1631–1634. [PubMed] [Google Scholar]
- 60.Burgess T, Coxon A, Meyer S, Sun J, Rex K, Tsuruda T, Chen Q, Ho SY, Li L, Kaufman S, et al. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res. 2006;66:1721–1729. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 61.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 62.Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17:1343–1352. doi: 10.1038/sj.onc.1202171. [DOI] [PubMed] [Google Scholar]
- 63.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37 Suppl 4:S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 64.Kopp R, Rothbauer E, Ruge M, Arnholdt H, Spranger J, Muders M, Pfeiffer DG, Schildberg FW, Pfeiffer A. Clinical implications of the EGF receptor/ligand system for tumor progression and survival in gastrointestinal carcinomas: evidence for new therapeutic options. Recent Results Cancer Res. 2003;162:115–132. doi: 10.1007/978-3-642-59349-9_10. [DOI] [PubMed] [Google Scholar]
- 65.Layfield LJ, Bernard PS, Goldstein NS. Color multiplex polymerase chain reaction for quantitative analysis of epidermal growth factor receptor genes in colorectal adenocarcinoma. J Surg Oncol. 2003;83:227–231. doi: 10.1002/jso.10272. [DOI] [PubMed] [Google Scholar]
- 66.Franic TV, Judd LM, Nguyen NV, Samuelson LC, Loveland KL, Giraud AS, Gleeson PA, van Driel IR. Growth factors associated with gastric mucosal hypertrophy in autoimmune gastritis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G910–G918. doi: 10.1152/ajpgi.00469.2003. [DOI] [PubMed] [Google Scholar]
- 67.Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 68.Iqbal S, Lenz HJ. Integration of novel agents in the treatment of colorectal cancer. Cancer Chemother Pharmacol. 2004;54 Suppl 1:S32–S39. doi: 10.1007/s00280-004-0884-0. [DOI] [PubMed] [Google Scholar]
- 69.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346 Pt 3:561–576. [PMC free article] [PubMed] [Google Scholar]
- 70.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 72.Legoffic A, Calvo E, Cano C, Folch-Puy E, Barthet M, Delpero JR, Ferrés-Masó M, Dagorn JC, Closa D, Iovanna J. The reg4 gene, amplified in the early stages of pancreatic cancer development, is a promising therapeutic target. PLoS One. 2009;4:e7495. doi: 10.1371/journal.pone.0007495. [DOI] [PMC free article] [PubMed] [Google Scholar]


