Abstract
OBJECTIVES
Lipid Emulsion (LE) has been shown to be effective in resuscitating bupivacaine-induced cardiac arrest but its mechanism of action is not clear. Here we investigated whether fatty acid oxidation is required for rescue of bupivacaine induced cardiotoxicity by LE in rats. We also compared the mitochondrial function and calcium threshold for triggering of mitochondrial permeability transition pore (mPTP) opening in bupivacaine-induced cardiac arrest before and after resuscitation with LE.
DESIGN
Prospective, randomized, animal study.
SETTING
University Research Laboratory.
SUBJECTS
Adult male Sprague-Dawley rats.
INTERVENTIONS
Asystole was achieved with a single dose of bupivacaine (10mg/kg over 20seconds, i.v.) and 20% LE infusion (5ml/kg bolus, and 0.5ml/kg/min maintenance) with cardiac massage started immediately. The rats in CVT group were pretreated with a single dose of fatty acid oxidation inhibitor CVT (0.5, 0.25, 0.125 or 0.0625mg/kg bolus i.v.) 5min prior to inducing asystole by bupivacaine overdose. Heart rate (HR), ejection fraction (EF), fractional shortening (FS), the threshold for opening of mPTP, oxygen consumption and membrane potential were measured. The values are Mean±SEM.
MEASUREMENTS AND MAIN RESULTS
Administration of bupivacaine resulted in asystole. ILP infusion improved the cardiac function gradually as the EF was fully recovered within 5min (EF=64±4% and FS=36±3%, n=6) and heart rate increased to 239±9 beats/min (71% recovery, n=6) within 10min. LE was only able to rescue rats pretreated with low dose of CVT (0.0625mg/kg) (HR=~181±11 beats/min at 10 min, recovery of 56%; EF=50±1%; FS=26±0.6% at 5min, n=3) but was unable to resuscitate rats pretreated with higher doses of CVT (0.5, 0.25 or 0.125mg/kg). The calcium retention capacity in response to Ca2+ overload was significantly higher in cardiac mitochondria isolated from rats resuscitated with 20% LE compared to the group that did not receive ILP after bupivacaine-overdose (330±42 vs. 180±8.2 nmol/mg-mitochondrial protein, p<0.05, n=3 in each group). The mitochondrial oxidative rate and membrane potential were similar in bupivacaine group before and after resuscitation with LE infusion.
CONCLUSIONS
Fatty acid oxidation is required for successful rescue of bupivacaine induced cardiotoxicity by LE. This rescue action is associated with inhibition of mitochondrial permeability transition pore opening.
Keywords: Lipid Emulsion, bupivacaine, CVT, fatty acid oxidation, mitochondrial permeability transition pore, cardiotoxicity, local anesthetic toxicity
Introduction
Bupivacaine is a long-acting, lipophilic local anesthetic agent that is broadly used for cutaneous infiltration, peripheral nerve blocks, epidural anesthesia, and spinal anesthesia. Systemic toxicity from local anesthetic overdose can occur from accidental intravascular injection, drug overdose, or rapid absorption from the administration site (1). Well-described cardiotoxic effects of bupivacaine include dysrhythmias, hypotension, and depression of cardiac output leading to cardiac arrest (2).
It has been shown by Weinberg et al. that Lipid Emulsion (LE) is effective in resuscitation from cardiac arrest as a result of bupivacaine overdose in rat and canine models (3–5). Successful resuscitation of bupivacaine cardiotoxicity by LE has also been reported in patients (6–8). In spite of the proven efficacy of LE in resuscitating bupivacaine-induced cardiac arrest, the underlying mechanism behind the interaction of LE and bupivacaine is not fully understood. Lipid sink theory was among one of the several mechanisms proposed for the rescue action LE (5;9;10). Based on this theory fat droplets in the blood form lipid compartments or ‘lipid sinks’, into which lipophilic drugs like bupivacaine might dissolve and therefore LE increases the clearance of local anesthetic from the aqueous plasma or cardiac tissue (5).
It was also suggested that LE might have metabolic effects since bupivacaine impairs the production of adenosine triphosphate (11), LE may therefore attenuate a component of cardiotoxicity resulting from bupivacaine’s inhibition of adenosine triphosphate synthesis (3;5). In fact the discovery of lipid rescue was based on the metabolic effects of bupivacaine on mitochondria and the role of lipid treatment (3). The failure of LE treatment five minutes after relatively high doses of epinephrine and vasopressin in swine model makes metabolic mechanism more likely (1).
The role of mitochondrial permeability transition pore (mPTP) has been also highlighted in myotoxicity induced by bupivacaine overdose in skeletal muscle, as mPTP was a major underlying cause of mitochondrial depolarization induced by bupivacaine (12). In fact, this event was shown to be essential for the subsequent dysfunction that leads to hypercontracture and cell death (12).
Here we examined the hypothesis that the protective action of LE i) is mediated through fatty acid oxidation pathway and ii) is associated with inhibition of mitochondrial permeability transition pore opening.
We used CVT-4325, which is a piperazine derivative that is known to inhibit fatty acid oxidation in cardiac mitochondria with a high potency in a dose dependant manner (13–17). We also compared the mitochondrial function and calcium threshold for triggering of mPTP opening in bupivacaine-induced cardiac arrest with and without resuscitation with LE infusion.
Materials and Methods
Animals and treatments
Adult male Sprague-Dawley rats (300–350 g) were used. Protocols have received institutional approval. Thirty nine rats were used for this study. The technician was assigning the rats randomly to different groups. Rats were anesthetized intraperitoneally with a mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg). Rats were intubated via tracheotomy using a 16-gauge angiocatheter and ventilated with a ventilator. The ventilation was continued throughout the resuscitation on 100% oxygen. The femoral vein was accessed through a 22 gauge intravenous catheter. To induce asystole rats were treated with a single injection of bupivacaine at 10 mg/kg. This dose has been used commonly by Weinberg group to achieve cardiac depression rapidly and decrease the variability in the time to reach asystole(18–20). The dose of LE used in this study to resuscitate the rats from bupivacaine-induced cardiotoxicity is also similar to the dose that has been used by Weinberg group (one bolus of 5ml/kg, and 0.5ml/kg/min for maintenance) (18;20). Some rats were pre-treated with a single bolus of fatty acid oxidation inhibitor CVT at different doses (0.5, 0.25, 0.125 or 0.0625mg/kg bolus i.v.). Body temperature was maintained at 37°C throughout the experiment. The rats which were resuscitated by LE were euthanized at the end of the experiments by quickly removing the heart before recovery from anesthesia according to the protocol approved by the UCLA institutional review and committee. The investigator injecting the medications was not blinded but those performing the echocardiography, or isolating mitochondria were blinded.
