Abstract
In tissues containing pre-existing collateral vessels, occlusion of an upstream supply artery results in diversion of blood flow through these vessels, protecting the distal tissue from ischemia. The sudden rise in blood flow through collateral vessels exerts shear stress upon the vessel wall, thereby providing the initial stimulus for arteriogenesis. Arteriogenesis, the structural expansion of collateral circulation, involves smooth muscle cell (SMC) proliferation which leads to increased vessel diameter and wall thickness. Since shear is sensed at the level of endothelial cells (EC), communication from EC to the underlying SMC must occur as part of this process. We previously reported that endothelial cells (EC) exposed to shear stress release hydrogen peroxide (H2O2), and that H2O2 can signal vascular SMC to increase gene and protein expression of placenta growth factor (PLGF), a known mediator of arteriogenesis. The purpose of the current study was to further elucidate the mechanism whereby PLGF is regulated by H2O2. We found that a single, physiological dose of H2O2 increases PLGF mRNA half-life, but has no effect on PLGF promoter activity, in human coronary artery SMC (CASMC). We further demonstrated that the H2O2–induced increase in PLGF mRNA levels partially relies on p38 MAPK, JNK and ERK1/2 pathways. Finally, we showed that chronic exposure to pathological levels of H2O2 further increases PLGF mRNA levels, but does not result in a corresponding increase in PLGF secreted protein. These data suggest that PLGF regulation has an important translational component. To our knowledge, this is the first study to characterize post-transcriptional regulation of PLGF mRNA by H2O2 in vascular SMC. These findings provide new insights into the regulation of this important growth factor and increase our understanding of PLGF-driven arteriogenesis.
Keywords: Reactive oxygen species, arteriogenesis, angiogenesis, PLGF, p38, JNK, ERK1/2
Introduction
A critical determinant for survival following an acute coronary event caused by atherosclerotic vascular occlusion is the ability to develop coronary collateral arteries (1). This coronary collateralization process (termed arteriogenesis) functions as a compensatory process which allows continued blood flow to tissues distal to an arterial occlusion. Arteriogenesis is distinct from angiogenesis, or capillary proliferation, in both its initial stimulus and functional response. Angiogenesis is primarily driven by ischemia, leading to increased vascular endothelial growth factor-A (VEGF-A) production followed by endothelial cell proliferation and migration. In contrast, arteriogenesis involves the structural enlargement of pre-existing collateral vessels that form intra-arterial connections. These vessels have low flow rates in the absence of an upstream occlusion, due to the lack of a pressure gradient across the vessel length. However, they experience a substantial increase in blood flow following an upstream occlusion due to the decrease in pressure in the occluded vessel. This hemodynamic change exerts increased shear stress on the collateral vessel wall. Increased shear stress is generally considered to be the key initiator of the arteriogenic process, and activates signaling processes that lead to eventual increases in collateral artery diameter and wall thickness. Since shear stress is sensed by the endothelial layer, but migration and proliferation of vascular smooth muscle are key components of arteriogenesis, it is evident that shear stress activates endothelium-dependent signaling to vascular smooth muscle cells (2, 3). Arteriogenesis also depends upon the recruitment and activation of monocytes, which penetrate the vessel wall and differentiate into macrophages, creating an inflammatory environment that is thought to be essential for collateral growth (4–7).
Placenta growth factor (PLGF), a member of the VEGF family, is an important mediator of arteriogenesis (8–10). Although PLGF was first identified in placental tissue (11), we and others have shown that it is also expressed in adult vasculature (12) by both endothelial cells (EC) and smooth muscle cells (SMC)(10). The ability of PLGF to induce arteriogenesis requires recruitment of monocytes to the vessel wall(4–6). Since PLGF is chemotactic for monocytes and the vascular wall is largely composed of SMC, we hypothesized that SMC PLGF expression may play an important role in arteriogenesis. We further hypothesized that regulation of PLGF expression in SMC would be endothelium-dependent, since the shear signal is sensed by endothelium as discussed above. In agreement with this hypothesis, we previously reported that physiological levels of hydrogen peroxide (H2O2) are released from EC in response to shear stress, and that H2O2 can act as a paracrine mediator to increase PLGF gene and protein expression in vascular SMC (13). H2O2 has received increasing attention as an important physiological signaling molecule in vascular biology. Our previous findings are consistent with the important role of H2O2 in coronary collateral growth (7).
Clinical trials of the use of growth factors to stimulate arteriogenesis have reported little or no beneficial effect of growth factor treatment (1). Better understanding of the complex and interrelated signaling pathways that govern arteriogenesis may allow development of more efficacious pro-arteriogenic therapies for patients with ischemic cardiovascular disease. Despite the clearly important role of PLGF in adaptive arteriogenesis, little is known about its regulation. In the current study, we sought to further characterize the mechanism by which H2O2 increases PLGF expression in human coronary artery smooth muscle cells (CASMC). We report that treatment of human CASMC with a single dose of a physiological level of H2O2 extends the PLGF mRNA half-life. This post-transcriptional effect is partially dependent on the p38 MAPK, JNK and ERK1/2 pathways. Furthermore, we found that sustained exposure to pathological levels of H2O2 further increases PLGF mRNA levels, in comparison to a single dose of H2O2. Despite the large increase in PLGF mRNA, PLGF secreted protein levels were not increased by sustained exposure to high levels of H2O2. This finding suggests that pathological levels of H2O2 may affect translation of PLGF . Overall, these results reveal novel features of the PLGF regulatory mechanism and suggest important avenues for further research.
Materials and Methods
Primary cells
Human coronary artery smooth muscle cells (CASMC) were purchased from Lonza (Walkersville, MD). Cells were grown in a humidified incubator containing 5% CO2 in Smooth Muscle Cell Basal Medium (SmBM, Lonza) plus Smooth Muscle Growth Medium (SmGM-2 SingleQuots, Lonza) until 80% confluent. Culture medium was changed every other day. CASMC used for experiments were between passages 4 and 8. Donors include a 12 year old male and a 56 year old female. Human coronary artery endothelial cells (CAEC) were purchased from Lonza. Cells were grown in a humidified incubator containing 5% CO2 in Endothelial Cell Basal Medium (EMB, Lonza) plus Endothelial Cell Growth Medium (SingleQuots, Lonza). Culture medium was changed every other day. CAEC used for experiments were at passage 5. Donor cells were from a 21 year old male.