Experimental protocols
1. Blocking of Fatty Acid Oxidation
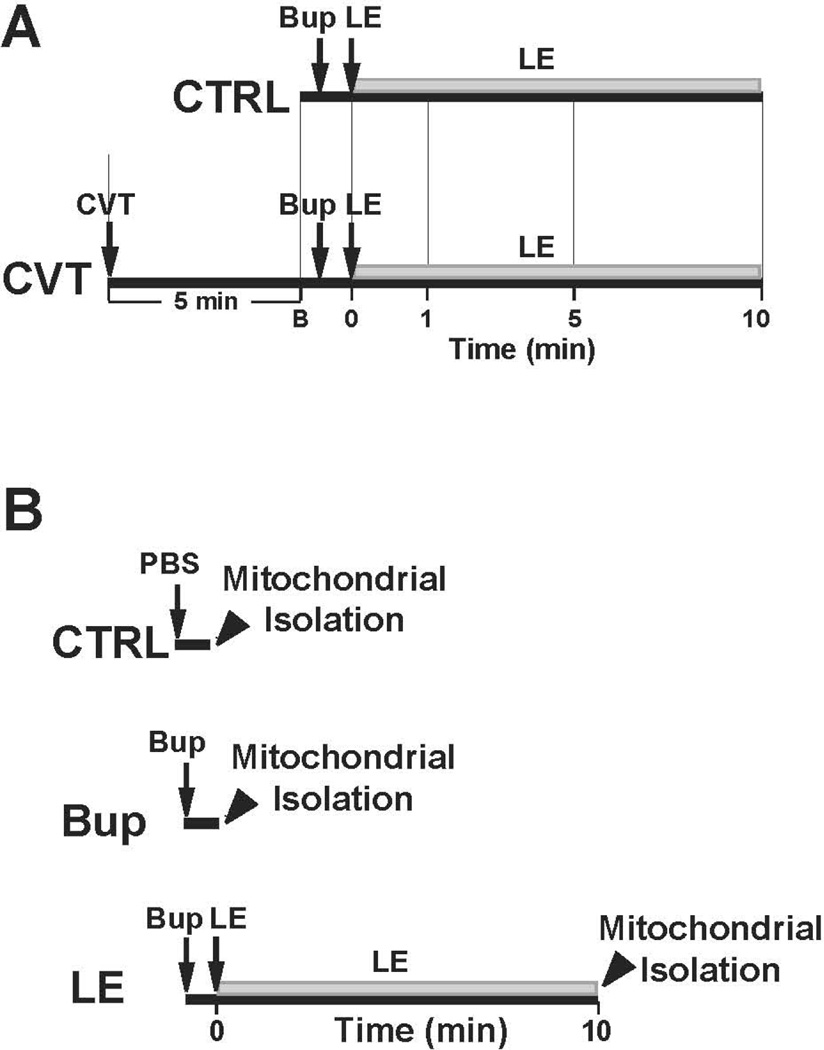
Rats were randomly divided in two groups of control (CTRL) and CVT (Fig. 1A). In control group (n=6 rats), after measuring the heart rate and ejection fraction at the baseline, asystole was achieved with a single dose of bupivacaine (10mg/kg over 20seconds, i.v.). The infusion of LE (20% intralipid (Sigma), 5ml/kg bolus, and 0.5ml/kg/min maintenance) with cardiac massage was started immediately. In the CVT group, the heart rate and ejection fraction were measured before and 5 min after a single bolus of fatty acid oxidation inhibitor CVT at different doses (0.5, 0.25, 0.125 or 0.0625mg/kg bolus i.v., n=3 rats per each dose of CVT). The asystole was then induced by bupivacaine overdose and LE infusion (20% intralipid (Sigma), 5ml/kg bolus, and 0.5ml/kg/min maintenance) along with cardiac massage was started immediately.
Figure 1. Experimental protocols.
A. In control (CTRL) group, asystole was achieved by a single injection of bupivacaine (10mg/kg over 20 seconds) and resuscitation with LE (5ml/kg bolus, and 0.5ml/kg/min maintenance) together with cardiac massage was started immediately (n=6 rats). In CVT group, the experimental protocol was identical to CTRL group, except rats were pre-treated with different doses of CVT-4325 (0.5, 0.25, 0.125, 0.0625 mg/kg) for 5 minutes (n=3 rats/each dose of CVT). B. The mitochondria were isolated from 3 groups i) CTRL group, rats only received a single injection of PBS; ii) Bup group, rats only received a single injection of bupivacaine (10mg/kg over 20 seconds) to induce asystole and iii) LE group, after inducing asystole with bupivacaine overdose, rats were resuscitated with 20% LE (5ml/kg bolus, and 0.5ml/kg/min maintenance). Rats were sacrificed in all 3 groups at the time indicated with arrowhead (within 2 min of PBS/Bup infusion in group 1 and 2 and 10 min after LE infusion in group 3 as indicated with arrowhead and the mitochondria were isolated immediately only from left ventricle. The mitochondria were used for measuring calcium retention capacity (in response to Ca2+ overload), oxygen consumption, and membrane potential.
2. Mitochondrial functional measurements
Rats were randomly divided in three groups. The mitochondria were isolated from i) CTRL group, rats were only treated with a single injection of PBS; ii) Bup group, rats were treated with a single injection of bupivacaine (10mg/kg over 20 seconds) to induce asystole and iii) LE group, after inducing asystole with bupivacaine overdose, rats were resuscitated with LE (20%, 5ml/kg bolus, and 0.5ml/kg/min for maintenance) along with cardiac massage for 10 min. Rats were sacrificed within 2 min of PBS/Bup infusion in group 1 and 2 (this is the time that usually takes for the heart to go to asystole after Bup infusion) and 10 min after LE infusion in group 3 as indicated with arrowhead. The isolation of mitochondria were started immediately only from left ventricle (LV). The freshly isolated mitochondria were used for measuring oxygen consumption (n=3 in CTRL, n=3 in LE, and n=4 in Bup), membrane potential (n=3 in CTRL, n=3 in LE and n=4 in Bup) and calcium retention capacity in response to Ca2+ overload (n=4 in LE and n=4 in Bup).
Mitochondrial isolation
Mitochondria were isolated only from the left ventricle as described previously (18). Briefly, LV myocardial sections of approximately 0.15–0.22 g were placed in isolation buffer A containing (mM: 70 sucrose, 210 mannitol, 1 EDTA and 50 Tris-HCl, pH 7.4 at 4°C. The tissue was finely minced with scissors and homogenized in the same buffer A (1 ml buffer/0.1g of tissue) using Kontes and Potter-Elvehjem tissue grinders. The homogenate was centrifuged at 1,300 g for 3 min; the supernatant was filtered through a cheesecloth and centrifuged at 10,000 g for 10 min. The mitochondrial pellet was resuspended in isolation buffer B containing in mM 70 sucrose, 210 mannitol, 0.1 EDTA and 50 Tris-HCl, pH 7.4. Mitochondrial protein concentration was measured using the Bradford assay. The freshly isolated mitochondria were used for measuring calcium retention capacity in response to Ca2+ overload, oxygen consumption and membrane potential. The investigator isolating mitochondria was blinded to the protocol.