H2O2 treatment of human CASMC
CASMC were grown in a CO2 incubator in 6-well plates containing SmBM plus SmGM-2 SingleQuots (Lonza, Walkersville, MD) until 80% confluent. Cells were then serum-starved (DMEM, 1% FBS) for 48 h followed by the addition of a single dose of H2O2 (50 µM) directly to the medium. For chronic exposure to pathological levels of H2O2, cells were treated with glucose oxidase (5 U/mL, Sigma, St. Louis, MO) which continually catalyzes the oxidation of β-D-glucose to form D-glucono-1,5-lactone and hydrogen peroxide (β-D-glucose + O2 + H2O → D-glucono-δ-lactone + H2O2). Following 8 h of treatment, medium was collected for ELISA and cells were lysed in TriZol (Invitrogen, Carlsbad, CA) for RNA isolation.
Plasmids
To assess PLGF promoter activity, the PLGF promoter sequence was inserted into the firefly luciferase pGL3 basic plasmid (Promega) at the at the Sst1 restriction site (14). This plasmid (PLGF-luc) was the generous gift of the late Dr. Brian Murphy (SRI International, Menlo Park, CA). The sequence of the PLGF promoter was confirmed by the OSU DNA sequencing facility. The pRL Renilla luciferase (pRL) plasmid (Promega) was used as the transfection efficiency control. A 50:1 ratio of firefly luciferase plasmid to Renilla luciferase plasmid was used, as recommended by the manufacturer.
Transfections
Human coronary artery smooth muscle cells (passage 5 – 8; 1 × 106 cells) were co-transfected with 2 µg PLGF-luc and 40 ng pRL using the Amaxa Nucleofector System, program A-033 (Lonza, Walkersville, MD). After transfection, cells were seeded in 6 well plates and left undisturbed for ~20 h. Next, 2 mL of fresh SmBM plus SmGM-2 was added to each well and co-transfected (PLGF-luc + pRL) cells were treated with H2O2 (50 µM) for 8 h. Medium was removed from the plates and 500 µL Passive Lysis Buffer (Promega) was added per well. Cells were scraped from the plate and immediately frozen at −20°C to ensure complete lysis. Frozen lysates were thawed and assayed for PLGF promoter activity using the Dual Luciferase assay system (Promega). Luminescence was measured using a Synergy HT multimode plate reader (BioTek).
Human coronary artery endothelial cells (passage 5; 5 × 105 cells) were co-transfected with 2 µg of PLGF-luc and 40 ng pRL using the Amaxa Nucleofector System, program S-005 (Lonza) to assess the basal PLGF promoter activity in endothelial cells, which constitutively produce relatively high levels of PLGF (data not shown) and thus serve as our positive control for the PLGF promoter activity assay. After transfection by electroporation, cells were seeded in 6 well plates and left undisturbed for ~20 h. Next, 1 mL of fresh EBM plus EGM-2 was added to each well. 24 h later, medium was removed from the plates and 500 µL Passive Lysis Buffer (Promega) was added per well. Cells were scraped from the plate and immediately frozen at −20°C to ensure complete lysis. Frozen lysates were thawed and assayed for PLGF promoter activity (as reported by luciferase protein expression) using the Dual Luciferase assay system (Promega). Luminescence was measured using a Synergy HT multimode plate reader (BioTek, Winooski, VT).
PLGF mRNA half-life assay following H2O2 treatment
CASMC were grown in 6 well plates until 80% confluent, serum starved for 48 h, and treated with a single dose of H2O2 (50 µM). Untreated and H2O2-treated CASMC were then exposed to the transcription inhibitor actinomycin D (10 µg/mL, Sigma, St. Louis, MO) for 0, 2, 4, 8 or 16 h. RNA was harvested using TriZol (Invitrogen) at each time point for measuring PLGF mRNA levels by real time PCR.
Inhibition of kinase pathways following H2O2 treatment
CASMC were grown until 80% confluent, serum-starved for 48 h, and treated with a single dose of H2O2 (50 µM). H2O2-treated CASMC were exposed to either a p38 MAPK inhibitor (SB202190, 10 µM, Tocris, Ellisville, MO), a JNK inhibitor (SU3327, 10 µM, Tocris) or an ERK1/2 inhibitor (FR180204, 10 µM, Tocris) for 8 h. RNA was then isolated using TriZol (Invitrogen) for real time PCR.
Anisomycin Treatment
CASMC were grown until 80% confluent, serum starved for 48 h, and treated with a single dose of H2O2 (50 µM). H2O2-treated CASMC were exposed to anisomycin (300 ng/mL, Sigma) or with anisomycin (300 ng/mL) alone for 8 h. RNA was then isolated using TriZol (Invitrogen) for real time PCR.
Analysis of gene expression
At the conclusion of the experimental treatments, culture medium was removed and total RNA was extracted using Trizol (Invitrogen) according to the manufacturer’s protocol. RNA was assayed for quality and quantity in a Take 3 plate in a Synergy HT multimode plate reader (BioTek, Winooski, VT). RNA was then treated to remove genomic DNA (Turbo DNAFree, Ambion, Austin, TX) and converted to cDNA (qScript cDNA SuperMix, Quanta BioSciences, Gaithersburg, MD) according to manufacturers’ protocols. Real time PCR was performed on an ABI 7500 Fast instrument for quantification of gene expression. Primers for human PLGF and β-actin were designed using Primer Express and custom-synthesized (Invitrogen) in accordance with our previous work (13). PCR product formation was detected using SYBR Green chemistry (PerfeCTa SYBR Green FastMix, Low ROX, Quanta BioSciences). ΔΔCt values were used to determine the RQ value (Applied Biosystems 7500 software) for PLGF gene expression relative to the expression of the internal housekeeping gene β-actin (results expressed as PLGF/β-actin mRNA, fold of control). Our lab has previously determined that β-actin gene expression is not significantly affected by H2O2 treatment (data not shown).
ELISA
CASMC were grown in 6 well plates until 80% confluent, serum starved for 48 h and treated with a single dose of H2O2 (50 µM) or glucose oxidase (5 U/mL, Sigma) for 8 h. To protect secreted protein from degradation during sample processing, protease inhibitor cocktail (Sigma) was added (1:250) to the medium during collection on ice following the 8 h treatment. Total protein was quantified using the BCA Protein Assay (Pierce, Rockford, IL). Secreted PLGF protein levels were measured using the Human PLGF ELISA System (R&D Systems, Minneapolis, MN).
Statistical analyses
The effects of H2O2, glucose oxidase, and kinase inhibitors on PLGF mRNA level following H2O2 treatment were detected with one-way ANOVA followed by the Holm-Sidak test. Significance of anisomycin treatment and co-transfections was determined using one-way analysis of variance (ANOVA) on ranks followed by Dunn’s post-hoc test. The effect of actinomycin D on PLGF mRNA half life and the effect of glucose oxidase on H2O2 levels in medium over time were assessed by two-way ANOVA followed by the Holm-Sidak test. Differences were considered significant at p<0.05. Statistical analyses were performed with SigmaStat software.