Measurement of Mitochondrial Respiration
Mitochondrial respiration was measured using a fiber optic oxygen sensor FOXY-AL300 (Ocean Optics) in a partially open continuously stirred quvette at 25°C as reported (21). Freshly isolated mitochondria were suspended in KME buffer (100 mM KCl, 50 mM Mops, 0.5 mM EGTA) and mitochondrial concentration was measured. Isolated mitochondria (500 µg) was added to the assay buffer containing 125 mmol/L KCl, 10 mmol/L Hepes, potassium phosphate (Pi, 2.5 mmol/L), pH 7.4 with Tris. To measure complex I-mediated respiration rates, substrates combinations of 1.6 mmol/L pyruvate plus 1.6 mmol/L L-malate and 1.6 mmol/L glutamate were added to the mitochondria. The respiration in state 3 (stimulated by 0.2 mM ADP) and state 4 (ADP-limited) were measured. The Respiratory Control Ratio (RCR) was measured by dividing the respiratory values in state 3 to state 4 and were normalized to mg of mitochondrial protein.
Determination of mitochondrial membrane potential (ΔΨm)
ΔΨm was monitored by transmembrane distribution of the fluorescence indicator tetramethylrhodamine methyl ester (TMRM) according to the Nernst equation (22). Briefly, 500 µg freshly isolated cardiac mitochondria were suspended in 2 ml of the respiration buffer containing 200 nM TMRM, and the fluorescence was recorded at 25 °C at excitation and emission wavelengths of 550 nm and 580 nm, respectively. After the equilibration period to reach state 2 (1.6 mM pyruvate/malate/glutamate added, without ADP), state 3 respiration was evoked by the addition of 200µM ADP. ΔΨm was assessed by comparing the fluorescence before and after addition of 2uM alamethacin, a mitochondrial uncoupler which fully depolarizes mitochondria.
Measurement of Mithocondrial Calcium Retention Capacity (CRC)
The onset of the mPTP opening was assessed in freshly isolated mithocndria following in-vitro Ca2+ overload. Free Ca2+ concentration outside the mitochondria was recorded with 0.5 µM calcium green-5N (Invitrogen) using excitation and emission wavelengths set at 500 and 530nm, respectively. Isolated mitochondria (1 mg of protein) were suspended in 2 ml buffer C (mM, 150 sucrose, 50 KCl, 2 KH2PO4, 5 succinic acid and 20Tris/HCl, pH 7.4). Samples were pre-incubated for 90 sec in the spectrofluorometer cuvette, and Ca2+ pulses (20 nanomoles (nmol)/mg of mitochondrial protein) were applied every 60sec in the spectrofluorometer. The Ca2+ pulses induced a peak of extra-mitochondrial Ca2+ concentration that returned to near-baseline level as Ca2+ entered the mitochondrial matrix via the Ca2+ uniporter. With increasing Ca2+ loading, the extra-mitochondrial Ca2+ concentration started accumulating, reflecting a lower capacity for mitochondria Ca2+ uptake, which was followed by a sustained Ca2+ increase indicating a massive release of the mitochondria Ca2+ by the mPTP opening. The Ca2+ retention capacity (CRC) was defined as the amount of Ca2+ required to trigger this massive Ca2+ release which was used here as an indicator of the mPTP sensitivity to Ca2+. CRC was expressed as nmol of CaCl2 per mg of mitochondrial protein.
Cardiac Hemodyanamics
Serial B-Mode and M-Mode echocardiography was performed at baseline and at 1, 5 and 10 minutes after LE treatment to measure the ejection fraction and fractional shortening using a VisualSonics Vevo 770 equipped with a 30-MHz linear transducer. Standard Lead II Electrocardiograms were acquired under anesthesia continuously throughout the experiment. The investigator performing echocardiography was blinded to the protocol.
Statistics
For HR, EF, FS, means were compared between group and over time using a repeated measure analysis of variance (ANOVA) model. The individual pairwise mean comparisons were evaluated using the (Tukey, Fisher LSD) criterion to adjust for multiple comparisons. For CRC (mPTP opening) and membrane potential measurements, means were compared between lipid emulsion vs. bupivacaine using t tests. For mitochondrial respiration, means were compared across the 3 groups of CTRL, bupivacaine and lipid emulsion using one way analysis of variance (ANOVA). P-values <0.05 were considered statistically significant. Means and standard error of the mean (mean +/− SEM) are reported. The error bars in the Figures are based on the SEM.
Results
The role of Fatty Acid Oxidation in reversal of Bupivacaine-induced cardiotoxicity by LE
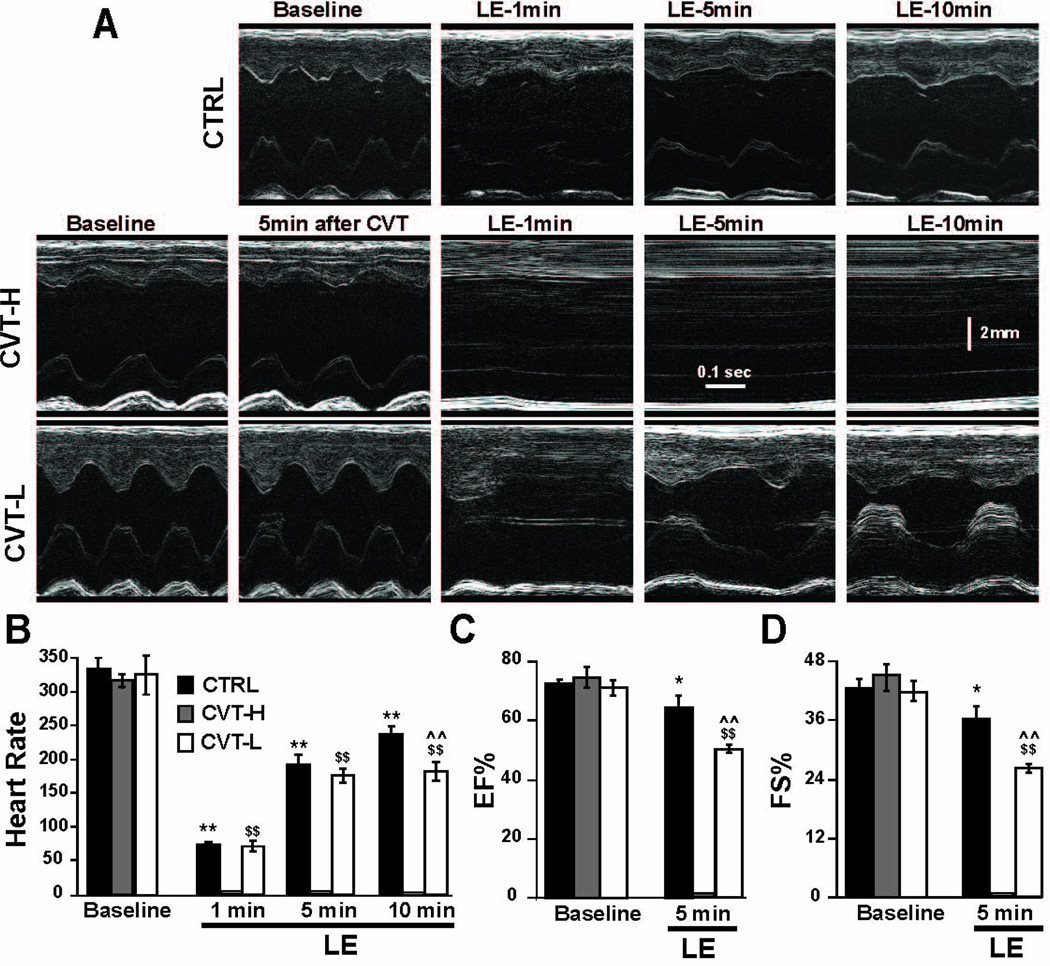
To examine whether rescue of bupivacaine-induced cardiac arrest by LE is mediated via fatty acid oxidation pathway, we used CVT-4325, which is a piperazine derivative that inhibits fatty acid oxidation in cardiac mitochondria with a high potency (13–17). M-mode echocardiography images in Fig. 2A from the same rat shows gradual resuscitation from bupivacaine-induced cardiotoxicity by LE in CTRL (See Fig.1A for experimental protocol). However, in the rats pretreated with CVT doses of 0.125 mg/kg or higher, LE was not able to rescue the rat and only partial recovery was observed in the presence of lower CVT dose (0.0625 mg/kg, Fig. 2A). The HR and EF at baseline in CTRL group before application of bupivacaine were 336.7±12.1 and 72.7±1.3%, respectively (Fig. 2B,C). Administration of bupivacaine resulted in asystole as expected and 20% LE resuscitation improved HR and cardiac function gradually within 10 min. The HR was, 74.7±4.6 beats/min at 1 min (22% recovery), increased to 197.5±13.2 beats/min at 5 min (59% recovery), and further to 239±9.5 beats/min 10 min after the initiation of LE therapy (71% recovery). The left ventricular systolic function was fully recovered in all rats within 5 min of ILP treatment (EF=64±4%, FS=36±3%, Fig. 2C,D)
Figure 2. LE can only restore the heart function following bupivacaine-induced cardiotoxicity in CVT-pretreated rats at very low dose.