Results
A single dose of hydrogen peroxide has no significant effect on PLGF promoter activity
We previously reported that PLGF mRNA levels are increased in human CASMC at 8 h following a single dose of exogenous H2O2 (13). In other cell types (human embryonic kidney cells, choriocarcinoma cells, placental villi), pro-inflammatory stimuli have been shown to increase PLGF promoter activity via metal responsive transcription factor (MTF-1), NF-κB, and CREB (14–16). It has also been well established that H2O2 activates transcription factors such as NF-κB and CREB (17, 18). In addition, the PLGF promoter region contained in the plasmid (PLGF-luc) used in this study was previously characterized and was reported to contain binding sites for MTF-1 and NF-κB (14). Therefore, we hypothesized that H2O2 treatment would increase PLGF mRNA levels in vascular smooth muscle cells by activation of PLGF gene transcription.
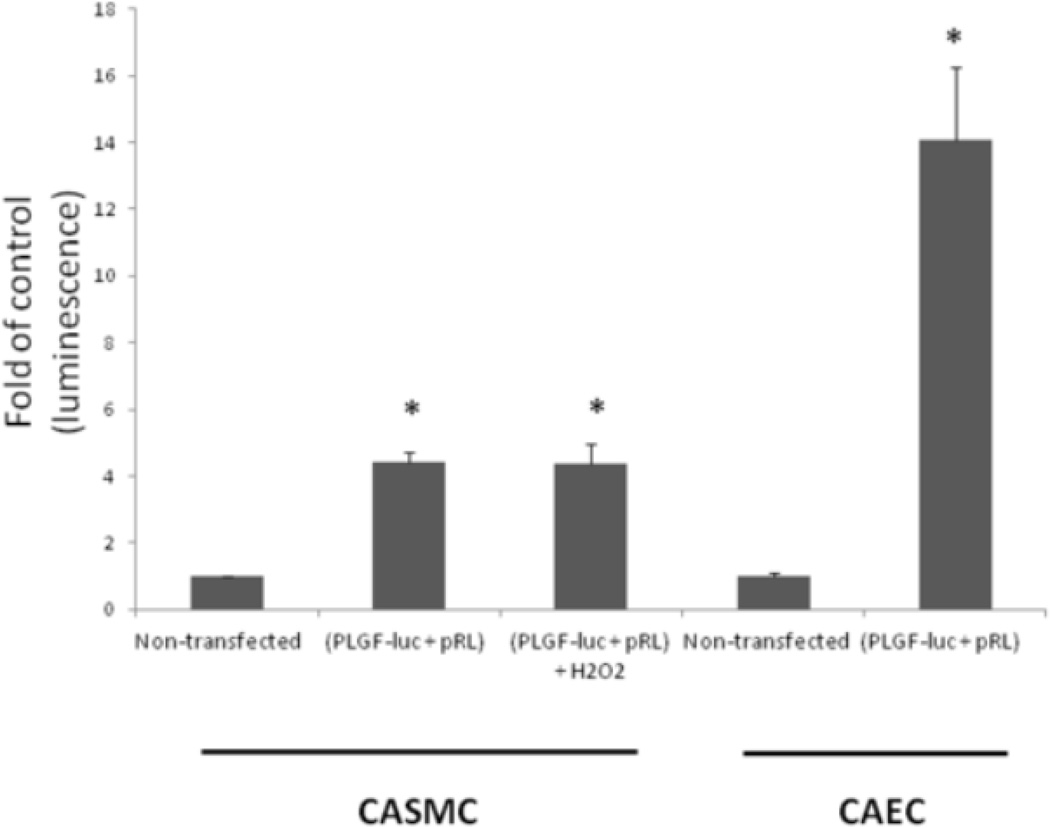
Currently, there are no positive controls known that drive PLGF promoter activity in smooth muscle cells. However, our lab has found that endothelial cells constituitively express PLGF mRNA and protein at levels higher than those found in smooth muscle cells (data not shown). Therefore, in order to demonstrate that the PLGF-luc plasmid can be used to detect changes in PLGF promoter activity, we transfected human coronary artery endothelial cells (CAEC) with PLGF-luc for comparison with CASMC. Co-transfected (PLGF-luc + pRL) CAEC had a higher basal level of PLGF promoter activity than CASMC co-transfected with PLGF-luc + pRL (Fig 1). Next, we assessed the effect of a single, physiological dose of H2O2 (50 µM) on PLGF promoter activity in CASMC. There was no significant difference in PLGF promoter activity between H2O2-treated CASMC and untreated CASMC at 8 h post treatment (Fig. 1). We conclude that a single physiological dose of H2O2 does not activate PLGF gene transcription in human CASMC.
Figure 1. Hydrogen peroxide has no significant effect on PLGF promoter activity.
Human CASMC were co-transfected with 2µg plasmid containing the PLGF promoter linked to the firefly luciferase reporter gene (PLGF-luc) and 40 ng Renilla luciferase control plasmid (pRL). Human CAEC (n=3) were also co-transfected with PLGF-luc and served as a positive control for PLGF promoter activity. PLGF promoter activity was not statistically different in co-transfected CASMC treated with H2O2 compared to the basal level of promoter activity observed in untreated co-transfected CASMC (n=6). (*p<0.05, non-transfected cells vs. respective transfected cell type).
Hydrogen peroxide increases the half-life of PLGF mRNA in human CASMC
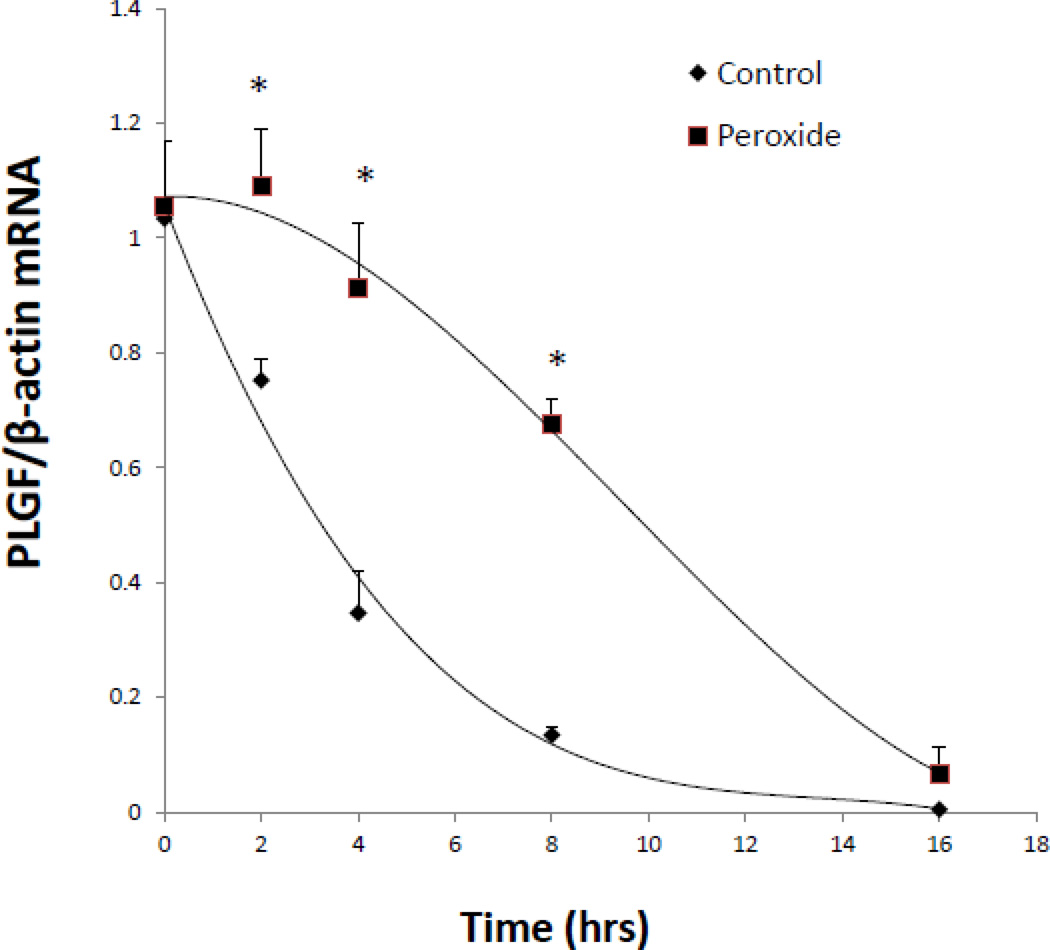
As shown in Fig. 1, there was no increase in PLGF promoter activity 8 h after treatment with a single dose of 50 µM H2O2. Therefore, we hypothesized that H2O2 acts to increase PLGF mRNA levels by extending the half-life of PLGF mRNA transcripts. To test this hypothesis, we treated human CASMC with either actinomycin D (10 µg/mL) alone to inhibit transcription or actinomycin D (10 µg/mL) plus a single dose of H2O2 (50 µM), then measured PLGF mRNA levels at 0, 2, 4, 8 and 16 h. We found that PLGF mRNA transcript half-life was significantly (p<0.01, n=3) increased by H2O2 at 2, 4, and 8 h post treatment (Fig. 2). There was no significant difference in PLGF mRNA levels between H2O2-treated and untreated groups at 0 or 16 h (Fig. 2).
Figure 2. Hydrogen peroxide increases the half-life of PLGF mRNA.
Human CASMC were treated with either actinomycin D (10 µg/mL) alone or with actinomycin D (10 µg/mL) plus a single dose of H2O2 (50 µM) and mRNA was collected at 0, 2, 4, 8 and 16 h later. PLGF mRNA transcript half-life was significantly (*p<0.01, n=3) increased at 2, 4, and 8 h post treatment with H2O2. There was no significant difference in PLGF mRNA levels at 0 and 16 h (n=3).
The level of H2O2 in the medium of CASMC following the addition of a single dose of 50 µM H2O2 had returned to baseline by 30 minutes post-treatment (data not shown). Therefore, brief exposure of CASMC to a single physiological dose of H2O2 appeared to exhibit a transient ability to stabilize PLGF mRNA that persisted for up to 8 h post-treatment and was no longer evident 16 h post treatment.
Anisomycin treatment increases the level of PLGF mRNA
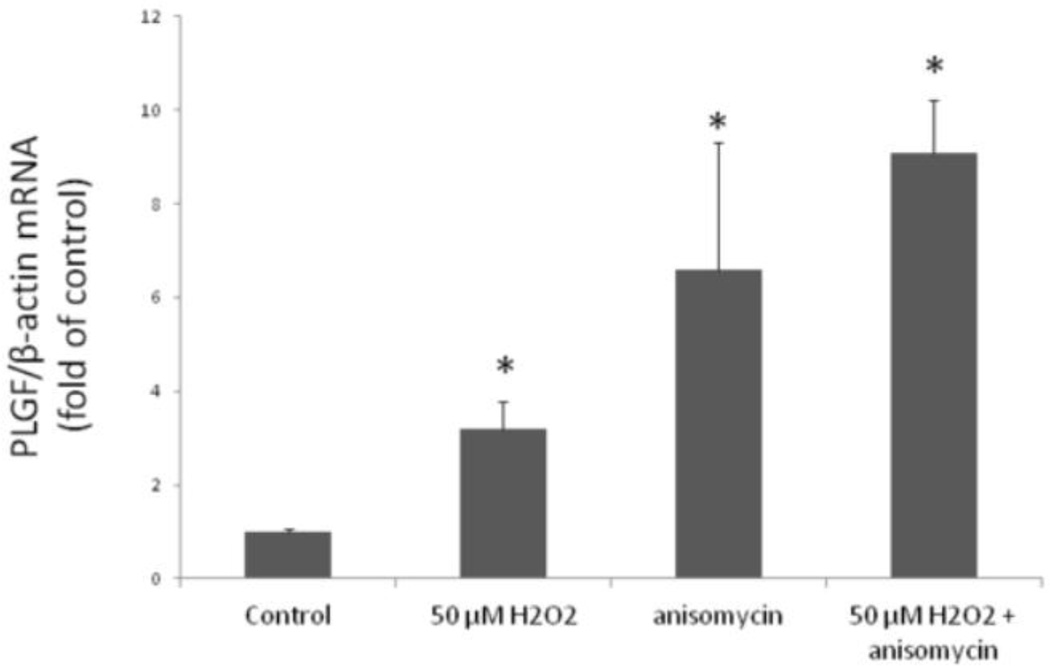
RNA stability often requires continuous translation of RNA stabilizing proteins that bind target transcripts and prevent degradation (19). Therefore, we hypothesized that ongoing protein translation might contribute to the increase in the PLGF mRNA levels following treatment with H2O2. To test this hypothesis, we treated cells with either anisomycin or cycloheximide, two inhibitors of protein synthesis. PLGF mRNA was significantly increased in CASMC treated with either H2O2 (50 µM) plus anisomycin (10 µg/mL) or anisomycin alone, compared to untreated control CASMC (Fig. 3). In contrast, neither cycloheximide alone nor H2O2 (50 µM) plus cycloheximide significantly affected PLGF mRNA levels, compared to the corresponding non-cycloheximide treated groups (data not shown).
Figure 3. Anisomycin increases PLGF mRNA.
Human CASMC treated with a single dose of H2O2 (50 µM), H2O2 plus anisomycin (10 µg/mL), and anisomycin alone showed a significant (*p<0.05, n=3) increase in PLGF mRNA, compared to control. There was no statistical difference in PLGF mRNA levels following treatment with H2O2 plus anisomycin vs. anisomycin alone.