A. M-mode echocardiographic images at baseline, 1, 5 and 10 min after ILP in CTRL (upper panels) and in CVT groups at baseline, 5 min after CVT (0.5, 0.25 and 0.125 mg/kg in middle panels and 0.0625 mg/kg in lower panels) and 1, 5 and 10 min after LE. B. Heart rate in CTRL (black bars, n=6 rats), in CVT high dose (0.5 mg/kg, n=3 rats) and low dose (0.0625 mg/kg, n=3 rats), white bars) at baseline, 1, 5 and 10 min after administration of LE. **P<0.0001 vs. baseline in CTRL; $$P<0.0001 vs. baseline in CVT-low; ^^P<0.0001 vs. CTRL at 10 min. C. Ejection fraction (EF) and D. fractional shortening (FS) in CTRL (black bars, n=6 rats) and in CVT high dose (0.5 mg/kg, n=3 rats, gray bars) and low dose (0.0625 mg/kg, n=3 rats, white bars) at baseline, 1, 5 and 10 min after administration of LE. *P<0.05 vs. baseline in CTRL; $$P<0.001 vs. baseline in CVT-low; ^^P<0.001 vs. CTRL at 5 min. The values are Mean±SEM
In CVT groups, the baseline HR and EF were similar before and 5 min after different doses of CVT (e.g. at 0.5mg/kg HR (beats/min)=316.7±8.8 vs. 311.7±12 5-min after CVT; EF=74.7± 3.5% vs. 74.1±4.9% 5-min after CVT), indicating that CVT-4325 alone is not affecting the heart rate or the cardiac function at all different doses of CVT. However, CVT pre-treatment at higher doses of 0.5, 0.25 or 0.125mg/kg (CVT-high) prevented the lipid rescue of bupivacaine overdose, as there was no recovery of cardiac function within 10 min of LE therapy. Resuscitation with 20% LE was only able to rescue bupivacaine-induced cardiotoxicity at lowest dose of 0.0625 mg/kg CVT (CVT-low) as cardiac function improved gradually within 10 min (HR from 71±5 at 1 min increased to 181±11 min at 10 min; EF=50±1%; FS=26±0.6% at 5min and QRS from 33 ms at 1 min to 27 ms at 10 min).
The Effect of LE on Mitochondrial oxidative rate and membrane potential
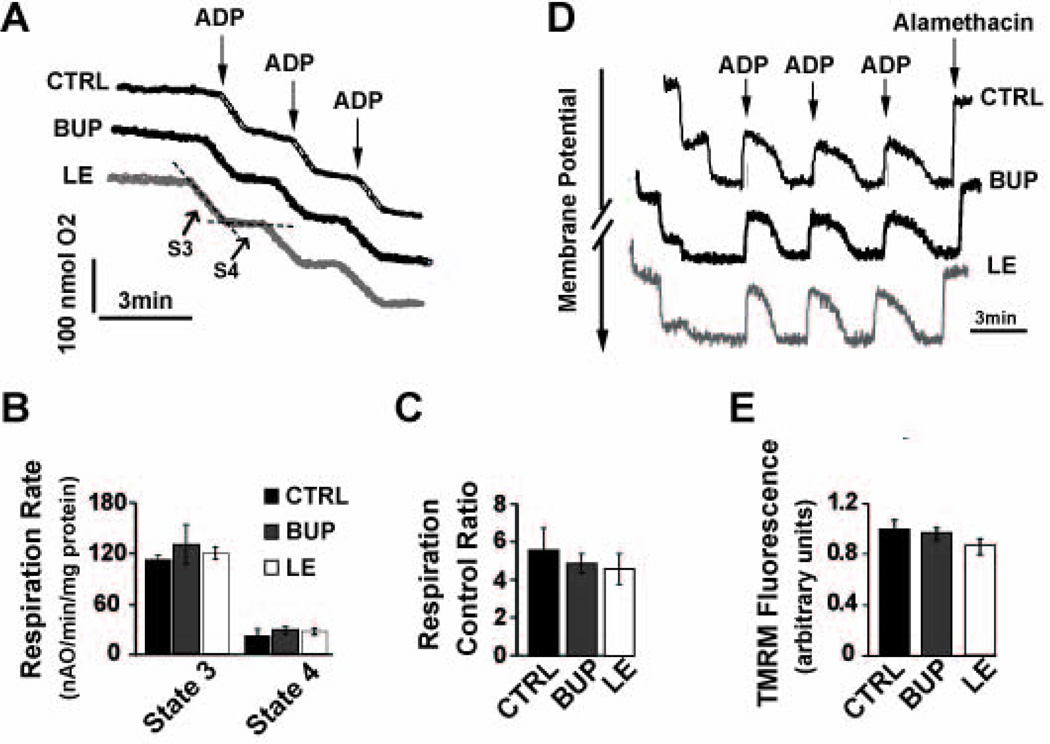
We compared the oxidative rates in cardiac mitochondria isolated from the hearts of CTRL, Bup and LE groups (See Figure 1B for experimental protocol). The visual inspection of oxygen electrode traces in isolated cardiac mitochondria from these 3 groups revealed similar slopes in state 3 (ADP-stimulated) and state 4 (resting state, ADP-limited, Fig. 3A). The slope of the state 3 and state 4 respiration in cardiac mitochondria isolated from CTRL rats which were only treated with a single dose of PBS were 112±4.8 and 23±6.5 nmol O/min/mg protein, respectively which resulted in a respiratory control ratio (RCR) value of 5.6±1.2 (Fig. 3B, C). The oxidative rates of state 3 and state 4 in Bup and LE group were not significantly different than CTRL (state 3: 133±24 in Bup vs. 121±6.8 nmol O/min/mg protein in LE, both P>0.05 vs. CTRL; state 4: 28±4.7 in Bup vs. 28±4.2 nmol O/min/mg protein in LE, both P>0.05 vs. CTRL, Fig. 3B). As a result, RCR values in Bup group (4.9±0.5), ILP group (4.6±0.8, Fig. 3C) and CTRL group were indistinguishable (P>0.05 vs. CTRL for both Bup and LE groups). LE also had no apparent effect on the mitochondrial membrane potential as the change in fluorescence intensity before and after addition of the uncoupler in LE group was not significantly different from Bup or CTRL group (0.85± 0.07 in LE vs. 0.96± 0.04 in Bup and 1±0.07 in CTRL, P>0.05 vs. CTRL for both Bup and LE groups, Fig. 3D,E).
Figure 3. Mitochondrial oxygen consumption or membrane potential is not affected by LE infusion.
A. Typical oxygen electrode traces showing the respiration in state 3 stimulated by 0.2 mM ADP (S3) and in resting state 4 (ADP-limited, S4) of complex-I in isolated mitochondria from CTRL, Bup, and LE group. B. Respiration rate of state 3 and state 4 and C. Respiratory control ratio (respiration rate of state 3/state 4) in CTRL (black bars, n=3 rats), Bup (gray bars, n=4 rats) and LE group (white bars (white bars, n=3 rats). P>0.05 vs. CTRL. D. Typical recording of the mitochondrial membrane potential in response to 200µM ADP (to stimulate state 3) and 2uM alamethacin (a mitochondrial uncoupler) in isolated mitochondria from the CTRL (black trace), bupivacaine overdose heart before (black trace) and after resuscitation with LE (gray trace). E. TMRM fluorescence (in arbitrary units) in CTRL group (black bar, n=3 rats), bupivacaine group before (gray bar, n=4 rats) and after resuscitation with LE (white bar, n=3 rats). P>0.05 vs. CTRL. The values are Mean±SEM.
The Effect of LE on Mitochondrial Permeability Transition Pore (mPTP) Opening
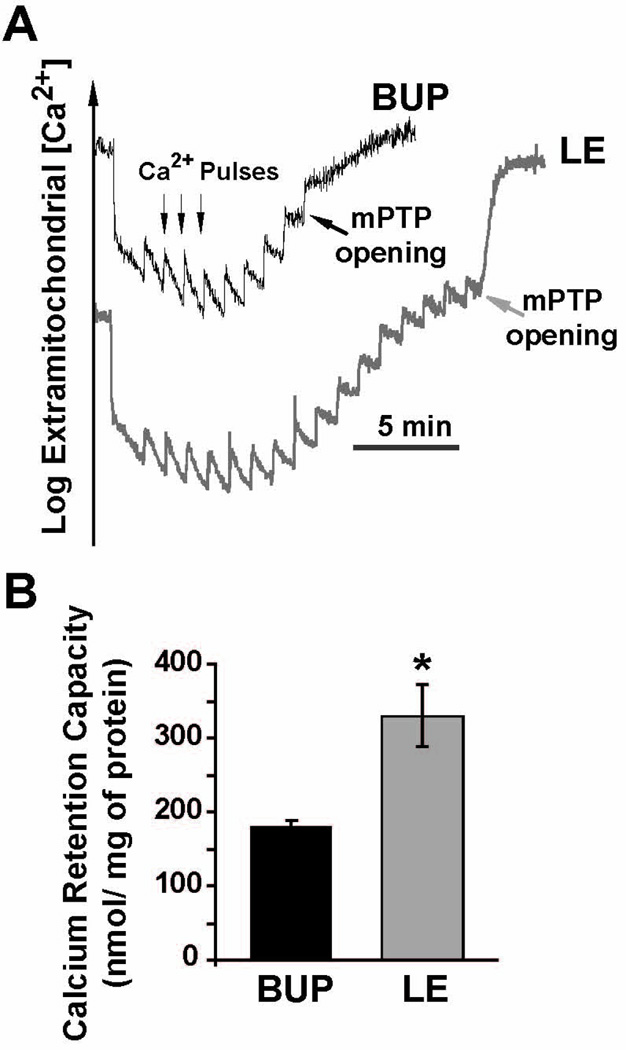
Next we compared the threshold for triggering of mPTP openings in response to calcium overload in isolated mitochondria from the hearts of Bup and LE groups (See Fig. 1B for experimental protocol). Fig. 4A shows a typical example of the time course of Ca2+ concentration in the mitochondrial external medium. In bupivacaine group, 9 pulses of 20 nmol Ca2+ were sufficient to trigger the opening of mPTP. Interestingly, the calcium load required for triggering mPTP opening significantly increased in mitochondria isolated from LE group as the number of calcium pulses (20 nmol) was increased to 17 pulses. The bar plot in Fig. 4B summarizes the calcium retention capacity (CRC); CRC was significantly higher in the LE group compared to bupivacaine group (330±42 in LE vs. 180±8.2 nmol /mg-mitochondrial protein in Bup, p<0.05).
Figure 4. Rescue of Bupivacaine Induced Cardiotoxicity by LE is associated with inhibition of the mitochondrial permeability transition pore opening.
A. Typical recording of the mitochondrial permeability transition pore (mPTP) opening in isolated mitochondria from the left ventricle in bupivacaine group (Bup, black trace) and in LE group (gray trace). B. Calcium retention capacity (CRC) in bupivacaine (Bup, black bar, n=4 rats) and LE (gray bar, n=4 rats). The values are Mean±SEM,*P<0.05 vs. Bup.
Discussion
It has been demonstrated previously that LE is very effective in resuscitation from cardiac arrest as a result of bupivacaine toxicity in rat and canine models as well as in patients (3–8). However the molecular mechanisms of this rescue action of LE are not fully understood. Here we show that fatty acid oxidation is required for successful rescue of bupivacaine induced cardiotoxicity by LE in rats. We also show that lipid infusion is associated with inhibition of mitochondrial permeability transition pore opening, without affecting mitochondrial respiration or membrane potential.