Although anisomycin is an inhibitor of protein translation, it is also widely accepted as a potent activator of MAP kinases (20). Since the protein synthesis inhibitor cycloheximide did not upregulate PLGF expression, we conclude that the increased PLGF mRNA levels seen following anisomycin treatment occurred secondary to activation of MAPK pathways. Combination of anisomycin and H2O2 (50 µM) did not further increase PLGF mRNA levels over either treatment alone, suggesting that anisomycin and H2O2 (50 µM) act via similar pathways to affect PLGF expression.
Kinase pathways contribute to the peroxide-induced increase in PLGF mRNA levels
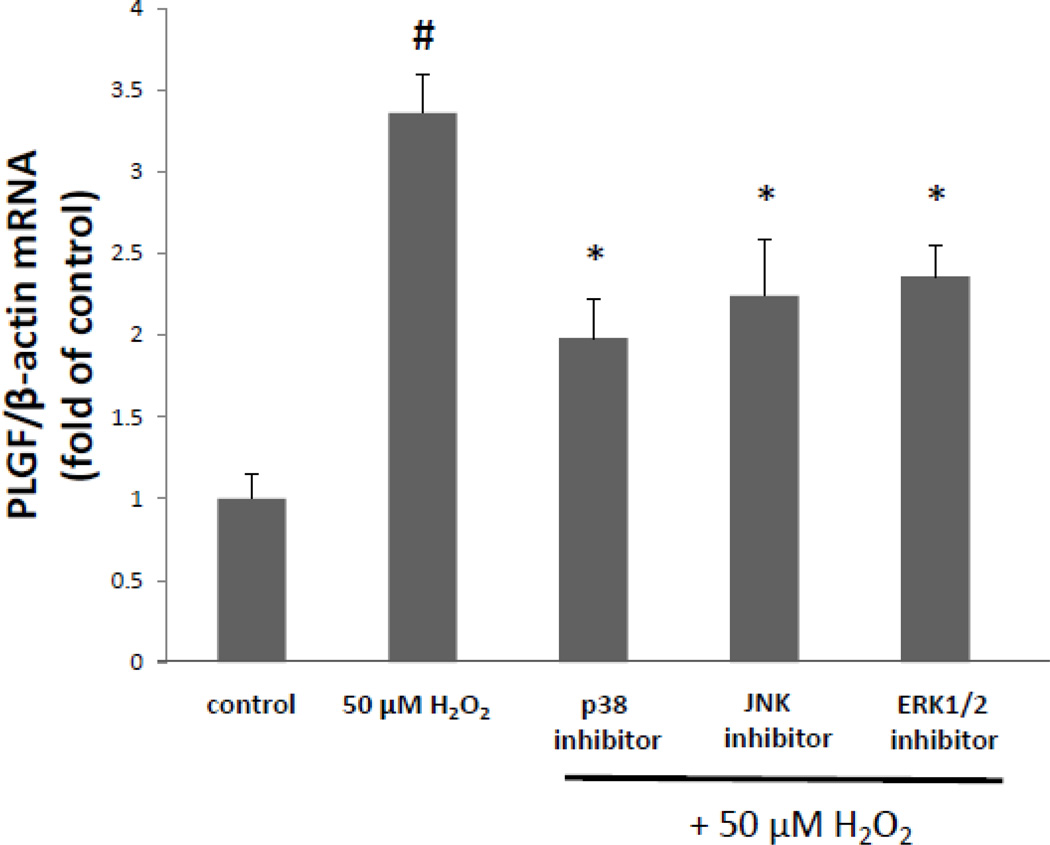
Since anisomycin treatment caused an increase in PLGF mRNA levels, we further characterized the role of kinase signaling in this mechanism. Oxidative stressors such as H2O2 are known to activate multiple kinase pathways which can affect transcription, RNA stability and translation (21). Thus, we hypothesized that kinases which are known to be activated by oxidative stress (such as p38 MAPK, JNK and/or ERK1/2), would contribute to the increase in PLGF mRNA following treatment with H2O2. Human CASMC were treated with a single dose of H2O2 (50 µM) in the presence of a p38 MAPK inhibitor (10 µM), a JNK inhibitor (10 µM) or an ERK1/2 inhibitor (10 µM). At 8 h post treatment, we found that all three kinase inhibitors significantly (p<0.05, n=3) inhibited (by ~40%) the peroxide-induced increase in PLGF mRNA levels (Fig. 4). We conclude that all three of these kinase pathways are partially responsible for the increase in PLGF mRNA levels following exposure to a single dose of a physiological level of H2O2.
Figure 4. Kinase pathways are involved in the peroxide-induced increase in PLGF mRNA.
Human CASMC were treated with a single dose of H2O2 (50 µM) in the presence of a p38 MAPK inhibitor (10 µM), JNK inhibitor (10 µM) or ERK1/2 inhibitor (10 µM) for 8 h. All three kinase inhibitors significantly reduced the H2O2-induced increase in PLGF mRNA, compared to treatment with H2O2 alone (*p<0.05, n=3; #p<0.05, H2O2 treated vs. untreated).