Fatty acid oxidation is required for the successful rescue of bupivacaine induced cardiotoxicity by LE
One of the dreaded complications of local anesthetics use in regional anesthesia is cardiovascular toxicity. Although systemic toxic reactions are not common, they can be life-threatening and resistant to treatment (23). Local anesthetics exert their toxic effects through a complex cellular mechanism and the ability of local anesthetics to interfere with multiple cellular functions may be the factor that makes overdose with these drugs potentially severe and refractory (23). Treatment of local anesthetic systemic toxicity includes airway management and circulatory support. Local anesthetic-induced cardiac arrest requires rapid restoration of coronary perfusion pressure to improve myocardial contractility and maintenance of cardiac output and oxygen delivery to tissues is critical for prevention and treatment of acidosis (24), but still the outcome is generally poor. In two case reports of patients surviving bupivacaine-induced cardiac arrest, circulation was supported by placing the patient on cardiopulmonary bypass until spontaneous circulation recurred (7). In 1997, investigations of Weinberg and colleagues led to the discovery that pretreatment with a lipid infusion increased the dose of bupivacaine required to induce asystole in rats. Further studies showed that early administration of lipid in bupivacaine-induced cardiotoxicity rescues the circulatory collapse (3–5).
In 2010, American Society of Regional Anesthesia Practice Advisory on local anesthetic systemic toxicity recommended that LE therapy is instrumental in facilitating resuscitation (24). Although more than a decade has elapsed since the findings of Weinberg et al., there are still several unanswered questions regarding LE therapy and its mechanism of action (24). Although “lipid sink theory” or “partition effect” was among one of the initial mechanisms proposed for the reversal of local anesthetic cardiotoxicity by LE (5;9;10), other mechanisms including a possible metabolic benefit has also been postulated (3;5). Here we present compelling data in support of a metabolic mechanism highlighting the view that fatty acid oxidation pathway is an important pathway in reversal of bupivacaine cardiotoxicity by LE. LE was only able to resuscitate cardiotoxicity resulted from bupivacaine overdose in rats pretreated with very low dose of CVT (0.0625 mg/kg). Lipid resuscitation failed if the rats were pre-treated with CVT at doses higher than 0.0625 mg/kg.
Although our data provides clear evidence in support of the metabolic theory, it does not exclude other suggested mechanisms postulated previously such as lipid sink theory (3). Since the time course of myocardial apoptosis seems too long to account for sudden fatal effects of bupivacaine toxicity, we speculate the rapid inhibitory action of bupivacaine on Na+ channel also play a role in inducing asystole. In this case, LE may also reverse acute effects of bupivacaine toxicity, by displacing bupivacaine from sodium channel to rescue the cardiac arrest.
The oxidative rate and membrane potential of mitochondria were similar in cardiac arrest before and after lipid resuscitation
Highly lipophilic local anesthetics also interfere with mitochondrial energy metabolism. These metabolic effects could in part explain some of the toxic effects of local anesthetics, such as bupivacaine-induced myocardial depression. Indeed, some studies suggest that bupivacaine-induced cardiotoxicity may be the result of the interference with mitochondrial energy transduction (25–29). In this study we show that lipid resuscitation from lipophilic drug overdose is not associated with alteration in mitochondrial oxidative rate or membrane potential. The basal and ADP-stimulated rates of respiration in the mitochondria isolated from the hearts resuscitated with LE were not significantly different from those of mitochondria prepared from the hearts of bupivacaine-overdose or from CTRL hearts perfused with PBS only (Fig. 3A–C). Since LE infusion had no effect on the mitochondrial respiration challenged by bupivacaine, the reversal of bupivacaine-induced cardiotoxicity by LE does not seem to act through stimulation of mitochondrial respiration.
Our finding is in contrast with the increased oxidative rates observed in skeletal muscle mitochondria following incubation with bupivacaine in-vitro with doses up to 1.5 mM (12). Whether the action of bupivacaine on the mitochondria isolated from skeletal muscle is different than the heart, or our in-vivo dose of 10mg/kg bupivacaine is not comparable with in-vitro dose used for skeletal muscle, remain to be seen in future studies.
Rescue of Bupivacaine Induced Cardiotoxicity by Lipid Emulsion is Associated with Inhibition of Mitochondrial Permeability Transition Pore Opening
mPTP is a large non selective conductance pore located in the inner membrane of mitochondria. The role of mPTP in the heart has been well studied in ischemia/reperfusion setting (See review (30)). mPTP has been shown to remain closed during ischemia, but opens during the reperfusion period (31;32). The opening of mPTP at reperfusion has been implicated in cell death(33;34). mPTP opening has also been shown to be a major underlying cause of mitochondrial depolarization induced by bupivacaine leading to hypercontracture and cell death in isolated flexor digitorum brevis fibers (12). Delaying the opening of the mPTP has been a potential target to reduce myocardial injury upon reperfusion. Here we show that the rescue of cardiac arrest by LE is associated with inhibition of the mPTP opening, as the mitochondrial calcium uptake required for the opening of the mPTP was significantly higher in hearts resuscitated by LE compared to the bupivacaine-overdose (Fig. 4). The lower Ca2+ threshold for triggering mPTP opening in bupivacaine group was not due to the lower quality of the mitochondrial preparation in this group, as the respiratory control ratio in both Bup and LE groups was greater than 4 (Fig. 3A–C)(35;36). Our results highlight the role of mPTP in reversal of cardiotoxicity by LE. We propose that LE enhances the homeostasis of cardiomyocytes to better regulate calcium overload and therefore increase the threshold for opening of the mPTP. Although LE increases the threshold of opening of mPTP, it has no effect on mitochondria respiration or membrane potential. This is often not the case in mitochondria isolated from the hearts that subjected to ischemia/reperfusion since the higher CRC is usually associated with higher oxidative phosphorylation and membrane potential (37).
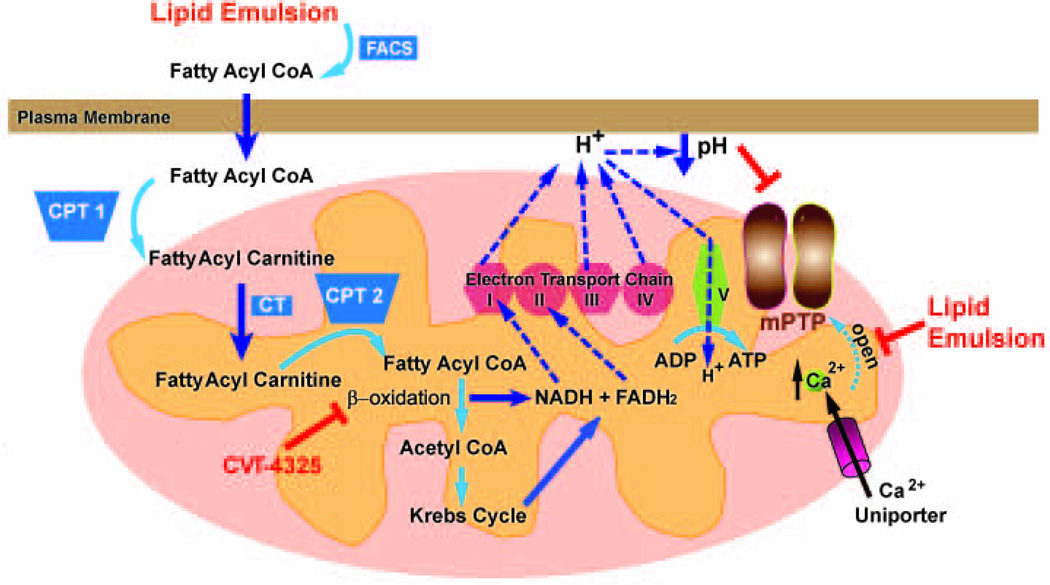
Fig. 5 summarizes our hypothetical scheme demonstrating fatty acid oxidation and calcium homeostasis are involved in rescue of bupivacaine induced cardiotoxicity by LE in rats.