Chronic exposure to highly elevated levels of peroxide further increases PLGF mRNA levels
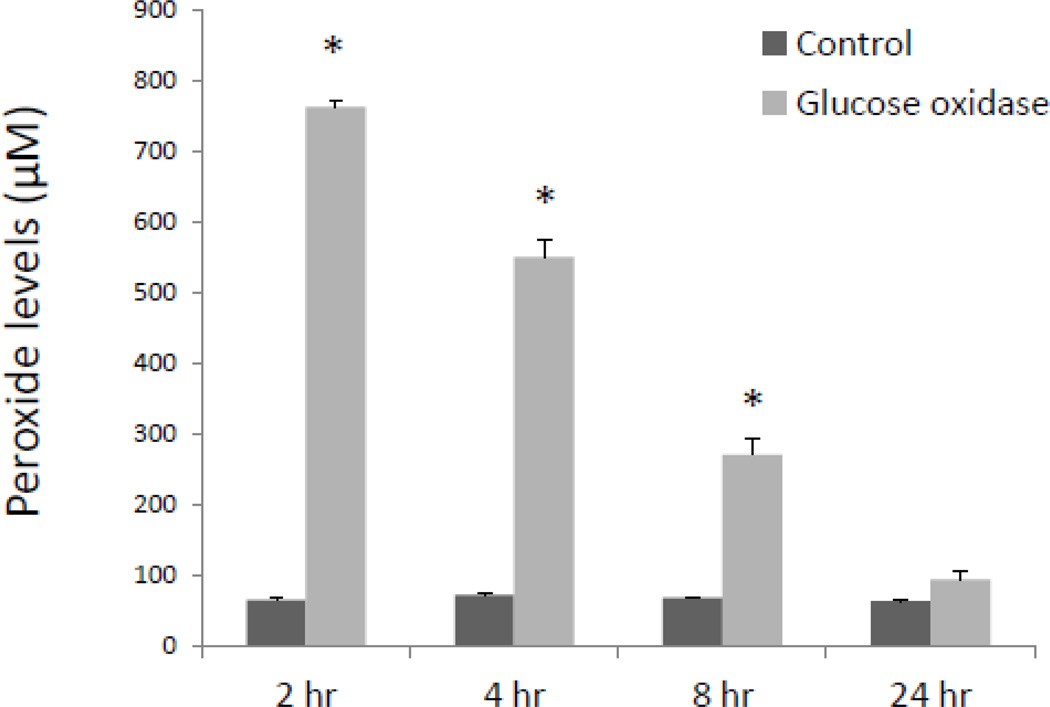
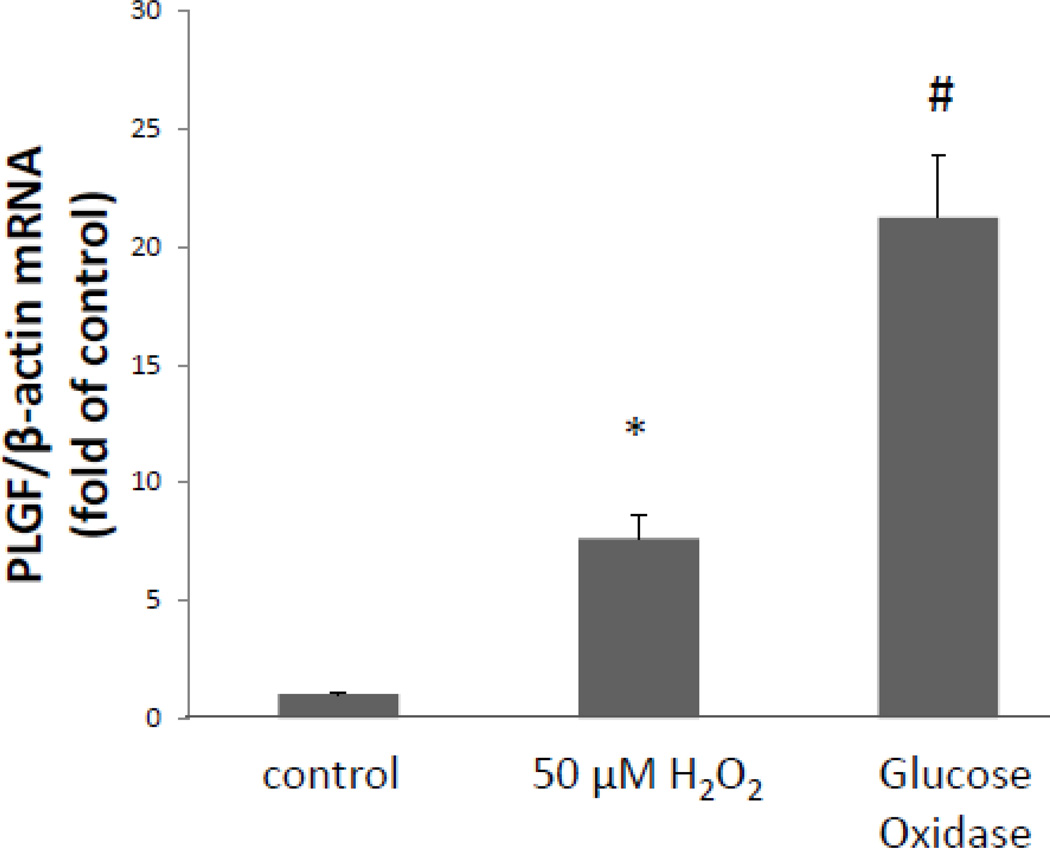
Exogenous treatment with a single dose of H2O2 (50 µM) reflects a mild burst of physiological oxidative stress, such as a short bout of moderate exercise. In the context of cardiovascular pathologies, however, H2O2 levels may be chronically elevated in diseased vasculature. We hypothesized that sustained exposure of CASMC to high levels of H2O2 would further increase PLGF mRNA levels. Human CASMC were treated with the glucose oxidase enzyme (5 U/mL) to provide a continuous source of H2O2 (by driving the enzymatic reaction β-D-glucose + O2 + H2O → D-glucono-δ-lactone + H2O2). Levels of H2O2 were highly elevated at 2 h (~750 µM) and gradually returned to control levels by 24 h as substrates for the enzymatic reaction were depleted (Fig 5). Whereas a single exogenous low dose of H2O2 caused a ~4–6 fold increase in PLGF mRNA levels, sustained exposure to highly elevated levels of H2O2 for 8 h resulted in a ~20-fold increase (p<0.001, n=3) in PLGF mRNA (Fig. 6). We conclude that chronic exposure to H2O2 further increases PLGF mRNA levels.
Figure 5. Time course of hydrogen peroxide concentration generated by glucose oxidase.
Medium was collected from wells containing human CASMC treated with glucose oxidase (5U/mL) for 2, 4, 8 and 24 h and assayed for peroxide. A large increase in hydrogen peroxide (~750 µM) was observed at 2 h and gradually declined, reaching ~250 µM by 8 h and ~60 µM by 24 h (*p<0.05, n=3, control vs. glucose oxidase).
Figure 6. Exposure to continuously elevated levels of peroxide further increases PLGF mRNA.
Human CASMC treated with glucose oxidase enzyme (5 U/mL) for 8 h exhibited a ~20-fold increase (#p<0.001, n=3) in PLGF mRNA compared to untreated cells, while a single dose of H2O2 (50 µM) produced a ~6 fold increase in PLGF mRNA (*p<0.05, n=3).
Chronic exposure to highly elevated levels of peroxide does not result in a corresponding increase in secreted PLGF protein
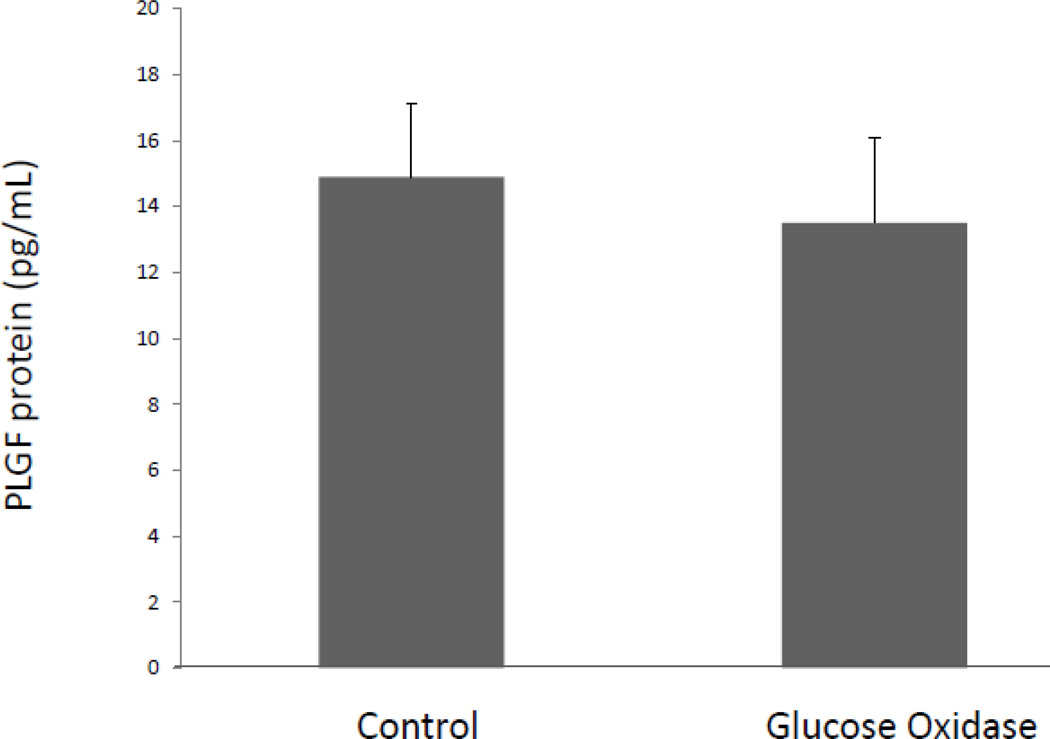
We next sought to determine whether the large increase in PLGF mRNA following glucose oxidase treatment resulted in a corresponding increase in PLGF protein. Previously, our lab reported a modest but significant increase in PLGF protein levels in human CASMC following treatment with a single physiological dose of H2O2 (50 µM) (13). Therefore, we hypothesized that chronic elevation of H2O2 would further increase PLGF protein levels. Surprisingly, we found that CASMC exposed to sustained high levels of H2O2 showed no significant change in secreted PLGF protein compared to untreated cells (Fig. 7), despite the substantial increase in PLGF mRNA (Fig. 6). These data raise the possibility that translation of PLGF mRNA may be inhibited in a highly oxidative environment.
Figure 7. Chronic exposure to highly elevated levels of peroxide does not increase PLGF protein level in CASMC medium.
Human CASMC exposed to a sustained high dose of H2O2 via treatment with glucose oxidase (5U/mL) showed no significant change in secreted PLGF protein, compared to untreated cells (n=3).
Discussion
PLGF is a VEGF-family protein that specifically mediates arteriogenesis rather than capillary proliferation (5, 9, 22). As such, modulation of PLGF expression may be useful in the setting of ischemic cardiovascular disease, where enlarged collateral arteries could provide an alternate route for blood flow to compensate for atherosclerotic obstruction of upstream arteries. Alternatively, inhibition of PLGF expression could potentially have an anti-tumor effect by preventing tumor blood vessel growth. However, despite the potential therapeutic utility of modulating PLGF expression, little is known about the signaling mechanisms that regulate its expression in adult vasculature.
We previously reported that conditioned medium from endothelial cells was able to induce PLGF gene expression in smooth muscle cells, and that this effect appeared to be mediated by H2O2 (13). Furthermore, exogenous H2O2 induced a dose-dependent increase in PLGF mRNA expression in both established and primary vascular SMC, along with a mild but significant increase in PLGF protein. This effect was specific for PLGF, as mRNA for VEGF-A was only transiently upregulated by exogenous H2O2, and VEGF-A protein did not increase following H2O2 treatment. The goal of the present study was to establish further details of this novel signaling pathway.
The results presented here demonstrate that a single dose of exogenous H2O2 acts to increase PLGF mRNA levels in SMC by increasing the half-life of PLGF mRNA, rather than by activating PLGF gene transcription. We also treated cells with anisomycin, which caused an increase in PLGF mRNA. In addition to its action as an inhibitor of protein synthesis, anisomycin is known to activate stress-related MAP kinases (20). Since cycloheximide did not upregulate PLGF, these results suggested that kinase signaling could be involved in the H2O2–induced increase in PLGF mRNA. Thus, we tested whether protein kinase inhibition could block the increase in PLGF mRNA following H2O2 treatment. Consistent with previous reports demonstrating that H2O2 upregulates p38 MAPK, Erk1/2, and JNK signaling (21), the effect of H2O2 on PLGF mRNA levels was found to be partially dependent on all three kinase pathways. Although H2O2 is more stable than superoxide (O2−) and is thus considered to be a more likely mediator of signaling in the vascular wall than O2−, it nonetheless has a relatively short half life. Analysis of H2O2 levels in media of cells treated with a single dose of 50 µM H2O2 demonstrated that H2O2 had returned to basal levels within 30 min of treatment (data not shown). To determine whether exposure to chronically elevated H2O2 produced similar effects to transient exposure, we utilized an enzymatic system to generate H2O2 in culture medium of SMC (glucose oxidase). Glucose oxidase treatment produced a marked increase in H2O2 levels (to ~750 µM after 2 h). This increase was associated with greatly elevated PLGF mRNA levels (~20 fold of untreated control at 8 h post-glucose oxidase). Surprisingly, however, we did not observe a corresponding increase in PLGF protein expression.
There are several possible explanations for the failure of increased PLGF mRNA to lead to a corresponding increase in PLGF protein. First, it is possible that the age of the primary human cells used in the glucose oxidase experiment influenced the results. In our previous paper (in which upregulation of PLGF mRNA led to a small, but significant increase in PLGF protein), we used primary human CASMC which were obtained from a young (12 yo) donor. When assessing PLGF expression in response to glucose oxidase, the source of the primary human CASMC was an older (56 yo) donor. Although we cannot draw any definitive conclusions about possible age effects on PLGF translation from this limited comparison, it is possible that aged cells fail to upregulate PLGF protein expression in response to increased PLGF mRNA levels. This hypothesis is consistent with the known reduction in arteriogenic capacity of aged animals (23–25), and with the observation that aged endothelium releases pathologically elevated levels of H2O2 (26). The question of whether age affects PLGF expression at the translational level therefore is an important area for further research.
Alternatively, there may be additional age-independent mechanisms controlling the translation of PLGF mRNA that are active in our model system. For instance, PLGF mRNA could potentially be protected from translation by microRNA (miRNA) binding to the transcript. It has recently been reported that H2O2 treatment (at concentrations similar to those used in our current study) upregulates numerous miRNA species in vascular SMC (27). It is unknown whether any of these miRNA regulate PLGF translation. If PLGF translation is regulated by miRNA, then H2O2 treatment may be able to simultaneously increase PLGF mRNA levels (by stabilizing the transcript), and inhibit its translation into protein (by upregulating inhibitory miRNA). The role of miRNA in regulation of PLGF translation is an interesting question for further investigation.