Figure 5. Proposed mechanisms underlying LE-induced rescue of cardiac arrest as a result of bupivacaine-overdose.
Oxidation of LE contains long chain fatty acids occurs in the mitochondria. Fatty acids are transported into the mitochondria via carnitine shuttle, and go through β-oxidation to produce NADH and FADH2. NADH and FADH2 enter the electron transport chain (ETC). ETC couples electron transfer between an electron donor (NADH and FADH2) and an electron acceptor (such as O2) to the transfer of H+ ions (protons) across a membrane. The resulting electrochemical proton gradient is used to generate energy in the form of adenosine triphosphate (ATP) in complex V. The release of H+ from complex I, III and IV lead to lowering PH in outer mitochondrial membrane which lead to inhibition of mPTP opening. Inhibition of β-oxidation pathway by CVT abolishes the production of NADH, FADH2, as well as H+ ions, leading to generation of less ATP. The rise of Ca2+ ions in mitochondria through Ca2+ uniporter will trigger the opening of mPTP. LE enhances the homeostasis of cardiomyocytes to better regulate calcium overload and therefore delay the opening of mPTP. FACS; Fatty Acyl CoA Synthase, CPT1, Carnitine Acyl Transferase 1, CT; Carnitine Acylcarnitine Translocase, CPT2, Carnitine Acyl Transferase 2.
Limitations
In our study we show that the rescue of bupivacaine induced cardiotoxicity by LE is associated with inhibition of mitochondrial permeability transition pore opening. The higher threshold for triggering mPTP opening could result from a reduced tissue bupivacaine content eg. as might occur due to the 'lipid sink' effect. Alternately, increased mPTP activation could merely serve as an indicator of irreparable myocardial damage secondary to the cardiac arrest and resulting hypoperfusion with ischemia/reperfusion injury. Further experiments are required to demonstrate a causal relationship for the inhibition of mPTP and lipid resuscitation.
Here using a fatty acid oxidation inhibitor we present compelling data in support of a metabolic mechanism for the phenomenon of lipid resuscitation from bupivacaine drug overdose. There are also several case reports indicating that LE can reverse signs and symptoms of CNS toxicity(38;39). The mechanisms in which LE reverses bupivacaine-induced toxicity in the heart may be different than in the CNS. Obviously a metabolic effect can not contribute to the reversal of toxicity in an organ that doesn't require fatty acid oxidation. As discussed in some cases of CNS toxicity following bupivacaine-overdose(38;39), the CNS symptoms could be short lived and self limiting and is not quite clear whether the recovery from CNS toxicity is related to the lipid treatment or not. Our study has only focused on the cardiotoxicity reversal and at present we can not explain the reversal of CNS toxicity by LE. Additional experiments in the future could unravel the mechanisms underlying reversal of CNS toxicity by lipid emulsion.
Failure of the study to accurately mimic the in vivo pharmacokinetics of clinical bupivacaine toxicity and lipid infusion in human is another limitation. In case reports both the bupivacaine dose that caused cardiotoxicity as well as the lipid emulsion dose used for rescue (1.5 ml/kg vs. 5 ml/kg) are lower than the dose in our rat study (24). In the clinical situation, however, the lipid infusion is not started right away and there is a lag by the time that the infusion is available and started. We have to consider that in our study rats are already intubated and ventilated with 100% oxygen which is different than human cases, as the bupivacaine toxicity appears while patient is awake or mildly sedated and the airway is not controlled at the time that toxicity appears.
Conclusions
We show here that Fatty acid oxidation is required for resuscitation of bupivacaine overdose by LE. Furthermore, the rescue of bupivacaine induced cardiotoxicity by LE is associated with inhibition of mitochondrial permeability transition pore opening independent of oxidative phosphorylation and membrane potential. Further research is needed to identify the exact role of LE in rescue of bupivacaine-induced cardiotoxicity.
Acknowledgment
The authors would like to thank Dr. Jeffrey Gornbein, DrPH Biostatistics, Senior Statistician, from Department of Biomathematics at UCLA, Los Angeles, CA, USA, for the statistical assistance.
Funding: Mansoureh Eghbali is supported by National Institutes of Health grants HL089876 and HL089876S1 (Bethesda, Maryland, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have not disclosed any potential conflicts of interest
Disclosures
None
References
- 1.Hicks SD, Salcido DD, Logue ES, Suffoletto BP, Empey PE, Poloyac SM, et al. Lipid emulsion combined with epinephrine and vasopressin does not improve survival in a swine model of bupivacaine-induced cardiac arrest. Anesthesiology. 2009 Jul;111(1):138–146. doi: 10.1097/ALN.0b013e3181a4c6d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levsky ME, Miller MA. Cardiovascular collapse from low dose bupivacaine. Can J Clin Pharmacol. 2005;12(3):e240–e245. [PubMed] [Google Scholar]
- 3.Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998 Apr;88(4):1071–1075. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003 May;28(3):198–202. doi: 10.1053/rapm.2003.50041. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg GL, Ripper R, Murphy P, Edelman LB, Hoffman W, Strichartz G, et al. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med. 2006 Jul;31(4):296–303. doi: 10.1016/j.rapm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Foxall G, McCahon R, Lamb J, Hardman JG, Bedforth NM. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007 May;62(5):516–518. doi: 10.1111/j.1365-2044.2007.05065.x. [DOI] [PubMed] [Google Scholar]
- 7.McCutchen T, Gerancher JC. Early intralipid therapy may have prevented bupivacaine-associated cardiac arrest. Reg Anesth Pain Med. 2008 Mar;33(2):178–180. doi: 10.1016/j.rapm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Lin EP, Aronson LA. Successful resuscitation of bupivacaine-induced cardiotoxicity in a neonate. Paediatr Anaesth. 2010 Oct;20(10):955–957. doi: 10.1111/j.1460-9592.2010.03406.x. [DOI] [PubMed] [Google Scholar]
- 9.Lokajova J, Laine J, Puukilainen E, Ritala M, Holopainen JM, Wiedmer SK. Liposomes for entrapping local anesthetics: a liposome electrokinetic chromatographic study. Electrophoresis. 2010 May;31(9):1540–1549. doi: 10.1002/elps.200900562. [DOI] [PubMed] [Google Scholar]
- 10.Young AC, Velez LI, Kleinschmidt KC. Intravenous fat emulsion therapy for intentional sustained-release verapamil overdose. Resuscitation. 2009 May;80(5):591–593. doi: 10.1016/j.resuscitation.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Eledjam JJ, de La Coussaye JE, Brugada J, Bassoul B, Gagnol JP, Fabregat JR, et al. In vitro study on mechanisms of bupivacaine-induced depression of myocardial contractility. Anesth Analg. 1989 Dec;69(6):732–735. [PubMed] [Google Scholar]
- 12.Irwin W, Fontaine E, Agnolucci L, Penzo D, Betto R, Bortolotto S, et al. Bupivacaine myotoxicity is mediated by mitochondria. J Biol Chem. 2002 Apr 5;277(14):12221–12227. doi: 10.1074/jbc.M108938200. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Belardinelli L, Fraser H. A novel partial fatty acid oxidation inhibitor decreases myocardial oxygen consumption and improves cardiac efficiency in demand-induced ischemic heart. J Cardiovasc Pharmacol. 2008 Apr;51(4):372–379. doi: 10.1097/FJC.0b013e318166803b. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Fraser H, Lloyd SG, McVeigh JJ, Belardinelli L, Chatham JC. A comparison between ranolazine and CVT-4325, a novel inhibitor of fatty acid oxidation, on cardiac metabolism and left ventricular function in rat isolated perfused heart during ischemia and reperfusion. J Pharmacol Exp Ther. 2007 Apr;321(1):213–220. doi: 10.1124/jpet.106.115519. [DOI] [PubMed] [Google Scholar]
- 15.Imai M, Rastogi S, Sharma N, Chandler MP, Sharov VG, Blackburn B, et al. CVT-4325 inhibits myocardial fatty acid uptake and improves left ventricular systolic function without increasing myocardial oxygen consumption in dogs with chronic heart failure. Cardiovasc Drugs Ther. 2007 Feb;21(1):9–15. doi: 10.1007/s10557-006-0496-5. [DOI] [PubMed] [Google Scholar]
- 16.Ashrafian H, Frenneaux MP. Metabolic modulation in heart failure: the coming of age. Cardiovasc Drugs Ther. 2007 Feb;21(1):5–7. doi: 10.1007/s10557-007-6000-z. [DOI] [PubMed] [Google Scholar]
- 17.Elzein E, Ibrahim P, Koltun DO, Rehder K, Shenk KD, Marquart TA, et al. CVT-4325: a potent fatty acid oxidation inhibitor with favorable oral bioavailability. Bioorg Med Chem Lett. 2004 Dec 20;14(24):6017–6021. doi: 10.1016/j.bmcl.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg GL, Di GG, Ripper R, Kelly K, Massad M, Edelman L, et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008 May;108(5):907–913. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 19.Hiller DB, Gregorio GD, Ripper R, Kelly K, Massad M, Edelman L, et al. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009 Sep;111(3):498–505. doi: 10.1097/ALN.0b013e3181afde0a. [DOI] [PubMed] [Google Scholar]
- 20.Di GG, Schwartz D, Ripper R, Kelly K, Feinstein DL, Minshall RD, et al. Lipid emulsion is superior to vasopressin in a rodent model of resuscitation from toxin-induced cardiac arrest. Crit Care Med. 2009 Mar;37(3):993–999. doi: 10.1097/CCM.0b013e3181961a12. [DOI] [PubMed] [Google Scholar]
- 21.Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res. 2008 Oct 10;103(8):873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaduto RC, Jr., Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999 Jan;76(1 Pt 1):469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renehan EM, Enneking FK, Varshney M, Partch R, Dennis DM, Morey TE. Scavenging nanoparticles: an emerging treatment for local anesthetic toxicity. Reg Anesth Pain Med. 2005 Jul;30(4):380–384. doi: 10.1016/j.rapm.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neal JM, Bernards CM, Butterworth JF, Di GG, Drasner K, Hejtmanek MR, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010 Mar;35(2):152–161. doi: 10.1097/AAP.0b013e3181d22fcd. [DOI] [PubMed] [Google Scholar]
- 25.Sztark F, Nouette-Gaulain K, Malgat M, Dabadie P, Mazat JP. Absence of stereospecific effects of bupivacaine isomers on heart mitochondrial bioenergetics. Anesthesiology. 2000 Aug;93(2):456–462. doi: 10.1097/00000542-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Sztark F, Malgat M, Dabadie P, Mazat JP. Comparison of the effects of bupivacaine and ropivacaine on heart cell mitochondrial bioenergetics. Anesthesiology. 1998 May;88(5):1340–1349. doi: 10.1097/00000542-199805000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Sztark F, Ouhabi R, Dabadie P, Mazat JP. Effects of the local anesthetic bupivacaine on mitochondrial energy metabolism: change from uncoupling to decoupling depending on the respiration state. Biochem Mol Biol Int. 1997 Dec;43(5):997–1003. doi: 10.1080/15216549700204811. [DOI] [PubMed] [Google Scholar]
- 28.Nouette-Gaulain K, Forestier F, Malgat M, Marthan R, Mazat JP, Sztark F. Effects of bupivacaine on mitochondrial energy metabolism in heart of rats following exposure to chronic hypoxia. Anesthesiology. 2002 Dec;97(6):1507–1511. doi: 10.1097/00000542-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Grishko V, Xu M, Wilson G, Pearsall AW. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010 Mar;92(3):609–618. doi: 10.2106/JBJS.H.01847. [DOI] [PubMed] [Google Scholar]
- 30.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004 Feb 15;61(3):372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 31.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998 Aug 10;1366(1–2):79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 32.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, et al. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002 Oct;4(5):769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 33.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999 Jul 15;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 34.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995 Jul 17;1241(2):139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 35.Haubenstricker ME, Holodnick SE, Mancy KH, Brabec MJ. Rapid toxicity testing based on mitochondrial respiratory activity. Bull Environ Contam Toxicol. 1990 May;44(5):675–680. doi: 10.1007/BF01701787. [DOI] [PubMed] [Google Scholar]
- 36.Kemp R, Castro-e-Silva, Santos JS, Sankarankutty AK, Correa RB, Baldo CF, et al. Evaluation of the mitochondrial respiration of cardiac myocytes in rats submitted to mechanical bile duct obstruction. Acta Cir Bras. 2008;23(Suppl 1):66–71. doi: 10.1590/s0102-86502008000700012. [DOI] [PubMed] [Google Scholar]
- 37.Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol. 2009 Jun;46(6):902–909. doi: 10.1016/j.yjmcc.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Spence AG. Lipid reversal of central nervous system symptoms of bupivacaine toxicity. Anesthesiology. 2007 Sep;107(3):516–517. doi: 10.1097/01.anes.0000278864.75082.72. [DOI] [PubMed] [Google Scholar]
- 39.Heavner JE, Arthur J, Zou J, McDaniel K, Tyman-Szram B, Rosenberg PH. Comparison of propofol with thiopentone for treatment of bupivacaine-induced seizures in rats. Br J Anaesth. 1993 Nov;71(5):715–719. doi: 10.1093/bja/71.5.715. [DOI] [PubMed] [Google Scholar]