Although the details of mechanisms regulating PLGF translation remain to be revealed, our present findings are consistent with reports in the literature showing that the dependence of arteriogenesis on oxidative signaling is finely balanced; adaptive collateral artery enlargement is prevented by both pathologically high levels of ROS and by insufficient levels of ROS (7). Thus, it is possible that mild oxidative stress results in increased PLGF protein expression and a resulting enhancement of arteriogenesis, whereas a greater degree of oxidative stress prevents production of PLGF protein and hinders arteriogenesis.
Conclusions
In summary, these studies expand on our previously published results to demonstrate that H2O2 treatment increases PLGF gene expression by increasing the half-life of PLGF mRNA, rather than by increasing the rate of PLGF mRNA transcription and that the H2O2-induced increase in PLGF mRNA is partially dependent upon kinase signaling. Finally, we found that a high level of oxidative stress induces even greater elevation of PLGF mRNA than does mild oxidative stress, without a corresponding increase in PLGF protein expression. These results suggest that regulation of PLGF expression has an important translational component. These data further increase our understanding of the regulation of this key arteriogenic protein, and generate important questions for further research.
Highlights.
A single, low dose of H2O2 increases the half-life of PLGF mRNA in human CASMC
The H2O2 induced increase in PLGF mRNA levels depend, in part, on kinase signaling
Chronic exposure to H2O2 induces an even greater elevation of PLGF mRNA
Elevated PLGF mRNA due to chronic exposure does not result in increased PLGF protein
Acknowledgements
These studies were supported by funding from the National Institutes of Health (NIH R01 HL084494, PL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None.
Disclosures: None declared.
Reference List
- 1.Schirmer SH, van Nooijen FC, Piek JJ, van RN. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart. 2009;95:191–197. doi: 10.1136/hrt.2007.136119. [DOI] [PubMed] [Google Scholar]
- 2.Osanai T, Fujita N, Fujiwara N, Nakano T, Takahashi K, Guan W, et al. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am J Physiol Heart Circ Physiol. 2000;278:H233–H238. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- 3.Coyle CH, Kader KN. Mechanisms of H2O2-induced oxidative stress in endothelial cells exposed to physiologic shear stress. ASAIO J. 2007;53:17–22. doi: 10.1097/01.mat.0000247157.84350.e8. [DOI] [PubMed] [Google Scholar]
- 4.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. Journal of Biological Chemistry. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 5.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, et al. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circulation Research. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- 6.Scholz D, Elsaesser H, Sauer A, Friedrich C, Luttun A, Carmeliet P, et al. Bone marrow transplantation abolishes inhibition of arteriogenesis in placenta growth factor (PlGF) −/− mice. Journal of Molecular and Cellular Cardiology. 2003;35:177–184. doi: 10.1016/s0022-2828(02)00304-8. [DOI] [PubMed] [Google Scholar]
- 7.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol. 2007;292:H2729–H2736. doi: 10.1152/ajpheart.01330.2006. [DOI] [PubMed] [Google Scholar]
- 8.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nature Medicine. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 9.Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, et al. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. Journal of Cell Science. 2002;115:2559–2567. doi: 10.1242/jcs.115.12.2559. [DOI] [PubMed] [Google Scholar]
- 10.Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: a historical review. Angiogenesis. 2008;11:215–221. doi: 10.1007/s10456-008-9114-4. [DOI] [PubMed] [Google Scholar]
- 11.Maglione D, Guerriero V, Viglietto G, li-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. American Journal of Physiology Heart and Circulatory Physiology. 2004;287:H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JH, Xiang L, Shah A, Yin W, Lloyd PG. Placenta growth factor expression is regulated by hydrogen peroxide in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2011;300:C349–C355. doi: 10.1152/ajpcell.00374.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer M, Nagy I, Murphy BJ, Gassmann M, Hottiger MO, Georgiev O, et al. NF-kappaB contributes to transcription of placenta growth factor and interacts with metal responsive transcription factor-1 in hypoxic human cells. Biol Chem. 2005;386:865–872. doi: 10.1515/BC.2005.101. [DOI] [PubMed] [Google Scholar]
- 15.Green CJ, Lichtlen P, Huynh NT, Yanovsky M, Laderoute KR, Schaffner W, et al. Placenta growth factor gene expression is induced by hypoxia in fibroblasts: a central role for metal transcription factor-1. Cancer Res. 2001;61:2696–2703. [PubMed] [Google Scholar]
- 16.Depoix C, Tee MK, Taylor RN. Molecular regulation of human placental growth factor (PlGF) gene expression in placental villi and trophoblast cells is mediated via the protein kinase a pathway. Reprod Sci. 2011;18:219–228. doi: 10.1177/1933719110389337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu S, Zhu H, Wei X, Zhang C, Jiang L, Liu Y, et al. Oxidative stress-mediated up-regulation of myocardial ischemic preconditioning up-regulated protein 1 gene expression in H9c2 cardiomyocytes is regulated by cyclic AMP-response element binding protein. Free Radic Biol Med. 2010;49:580–586. doi: 10.1016/j.freeradbiomed.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 19.Masuda K, Abdelmohsen K, Gorospe M. RNA-binding proteins implicated in the hypoxic response. J Cell Mol Med. 2009;13:2759–2769. doi: 10.1111/j.1582-4934.2009.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barros LF, Young M, Saklatvala J, Baldwin SA. Evidence of two mechanisms for the activation of the glucose transporter GLUT1 by anisomycin: p38(MAP kinase) activation and protein synthesis inhibition in mammalian cells. J Physiol. 1997;504(Pt 3):517–525. doi: 10.1111/j.1469-7793.1997.517bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat NR, Zhang P. Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J Neurochem. 1999;72:112–119. doi: 10.1046/j.1471-4159.1999.0720112.x. [DOI] [PubMed] [Google Scholar]
- 22.Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003;1:1356–1370. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 23.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan KM, Ferguson MJ, Distasi MR, Witzmann FA, Dalsing MC, Miller SJ, et al. Impact of genetic background and aging on mesenteric collateral growth capacity in Fischer 344, Brown Norway, and Fischer 344 x Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2007;293:H3498–H3505. doi: 10.1152/ajpheart.00040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westvik TS, Fitzgerald TN, Muto A, Maloney SP, Pimiento JM, Fancher TT, et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg. 2009;49:464–473. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller-Delp JM, Gurovich AN, Christou DD, Leeuwenburgh C. Redox Balance in the Aging Microcirculation: New Friends, New Foes, and New Clinical Directions. Microcirculation. 2011 doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]